Abstract
Background
Depression in informal caregivers of persons with dementia is a major, costly and growing problem. However, it is not yet clear which caregivers are at increased risk of developing depression. With this knowledge preventive strategies could focus on these groups to maximize health gain and minimize effort.
Methods
The onset of clinically relevant depression was measured with the Center for Epidemiologic Studies - Depression Scale in 725 caregivers who were not depressed at baseline and who were providing care for a relative with dementia. Caregivers were followed over 18 months. The indices calculated to identify the most important risk indicators were: odds ratio, attributable fraction, exposure rate and number needing to be treated.
Results
The following significant indicators of depression onset were identified: increased initial depressive symptoms, poor self-rated health status and white or Hispanic race/ethnicity. The incidence of depression would decrease by 72.3% (attributive fraction) if these risk indicators together are targeted by a completely effective intervention. Race/ethnicity was not a significant predictor if caregivers of patients who died or were institutionalized were left out of the analyses.
Conclusion
Detection of only a few characteristics makes it possible to identify high-risk groups in an efficient way. Focusing on these easy-to-assess characteristics might contribute to a cost-effective prevention of depression in caregivers.
Keywords: incidence, high-risk groups, risk factors, carers, Alzheimer’s disease
Introduction
Depression is a common disorder in informal caregivers (Dura et al., 1991; Coope et al., 1995; Cohen, 2000). Caregivers of dementia patients, in particular, experience higher levels of depressive symptoms compared to non-caregivers, but also compared to caregivers of physically-impaired older adults (Ory et al., 1999). A systematic review reported that almost half of the caregivers develop a depressive disorder within a year (Cuijpers, 2005). A recent cohort study estimated that the incidence of depression in spouses of patients with dementia was more than four times higher than in persons with a spouse with no dementia (Joling et al., 2010).
Depression in caregivers not only reduces their quality of life, but also leads to less optimal care for their relatives, which begs the question how the incidence of depression in caregivers can be lowered. A meta-analysis of 19 randomized prevention trials (among non-caregiving populations) showed that the incidence of depression can be reduced by 22% (Cuijpers et al., 2008b). Depression prevention programs might be a successful strategy to improve the health of dementia caregivers.
Current data suggest that focusing attention on high-risk groups is likely to be more fruitful than adopting preventive strategies aimed at the whole population (Beekman et al., 2010). Therefore, it is important to know how to select groups of caregivers at increased risk of developing depression to target preventive efforts effectively.
With more precise information about the risk indicators of the onset of depression in caregivers, we could identify high-risk groups in which prevention programs might generate substantial health gains for the least effort. Since long-term institutionalization of patients is extremely costly and the mental health of the informal caregiver is often decisive in the timing of nursing home placement (Mittelman et al., 2006), targeted prevention of depression may be likely to be cost-effective as well. The aim of this study was to identify target groups for prevention of depression among caregivers of persons with dementia.
Methods
Design and procedures
Data were derived from the Resources for Enhancing Alzheimer’s Caregiver Health study (REACH). The design of REACH has been described elsewhere (Gitlin et al., 2003; Wisniewski et al., 2003). Briefly, REACH tested the effectiveness of a series of interventions aimed at improving the health and well-being of family caregivers living with and caring for persons with dementia. Data for 1229 dyads of caregivers and care recipients recruited from multiple community sites and health services were collected at six sites in the USA. Follow-up assessments were administered at 6, 12 and 18 months.
Sample
For the present study, caregivers of the intervention and control group were combined into a single cohort. Of the 1229 caregivers of dementia patients interviewed at baseline, 504 persons had clinically significant depressive symptoms at baseline (defined as a score ≥16 on the Center for Epidemiologic Studies - Depression Scale (CES-D)). The cohort at risk of becoming incident cases of depression thus consisted of 725 persons who at baseline had not met the criteria for depression. The longitudinal data of 725 of the 1229 caregivers were available for this analysis based on the baseline and all follow-up assessments of REACH.
Depression
Depression was measured with the CES-D scale. This instrument was designed for screening and monitoring depression and consists of 20 items with total scores ranging between 0 and 60 with higher scores indicating greater depressive symptom severity. Scores of 16 and higher indicate the presence of clinically significant depression (Radloff, 1977). The CES-D was measured at baseline and at all follow-up interviews.
A person was deemed to be an incident case when two criteria were met: (1) presence of depression at follow-up (CES-D score ≥16), and (2) significant increase in depression severity between two follow-up measurements (change score on the CES-D ≥ 5). Criterion 1 was used to ascertain depression status at one of the three follow-up measurements, and criterion 2 to prevent false-positive cases due to measurement error in the CES-D. A change of 5 scale points on the CES-D was chosen, because it represents, in clinical terms, a medium to large change (Lipsey and Wilson, 1993; Beekman et al., 2002; Smit et al., 2004).
Putative risk indicators
To identify variables that predict the onset of depression, several putative predictors were assessed at baseline interview, including sociodemographics, clinical variables and characteristics of the caregiver context. We based the selection of predictors on previous cross-sectional studies on risk factors for depression in caregivers (Schulz and Williamson, 1991; Schulz et al., 1995; Livingston et al., 1996; Donaldson et al., 1997; Clyburn et al., 2000; Janevic and Connell, 2001) All risk indicators were dichotomized at the median such that the index category (coded 1) was the assumed higher risk compared with the reference category (coded 0). Dichotomization was carried out before the analysis.
Socio-demographics of the caregiver included: gender, age, race/ethnicity (black = reference category, as black persons in our sample had the lowest risk of developing depression), education (primary school vs. secondary school or higher), being spouse of the patient. Age and gender of the care recipient were also measured.
Clinical characteristics included self-rated poor health (1 = poor/fair, 0 = good- excellent), depressive symptoms (1 = CES-D scores between 7 and 15, 0 = CES-D <7, i.e. below 50th percentile), anxious symptoms as measured with the Anxiety Inventory (Spielberger et al., 1983) (1 = score > 18, 0 = scores ≤ 18), antidepressant use.
Severity of dementia-related problems was assessed using threes measures of the severity of the care recipients’ problems: physical health of the care recipient was rated by the caregiver (1 = poor/fair, 0 = good- excellent); cognitive functioning was assessed with the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), a higher score indicating better cognitive functioning (1 = MMSE below 13, 0 = MMSE 13 or higher); and behavioral problems were measured with the disruptive behavior subscale of the Revised Memory and Behavior Problems Checklist (Teri et al., 1992) (1 = 2 or more disruptive behaviors, 0 = less than 2 disruptive behaviors).
Characteristics of the caregiving context included: hours a day “doing things for the patient” (1 = 6 or more, 0 = less than 6), how long caregivers had taken care of their relative (1 = 3 years or more, 0 = less than 3 years) and social support (help from family and friends last week yes/no), distress or burden caused by the presence of memory and behavior problems as measured with the Revised Memory and Behavior Problems Checklist (RMBPC) burden score (1 = high burden, 0 = low burden).
Methodology for selecting high-risk groups
The methodology used to select high-risk groups was developed by Smit et al. (2004), and has been used in previous research (Schoevers et al., 2006; Smit et al., 2006; 2007; Batelaan et al., 2010). First, the risk indicators that are most strongly related to the onset of depression were selected from our set of putative risk indicators, based on the magnitude of their odds ratio (OR). This set of strongest risk indicators was used as a starting point for the selection of the best target groups for preventive interventions: i.e. those in which the expected health gain of a preventive intervention is as large as possible, the effort to generate that health gain as small as possible, and in which prevention is likely to be efficient. This selection process was based on the OR, the attributable fraction (AF), the exposure rate (ER), and the number needed to be treated (NNT). The OR describes the strength of the association between the risk indicator and the subsequent risk of developing depression. The AF represents the percentage by which the incidence rate of depression can be reduced when the adverse effect of a risk factor is completely eliminated (Miettinen, 1974; Rothman and Greenland, 1998). The ER represents the percentage of the caregiver sample exposed to the risk indicator. Finally, the NNT indicates how many caregivers would have to receive a preventive intervention to avoid one new case of depression, assuming that the adverse effect of the risk factor can be completely blocked by the preventive intervention, and is thus an indicator of the maximum efficiency of the intervention. Thus, risk indicators with higher ORs and AFs, and lower ERs and NNTs are likely to have greater value for the prevention of depression. These metrics can be computed for each risk indicator separately, but also for combinations of risk indicators.
To select the best target groups for prevention risk profiles were generated using the significant risk indicators from the complete multivariate model. From these indicators, the most promising risk indicator was selected (with the highest OR and AF, and lowest ER and NNT). This was followed by consecutively selecting and adding risk indicators in such a way that the values for the potential health benefit (OR and AF) were kept as high as possible and the values for effort and cost (ER and NNT) as low as possible.
Statistical analyses
All analyses were controlled for group assignment because active REACH interventions were superior to control conditions for specific sites and subgroups. First, ORs were calculated in a multivariate logistic regression model including all putative risk indicators. Using conventional back-stepping procedures, the smallest set of statistically significant risk indicators was retained (p<0.05). In both models, a maximum-likelihood estimate of the AF was calculated using Stata’s downloadable aflogit procedure for each of the risk indicators under a logistic regression model (Greenland and Drescher, 1993). Second, the best target groups for preventive interventions were selected, based on the OR, AF, ER and NNT, calculated in bivariate analyses. The NNT was calculated as the inverse of the risk difference. The risk difference was obtained by regressing the outcome on a risk indicator in a linear probability model, e.g. a generalized linear model with a binomial distribution for its outcome and identity as its link function. Because placement and bereavement of the patient might affect reported depressive symptoms on the CES-D, the analysis was replicated removing the caregivers where the patient either died or was institutionalized during the study. Missing CES-D data points on the follow-up measurements were imputed with the Maximum Likelihood Estimation (MLE) procedure, as implemented by the EM algorithm in SPSS. In order to replace missing values on the CES-D follow-up measurements by their most likely values while also taking into account the mechanism that generated the missing values, statistically significant predictors of the CES-D score and missing values were used in the MLE imputation procedure to obtain the required predicted values. All statistical analyses were performed using SPSS version 15 or Stata version 9 (StataCorp LP, College Station, TX).
Results
Incidence and persistence of depression
The caregivers at risk for depression who were included in the study sample scored significantly better on most of the baseline variables compared with the excluded caregivers with prevalent clinically depressive symptoms at baseline. The baseline characteristics of the study sample are described in Table 1. Of the 725 caregivers at risk, 180 (24.8%) developed depression during the 18-month follow-up. Fifty-eight cases had high levels of CES-D (≥16) for all following measurement points. Forty caregivers only became a case at the last measurement point. Depression was not persistent in 82 cases.
Table 1.
Baseline characteristics of sample at risk and the incident sample
| TOTAL SAMPLE AT RISK (%) | INCIDENT CASES (%) | |
|---|---|---|
| RISK INDICATOR | N = 725 | N = 180 |
| Socio-demographics: caregiver | ||
| Young age (<65 yrs) | 51.7 | 53.3 |
| Female gender | 77.1 | 78.3 |
| Ethnicity (reference = Black) | ||
| Hispanic | 14.5 | 18.9 |
| White | 57.7 | 60.6 |
| Low educational level | 40.7 | 46.7 |
| Being spouse of the patient | 46.9 | 47.8 |
| Socio-demographics: patient | ||
| Old age (>80 yrs) | 50.1 | 52.2 |
| Male gender | 40.4 | 43.9 |
| Clinical variables: caregiver | ||
| Poor self-rated health | 26.9 | 41.7 |
| Increased depressive symptoms (CES-D>7) | 55.9 | 79.4 |
| Antidepressant use | 9.5 | 15.6 |
| High anxiety level | 57.0 | 71.7 |
| Severity of dementia-related problems | ||
| Worse physical health | 42.9 | 51.7 |
| Cognitive impairment (MMSE >13) | 52.2 | 54.3 |
| Behavior problems | 58.3 | 64.4 |
| Caregiving context | ||
| Caring for more than 3 yrs | 58.6 | 59.4 |
| No help from family and friends last week | 45.2 | 48.3 |
| Hours a day “doing things for patient” | 54.2 | 58.9 |
CES-D = Center for Epidemiologic Studies - Depression scale; MMSE = Mini-Mental State Examination.
Selecting a small set of risk indicators
Table 2 shows the complete multivariate model for incident depression, including all 18 putative risk indicators. The total AF of the complete multivariate model was 87.8%. Using conventional back-stepping procedures, the smallest set of significant risk indicators was selected (Table 3). Only four risk indicators were retained in this parsimonious model: increased initial depressive symptoms in the caregiver, poor self-rated health of the caregiver, and Hispanic or white ethnicity/race of the caregiver. Using the selected risk indicators, 72.3% of future cases of clinically relevant depression can be identified (total AF in Table 3). We also calculated the attributive fraction for the two risk factors that are amenable to change (poor self-rated health and increased depressive symptoms) and this resulted in an attributive fraction of 58.72% (95% CI 45.46–68.76). This implies that it is possible to achieve a substantial health gain when using a much smaller set of risk indicators.
Table 2.
Complete multivariate model of the risk indicators for 725 caregivers at risk for developing depression
| RISK INDICATOR | OR | 95% CI | AF % | 95% CI |
|---|---|---|---|---|
| Socio-demographics: caregiver | ||||
| Young age (<65 yrs) | 1.49 | 0.86–2.55 | 12.29 | −5.26–26.91 |
| Female gender | 0.83 | 0.46–1.49 | −9.68 | −*** |
| Ethnicity (reference = Black) | ||||
| Hispanic | 2.03 | 1.11–3.71* | 7.28 | 0.77–13.36 |
| White | 1.87 | 1.13–3.09* | 21.39 | 3.75–35.80 |
| Low educational level | 0.88 | 0.59–1.30 | 3.74 | −7.99–14.19 |
| Being spouse of the patient | 0.97 | 0.49–1.90 | −1.14 | −*** |
| Socio-demographics: patient | ||||
| Old age (>80 yrs) | 1.11 | 0.74–1.66 | 3.26 | −10.55–15.34 |
| Male gender | 1.15 | 0.67–1.98 | 3.93 | −12.02–17.62 |
| Clinical variables: caregiver | ||||
| Poor self-rated health | 2.10 | 1.38–3.19** | 15.54 | 6.21–23.94 |
| Increased depressive symptoms (CES-D>7) | 3.04 | 1.92–4.81** | 43.28 | 25.25–56.97 |
| Antidepressant use | 1.41 | 0.78–2.56 | 2.58 | −2.06–7.02 |
| High anxiety level | 1.43 | 0.93–2.21 | 15.04 | −4.44–30.89 |
| Severity of dementia-related problems | ||||
| Worse physical health | 1.23 | 0.84–1.82 | 6.24 | −6.03–17.09 |
| Cognitive impairment (MMSE >13) | 1.16 | 0.78–1.71 | 4.86 | −9.34–17.22 |
| Behavior problems | 1.21 | 0.82–1.77 | 7.35 | −9.07–21.31 |
| Caregiving context | ||||
| Caring for more than 3 yrs | 0.88 | 0.60–1.30 | −4.92 | −*** |
| No help from family and friends last week | 1.26 | 0.86–1.84 | 6.84 | −5.10–17.42 |
| Hrs a day ‘doing things for patient’ | 1.24 | 0.84–1.84 | 7.80 | −7.21–20.70 |
| Total AF | 87.71 | 67.84–95.30 |
CI = confidence interval; CES-D = Center for Epidemiologic Studies - Depression scale; MMSE = Mini-Mental State Examination; OR = odds ratio; AF = attributable fraction; NNT = number needed to be treated.
p<0.05,
p<0.001.
A confidence interval of a negative AF cannot be computed.
Table 3.
Significant predictors of incident depression from the multivariate model (parsimonious model)
| RISK INDICATOR | ER (%) | OR | 95% CI | AF (%) | 95% CI | NNT |
|---|---|---|---|---|---|---|
| Increased depressive symptoms in the CG | 55.86 | 3.56 | 2.36–5.36** | 48.43 | 33.11–60.24 | 4.9 |
| Poor self-rated health of the CG | 26.90 | 2.15 | 1.46–3.16** | 16.24 | 7.41–24.24 | 6.8 |
| Hispanic caregiver ethnicity | 14.48 | 1.97 | 1.10–3.53* | 6.87 | 0.69–12.67 | 8.7 |
| White caregiver ethnicity | 57.66 | 1.74 | 1.11–2.73* | 19.92 | 3.41–33.60 | 11.5 |
| Total AF | 72.25 | 57.14–82.03 |
CG = caregiver; ER = exposure rate; OR = odds ratio; CI = confidence interval; AF = attributable fraction; NNT = number needed to be treated.
p<0.05;
p<0.001.
Selecting risk profiles for indicated prevention
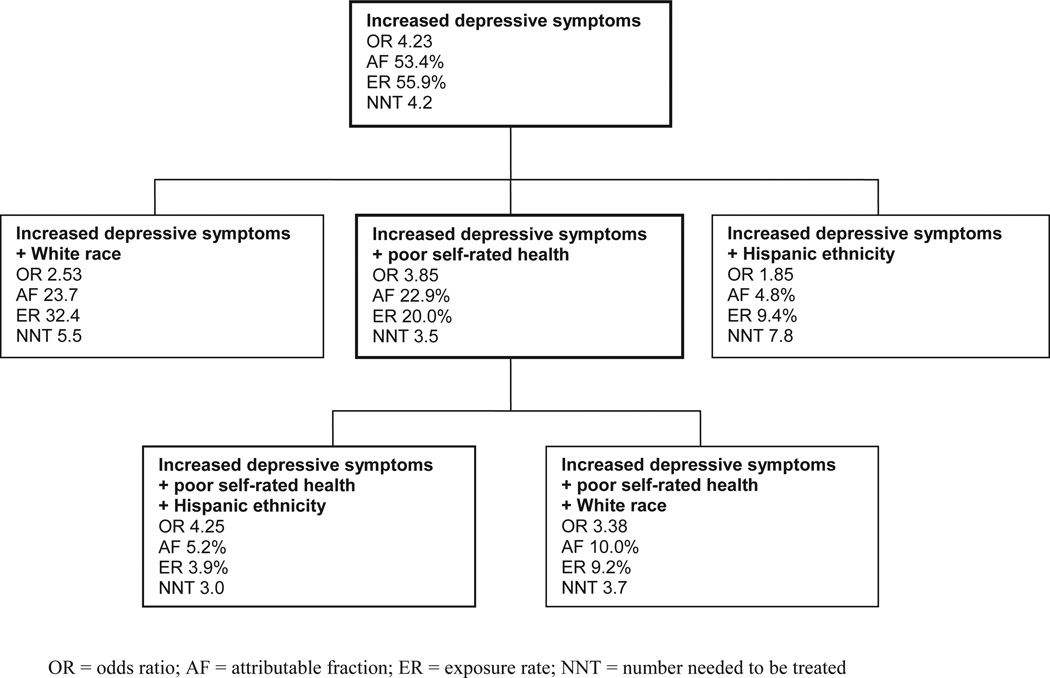
Having “increased initial depressive symptoms” was the strongest predictor of depression onset (Table 3) and was the best candidate to be selected as a starting point for identifying the “best” high-risk group for prevention. Caregivers with slightly increased depressive symptoms are associated with a high risk. The NNT suggests that five caregivers would have to receive an intervention blocking the adverse effect of increased initial depressive symptoms, to avoid depression onset in one caregiver. But this group is still large (56% of the caregivers) and prevention might be difficult to deliver. Next, three risk indicators can be added to the risk profile (Figure 1). Poor self-rated health was the best candidate to be added. This step reduced the OR and the AF, but the exposure rate declined which means a considerably smaller group would need to be targeted. When adding ethnicity/race as a third risk indicator to the risk profile a much smaller group would have to be targeted. The OR increased, but the AF has dropped significantly while the NNT remained more or less the same.
Figure 1.
Selecting high-risk groups for depression prevention.
Model without caregivers where the patient died or was institutionalized
During the study period, 135 (18.6%) patients were institutionalized, 78 (10.8%) died and 38 (5.2%) died after placement. A separate analysis was carried out using only caregivers where the patient survived and lived at home during the entire study period. The model based on the smallest subset of statistically significant risk indicators (at p<0.05) only consisted of the risk indicators “increased depressive symptoms” (OR = 3.67, p = 0.000, 95% CI 2.16–6.22) and “poor self-rated health” (OR = 1.87, p = 0.011, 95% CI 1.15–3.05). The total AF of this model was 58.04% (95% CI 39.84–70.74). Caregiver race/ethnicity was no longer a significant independent predictor of incident depression (for Hispanic ethnicity: OR = 1.98, p = 0.070, 95% CI 0.95–4.13 and for White race: OR = 1.55, p = 0.185, 95% CI 0.81–2.99)
Discussion
In this study we tried to identify the high risk groups of dementia caregivers in which prevention is likely to generate substantial health gains for the least effort and hence for the lowest costs. We used an innovative method to determine which persons are at risk for developing depression, the condition that was the focus of this study.
Main findings
Results suggested that targeting people with some signs or symptoms but no disorder (indicated prevention) is the most effective strategy to prevent the onset or development of a full-blown disorder and may thus offer a good starting point for preventive efforts.
A recent meta-analysis demonstrated that prevention programs are actually capable of reducing the incidence of depression in different target populations and settings (Cuijpers et al., 2008a). Indicated prevention could become more specific and cost-effective by expanding the risk profile with other risk indicators. Beyond an increased level of depressive symptoms, we found poor self-rated health as a strong predictor of incident depression in the caregiver. Finally, there were race-ethnic differences in the risk. However, race/ethnicity was no longer a significant predictor when analyzing the sample without the caregivers whose patient was placed in an institution or died. Therefore, it appears that the race/ethnicity factor is not a predictor for depression unless the patient is institutionalized or deceased. A reason for race/ethnic differences in placement might be attributed to cultural norms regarding family care. It would be worth exploring in future studies how various ethnic caregivers deal with such events and how this relates to the development of depression.
When targeting the caregivers with the first two indicators the attributable fraction was 22.9%. When targeting only the caregivers with the strongest single risk factor (increased initial depressive symptoms) the incidence of depression could even have been halved. On the other hand, the target group would then have to increase from 20% to almost 56%. We emphasize that these estimates are upper limits and will only be reached if an intervention is completely effective, which is not realistic.
Placing our findings in the context of the literature
Depressive symptoms on self-report scales are short-term predictors of major depressive episodes (Cuijpers and Smit, 2004). Poor self-rated health has also been reported to be a good predictor (Beekman et al., 1995; Cole and Dendukuri, 2003). The risk indicators we identified are well known in the existing depression literature. We had expected that risk factors reflecting the contextual distress of the caregivers would have played a much larger role in the development of depression. However, we did not find a significant contribution of patient-related indicators and indicators related to the specific caregiving context to the onset of depression in the caregivers. This may suggest that depression in caregivers is not necessarily a contextual problem but that the same causal pathways as in other populations may be involved.
Strengths and limitations
Our study has several strengths. It is the first study to apply this methodological approach of identifying risk indicators for prevention and goes much further than the conventional risk factor studies. Statistics were used to quantify potential health benefits and the efforts required to generate these benefits. The risk profiles also give guidance on the type of intervention that needs to be offered to the intended target group. The use of a large sample size and long follow-up period should be regarded as the other strengths of the study.
Our study also has some limitations. First, the US caregivers included in this study may not be representative of the total population of caregivers of dementia patients. They were already caring for persons with relatively severe dementia. In addition, subjects were willing to participate in a trial to test psychosocial interventions and so might have been help-seeking and at a higher risk of developing depression. On the other hand, they may represent persons willing to accept a preventive intervention. Secondly, we could examine only a selection of relevant risk indicators. For example, we lacked information on history of depression, an important predictor of new episodes. Also some other risk factors already found to be related with depression, such as personality of the caregiver and dysfunctional coping style, were not included in this study (Clark and Diamond, 2010). Finally, we used the CES-D to measure depression, which has good psychometric properties but is not a diagnostic instrument.
Implications and conclusions
With the methodology we employed, we were able to select a very small set of risk indicators to identify groups at high risk for the onset of depression. Instead of measuring a variety of variables, the use of a simple checklist of the relevant risk indicators (depressive symptoms, self-rated health and ethnicity of the caregiver) will be required to recognize the subgroups in which preventive efforts should be made. The two strongest risk indicators we found can be measured relatively quickly with the help of short self-rated scales. Use of this strategy is feasible, might yield substantial health gains, and hence might prove to be cost-effective.
Acknowledgments
This research was supported through the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) project, which is supported by the National Institute on Aging and the National Institute of Nursing Research (Grants U01-NR13269, U01-AG13313, U01-AG13297, U01-AG13289, U01-AG13265, U01-AG13255 and AG13305). The National Institute on Aging and the National Institute of Nursing Research had no further role in the study design, in the collection, analysis and interpretation of the data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
None.
Description of authors’ roles
KJ, FS, HM and HH formulated the research questions and designed the study. RS supervised the data collection. FS was responsible for the statistical design of the data. KJ carried out the statistical analysis and wrote the paper. All authors assisted with writing the paper and approved the final version.
References
- Batelaan NM, Smits F, de GR, van Balkom AJ, Vollebergh WA, Beekman AT. Identifying target groups for the prevention of anxiety disorders in the general population. Acta Psychiatrica Scandinavica. 2010;122:56–65. doi: 10.1111/j.1600-0447.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, van Tilburg T, Smit JH, Hooijer C, van Tilburg W. Major and minor depression in later life: a study of prevalence and risk factors. Journal of Affective Disorders. 1995;36:65–75. doi: 10.1016/0165-0327(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Beekman AT, et al. The natural history of late-life depression: a 6-year prospective study in the community. Archives of General Psychiatry. 2002;59:605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Smit F, Stek ML, Reynolds CF, Cuijpers PC., III Preventing depression in high-risk groups. Current Opinion in Psychiatry. 2010;23:8–11. doi: 10.1097/YCO.0b013e328333e17f. [DOI] [PubMed] [Google Scholar]
- Clark MC, Diamond PM. Depression in family caregivers of elders: a theoretical model of caregiver burden, sociotropy, and autonomy. Research in Nursing and Health. 2010;33:20–34. doi: 10.1002/nur.20358. [DOI] [PubMed] [Google Scholar]
- Clyburn LD, Stones MJ, Hadjistavropoulos T, Tuokko H. Predicting caregiver burden and depression in Alzheimer’s disease. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2000;55:S2–S13. doi: 10.1093/geronb/55.1.s2. [DOI] [PubMed] [Google Scholar]
- Cohen D. Caregivers for persons with Alzheimer’s disease. Current Psychiatry Reports. 2000;2:32–39. doi: 10.1007/s11920-000-0039-x. [DOI] [PubMed] [Google Scholar]
- Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. American Journal of Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- Coope B, et al. The prevalence of depression in the carers of dementia sufferers. International Journal of Geriatric Psychiatry. 1995;10:237–242. [Google Scholar]
- Cuijpers P. Depressive disorders in caregivers of dementia patients: a systematic review. Aging and Mental Health. 2005;9:325–330. doi: 10.1080/13607860500090078. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Smit F. Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatrica Scandinavica. 2004;109:325–331. doi: 10.1111/j.1600-0447.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van SA, Smit F, Mihalopoulos C, Beekman A. Preventing the onset of depressive disorders: a meta-analytic review of psychological interventions. American Journal of Psychiatry. 2008a;165:1272–1280. doi: 10.1176/appi.ajp.2008.07091422. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van SA, Smit F, Mihalopoulos C, Beekman A. Preventing the onset of depressive disorders: a meta-analytic review of psychological interventions. American Journal of Psychiatry. 2008b;165:1272–1280. doi: 10.1176/appi.ajp.2008.07091422. [DOI] [PubMed] [Google Scholar]
- Donaldson C, Tarrier N, Burns A. The impact of the symptoms of dementia on caregivers. British Journal of Psychiatry. 1997;170:62–68. doi: 10.1192/bjp.170.1.62. [DOI] [PubMed] [Google Scholar]
- Dura JR, Stukenberg KW, Kiecolt-Glaser JK. Anxiety and depressive disorders in adult children caring for demented parents. Psychology and Aging. 1991;6:467–473. doi: 10.1037//0882-7974.6.3.467. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, et al. Effect of multicomponent interventions on caregiver burden and depression: the REACH multisite initiative at 6-month follow-up. Psychology and Aging. 2003;18:361–374. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–872. [PubMed] [Google Scholar]
- Janevic MR, Connell CM. Racial, ethnic, and cultural differences in the dementia caregiving experience: recent findings. Gerontologist. 2001;41:334–347. doi: 10.1093/geront/41.3.334. [DOI] [PubMed] [Google Scholar]
- Joling KJ, et al. Incidence of depression and anxiety in the spouses of patients with dementia: a naturalistic cohort study of recorded morbidity with a 6-year follow-up. American Journal of Geriatric Psychiatry. 2010;18:146–153. doi: 10.1097/JGP.0b013e3181bf9f0f. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment: confirmation from meta-analysis. American Psychologist. 1993;48:1181–1209. doi: 10.1037//0003-066x.48.12.1181. [DOI] [PubMed] [Google Scholar]
- Livingston G, Manela M, Katona C. Depression and other psychiatric morbidity in carers of elderly people living at home. BMJ. 1996;312:153–156. doi: 10.1136/bmj.312.7024.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. American Journal of Epidemiology. 1974;99:325–332. doi: 10.1093/oxfordjournals.aje.a121617. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67:1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- Ory MG, Hoffman RR, Yee JL, III, Tennstedt S, Schulz R. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177–185. doi: 10.1093/geront/39.2.177. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia: Lippincott Raven; 1998. [Google Scholar]
- Schoevers RA, et al. Prevention of late-life depression in primary care: do we know where to begin? American Journal of Psychiatry. 2006;163:1611–1621. doi: 10.1176/ajp.2006.163.9.1611. [DOI] [PubMed] [Google Scholar]
- Schulz R, Williamson GM. A 2-year longitudinal study of depression among Alzheimer’s caregivers. Psychology and Aging. 1991;6:569–578. doi: 10.1037//0882-7974.6.4.569. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Smit F, Beekman A, Cuijpers P, de GR, Vollebergh W. Selecting key variables for depression prevention: results from a population-based prospective epidemiological study. Journal of Affective Disorders. 2004;81:241–249. doi: 10.1016/j.jad.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Smit F, Ederveen A, Cuijpers P, Deeg D, Beekman A. Opportunities for cost-effective prevention of late-life depression: an epidemiological approach. Archives of General Psychiatry. 2006;63:290–296. doi: 10.1001/archpsyc.63.3.290. [DOI] [PubMed] [Google Scholar]
- Smit F, Comijs H, Schoevers R, Cuijpers P, Deeg D, Beekman A. Target groups for the prevention of late-life anxiety. British Journal of Psychiatry. 2007;190:428–434. doi: 10.1192/bjp.bp.106.023127. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA. State-Trait Anxiety Inventory for Adults (STAI-AD) Manual. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychology and Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, et al. The Resources for Enhancing Alzheimer’s Caregiver Health (REACH): project design and baseline characteristics. Psychology and Aging. 2003;18:375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]