Abstract
Reduced skeletal muscle mitochondrial density is proposed to lead to impaired muscle lipid oxidation and increased lipid accumulation in sedentary individuals. We assessed exercise-stimulated lipid oxidation by imposing a prolonged moderate-intensity exercise in men with variable skeletal muscle mitochondrial density as measured by citrate synthase (CS) activity. After a 2-day isoenergetic high-fat diet, lipid oxidation was measured before and during exercise (650 kcal at 50% VO2 max) in 20 healthy men with either high (HI-CS = 24 ± 1; mean ± s.e.) or low (LO-CS = 17 ± 1 nmol/min/mg protein) muscle CS activity. Vastus lateralis muscle biopsies were obtained before and immediately after exercise. Respiratory exchange data and blood samples were collected at rest and throughout the exercise. HI-CS subjects had higher VO2 max (50 ± 1 vs. 44 ± 2 ml/kg fat free mass/min; P = 0.01), lower fasting respiratory quotient (RQ) (0.81 ± 0.01 vs. 0.85 ± 0.01; P = 0.04) and higher ex vivo muscle palmitate oxidation (866 ± 168 vs. 482 ± 78 nmol/h/mg muscle; P = 0.05) compared to LO-CS individuals. However, whole-body exercise-stimulated lipid oxidation (20 ± 2 g vs. 19 ± 1 g; P = 0.65) and plasma glucose, lactate, insulin, and catecholamine responses were similar between the two groups. In conclusion, in response to the same energy demand during a moderate prolonged exercise bout, reliance on lipid oxidation was similar in individuals with high and low skeletal muscle mitochondrial density. This data suggests that decreased muscle mitochondrial density may not necessarily impair reliance on lipid oxidation over the course of the day since it was normal under a high-lipid oxidative demand condition. Twenty-four-hour lipid oxidation and its relationship with mitochondrial density need to be assessed.
INTRODUCTION
Accumulation of intramyocellular tryacylglyceride, diacylglyceride, and ceramide is inversely related to insulin resistance (IR) in sedentary humans (1). In fact, such lipid species can impair insulin signaling activity (2,3). Intramyocellular lipid accumulation has been proposed to be the result of impaired muscle mitochondrial lipid oxidative metabolism (4), although lipid overload can also impair mitochondrial metabolism (5,6). Indeed, IR or type 2 diabetes mellitus are often accompanied by reduced skeletal muscle mitochondrial enzyme activity (e.g., citrate synthase (CS) and cytochrome c oxidase), mitochondrial density and protein content, mitochondrial respiration, Krebs cycle flux, and ATP synthesis rate as reviewed by Turner & Heilbronn (7). In addition, IR is characterized by impaired fasting lipid oxidation and a blunted ability to increase glucose oxidation during glucose/insulin stimulation called “metabolic inflexibility” (8). These findings are considered a feature of muscle mitochondrial deficiency and a key mechanism involved in increased intramyocellular lipid accumulation and IR (4).
There are, however, several factors which challenge this hypothesis. First, skeletal muscle energy needs under resting conditions are well below the capacity of muscle to oxidize substrate even in sedentary individuals. Thus, the often reported decrease in mitochondrial density in insulin-resistant subjects should have no effect on the ability of resting muscle to oxidize fat (9). Second, “metabolic inflexibility” to glucose/insulin stimulation described in type 2 diabetic obese subjects is mainly due to impaired glucose disposal rate as the result of defective glucose transport into the cells (10) rather than a mitochondrial defect (8). Third, animal models exhibiting mitochondrial deficiency show increased insulin sensitivity vs. wild-type animals (11–13) and even enhanced lipid oxidation (12). Fourth, it has been argued that most of the differences in muscle mitochondrial markers are due to differences in physical fitness (14). Finally, the impact of muscle mitochondrial density on lipid oxidation under a condition of high energy and lipid oxidative demands has not been conducted. However, some studies assessed exercise-induced lipid oxidation between insulin-resistant and insulin-sensitive subjects (15–17). Unfortunately, from those studies it is difficult to draw conclusions about the role of muscle mitochondrial density on lipid oxidation because muscle mitochondrial density was not determined as well as plasma/muscle substrate concentrations. Furthermore, energy expenditure during exercise was not similar between groups.
Our main goal was therefore to compare the reliance on lipid oxidation during an exercise bout of similar intensity and energy demand in subjects with variable and contrasting skeletal muscle mitochondrial density (by CS activity). We hypothesized that individuals with lower muscle mitochondrial density would have impaired ability to switch lipid oxidation on in response to increased lipid oxidative demand compared with individuals having greater muscle mitochondrial density.
METHODS AND PROCEDURES
Subjects
Twenty healthy, normoglycemic, nonsmoking men between 18–35 years with a BMI 21–26 kg/m2 were recruited. None of the participants were engaged in sports at a competitive level and none had physically demanding employment. After completion of the entire protocol, volunteers were retrospectively divided into two groups based on their skeletal muscle mitochondrial density estimated by CS activity. The protocol was approved by the institutional review board of the Pennington Biomedical Research Center (PBRC). All subjects provided written informed consent prior to study participation.
Experimental design
After screening, body composition and maximal aerobic capacity (VO2max) were measured. Energy requirements were calculated according to published equations (18). One week after screening, participants presented at PBRC on 2 successive days to receive an isoenergetic high-fat diet (67% fat, 25% carbohydrate). Breakfasts and dinners were consumed at PBRC, whereas lunch and a snack were packaged and consumed outside the Center. Volunteers were admitted to the in-patient unit the evening before the testing day. After a 10-h overnight fast, resting metabolic rate was measured for 30 min, a blood sample was drawn (for glucose, lactate, free-fatty acids (FFA), insulin, and catecholamines) and a vastus lateralis muscle biopsy was obtained. Participants then exercised on a cycle ergometer at 50% VO2max until reaching a total energy expenditure of 650 kcal. Gas exchange was measured and a blood sample was drawn after completion of 20, 40, 60, 80, and 100% of the exercise period. Another muscle biopsy was obtained immediately after exercise.
Body composition
Percent body fat was measured on a Hologic Dual Energy X-ray Absorptiometer (DXA; QDR 4500; Hologic, Waltham, MA) and fat-free mass and fat mass calculated from weight obtained on a scale.
Maximal aerobic capacity (VO2 max)
Maximal aerobic capacity was measured during an incremental protocol on a cycle ergometer (Lode Excalibur, Gronig, Netherlands). The test contained two phases: a linearization and a maximal phase. During the linearization phase, the workload started at 35 W and increased by 35 W every 3 min until 140 W. After the initial linearization phase, the work rate was increased by 35 W each minute until volitional fatigue (maximal phase). Gas exchange was measured during the entire testing period with a metabolic cart (TruOne 2400; ParvoMedics, Sandy, UT). The data from the linearization phase was used to calculate the workload necessary to elicit 50% of VO2max.
Exercise protocol
After the baseline skeletal muscle biopsy, subjects completed a 2-min warm-up by cycling at 0 W. After that, a workload equivalent to 50% of VO2max was imposed. Gas exchange measurements were simultaneously initiated. Steady-state oxygen consumption between 5 and 8 min of exercise was compared with the expected 50% VO2max. Workload was immediately adjusted to obtain the expected oxygen consumption necessary for the entire duration of exercise to reach the 650 kcal. Gas exchange was measured for 3–5 min before ending 20, 40, 60, 80, and 100% of the exercise bout, equivalent to 130, 260, 390, 520, and 650 kcal, respectively. After each fraction of exercise heart rate and rate of perceived exertion (RPE) were measured (19).
Muscle biopsy
Vastus lateralis muscle biopsies (350 mg) were obtained before and immediately after exercise using a 5-mm Bergstrom needle. Two incisions (3–4 cm apart) were made before exercise and the lower and upper incisions were used to take the muscle biopsy before and after exercise, respectively. The skin, adipose tissue and skeletal muscle fascia were anesthetized using 5 ml of a 1:1 mixture of 0.5% bupivicaine/2% lidocaine.
Indirect calorimetry
Gas exchange measurements under resting conditions were performed as described previously (20) whereas measurements during exercise were obtained using the TruOne 2400 metabolic cart. Steady-state VO2 and VCO2 values during the 5 min of gas collection were averaged and used for analysis. Standard equations were used to determine substrate oxidation (21).
Blood analysis
Glucose, FFA, and lactate were analyzed using Beckman Coulter DXC 600 commercial kits (Beckman Coulter, Brea, CA). Insulin was measured using a Siemens 200 (Siemens, Los Angeles, CA). Catecholamines were measured via Bio-Rad HPLC with electrochemical detection. Homeostasis model assessment of insulin resistance was calculated as (glucose (mg/dl) × 0.05551 × insulin (mIU/l))/22.5.
Skeletal muscle mitochondrial density
Maximal CS activity
About 80 mg skeletal muscle was diluted 20-fold in extraction buffer (0.1 mol/l KH2PO4/Na2PHO4, 2 mmol/l EDTA (pH = 7.2)) and then homogenized (Glas Col, Terre Haute, IN). Activity was measured at 37 °C in 0.1 mol/l Tris-HCl (pH 8.3) assay buffer containing 0.12 mmol/l 5,59-dithio-bis 2-nitrobenzoic acid and 0.6 mmol/l oxaloacetate. After an initial 2-min absorbance reading at 412 nm, the reaction was initiated by adding 3 mmol/l acetyl-CoA, and the change in absorbance was measured every 10 s for 7 min. Values were adjusted for total protein (mg/ml).
Real-time PCR for mitochondrial DNA
DNA was extracted from frozen muscle tissue (DNeasy Blood and Tissue Kit; Qiagen, Valencia, CA). Relative amounts of mitochondrial DNA (mtDNA) and nuclear DNA were determined by qRT-PCR as described previously (22,23).
Muscle mitochondrial complexes
Skeletal muscle was homogenized by Kontes Duall tissue grinders in RIPA buffer with protease inhibitor and phosphatase inhibitor cocktails (Sigma, St Louis, MO). Twenty μg of protein of each sample was run on a 12.5% Criterion Tris-HCl gel (Bio-Rad, Hercules, CA) and transferred to a PVDF membrane (Millipore, Billerica, MA). OXPHOS (1:208, MS601; Mito-Sciences, Eugene, OR), a primary antibody cocktail was used for complexes I-V expression at the same time, and GAPDH (1:1,000, ab9484; Abcam, Cambridge, MA) was used as loading control. Membranes were incubated with primary antibodies overnight at 4 °C, and then probed with goat anti-mouse IgG Alexa Fluor 680 (1:10,000; Invitrogen, Carlsbad, CA). Bands were visualized and densitometry value was quantified by using an Odyssey 9120 Infrared Imaging System (LI-COR, Lincoln, NE).
Skeletal muscle palmitate oxidation
Muscle tissue (~80 mg) was minced and homogenized in a modified sucrose-EDTA medium (250 mmol/l sucrose, 1 mmol/l EDTA, 1 mol/l Tris·HCl and 2 mmol/l ATP, pH 7.4). Palmitate oxidation rate was determined by measuring production of 14C-labeled acid-soluble metabolites and 14CO , by use of a custom made 24-well microtiter plate (24). Reactions were initiated by adding 310 μl of the incubation buffer (pH 7.4) to 80 μl of whole homogenate, yielding final concentrations of 0.17 μmol/l total palmitate and 0.8 μCi/ml [1-14C]-palmitate (Perkin Elmer, Waltham, MA), 102 mmol/l sucrose, 80 mmol/l potassium chloride, 10 mmol/l potassium phosphate, 8 mmol/l Tris·HCl, 2 mmol/l ATP, 1 mmol/l magnesium chloride hexahydrate, 1 mmol/l L-carnitine, 1 mmol/l dithiothreitol, 0.2 mmol/l EDTA, 0.1 mmol/l malate, 0.1 mmol/l NAD+, 0.05 mmol/l CoA, and 0.5% fatty acid-free BSA. After incubation for 120 min at 37 °C, reactions were terminated by adding 40 μl of 70% perchloric acid, and the CO2 produced during the incubation was trapped in 200 μl of 1 N NaOH added to adjacent wells. The acidified medium was stored at 4 °C overnight, and then acid-soluble metabolites assayed in supernatants. Radioactivity of 14CO and acid-soluble metabolites were determined by liquid scintillation counting and normalized for muscle wet weight.
Skeletal muscle glycogen
Muscle tissue was homogenized in PBM buffer (20 mmol/l KH2PO4, 10 μmol/l CaCl2, 1 mmol/l MgCl2, pH 6.1) using a hand-held homogenizer (Kontes) at a ratio of 1 mg of tissue to 10 μl of buffer. Cells were lysed by freeze-thaw followed by sonication. Samples were then boiled for 20 min in 30% KOH saturated with anhydrous Na2SO4. Glycogen was precipitated with 95% ethanol, dissolved in double-distilled H2O and incubated at 100 °C for 20 min with 0.2% anthrone in H2SO4. Glycogen concentration was determined spectrophotometrically at 620 nm relative to an oyster glycogen standard curve, and normalized to protein content.
Skeletal muscle fiber composition and intramyocellular lipid content
Skeletal muscle fiber type and IMCL content were determined via immunohistochemistry (25). Muscle tissue was mounted in a mixture of optimal cutting temperature compound and tragacanth gum powder and frozen in isopentane cooled over liquid nitrogen. Twelve sections were obtained and processed for immunohistochemical staining. Muscle sections were incubated overnight at 4 °C in blocking buffer (donkey serum) for 2 h followed by primary antibodies specific for slow muscle myosin (MAB1628, Chemicon) and laminin (AB2500, Abcam). After washing with phosphate-buffered saline, sections were incubated in the secondary antibodies (Alexa fluor 680 donkey anti-mouse and Alexa fluor 350 donkey anti-rat; Invitrogen, Carlsbad, CA). Bodipy green (1:100) was applied for 20 min. The sections were washed and mounted in citifluor mounting media (TedPella, Redding, CA) and a coverglass was applied. Type I fibers were counted using a confocal microscope (Leica SP5). IMCL was determined by measuring the intensity of bodipy staining using Sigma Scan Pro software version 5.0.0 (SPSS, Chicago, IL).
Statistical analysis
Analyses were performed using SAS software version 9.1.3 (SAS Institute, Cary, NC). Differences between groups were analyzed using 2-tailed t-test. Covariance analysis was used to assess for interactions with group on dependent variables. Association between variables was assessed by Pearson test. Multivariate regression analysis was used to identify determinants of exercise-induced lipid oxidation. Data are expressed as means ± s.e. P < 0.05 was considered statistically significant.
RESULTS
Skeletal muscle mitochondrial density
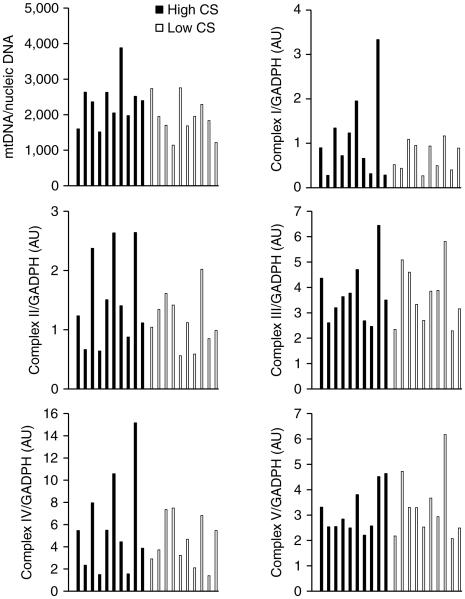
We first evaluated to what extent skeletal muscle mitochondrial density surrogates related to each other. Skeletal muscle CS activity and mitochondrial DNA content or any of the other surrogates of mitochondrial density (Complexes I to V) were weakly or not associated (r < 0.29). To decide which marker would allow us to better discriminate between individuals with high and low muscle mitochondrial density, we estimated that any acceptable surrogate should discriminate between individuals with high and low VO2max, ex vivo muscle palmitate oxidation rate and fiber composition. We tested this prediction by dividing the group (n = 20) into two groups on the basis of the median value for each surrogate, and then look at differences in VO2max, ex vivo muscle palmitate oxidation rate and fiber composition. Muscle CS activity was the only mitochondrial marker able to discriminate for these physiological variables (Figure 1), whereas mitochondrial DNA content or mitochondrial complexes (I to V) did not (data not shown). We therefore choose muscle CS activity as our biomarker of skeletal muscle mitochondrial density. The distribution of other mitochondrial measures according to high- and low-CS group is shown in Figure 2.
Figure 1.
Muscle maximal citrate synthase activity, (a) maximal aerobic capacity (per kg fat-free mass (FFM)), (b) in vitro skeletal muscle palmitate oxidation rate (c) and type I skeletal muscle fiber content (d) in subjects with high and low skeletal muscle citrate synthase activity (Means ± s.e.). Maximal aerobic capacity per kg of body weight in HI-CS vs. LO-CS were: 42 ± 1 vs. 37 ± 2 ml O /kg BW/min (P = 0.01). HI-CS, high skeletal muscle citrate synthase activity; LO-CS, low skeletal muscle citrate synthase activity.
Figure 2.
Distribution of skeletal muscle mitochondrial DNA and mitochondrial protein complexes I to V in individuals with high and low skeletal muscle citrate synthase (CS) activity.
Subjects’ characteristics
Subjects with a muscle CS activity above or below the median value (21 nmol/min/mg protein (range: 13–29)) were assigned to high (HI-CS) or low (LO-CS) CS groups (Figure 1 and Table 1). Individuals with HI-CS vs. LO-CS activity showed similar characteristics (Table 1) except for the already mentioned differences in VO2max and ex vivo muscle palmitate oxidation (Figure 1).
Table 1.
Characteristics of study subjects (Means ± s.e.)
| HI-CS | LO-CS | |
|---|---|---|
| n | 10 | 10 |
| Age (years) | 25.1 ± 1.5 | 22.9 ± 1.3 |
| Body weight (kg) | 76.4 ± 1.4 | 77.0 ± 2.6 |
| BMI (kg/m2) | 24.0 ± 0.5 | 23.2 ± 0.6 |
| Body fat (%) | 16.0 ± 1.1 | 17.2 ± 1.0 |
| Fat-free mass (kg) | 64.2 ± 1.3 | 63.6 ± 1.7 |
| Fasting glucose (mmol/l) | 4.9 ± 0.1 | 4.9 ± 0.1 |
| Fasting insulin (pmol/l) | 21 ± 4 | 22 ± 2 |
| HOMAIR | 0.77 ± 0.14 | 0.78 ± 0.10 |
| Muscle CS activity (nmol/min/mg protein) |
24.2 ± 0.8 | 17.0 ± 0.7a |
| mtDNA/nDNA | 2,351 ± 211 | 1,926 ± 174 |
| Compiex I (AU) | 1.1 ± 0.3 | 0.7 ± 0.1 |
| Compiex II (AU) | 1.5 ± 0.2 | 1.2 ± 0.1 |
| Compiex III (AU) | 3.7 ± 0.4 | 3.7 ± 0.4 |
| Compiex IV (AU) | 5.8 ± 1.4 | 4.5 ± 0.7 |
| Compiex V (AU) | 3.1 ± 0.3 | 3.3 ± 0.4 |
By design, muscle CS activity is different. HI-CS, high skeletal muscle citrate synthase activity; HOMAIR, homeostasis model assessment of insulin resistance; LO-CS, low skeletal muscle citrate synthase activity; mtDNA, mitochondrial DNA.
Whole-body energy metabolism
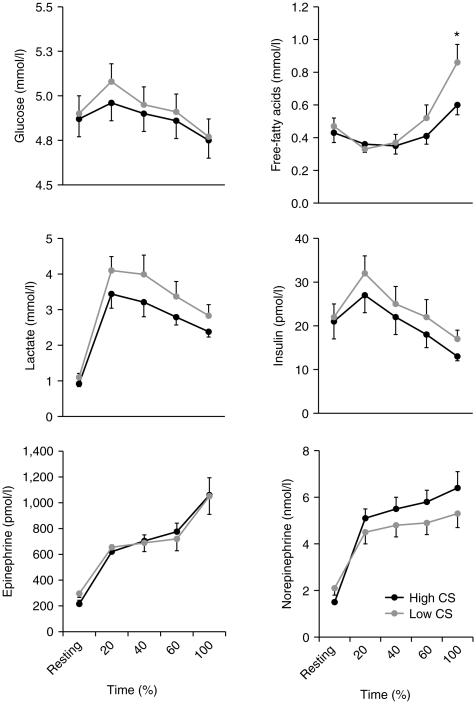
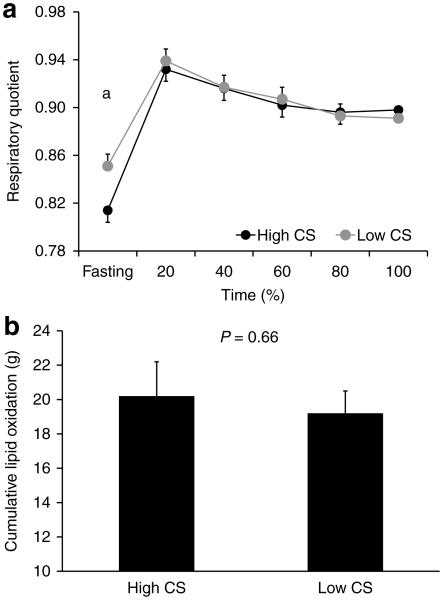
After an overnight fast, participants from both groups had similar plasma metabolites and hormones concentrations (i.e., glucose, lactate, FFA, insulin and catecholamines; Figure 3) and similar skeletal muscle glycogen content (0.39 ± 0.04 vs. 0.34 ± 0.02 μg per μg protein; P = 0.32; HI-CS and LO-CS, respectively) and intramyocelullar lipid content (29.7 ± 9.5 vs. 25.8 ± 8.3 AU; P = 0.76 Supplementary Figure S1). However, individuals with HI-CS had lower resting respiratory quotient (RQ) than individuals with LO-CS (Figure 4) resulting in greater lipid oxidation (83 ± 7 vs. 61 ± 3 mg/min; P = 0.01).
Figure 3.
Plasma glucose, lactate, free-fatty acids, insulin, epinephrine, and norepinephrine concentrations before and during exercise (Means ± s.e.) in subjects with high (black line) or low (gray line) skeletal muscle citrate synthase activity.
Figure 4.
Whole-body respiratory quotient (a) and cumulative lipid oxidation (b) before and during exercise (Means ± s.e.) in subjects with high or low skeletal muscle citrate synthase activity. aP < 0.05.
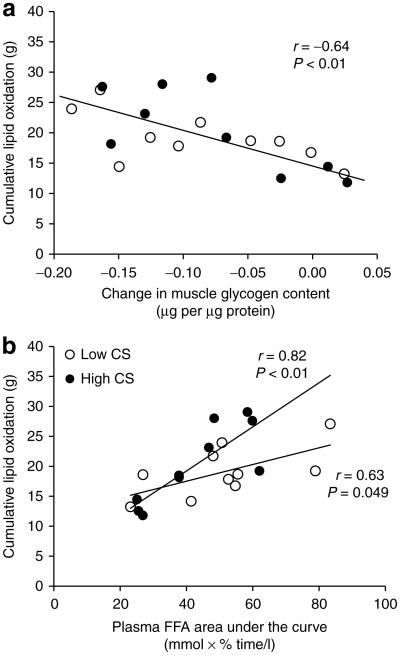
Such decreased resting lipid oxidation observed in individuals with low muscle mitochondrial density has often been interpreted as a feature of poor capacity to rely on lipid as an energy source. To further explore this hypothesis, we imposed a condition of increased lipid oxidation demand by exercise with similar intensity and total volume (50% VO2max and 650 kcal; Table 1). Because we set similar intensity and total energy expenditure across individuals, the time to finish the exercise tended to be shorter in HI-CS vs. LO-CS subjects (Table 2). We observed similar plasma glucose, lactate, insulin and catecholamine responses during exercise between groups (Figure 3), although plasma FFA concentration at the end of exercise was higher in the LO-CS vs. HI-CS group. Intramyocellular lipid content was similar between groups (P = 0.83) and was not altered in response to exercise (P = 0.41). In contrast, muscle glycogen content was decreased after exercise (P = 0.0002) but this reduction was similar in both group (HI-CS: −0.08 ± 0.02 and LO-CS: −0.09 ± 0.02 μg per μg protein; P = 0.78). We then evaluated the impact of muscle mitochondrial density on lipid oxidation, our main study goal. We observed that RQ and cumulative lipid oxidation during exercise were similar between groups (Figure 4). While exercise-induced lipid oxidation related to the change in muscle glycogen content (Figure 5a) and plasma FFA area under the curve (Figure 5b), the latter association appeared to be stronger in subjects with high (vs. low) muscle CS activity, with a significant interaction (P = 0.04; Figure 5b). By stepwise regression model, approximately half of the variance in cumulative lipid oxidation during exercise was explained by the reduction in muscle glycogen content (R2 = 0.41; P = 0.003) and plasma FFA area under the curve (R2 = 0.10; P = 0.09).
Table 2.
Work load, energy expenditure, intensity, and duration of the exercise bout (Means ± s.e.)
| HI-CS | LO-CS | |
|---|---|---|
| Energy expenditure (kcal) | 651 ± 1 | 647 ± 1* |
| Duration (min) | 77 ± 4 | 86 ± 4** |
| Heart rate, end of exercise (bpm) | 147 ± 3 | 149 ± 5 |
| RPE, end of exercise | 13.6 ± 0.5 | 13.3 ± 0.5 |
| Intensity at 20% exercise duration (%VO2max) |
52 ± 1 | 53 ± 1 |
| Intensity at 100% exercise duration (%VO2max) |
57 ± 1 | 59 ± 2 |
| Work load (W) | 98 ± 7 | 82 ± 5** |
P = 0.02;
P = 0.07.
HI-CS, high skeletal muscle citrate synthase activity; LO-CS, low skeletal muscle citrate synthase activity; RPE, rate of perceived exertion.
Figure 5.
Association between cumulative lipid oxidation and exercise-induced muscle glycogen change (a) and plasma free-fatty acid (FFA) area under the curve (b). HI-CS, high skeletal muscle citrate synthase activity; LO-CS, low skeletal muscle citrate synthase activity.
DISCUSSION
In the present study, we estimated skeletal muscle mitochondrial density by maximal CS activity, a widely accepted marker of mitochondrial density (26,27). As expected, we observed that volunteers with increased muscle CS activity had higher VO2max, higher ex vivo muscle palmitate oxidation, and a tendency towards increased type I muscle fiber content. Moreover, we observed that subjects with lower muscle CS activity had reduced resting lipid oxidation. This finding is consistent with observations of decreased muscle mitochondrial activity and increased fasting RQ in insulin-resistant individuals (28,29). Additionally, both VO2max and type I fiber content have been associated with increased expression of several mitochondrial electron transport chain genes (30). This evidence lends support to our selection of CS as a surrogate of muscle mitochondrial density. In contrast, mitochondrial DNA and mitochondrial protein complexes content did not discriminate between individuals with high or low VO2max, ex vivo muscle palmitate oxidation or type I muscle fiber content. The lack of association between mitochondrial density surrogates has been previously reported. For instance, Morino et al. (31) found reduced muscle mitochondrial density in insulin-resistant vs. control subjects when assessed by electron microscopy, but not by mtDNA.
The hypothesis that reduced muscle mitochondrial density has a critical role on impaired lipid oxidation, lipid accumulation and IR is mainly based on studies reporting lower fasting lipid oxidation in the resting state in individuals with obesity-associated IR (28), family history of type 2 diabetes (32) or type 2 diabetes (29). Consistently, obese subjects after weight loss and physical training showed enhanced fasting resting lipid oxidation and improved insulin sensitivity (33). However, several arguments challenge this hypothesis:
The metabolic challenge imposed on muscle mitochondria under resting conditions is minimal. Then, it is unlikely that decreased mitochondrial density can be severe enough to impair resting lipid oxidation (9).
Impaired muscle mitochondrial density may not necessarily lead to impaired lipid oxidation and IR. In fact, mice having impaired muscle mitochondrial activity showed enhanced lipid oxidation (12) and even higher insulin sensitivity vs. wild-type animals (11–13).
In most studies, resting lipid oxidation has been assessed by whole-body indirect calorimetry. This methodology does not necessarily reflect skeletal muscle lipid oxidation since skeletal muscle accounts at most for one-quarter of whole-body oxygen consumption (34). Resting lipid oxidation measured for a short period of time does not represent 24-h lipid oxidation, which in combination with daily lipid intake determine for lipid balance. In a previous study in our laboratory, 24-h lipid oxidation was unrelated to muscle mitochondrial density in humans (22).
Our results somewhat challenge the concept that individuals with low muscle mitochondrial density have an impaired ability to up-regulate muscle lipid oxidation. Indeed, we here clearly demonstrate that during a moderate-intensity exercise, subjects with high and low mitochondrial density have similar lipid oxidation rates. In addition, our findings are in line with other studies in which total lipid oxidation was assessed during exercise in type 2 diabetic and nondiabetic subjects (15,17). These studies reported similar reliance on lipid oxidation during exercise in diabetic and nondiabetic individuals. Even though mitochondrial density was not measured in these two studies, it is reasonable to speculate that these groups likely differed in their muscle mitochondrial density/activity as often reported (35–39). However, differences in plasma glucose concentration during exercise and the lack of measures of muscle energy substrate content (glycogen and triglycerides) may have influenced the results. In addition, VO2max differed by at least 10% between groups, which will lead to differences in energy expenditure and lipid oxidation when relative intensity and exercise duration are kept fixed among individuals. Furthermore, a potential impairment in lipid oxidation capacity would lead to a compensatory increase in muscle anaerobic glycolytic flux, and therefore plasma lactate concentration. While Mensink et al. did not measure plasma lactate concentration during exercise (17), Blaak et al. (15) did not observe any difference in plasma lactate concentration between groups. Together, these observations do not support the widely accepted notion that reliance on lipid oxidation is impaired in insulin-resistant and/or type 2 diabetic individuals.
The observation that whole-body lipid oxidation at rest was lower in LO-CS vs. HI-CS subjects is in line with previous studies (28,29). As discussed elsewhere (40), RQ is mostly affected by energy balance and diet composition. In the current study, we provided a standardized diet to our participants for 2 days prior to metabolic testing and the participants were instructed to maintain their usual physical activity pattern. However, since energy expenditure was not measured in these participants, it is impossible to determine whether all of them were in energy balance despite our controlled feeding design. Furthermore, liver is an important energy-demanding tissue under resting conditions (41) which also influence whole-body lipid oxidation rate.
The cross sectional nature of our study may be seen as a weakness compared to intervention studies in which muscle mitochondrial density is modified. For instance, Rimbert et al. (42) found in humans that a 4-week training cessation period decreased mitochondrial enzyme activities and lowered the reliance on lipid oxidation during a maximal aerobic test. A similar study with a longer exercise training protocol and with careful control of plasma/muscle fuel availability would be insightful.
Our data emphasize the importance of taking fuel availability into account when fuel oxidation is assessed in response to a particular challenge. Indeed, muscle glycogen content and plasma FFA concentration were the main determinant of lipid oxidation during exercise whereas differences in skeletal muscle mitochondrial density showed no influence on total lipid oxidation. In conclusion, the hypothesis that impaired muscle mitochondrial density leads to impaired reliance on lipid oxidation and eventually increased muscle lipid accumulation and IR needs more assessments of lipid balance using metabolic chambers over days.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the participants, research associates and nursing staff of The Pennington Biomedical Research Center. This study was partly funded by a young Scientist Award 2008 from The Obesity Society (to j.E.G.), with additional support from P30-DK-072476 and R01-DK-060412 (to E.R). j.E.G. was supported by a fellowship from The International Nutrition Foundation/Ellison Medical Foundation. This work utilized the Cell Biology and Bioimaging Core facilities that are supported in part by COBRE (NIH P20-RR021945) and CNRU (NIH 1P30-DK072476) center grants from the National Institutes of Health.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http:// www.nature.com/oby
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Moro C, Galgani JE, Luu L, et al. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J Clin Endocrinol Metab. 2009;94:3440–3447. doi: 10.1210/jc.2009-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 4.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–395. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 7.Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008;19:324–330. doi: 10.1016/j.tem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 9.Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr. 2009;89:463S–466S. doi: 10.3945/ajcn.2008.26717C. [DOI] [PubMed] [Google Scholar]
- 10.Galgani JE, Heilbronn LK, Azuma K, et al. Look AHEAD Adipose Research Group. Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes. 2008;57:841–845. doi: 10.2337/db08-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han DH, Nolte LA, Ju JS, et al. UCP-mediated energy depletion in skeletal muscle increases glucose transport despite lipid accumulation and mitochondrial dysfunction. Am J Physiol Endocrinol Metab. 2004;286:E347–E353. doi: 10.1152/ajpendo.00434.2003. [DOI] [PubMed] [Google Scholar]
- 12.Handschin C, Choi CS, Chin S, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wredenberg A, Freyer C, Sandström ME, et al. Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochem Biophys Res Commun. 2006;350:202–207. doi: 10.1016/j.bbrc.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Rabøl R, Boushel R, Dela F. Mitochondrial oxidative function and type 2 diabetes. Appl Physiol Nutr Metab. 2006;31:675–683. doi: 10.1139/h06-071. [DOI] [PubMed] [Google Scholar]
- 15.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes. 2000;49:2102–2107. doi: 10.2337/diabetes.49.12.2102. [DOI] [PubMed] [Google Scholar]
- 16.Braun B, Sharoff C, Chipkin SR, Beaudoin F. Effects of insulin resistance on substrate utilization during exercise in overweight women. J Appl Physiol. 2004;97:991–997. doi: 10.1152/japplphysiol.00231.2004. [DOI] [PubMed] [Google Scholar]
- 17.Mensink M, Blaak EE, van Baak MA, Wagenmakers AJ, Saris WH. Plasma free Fatty Acid uptake and oxidation are already diminished in subjects at high risk for developing type 2 diabetes. Diabetes. 2001;50:2548–2554. doi: 10.2337/diabetes.50.11.2548. [DOI] [PubMed] [Google Scholar]
- 18.Redman LM, Heilbronn LK, Martin CK, et al. Pennington CALERIE Team. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 20.Galgani JE, Ryan DH, Ravussin E. Effect of capsinoids on energy metabolism in human subjects. Br J Nutr. 2010;103:38–42. doi: 10.1017/S0007114509991358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 22.Ukropcova B, Sereda O, de Jonge L, et al. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007;56:720–727. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- 23.Costford SR, Bajpeyi S, Pasarica M, et al. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298:E117–E126. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 25.Bajpeyi S, Tanner CJ, Slentz CA, et al. Effect of exercise intensity and volume on persistence of insulin sensitivity during training cessation. J Appl Physiol. 2009;106:1079–1085. doi: 10.1152/japplphysiol.91262.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holloszy JO, Oscai LB, Don IJ, Molé PA. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun. 1970;40:1368–1373. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- 27.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9:273–278. [PubMed] [Google Scholar]
- 28.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 29.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–2356. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh H, Nilsson E, Ling C, et al. Molecular correlates for maximal oxygen uptake and type 1 fibers. Am J Physiol Endocrinol Metab. 2008;294:E1152–E1159. doi: 10.1152/ajpendo.90255.2008. [DOI] [PubMed] [Google Scholar]
- 31.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lattuada G, Costantino F, Caumo A, et al. Reduced whole-body lipid oxidation is associated with insulin resistance, but not with intramyocellular lipid content in offspring of type 2 diabetic patients. Diabetologia. 2005;48:741–747. doi: 10.1007/s00125-005-1686-6. [DOI] [PubMed] [Google Scholar]
- 33.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 34.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–1427. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang H, Bowen BP, Lefort N, et al. Proteomics analysis of human skeletal muscle reveals novel abnormalities in obesity and type 2 diabetes. Diabetes. 2010;59:33–42. doi: 10.2337/db09-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mogensen M, Sahlin K, Fernström M, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–1599. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- 37.Phielix E, Schrauwen-Hinderling VB, Mensink M, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritov VB, Menshikova EV, Azuma K, et al. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab. 2010;298:E49–E58. doi: 10.1152/ajpendo.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–120. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 40.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E1009–E1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 42.Rimbert V, Vidal H, Duché P, et al. Rapid down-regulation of mitochondrial fat metabolism in human muscle after training cessation is dissociated from changes in insulin sensitivity. FEBS Lett. 2009;583:2927–2933. doi: 10.1016/j.febslet.2009.07.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.