Abstract
Visual field (VF) test results are often unreliable in visually impaired patients, but continue to be a cornerstone of clinical trials and play a vital role in clinical decision making since they are the primary method to determine patients’ functional vision loss or progression. Currently, patients are typically asked to perform VF tasks with minimal instruction or consideration of their psychological experience during the test. The gradual loss of vision due to retinal diseases, such as retinitis pigmentosa (RP), age-related macular degeneration (AMD), or glaucoma can contribute to the experience of negative psychosocial states, such as anxiety, stress, and depression, as well as diminished quality of life. We hypothesize that VF testing elicits test performance anxiety and perception of functional losses of vision, which induces distracting negative thoughts that result in increased VF test variability. Resources for processing and responding to vision-related information may be diverted from task-relevant VF stimuli to task-irrelevant ones, such as internal worry and test anxiety, thereby resulting in VF test performance decrements. We present a theoretical model to support the hypothesis that VF variability is linked to patients’ negative thoughts during VF testing. This conceptual framework provides a basis for the development of coping strategies and mindfulness-based interventions to be evaluated in future research aimed at improving psychosocial states and VF reliability in visually-impaired patients. It would be highly significant to intervene by modifying negative thoughts during VF testing to reduce test variability in glaucoma patients who are progressively losing vision to a blinding eye disease, but whose vision loss has not been accurately identified and treated early enough due to variable VF results. In clinical trials of potential interventions for RP and non-neovascular AMD, reducing VF variability would effectively increase the precision for detecting treatment effects and allow a reduction in the number of VF tests needed to estimate the treatment responses, thus reducing burden on investigators and patients, as well as saving time and money.
Introduction
VF Variability in Glaucoma
Visual field (VF) testing using static automated perimetry (SAP) is the cornerstone of clinical care and trials in glaucoma. SAP presents individual point stimuli of varying intensities to patients throughout their central visual field up to 24° (54 points) or 30° (76 points) radius. The threshold intensity for which patients are able to see the stimuli is then compared against a database of normally-sighted individuals of similar age. The score for each individual location is then calculated as a deviation from the value in normally-sighted individuals. An average of the deviation for all points produces a mean deviation (MD) value, which is negative for individuals with worse sight than normal and positive for those with better sight than normal. A MD of −30 dB corresponds to complete vision loss.
SAP is a useful tool in assessing vision loss in glaucoma, but it is plagued by lack of reliability in many individuals with vision loss. Frequently, VF retests fail to confirm defects found on previous exams. In the Ocular Hypertension Treatment Study, VF defects were not confirmed upon retest for 604 (86%) of the 703 originally reliable VFs that showed some potential loss of vision.1 Another study indicated that two-thirds of glaucoma patients had at least one unreliable VF out of 3–4 VF tests over several years.2 Other research has indicated that up to 22% of early glaucoma patients had >20% fixation losses, which was the most common source of unreliable results.3 Approximately 22% of glaucoma patients exhibited high variability >1.5 dB for the mean deviation in a large, longitudinal study.4
VF test variability greatly diminishes physicians’ ability to determine progression of vision loss or treatment efficacy. Assessment of the glaucoma progression rate should help predict blindness occurring during the patient’s lifetime and provide information on the need for treatment and its intensity. In glaucoma patients with moderate progression rates of vision loss (−0.5 dB/year) and highly variable VF test results (SD of MD=2 dB), it would take 19 years to determine their vision loss if vision tests were performed once a year.4 Even if tests were performed twice a year, it would still take 8.5 years to determine the same rate of vision loss.4 If, however, the same population exhibited low VF variability (SD of MD=0.5 dB), it would take 9 or 4.5 years, respectively, to determine the rate of vision loss if testing was completed once or twice a year. The VF progression rate cannot be definitively established with a reasonable number of tests (i.e., twice a year) within a 4 year period in patients with moderate to high test variability (SD of MD=1–2 dB) and low to moderate progression rates (−0.25 to −0.5 dB/year). It is therefore critical to reduce VF variability in order to more quickly diagnose and treat patients earlier before permanent vision loss occurs. Greater VF variability increases the burden on providers, researchers, patients, and society; therefore it is important to identify potentially modifiable factors.
It is either impossible or at least impractical to modify the Humphrey SAP test-related factors that contribute to increased VF variability, such as small stimuli that are widely spaced and increased eccentricity, which correlates with increased reaction time.5 Such changes would necessitate the adoption and validation of new instrumentation and test protocols (e.g. with blurry, larger stimuli), which would be challenging to implement given the widespread and long established use of Humphrey SAP. Some factors contributing to VF variability in glaucoma patients are inherent with disease severity, such as diminished VF sensitivity due to reduced functional ganglion cell density, in which remaining cells may not respond optimally and consistently.6 Unfortunately, these factors cannot be altered, and determination of VF change is most difficult for important regions that are losing sensitivity at the edges of vision loss and are prone to further progressive loss. Patient-related factors, such as negative thoughts during testing, have hardly been considered, much less measured, as contributors to substantial VF variability.
Variability of Vision in Retinitis Pigmentosa (RP)
Retinitis Pigmentosa (RP) is a retinal degenerative disease that primarily affects night vision and peripheral vision, and thus VF tests are often used as the primary outcomes measure to determine disease severity and progression; therefore, reliable VF estimates are needed to estimate changes in peripheral vision between clinical visits and during future treatment clinical trials for RP. We previously determined that test-retest variability in VF area can be limited to <20% for most patients by using a single experienced VF operator to administer the test,7 but others have found VF variability in RP up to 50%8. Examination of the study’s published data indicates that as the VF area decreased, some subjects’ eyes demonstrated increased variability between sessions, whereas others maintained low variability, but potential sources or factors contributing to increased variability were not elucidated. Our research group has examined the relationship between severity of vision loss and variability in vision among patients with RP. We recently reported that the severity of RP patients’ vision loss was a major factor in determining how reliable or consistent their visual acuity (VA) and VF tests were on a day-to-day basis.9 We also found that patients in later stages of RP with more vision loss experienced more frequent significant fluctuations in their VF size or area on a day-to-day basis,10 similar to the findings of previous research. RP patients with more severe VF losses will likely be among those enrolled in early-stage clinical trials of potential therapeutic interventions, and thus it is critical to identify and reduce any modifiable factors related to increased VF variability.
Variability in fixation stability and macular sensitivity during microperimetry in age-related macular degeneration (AMD)
In early stages, many macular diseases do not affect VA. An alternative approach to assess visual function in age-related macular degeneration (AMD)is to measure paracentral retinal sensitivity with microperimetry, which is a type of VF testing. A previous study found no significant increase in test-retest variability of MP1 microperimetry thresholds with decreasing macular sensitivity, among macular disease subjects with relatively good acuity.11 Unlike glaucoma and RP, factors other than loss of retinal sensitivity appear to influence the reliability of microperimetry in AMD subjects. For example, the authors of this study commented that during the examination, some patients complained that the trigger failed to register their responses. This may have occurred if the patients were distracted or had slow reaction times, since the current software only recognizes responses within 1.5 seconds after the stimulus presentation. Decreased fixation stability, measured by bivariate contour ellipse area(BCEA) in AMD has been associated with poor visual performance (e.g. reading12,13, VA variability). However, large intersubject variability exists for fixation stability (BCEA) despite similar levels of reduced VA.14 Therefore, a likely scenario is that patient-related factors such as cognitive status, attention and reaction time may influence the variability of retinal sensitivity measures in AMD. However, these factors have not yet been systematically studied in AMD patients undergoing microperimetry testing.
Psychosocial Consequences of Vision Loss
Our interest in the influence of psychological factors on the variability of vision measurement derives in part from the known impact vision loss has on patients, including the experience of stress in response to loss of function and activity restrictions, as well as the uncertainty of future independence. Vision loss in RP, AMD and glaucoma can be unpredictable and inexorable, thus forming a continuous threat to patients’ independence. As vision loss gradually progresses, patients may lose their ability to perform valued activities, as well as their sense of independence and self-confidence, causing them to view themselves and their future negatively and experience negative psychosocial states.
Patients with visual impairments such as RP have been reported to have increased distress associated with severity of the impairment.15 RP patients with higher Beck Depression Inventory scores had worse subjective visual function measured by the National Eye Institute Visual Functioning Questionnaire - 25(NEI-VFQ-25), as compared to RP patients with lower depression scores.16 Depression occurs in a third of AMD patients17 and can be predicted by an inability to perform valued activities such as face recognition, reading, and crafts requiring fine manipulations.18,19 Patients with AMD are prone to both depression and anxiety possibly because vision loss may inhibit individuals from being able to perform valued leisure activities20 and driving.21,22 Glaucoma patients are more likely to have emotional instability,23,24 nervousness,25 anxiety or depression when compared to normally-sighted controls.26 For glaucoma patients, younger age was a risk factor for anxiety, whereas older patients were at greater risk for depression.27 Self-reported visual ability has also been reported as a significant risk factor for depression in glaucoma.28 Depression is positively correlated with glaucoma severity and with difficulty performing everyday vision-related tasks,29 and this relationship is not explained by (VA).16
RP, AMD and glaucoma patients are at an increased risk for negative psychosocial states.17,18,19,20,28,29 Currently, most research relates visual function to psychosocial factors, finding in some cases that increased disease severity is associated with increased negative psychosocial factors,15,29 but not all studies have found evidence of this relationship. AMD patients with monocular severe vision loss report increased emotional distress (i.e. anxiety, anger, fatigue, confusion)30 despite having good visual function in their better seeing eye. Negative moods are common in early stages of glaucoma, with roughly a third of newly diagnosed glaucoma patients reporting nervousness, anxiety, stress, or restless sleep,31 or being afraid of going blind,32 even though most reported excellent or good vision and half had no vision loss.33 In a study involving participants with RP, those with more severe vision loss were not significantly more likely to have increased mean perceived stress, negative moods or depressive symptoms.9
Hypothesis / Theoretical Framework
We hypothesize that VF testing provokes negative thoughts about vision loss and/or perceived performance during testing, which results in: (1) mental processing resources being diverted from responding to VF stimuli to internal worry and test anxiety34; and (2) decreased efficiency with which vision-related information is processed and acted upon, resulting in poor VF performance and reliability. This hypothesis is supported by a strong theoretical framework from the behavioral science literature, which includes two theories. Attentional control theory stipulates that anxiety or negative thoughts impair processing efficiency more than performance effectiveness.35 Thus, there is an increased probability that mental processing resources will be diverted from task-relevant stimuli to task-irrelevant ones, such as negative thoughts. Processing efficiency theory indicates that when confronted by anxiety-inducing circumstances (state, not trait anxiety), the efficiency with which information is processed and acted upon decreases,15 potentially resulting in performance decrements. It assumes that worry is the component of state anxiety responsible for the changes in performance.
Figure 1 depicts how patients’ negative thoughts, worry, anxiety and/or stress may affect their responses to VF stimuli and thus test reliability indices. In figure 1, decision criterion refers to the person’s willingness or reluctance to say that a stimulus is present, such that if a person expects a stimulus to be present, then his or her decision criterion will be lowered. Our conceptual model showing the proposed relationships between psychosocial factors, VF variability, clinical outcomes and quality of life is presented in Figure 2. For the conceptual model in figure 2, examples of negative moods that may affect VF test reliability are feeling irritable, downhearted, weak, or sluggish. Examples of negative thoughts that may influence VF test reliability are being worried about failing or doing poorly on the VF test, or thinking about whether something serious may happen because of the reduced VF results.
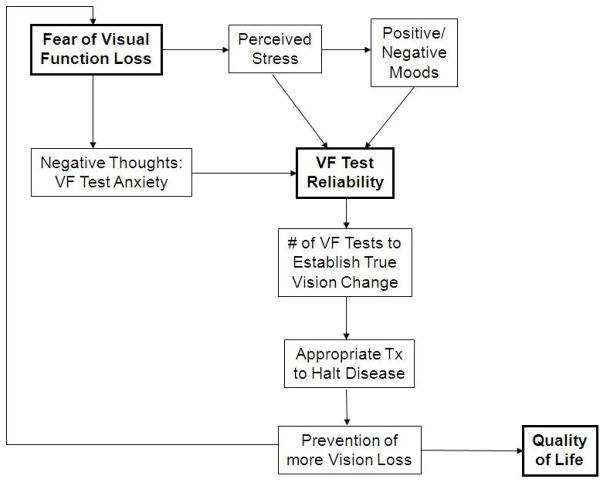
Figure 1.
Conceptual framework of hypotheses for how patients’ negative thoughts, worry, anxiety and/or stress may affect their responses to VF stimuli and thus test reliability indices. Abbreviations: Neg.=negative; Inc.=increased; Pos.=positive; Assoc.=associated; BCEA= bivariate contour ellipse area
Figure 2.
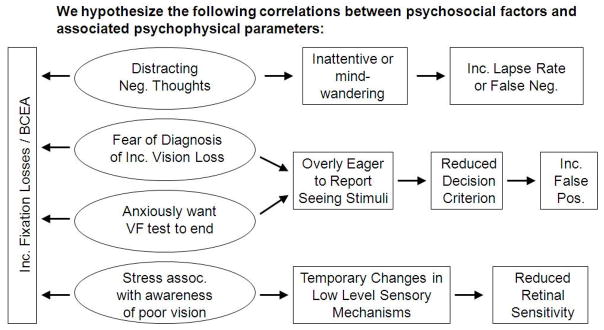
Conceptual model demonstrating the relationships between negative psychosocial factors, VF reliability, clinical outcomes and quality of life.
Evaluation of the Hypothesis
A few previous research studies in non-visually impaired individuals support the hypothesis that apparent VF narrowing is associated with negative thoughts, such as stress and anxiety. Normally-sighted individuals with higher life-event stress experienced greater vision reduction and variability in peripheral VF during a laboratory induced stress condition than those with lower life-event stress (mean change of 14° vs. 2°),36 and have been shown to experience attentional narrowing with external distraction.37 Real-life stressful situations produce even greater peripheral VF defects than lab-induced situations, possibly due to increased anxiety.38 Previous research has suggested that positive mood states are associated with a broadening of attentional processes, and we expect that these factors are essential for reliable VF measures since they control information processing, thought and behavior.39
Vision scientists have historically paid limited attention to patient-related sources of VF variability that may be accounted for or modified. There is currently little published research relating negative psychosocial factors to vision test variability in visually impaired patients. For the past few years, our research group has been examining the relationship between a range of psychosocial factors and variability in vision among patients with RP. We found that negative psychological symptoms of depression and negative moods (i.e., increased irritability or anger due to uncontrollable situations, as well as reductions in positive emotions such as feeling less “active, strong and proud”) were related to significantly more variability in the VF area from one test to the next, after accounting for the severity of VF loss.9 Our group also investigated the effects of RP patients’ day-to-day changes in their general health on their measured level of vision.10 We found that patients who reported reductions in general health at a test session (e.g. not feeling as well as usual due to headache, temporary illness, fatigue, etc.) were significantly more likely to have reductions in their VF size.10 However, day-to-day changes in the tests of central vision (i.e. visual acuity and contrast sensitivity) were not significantly related to general health status or psychological state.10
Studies have also reported that RP patients experience potentially vision-hindering photopsias or spontaneous light phenomena.40 A common type is the phosphene, i.e., dots or shapes slowly moving across the VF. Other types reported by RP patients include a pattern of quick flashes of light, static noise (i.e. similar to the static or “snow” on a television with no reception) or a background glow or fluorescence. These photopsias can interfere with VF testing and are an added complication with which RP patients must deal. They have also been shown to be correlated with psychological factors: decreased positive mood and increased perceived stress have been associated with these phenomena.41 As with the rest of RP symptoms, there is no current proven treatment option for photopsias, another aspect of the disease which may cause patients to tend towards negative thoughts.
If our proposed hypothesis is supported by further evidence, one should be able to: (1) predict which patients will have unreliable VF results based on the extent and/or type of negative thoughts they report having while completing a VF test, and (2) intervene to reduce negative thoughts and thus improve the reliability of VF testing in at risk individuals. Prediction could be valuable in helping to identify reliable candidates to participate in clinical trials, as well as to help explain potential causes for large deviations in VFs obtained in the clinic, which would require retesting prior to indicating to patients with negative thoughts that they had a major, devastating decline in vision. Effective intervention to reduce VF variability could help reduce the number of tests needed to determine significant changes. The first step in testing our hypothesis will require the validation of a questionnaire to determine which negative thoughts are associated with increased VF variability. One possible approach would be to identify one or more powerful constructs from the behavioral science literature that have been repeatedly correlated to several other health-related outcomes, and apply them to the experience of visually-impaired patients during VF testing by adapting existing instruments.
Potential Strategies and Interventions to Help Alleviate Negative Psychosocial States
Some studies have investigated whether patients’ coping strategies influence their adjustment to vision loss. Previously reported coping strategies used by AMD patients’ include accepting what they can and cannot do, cherishing independence, creating novel methods to complete a goal, acknowledging the progression of their visual impairment, confronting the uncertainty of their future, and maintaining optimism.42 In another study of AMD patients, researchers found three factors related to how well patients were adapting to vision loss: (1) acceptance of vision loss, (2) the effect of vision loss on interpersonal relationships, and (3) attitudes towards compensating for their vision loss.43 In a previously published review paper of vision loss due to various diseases including glaucoma and AMD, researchers found that loss of independence and the fear of future vision loss were also important considerations of patients with low-vision.21 They also found that some factors indicating psychological well-being were the acceptance of vision loss and positive attitudes employing humor and laughter.22
During focus group interviews with RP patients, we found that they developed different strategies to cope with their RP, ranging from a “kicking and screaming” mentality as they fight to maintain independence to an attitude that “it could be worse”.44 Such varying perspectives show that some patients with RP are in need of support, while others are highly resilient when faced with the uncertainties of variable and slowly deteriorating vision. RP patients have also reported the use of humor and laughter, social support from other people with RP, “letting go” of things beyond their control, and appreciating the vision and function they still have as some positive strategies to help them cope with their disease.44
In a previous survey in 2006, RP patients have also reported receiving help to manage their stress and anxiety from complementary and alternative medicine (CAM) interventions such as yoga, meditation, and mind-body therapies.45 In a 1998 survey, only 1.8% of glaucoma patients reported using meditation specifically for glaucoma;46 it is possible that this proportion would be slightly greater today given the increasing popularity of alternative medicine. A literature search of PubMed and similar sources revealed no publications related to the prospective study of mindfulness-based therapies for low vision patients, although the benefits of these interventions47 are now being demonstrated for several other chronic diseases.48,49,50,51,52 Mindfulness is a form of meditation in which one intentionally regulates attention to achieve a state of detached moment to moment awareness, which may include at times proprioceptive input, sensory perceptions, cognitions, emotions and situational factors.53
There is good evidence that psychological characteristics,15 distress,54 and test anxiety55 may be altered by mindfulness-based interventions. Reported benefits of mindfulness training in depressed and anxious patients include improved mood, sleep, relaxation, sense of self-worth and self-awareness, and new ways of working with negative thoughts and emotions.56 Normally-sighted meditation practitioners, on the other hand, have been shown to be capable of detecting light flashes of shorter duration than non-meditators,57 and do so more quickly.58 These findings suggest that mindfulness training leads to better visual perceptual sensitivity, potentially due to subjects’ decreased stress, improved attention and relaxed state that allows them to be more responsive to the stimuli, and suggest a possible effect on central nervous system factors underlying perceptual and sensory processing. Previous research has suggested that mindfulness is intimately linked to improvements of attentional functions and cognitive flexibility.59,60
According to our model, coping strategies and mindfulness techniques that focus on patients’ acceptance of their vision loss and the uncertainty it casts on their future,42,43 may affect VF variability by reducing the fear of losses in visual function. Another potential strategy to reduce VF variability would be to teach patients to be mindful in the present moment during VF testing, i.e. continually focus their attention to simply respond to the test stimuli and dismiss any negative ruminating thoughts regarding their test performance or perceived vision loss. Following the support of our hypothesis with additional studies to correlate negative thoughts with VF variability, clinical trials will be needed to confirm the effects these strategies would have on VF variability, and determine appropriate dosing and timing of the intervention.
Consequences of the Hypothesis and Discussion
The high variability of VF tests in visually-impaired patients remains a major issue for clinical trials and patient management1,61 since timely treatment decisions in patients who may be progressively losing vision are dependent upon these tests.62,63 The current practice standard is for patients to perform VF tasks with minimal instruction or consideration of their psychological experience during the test; however, the end results can be unreliable and therefore practically useless in the treatment and management process. Relatively weak correlations between retinal structure and visual function similarly imply that our current objective ocular imaging techniques cannot serve as adequate surrogate assessments of patients’ visual function in many cases. Therefore, the time has come to adopt a conceptual framework that yields testable hypotheses focused on reducing variability in VF testing. This framework can guide the design of studies, including clinical trials, to evaluate whether modifying patient-level psychosocial factors enhances our ability to reliably determine patients’ visual status. Evaluating the impact of psychological factors on VF test reliability has not been previously attempted due to its challenging nature. If it is possible to reduce VF test anxiety and variability, we hypothesize that the results would translate to a reduction in the number of test sessions and time needed to determine true changes in vision, which has economic implications for both clinical practice and trials. In addition, we anticipate that more accurate VF results will lead to improvements in physicians’ treatment decision making and thus the prevention of further vision loss by delayed treatment, and similarly a reduction in unnecessary treatments or surgeries.
Acknowledgments
This work was funded by NIH grant K23 EY018356 to AKB.
The grant sponsor had no involvement in this paper.
Footnotes
Conflict of interest statement
None of the authors have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) the work presented in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Collin Rozanski, Johns Hopkins University.
Jennifer A. Haythornthwaite, Johns Hopkins University; Dept. of Psychiatry & Behavioral Science.
Gislin Dagnelie, Johns Hopkins University; Dept. of Ophthalmology; Wilmer Eye Institute.
Ava K. Bittner, Nova Southeastern University; College of Optometry.
References
- 1.Keltner JL, Johnson CA, Quigg JM, Cello KE, Kass MA, Gordon MO. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Ocular Hypertension Treatment Study Group. Arch Ophthalmol. 2000;118:1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 2.Katz J, Sommer A, Witt K. Reliability of visual field results over repeated testing. Ophthalmology. 1991;98:70–75. doi: 10.1016/s0161-6420(91)32339-x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CA, Nelson-Quigg JM. A prospective three-year study of response properties of normal subjects and patients during automated perimetry. Ophthalmology. 1993;100:269–274. doi: 10.1016/s0161-6420(93)31660-x. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–573. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall M, Maw RJ, Stanek KE, Chauhan BC. The psychometric function and reaction times of automated perimetry in normal and abnormal areas of the visual field in patients with glaucoma. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):878–85. [PubMed] [Google Scholar]
- 6.Henson DB, Chaudry S, Artes PH, Faragher EB, Ansons A. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41:417–421. [PubMed] [Google Scholar]
- 7.Bittner AK, Iftikhar MH, Dagnelie G. Test-retest, Within-visit Variability of Goldmann Visual Fields in Retinitis Pigmentosa. Invest Ophthalmol Vis Sci. 2011;52(11):8042–6. doi: 10.1167/iovs.11-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross DF, Fishman GA, Gilbert LD, Anderson RJ. Variability of visual field measurements in normal subjects and patients with retinitis pigmentosa. Arch Ophthalmol. 1984;102(7):1004–1010. doi: 10.1001/archopht.1984.01040030806021. [DOI] [PubMed] [Google Scholar]
- 9.Bittner AK, Ibrahim M, Haythorthwaite JA, Diener-West M, Dagnelie G. Vision test variability in retinitis pigmentosa and psychosocial factors. Optom Vis Sci. 2011;88:1496–506. doi: 10.1097/OPX.0b013e3182348d0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bittner AK, Haythornthwaite JA, Diener-West M, Dagnelie G. Worse-than-usual visual fields measured in retinitis pigmentosa related to episodically decreased general health. Br J Ophthalmol. 2013;97:145–148. doi: 10.1136/bjophthalmol-2012-302116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen FK, Patel PJ, Xing W, et al. Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50:3464–72. doi: 10.1167/iovs.08-2926. [DOI] [PubMed] [Google Scholar]
- 12.Schuchard RA. Preferred retinal loci and macular scotoma characteristics in patients with age-related macular degeneration. Can J Ophthalmol. 2005;40:303–312. doi: 10.1016/S0008-4182(05)80073-0. [DOI] [PubMed] [Google Scholar]
- 13.Crossland MD, Dunbar HM, Rubin GS. Fixation stability measurement using the MP1 microperimeter. Retina. 2009;29:651–656. doi: 10.1097/IAE.0b013e318196bd65. [DOI] [PubMed] [Google Scholar]
- 14.Tarita-Nistor L, González EG, Markowitz SN, Steinbach MJ. Fixation characteristics of patients with macular degeneration recorded with the mp-1 microperimeter. Retina. 2008;28:125–133. doi: 10.1097/IAE.0b013e3180ed4571. [DOI] [PubMed] [Google Scholar]
- 15.Ormel J, Kempen GI, Penninx BW, Brilman EI, Beekman AT, van Sonderen E. Chronic Medical conditions and mental health in older people: disability and psychosocial resources mediate specific mental health effects. Psychol Med. 1997;27:1065–1077. doi: 10.1017/s0033291797005321. [DOI] [PubMed] [Google Scholar]
- 16.Hahm BJ, Shin YW, Jeon HJ, Seo JM, Chung H, Yu HG. Depression and the vision-related quality of life in patients with retinitis pigmentosa. Br J Opthalmol. 2008;92:650–654. doi: 10.1136/bjo.2007.127092. [DOI] [PubMed] [Google Scholar]
- 17.Brody BL, Gamst AC, Williams R, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108:1893–1901. doi: 10.1016/s0161-6420(01)00754-0. [DOI] [PubMed] [Google Scholar]
- 18.Rovner BW, Casten RJ, Hegel MT, Hauck WW, Tasman WS. Dissatisfaction with performance of valued activities predicts depression in age-related macular degeneration. Int J Geriatr Psychiatry. 2007;22:789–793. doi: 10.1002/gps.1742. [DOI] [PubMed] [Google Scholar]
- 19.Slakter JS, Stur M. Quality of life in patients with age-related macular degeneration: impact of the condition and benefits of treatment. Surv Opthalmol. 2005;50:263–273. doi: 10.1016/j.survophthal.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Šiaudvytytė L, Mitkutė D, Balčiūnienė J. Quality of life in patients with age-related macular degeneration. Medicina (Kaunas) 2012;48:109–111. [PubMed] [Google Scholar]
- 21.Suzukami Y, Oshika T, Yuzawa M, et al. Psychometric properties of the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25), Japanese version. Health Qual Life Outcomes. 2005;3(65):1–11. doi: 10.1186/1477-7525-3-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyman SR, Dibb B, Victor CR, Gosney MA. Emotional well-being and adjustment to vision loss in later life: a meta-synthesis of qualitative studies. Disabil Rehabil. 2012;34:971–981. doi: 10.3109/09638288.2011.626487. [DOI] [PubMed] [Google Scholar]
- 23.Erb C, Batra A, Flammer J, et al. Psychological characteristics of patients with normal tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 1999;237:753–757. doi: 10.1007/s004170050308. [DOI] [PubMed] [Google Scholar]
- 24.Mabuchi F, Yoshimura K, Kashiwagi K, et al. Personality assessment based on the fivefactor model of personality structure in patients with primary open-angle glaucoma. Jpn J Ophthalmol. 2005;49:31–35. doi: 10.1007/s10384-004-0134-3. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi Y, Sato E, Ito A, et al. Comparison of Yatabe-Guilford personality test results in retinitispigmentosa and glaucoma patients. Jpn J Ophthalmol. 2003;47:1–5. doi: 10.1016/s0021-5155(02)00621-4. [DOI] [PubMed] [Google Scholar]
- 26.Mabuchi F, Yoshimura K, Kashiwagi K, et al. High prevalence of anxiety and depression in patients with primary open-angle glaucoma. J Glaucoma. 2008;17:552–557. doi: 10.1097/IJG.0b013e31816299d4. [DOI] [PubMed] [Google Scholar]
- 27.Mabuchi F, Yoshimura K, Kashiwagi K, et al. Risk factors for anxiety and depression in patients with glaucoma. Br J Opthalmol. 2012;96:821–825. doi: 10.1136/bjophthalmol-2011-300910. [DOI] [PubMed] [Google Scholar]
- 28.Wang SY, Singh K, Lin SC. Prevalence and predictors of depression among paticipants with glaucoma in a nationally representative population sample. Am J Opthalmol. 2012;154:436–444. doi: 10.1016/j.ajo.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skalicky S, Goldberg I. Depression and Quality of Life in Patients With Glaucoma: A Cross-sectional Analysis Using the Geriatric Depression Scale-15, Assessment of Function Related to Vision, and the Glaucoma Quality of Life-15. J Glaucoma. 2008;17:546–551. doi: 10.1097/IJG.0b013e318163bdd1. [DOI] [PubMed] [Google Scholar]
- 30.Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The psychosocial impact of macular degeneration. Arch Opthalmol. 1998;116:514–520. doi: 10.1001/archopht.116.4.514. [DOI] [PubMed] [Google Scholar]
- 31.Jampel HD, Frick KD, Janz NK, Wren PA, Musch DC, Rimal R, Lichter PR CIGTS Study Group. Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol. 2007;144:238–244. doi: 10.1016/j.ajo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 32.Janz NK, Wren PA, Guire KE, Musch DC, Gillespie BW, Lichter PR Collaborative Initial Glaucoma Treatment Study. Fear of blindness in the Collaborative Initial Glaucoma Treatment Study: patterns and correlates over time. Ophthalmology. 2007;114:2213–2220. doi: 10.1016/j.ophtha.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Odberg T, Jakobsen JE, Hultgren SJ, Halseide R. The impact of glaucoma on the quality of life of patients in Norway. I. Results from a self-administered questionnaire. Acta Ophthalmol Scand. 2001;79:116–120. doi: 10.1034/j.1600-0420.2001.079002116.x. [DOI] [PubMed] [Google Scholar]
- 34.Janelle CM, Singer RN, Williams AM. External distraction and attentional narrowing: visual search evidence. Journal of Sport and Exercise Psychology. 1999;21:70–91. [Google Scholar]
- 35.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–53. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 36.Williams JM, Tonymon P, Andersen MB. Effects of life-event stress on anxiety and peripheral narrowing. Behav Med. 1990;16:174–181. doi: 10.1080/08964289.1990.9934606. [DOI] [PubMed] [Google Scholar]
- 37.Janelle CM. Anxiety, arousal and visual attention: a mechanistic account of performance variability. J Sports Sci. 2002;20:237–251. doi: 10.1080/026404102317284790. [DOI] [PubMed] [Google Scholar]
- 38.Rogers TJ, Alderman BL, Landers DM. Effects of life-event stress and hardiness on peripheral vision in a real-life stress situation. Behav Med. 2003;29:21–26. doi: 10.1080/08964280309596171. [DOI] [PubMed] [Google Scholar]
- 39.Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proc Natl Acad Sci USA. 2007;104:383–388. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bittner AK, Diener-West M, Dagnelie G. A survey of photopsias in self-reported retinitis pigmentosa: location of photopsias is related to disease severity. Retina. 2009;29:1513–1521. doi: 10.1097/IAE.0b013e3181af0d57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bittner AK, Haythorthwaite JA, Diener-West M, Dagnelie G. Photopsias are related in part to Perceived Stress and Positive Mood in Retinitis Pigmentosa. Eye. 2012;26:101–108. doi: 10.1038/eye.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore LW, Miller M. Older men’s experiences of living with severe visual impairment. J Adv Nurs. 2003;43:10–18. doi: 10.1046/j.1365-2648.2003.02668.x. [DOI] [PubMed] [Google Scholar]
- 43.Tolman J, Hill RD, Kleinschmidt JJ, Gregg CH. Psychosocial adaptation to visual impairment and its relationship to depressive affect in older adults with age-related macular degeneration. The Gerontologist. 2005;45:747–753. doi: 10.1093/geront/45.6.747. [DOI] [PubMed] [Google Scholar]
- 44.Bittner AK, Edwards L, George M. Coping Strategies to Manage Stress related to Vision Loss and Fluctuations in Retinitis Pigmentosa. Optometry. 2010;81:461–468. doi: 10.1016/j.optm.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiser AK, Dagnelie G. Reported effects of non-traditional treatments and complementary and alternative medicine by retinitis pigmentosa patients. Clin Exp Optom. 2008;91:166–176. doi: 10.1111/j.1444-0938.2007.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhee DJ, Spaeth GL, Myers JS, et al. Prevalence of the use of complementary and alternative medicine for glaucoma. Ophthalmology. 2002;109:438–443. doi: 10.1016/s0161-6420(01)01030-2. [DOI] [PubMed] [Google Scholar]
- 47.Astin JA, Shapiro SL, Eisenberg DM, Forys KL. Mind-body medicine: state of the science, implications for practice. J Am Board Fam Pract. 2003;16:131–147. doi: 10.3122/jabfm.16.2.131. [DOI] [PubMed] [Google Scholar]
- 48.Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a metaanalysis. J Psychosom Res. 2010;68:539–544. doi: 10.1016/j.jpsychores.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Gayner B, Esplen MJ, Deroche P, et al. A randomized controlled trial of mindfulness-based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. J Behav Med. 2012;35:272–285. doi: 10.1007/s10865-011-9350-8. [DOI] [PubMed] [Google Scholar]
- 50.Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75:1141–1149. doi: 10.1212/WNL.0b013e3181f4d80d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lovas DA, Barsky AJ. Mindfulness-based cognitive therapy for hypochondriasis, or severe health anxiety: a pilot study. J Anxiety Disord. 2010;24:931–935. doi: 10.1016/j.janxdis.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Vølestad J, Sivertsen B, Nielsen GH. Mindfulness-based stress reduction for patients with anxiety disorders: evaluation in a randomized controlled trial. Behav Res Ther. 2011;49:281–288. doi: 10.1016/j.brat.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Allen NB, Chambers R, Knight W. Mindfulness-based psychotherapies: a review of conceptual foundations, empirical evidence and practical considerations. Aust N Z J Psychiatry. 2006;40:285–294. doi: 10.1080/j.1440-1614.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 54.Lehrer PM, Carr R, Sargunaraj D, Woolfolk RL. Stress Management Techniques: Are they all Equivalent, or do they have Specific Effects? Biofeedback Self Regul. 1994;19:353–401. doi: 10.1007/BF01776735. [DOI] [PubMed] [Google Scholar]
- 55.Brown LA, Forman EM, Herbert JD, Hoffman KL, Yuen EK, Goetter EM. A randomized controlled trial of acceptance-based behavior therapy and cognitive therapy for test anxiety: a pilot study. Behav Modif. 2011;35:31–53. doi: 10.1177/0145445510390930. [DOI] [PubMed] [Google Scholar]
- 56.Finucane A, Mercer SW. An exploratory mixed methods study of the acceptability and effectiveness of Mindfulness-Based Cognitive Therapy for patients with active depression and anxiety in primary care. BMC Psychiatry. 2006;6:14. doi: 10.1186/1471-244X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown D, Forte M, Dysart M. Differences in visual sensitivity among mindfulness meditators and non-meditators. Percept Mot Skills. 1984;58:727–733. doi: 10.2466/pms.1984.58.3.727. [DOI] [PubMed] [Google Scholar]
- 58.Hodgins HS, Adair KC. Attentional processes and meditation. Conscious Cogn. 2010;19:872–878. doi: 10.1016/j.concog.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Conscious Cogn. 2009;18:176–186. doi: 10.1016/j.concog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 60.van den Hurk PA, Giommi F, Gielen SC, Speckens AE, Barendregt HP. Greater efficiency in attentional processing related to mindfulness meditation. Q J Exp Psychol (Hove) 2010;63:1168–1180. doi: 10.1080/17470210903249365. [DOI] [PubMed] [Google Scholar]
- 61.Schulzer M. Errors in the diagnosis of visual field progression in normal-tension glaucoma. Ophthalmology. 1994;101:1589–1595. doi: 10.1016/s0161-6420(94)31133-x. [DOI] [PubMed] [Google Scholar]
- 62.Jansonius NM. On the accuracy of measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2010;94:1404–1405. doi: 10.1136/bjo.2009.164897. [DOI] [PubMed] [Google Scholar]
- 63.Nouri-Mahdavi K, Zarei R, Caprioli J. Influence of Visual Field Testing Frequency on Detection of Glaucoma Progression With Trend Analyses. Arch Ophthalmol. 2011;129:1521–1527. doi: 10.1001/archophthalmol.2011.224. [DOI] [PubMed] [Google Scholar]