Abstract
The hemicholinium-3 (HC-3) sensitive, high-affinity choline transporter (CHT) sustains cholinergic signaling via the presynaptic uptake of choline derived from dietary sources or from acetylcholinesterase (AChE)-mediated hydrolysis of acetylcholine (ACh). Loss of cholinergic signaling capacity is associated with cognitive and motor deficits in humans and in animal models. Whereas genetic elimination of CHT has revealed the critical nature of CHT in maintaining ACh stores and sustaining cholinergic signaling, the consequences of elevating CHT expression have yet to be studied. Using bacterial artificial chromosome (BAC)-mediated transgenic methods, we generated mice with integrated additional copies of the mouse Slc5a7 gene. BAC–CHT mice are viable, appear to develop normally, and breed at wild-type (WT) rates. Biochemical studies revealed a 2 to 3-fold elevation in CHT protein levels in the CNS and periphery, paralleled by significant increases in [3H]HC-3 binding and synaptosomal choline transport activity. Elevations of ACh in the BAC–CHT mice occurred without compensatory changes in the activity of either choline acetyltransferase (ChAT) or AChE. Immunohistochemistry for CHT in BAC–CHT brain sections revealed markedly elevated CHT expression in the cell bodies of cholinergic neurons and in axons projecting to regions known to receive cholinergic innervation. Behaviorally, BAC–CHT mice exhibited diminished fatigue and increased speeds on the treadmill test without evidence of increased strength. Finally, BAC–CHT mice displayed elevated horizontal activity in the open field test, diminished spontaneous alteration in the Y-maze, and reduced time in the open arms of the elevated plus maze. Together, these studies provide biochemical, pharmacological and behavioral evidence that CHT protein expression and activity can be elevated beyond that seen in wild-type animals. BAC–CHT mice thus represent a novel tool to examine both the positive and negative impact of constitutively elevated cholinergic signaling capacity.
Keywords: Acetylcholine, Choline, Transport, Slc5a7, Hemicholinium-3
1. Introduction
The high-affinity choline transporter (CHT, SLC5A7) is a Na+/Cl− symporter that imports extracellular choline into presynaptic terminals, thereby supporting the synthesis of the neurotransmitter acetylcholine (ACh) (Apparsundaram et al., 2000; Haga and Noda, 1973; Iwamoto et al., 2006; Okuda et al., 2000). The Ach synthesizing enzyme, choline acetyltransferase (ChAT), has a KM for choline that is higher than intracellular free choline levels, whereas the KM of CHT for choline indicates the transporter is likely saturated with substrate under most physiological conditions (Cooper et al., 2003; Ferguson et al., 2003; Klein et al., 1993, 1991). Consequently, the plasma membrane uptake of choline and not ChAT enzymatic activity is thought to be rate-limiting for ACh synthesis. Consistent with this idea, application of the competitive CHT-inhibitor hemicholinium-3 (HC-3) eliminates the ability of cholinergic terminals to maintain ACh production and release (Apparsundaram et al., 2000; Guyenet et al., 1973; Mulder et al., 1974), and when the drug is peripherally administered, paralysis and death can ensue (Freeman et al., 1982). Additionally, choline uptake capacity can be upregulated during periods of elevated firing by the delivery of additional transporter molecules to the plasma membrane (Antonelli et al., 1981; Apparsundaram et al., 2005; Ferguson et al., 2003) due to the intracellular localization of CHT arises on a subset of cholinergic synaptic vesicles (Ferguson et al., 2003). Thus, when ACh release is augmented, fusion of CHT-containing vesicles during exocytosis insures sufficient recapture of choline to maintain adequate rates of ACh synthesis.
Previously, we generated and characterized a CHT knockout (KO) mouse as a transgenic model of constitutive cholinergic hypofunction. Homozygous deletion of CHT caused perinatal death (Ferguson et al., 2004) but heterozygous CHT KO (CHT+/−) mice are a viable model of cholinergic hypofunction, with significant reductions in CHT protein expression and ACh levels in tissues that receive cholinergic innervation (Bazalakova et al., 2007; English et al., 2010; Parikh et al., 2013). CHT+/− mice also display physiological and behavioral deficits under conditions where cholinergic transmission is taxed, including treadmill endurance (Bazalakova et al., 2007), cardiovascular performance (English et al., 2010), and during a sustained attention task that requires phasic cortical ACh transmission (Parikh et al., 2013).
Efforts to remedy cholinergic hypofunction in conditions such as myasthenia or Alzheimer’s disease have traditionally focused on inhibiting AChE, but such treatments prevent the enzyme from restricting the overflow of ACh to neighboring synapses and are difficult to tailor to the activity states of different cholinergic terminals. The coupling of CHT trafficking and function to neuronal activity suggest that novel therapies could target the elevation of CHT protein expression, membrane-trafficking or functional transport. CHT-targeted therapies could allow for an increase in the capacity for ACh release while retaining normal temporal and spatial dynamics of ACh signaling, thus providing significant improvement over current pharmacological agents. At present it is not known whether elevated CHT protein expression necessarily leads to increased choline uptake capacity and ACh synthesis, particularly as the majority of transporters are located intracellularly, where they cannot function. We tested whether elevation of CHT protein expression could enhance high-affinity choline uptake by engineering mice that constitutively overexpress CHT using bacterial artificial chromosome (BAC)-mediated transgenic methods. Here we present evidence that BAC–CHT mice overexpress CHT protein in cholinergic neurons without apparent ectopic expression, and that the overexpression of CHT protein leads to functional enhancement of high-affinity choline uptake, increased ACh synthesis, and behavioral changes consistent with augmented ACh transmission.
2. Materials and methods
2.1. Animal care and husbandry
All procedures were performed under an approved protocol reviewed annually by the Vanderbilt Institutional Animal Care and Use Committee (IACUC). All animals tested were congenic on a C57BL/6J background and housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved mouse housing facility on a 12:12 light-dark cycle with food and water provided ad libitum.
2.2. Generation of BAC–CHT transgenic mice
The BAC construct used for transgenic animal production was obtained from the BACPAC Resources Center at the Children’s Hospital of Oakland Research Institute (http://bacpac.chori.org). The selected clone, RP24-290K23 (C57BL/6J background), houses a portion of mouse chromosome 17 inclusive of the full Slc5a7 gene, as well as genomic regions both upstream and downstream of the CHT coding sequence. No other gene is known to lie within the BAC genomic insert. Following purification of the BAC–CHT construct by CsCl gradient centrifugation, DNA was injected into single cell embryos in the Vanderbilt Center for Transgenic/Embryonic Stem Cell Shared Resource Core. Surviving embryos were implanted into pseudo-pregnant C57BL/6J mice. Progeny were screened for BAC integration by PCR performed using genomic DNA obtained from tail samples with the following primers: sense-5′-GCC TAC TCG TAA GTA GTC C-3′ antisense-5′-TGG AAG CCA TCA CAA AGG-3′. Both primers anneal to sequences within the CmR gene of the bacterial vector. The resulting ~400 bp PCR product is therefore only amplified from BAC–CHT positive animals. Both copy number and transmission profiles obtained from genotyped breeders were used to discern single (hemizygous) vs. double (homozygous) chromosome integration. Control primers (3′-TCC ATC AAG CCT GTA TCA GC-5′ sense, 3′-AGA GGA GCA GAC TGT GGA AAC-5′ antisense) directed against the unrelated proline transporter were included in the reaction to distinguish between CHT WT animals and failed reactions. Two founder animals were generated that genotyped positive for multi-copy BAC integration, though one died prior to breeding. Multiple, additional attempts to generate other founders proved unsuccessful.
2.3. Estimation of BAC genomic copy number in BAC-CHT Mice
BAC copy number was estimated by qPCR using a LightCycler 480 Real-Time PCR System (Roche Applied Science, Indianapolis, IN, USA). Oligonucleotide primers were designed to amplify sequences within exon 9 and within exon 3 of Slc5a7 or to match BAC vector sequences. qPCR amplification of Slc5a7 sequences were performed using DNA from both BAC–CHT and WT C57BL/ 6J mice.
2.4. Immunoblotting of CHT protein
To quantify CHT protein levels, age-matched, adult BAC–CHT and WT mice were rapidly decapitated and brains and peripheral tissues were removed and dissected on ice. Tissues were homogenized in ice-cold buffer (0.32 M sucrose, 5 mM HEPES, pH 7.4) and either taken as total tissue extracts or further processed as synaptosomes by centrifugation at 1000g for 15 min at 4 °C. Supernatants were centrifuged at 15,000g for 15 min at 4 °C and the resulting pellets (P2) were resuspended in lysis buffer (100 mM Tris-HCl, 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.1% SDS, and 1% Triton X-100) +protease inhibitor cocktail (PI, Sigma P8340). Peripheral tissues were disrupted in ice-cold buffer using a Polytron homogenizer (Brinkmann Instruments, Westbury, NY) and centrifuged at 3650g for 20 min. The tissue pellet was resuspended in lysis buffer + PI and incubated overnight at 4 °C. Solubilized protein concentrations were determined using the bicinchoninic acid method (Pierce BCA Protein Assay). Samples containing equal amounts of total protein for SDS–PAGE were prepared in Laemmli loading buffer (10% glycerol, 1% SDS, 0.004% bromophenol blue), incubated overnight at 4 °C, and heated to 50 °C for 15 min prior to loading. Samples were resolved on Novex 4–12% Bis-Tris gradient acrylamide gels in MOPS running buffer (Life Technologies, Piscataway, NJ) and transferred to PVDF membrane (1 mM CAPS + 10% methanol, 50 V, 2 h) for immunoblotting. Following transfer, membranes were pretreated for 15 min at 55 °C in stripping buffer (25 mM glycine + 0.1% SDS, pH 2.0) and rinsed 3×5 min in 0.1 M phosphate buffered saline, pH 7.4 (PBS) + 0.5% Tween-20 (PBS-T). Blots were blocked in PBS-T +5% non-fat dry milk (PBS–TM) for at least 30 min and incubated in PBS containing 5% BSA and primary antibody (mouse anti-CHT, 1:1000, or rabbit anti-CHT 1:1000 (Ferguson et al., 2003)) overnight at 4 °C. Blots were rinsed 3 × 5 min in PBS−TM and incubated in HRP-conju-gated secondary antibody (goat anti-mouse, 1:5000 in PBS−TM, Jackson Immunoresearch, West Grove, PA) for 30 min at room temperature. Blots were rinsed 3 × 5 min in PBS-TM, 2 × 5 min in PBS–T, 3 × in PBS, and developed by chemiluminescence (Western Lightning Enhanced Chemiluminescence kit, Perkin Elmer, Waltham, MA). Blots were exposed to photographic film (GE Hyperfilm, GE Healthcare, Buckinghamshire, UK) and developed using a Konica Minolta SRX101A development system (Konica Minolta Medical Graphic Inc., USA). After imaging CHT-immunoreactive bands, blots were stripped for 15 min at 50 °C, rinsed 3 × 5 min in PBS–T, and re-probed for synaptophysin (brain samples) or PP2A (peripheral tissue) using rabbit anti-synaptophysin, (1:10,000, EMD Millipore Corporation, Billerica, MA) or mouse anti-PP2A (1:1000, BD Biosciences, San Jose, CA), developed with HRP-conjugated goat anti-rabbit or goat-anti-mouse antibodies (1:5000, Jackson Immunoresearch). Films were scanned and digitized using a CanoScan 8800F (Canon, Lake Success, NY) and band density was quantified using ImageJ software. Multiple exposures were taken to ensure quantitation within the linear range of the film. In order to compensate for inter-experiment variability, CHT bands were normalized to synaptophysin or PP2A bands and the CHT/SYPH (or CHT/PP2A) ratio of BAC animals was then expressed as a percentage of the WT control in each blot. To determine whether normalized CHT levels were higher in BAC–CHT animals than in WT, these percentages were transformed to a log scale, and significant increases in the CHT/SYPH or CHT/PP2A ratios were detected by testing the hypothesis that the log (% WT ratio) > 2.0 (i.e., that the BAC CHT/SYPH or CHT/PP2A, expressed as % WT was greater than 100%), using GraphPad Prism. The log transformation was necessary in order to use the parametric t-test without violating assumptions of normality of the underlying distributions.
2.5. Hemicholinium-3 (HC-3) radioligand binding assays
Mice were rapidly decapitated and the brains were removed and transferred to an iced dissecting platform. Brain regions were dissected and homogenized in 8 vol. of 0.32 M sucrose. Homogenates were centrifuged for 15 min at 1000g and supernatant were further centrifuged at 20,000g for 20 min. The resulting pellets were resuspended in 10 vol. of distilled water and disrupted with a Polytron. Lysates were then centrifuged at 8000g for 20 min and the supernatant and buffy coat were collected and membranes were pelleted at 48,000g for 15 min. Pellets were resuspended in 5 vol. of glycylglycine buffer (0.05 M gly–gly, 0.2 M NaCl, pH 7.8), disrupted by Polytron homogenization and centrifuged at 48,000g for 15 min. The latter steps were repeated 3 ×, and the final pellets were resuspended in 0.7 vol. gly–gly buffer. Membranes were incubated for 30 min at room temperature in 10 nM methyl-[3H]HC−3 diacetate (Perkin Elmer, 120 Ci/mmol)) ± 10 µM unlabeled HC−3 (Sigma)). Binding was quenched by the addition of 10 vol. ice-cold gly–gly buffer. Membrane were harvested on glass fiber filters (Whatman GF/B, Brandel, Gaithersburg, MD) that were pretreated with 0.3% polyethylenimine and 0.2% BSA and rinsed 4 × with 50 mM Tris–HCl/200 mM NaCl. Filters were airdried overnight, solubilized in EcoScint H (National Diagnostics, Atlanta, GA), and radiolabel quantified by liquid scintillation counting (TriCarb 2900TR, Perkin Elmer). Protein content of membrane samples was determined by the BCA protein assay (Pierce).
2.6. Synaptosomal choline transport assays
WT and BAC–CHT mice were decapitated under urethane anesthesia and brains were removed and tissues dissected on an ice-cold petri dish. Crude synaptosomes (P2) were prepared from isolated tissue as described above (Apparsundaram et al., 2005). Right frontal cortices and striatal tissue from two mice were pooled. Aliquots (50 µL) of crude synaptosomes were incubated with 100 µL [3H]-methyl choline chloride (0.02–6.0 µM, Perkin Elmer) in Kreb’s bicarbonate buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 25 mM NaHCO3, 1.7 mM CaCl2, 10 mM glucose, 100 mM ascorbic acid, and 10 mM physostigmine) in the presence and absence of 10 µM HC-3 for 5 min at 37 °C. Transport assays were terminated by transferring the tubes to an ice bath followed by rapid filtration over a Brandel cell harvester (Brandel Inc., Gaithersburg, MD). Accumulated radioactivity was determined by scintillation spectrometry. Protein concentrations were measured using the Pierce BCA protein assay kit (Thermo Fisher Scientific Inc., Rockford, IL). CHT-mediated choline uptake was determined as total choline uptake minus the uptake obtained in the presence of 10 µM HC-3. Maximum transporter velocity (VMax) and affinity for choline (KM) were determined using nonlinear Michaelis-Menten curve fits in Prism 5 (GraphPad).
2.7. Assay of ACh and choline levels
Fresh brain tissues were dissected as for HC-3 binding and choline uptake. Samples of crude homogenates and the P2 synaptosomal fraction were briefly centrifuged to remove the sucrose solution and the resulting pellets were frozen on dry ice and stored at −80 °C until HPLC analysis of ACh and choline content by the Neurochemistry Core of the Vanderbilt Brain Institute. Prior to analyses, frozen pellets were homogenized in 250 µL acetonitrile using an ultrasonic dismembrator and then centrifuged at 13000g for 30 min. Acetonitrile extracts were transferred to a clean tube and washed with twice with 125 µL heptane. The acetonitrile layer was then evaporated under nitrogen gas and mixed with 75 µL of the HPLC mobile phase (37.5 mM H3PO4, pH 8.5). From these samples, 50 µL was injected into the equilibrated HPLC, composed of a Waters 717 + autosampler, Water model 515 pump and Antec Decade electrochemical detector. ACh and choline were separated on a BASi MF-6150 analytical column (Bioanalytical Systems, West Lafayette, IN) and then subjected to an AChE/choline oxidase, post-column, immobilized enzyme reactor. H2O2 generated by choline oxidase was detected amperometrically with a platinum working electrode set at +400 mV.
2.8. ChAT and AChE activity assays
2.8.1. ChAT assays
ChAT activity was evaluated by measuring the formation of [14C] ACh from [14C] acetyl CoA and unlabeled choline chloride. Brain regions were rapidly dissected on ice and disrupted with a Polytron in 50 mM Tris–HCl containing 0.02% Triton X-100 and protease inhibitor cocktail (Sigma). Crude extracts were added to an equal volume of reaction buffer containing 600 mM NaCl, 100 mM NaHPO4 (pH 7.4), 20 mM EDTA, 0.2 mM eserine, 0.1 mg/ mL BSA, 16 mM choline chloride, 0.4 mM acetyl [14C] CoA (40–60 Ci/mmol), Perkin Elmer). Reactions proceeded for 20 min at 37 °C and were then quenched by the addition of 80 µL of a 17:3 mixture of toluene:acetonitrile with 5 g/L of sodium-tetra-phenylboron. Samples were centrifuged briefly at 13,000 rpm to separate the organic phase containing the [14C] ACh product. The upper layer was removed to scintillation vials, mixed with scintillation fluid and counted on a TriCarb liquid scintillation counter. Assays using no tissue were performed in parallel and counts subtracted to yield specific enzyme activity.
2.8.2. AChE assays
AChE activity was measured using the Amplex Red ACh/AChE Assay Kit (Sigma, A12217). Crude extracts of brain tissue were obtained as detailed above. Equal volumes of extract and reaction buffer containing 400 µM Amplex Red reagent, 0.2 U/mL choline oxidase, and 100 µM ACh were incubated together at room temp for 30 min with room light shielding. Product was detected via 590 nm fluorescence elicited with 550 nm excitation on a Flexstation fluorescence microplate reader (Molecular Devices Corp., Sunnyvale, CA).
2.9. Immunohistochemical analysis of CNS–CHT expression
Adult mice were terminally anesthetized with sodium pentobarbital (Nembutal, 100 mg/kg i.p.) and perfused transcardially with heparinized saline (10 U/mL in 0.9% NaCl) followed by paraformaldehyde, lysine and sodium meta-periodate fixative (PLP fix: 4% paraformaldehyde, 1.4% lysine acetate, 0.1% sodium meta-periodate (McLean and Nakane, 1974)). Brains were removed, post-fixed overnight in the same fixative, then cryoprotected in 30% sucrose and cut to 30 µm on a freezing microtome. Sections were collected in 0.1 M phosphate buffer, pH 7.4 (PB) and treated for 30 min in 1% sodium borohydride, followed by several rinses in PB.
2.9.1. Immunoperoxidase staining
Sections were rinsed 3 × 5 min in 0.1 M Trizma-buffered saline, pH 7.6 (TBS), then incubated for 30 min in a blocking solution containing 3% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.5% Triton X-100. Sections were then incubated in primary antibody diluted in the same blocking solution (rabbit anti-CHT, 1:2000; Ferguson et al., 2003) overnight at 4 °C. Sections were rinsed in TBS 3 × 5 min, then incubated in biotinylated secondary antibody (goat anti-rabbit, 1:400, Jackson Immunoresearch), diluted in blocking solution, for 30 min. Sections were rinsed 3 × 10 min in TBS, and then incubated in an avidin-biotin peroxidase complex (VectaStain kit, Vector Labs, Burlingame, CA) for 30 min. Sections were rinsed 3 × 5 min in TBS prior to development in diaminobenzidine (DAB substrate kit, Vector Labs) for 3–5 min. Reactions were quenched by rinsing several times in TBS. Finished sections were rinsed in PBS 2 × 5 min before mounting on glass slides (Superfrost, ThermoFisher Scientific, Pittsburgh, PA) and coverslipping with Aqua Poly/Mount (Polysciences, Inc., Warrington, PA).
2.9.2. Immunofluorescence staining
Sections were rinsed 3 × 5 min in 0.01 M PBS, then incubated for 30 min in a blocking solution containing 5% non-fat dry milk, 0.1% lysine acetate, 0.1% glycine, 3% normal donkey serum and 0.5% Triton X-100. Sections were then incubated in primary antibodies overnight at 4 °C: rabbit anti-CHT (1:2000) and mouse anti-GAD-67 (MAB5406 1:2000; Millipore, Billerica, MA); mouse anti-CHT (1:1000) and rabbit anti-VAChT (1:2000; Synaptic Systems, Göttingen, Germany); or mouse anti-CHT (1:1000) and goat anti-ChAT (AB144P, 1:500; Millipore). Sections were rinsed 3 × 10 min in blocking solution, then incubated for 1 h at room temperature in the appropriate secondary antibodies: Alexa 594-conjugated Donkey anti-rabbit, Alexa 488-conjugated Donkey anti-mouse, Alexa 594-conjugated Donkey anti-goat (1:400, Life Technologies, Grand Island, NY). During these and subsequent steps, sections were protected from light to prevent bleaching of the fluorophores. Excess secondary antibodies were removed by rinsing 4 × 5 min in PBS prior to mounting and coverslipping stained sections.
2.9.3. Microscopy and image processing
Peroxidase sections were examined on a Zeiss Axiophot upright microscope and digitally photographed with a CoolSnap cf CCD camera (Photometrics, Tucson, AZ) and image acquisition software (Open Lab 5.5.0, Perkin Elmer) running on an Apple Mac Pro computer (Apple, Cupertino, CA). Dual immunofluorescence images were acquired using a Zeiss LSM 510 confocal microscope provided by the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY008126). All files were exported in TIFF format and adjusted in GIMP (Gnu Image Manipulation Program, v 2.8) for optimal contrast and brightness and to generate final figures.
2.10. Behavioral analyses
All behavioral experiments were performed in the Vanderbilt Laboratory for Neurobehavior Core Facility operated by the Vanderbilt Brain Institute, using animals generated from crosses of hemizygous BAC–CHT × WT C57 mice. BAC–CHT and WT littermates were weighed weekly from P14 until P63. Average mass was plotted separately for male and female animals and analyzed, as were all subsequent behavioral tests, using GraphPad Prism (v 5.0, GraphPad Software, Inc., La Jolla, CA), with post hoc comparisons noted in the figure legends. Behavioral tests were performed with male, adult animals (10–20 weeks). Each testing apparatus was cleaned thoroughly with 70% ethanol between each subject and unless otherwise specified, all testing was carried out under normal lighting conditions (250–300 lux).
2.11. Behavioral procedures
2.11.1. Wire hang
Gross motor function was examined in a basic wire hang test. Mice were allowed to hang via their forepaws on a plastic wire (14 gauge) suspended 35 cm above the surface. Mice were able to use their back paws for balance and support during the test. Latency to fall into an arena with fresh bedding was recorded for up to 120 s, at which point mice were removed from the wire and returned to the home cage. All mice were tested on 3 trials, with a 5 min inter-trial interval, and the average of all trials used for comparative analysis.
2.11.2. Grip strength
Mice were allowed to grip with their forepaws a wire grid attached to a force gauge (San Diego Instruments, San Diego, CA). The experimenter then gently pulled on the mouse’s tail until grip was released. Grip strength was recorded via a digital gauge that locked in the peak force recorded for each trial. The average of five consecutive trials per mouse was used for analysis.
2.11.3. Treadmill testing
Mice were tested for running endurance on a 6 lane motorized treadmill with an electric shock grid at one end (Columbus Instruments; Columbus OH). On the first test day, mice were habituated to the test apparatus for 5 min with the treadmill and shock turned off. The shock grid was turned on for 5 min, followed by activation of the treadmill at 5 m/min. The speed was increased by 1 m/min every 30 s to a maximum speed of 15 m/min. After 1 h, speed was again increased 1 m/min every 30 s to a maximum of 18 m/ min. The second day of testing was identical except the maximum speed for the first hour was 18 m/min, and the final maximum speed was 21 m/min. Exhaustion was defined as resting on the electric grid for more than 15 s/min or falling back onto the grid more than 15times/min. The time to exhaustion and maximum speed achieved were recorded and analyzed for both days using Prism software.
2.11.4. Open field locomotion
Mice were placed in activity monitoring chambers (27 × 27 × 20 cm, ENV-510, Med Associates Inc., St. Albans, VT) equipped with 16 infrared beams to monitor movement and position in the x, y, and z planes at 50 ms intervals. Locomotor activity was recorded for 30 min and analyzed in 5 min epochs using activity monitor software (Med Associates) and microsoft access and excel.
2.11.5. Spontaneous alternation
Each mouse was placed in the middle of a 3-armed Y-maze with 30 cm × 5 cm arms radiating from the center at equal angles. Walls were 10 cm high and made of clear Plexiglas with a clear Plexiglas top. Arm entries were defined by the presence of all four-paws in an arm. Each trial lasted 7 min and was scored via live video feed in an adjacent room by an observer blinded to genotype. The maze was washed with 70% ethanol between trials.
2.11.6. Elevated plus maze (EPM)
Mice were between 12 and 13 weeks old during the EPM test. Each mouse was placed in the middle of a 4-armed maze with arms radiating from the center in the shape of a plus symbol (+). Opposite arms were identical and either without walls (open arms) or with black plastic walls 15 cm high (closed arms). Each arm was 30 cm long and 5 cm wide. The maze was divided into 5 zones (each arm, and the center) and the mouse was considered to be in an arm zone only when it had all four paws in the arm; otherwise the mouse was considered to be in the center zone. Data were analyzed as percentage of time spent in open arms, closed arms, or the center zone relative to the total trial length. Each trial lasted 5 min and was scored by the ANY-maze software package (Stoelting, Wood Dale, IL). Automatic scoring was validated and corrected as necessary by an observer blinded to genotype.
3. Results
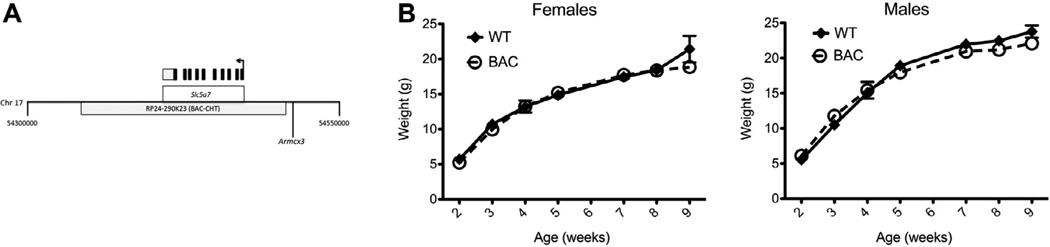
In order to maximize the likelihood of including relevant regulatory sequences and thereby limit the sites of transgene expression to endogenous cells expressing CHT, we pursued transgenic overexpression of the transporter using a C57BL/6J BAC that encompasses the Slc5a7 gene with 45 kb of sequence 5′ and 90 kb of sequence 3′ of the CHT transcription site, as illustrated in Fig. 1A. This BAC was selected over others to avoid a neighboring gene, Armcx3, present 50 kb 5′of Slc5a7. Quantitative PCR for estimation of BAC copy number in the CHT founder revealed ~20 copies of the transgene integrated in hemizygous animals (data not shown). BAC–CHT mice appeared morphologically to be normal at birth, and underwent similar postnatal weight gain as their WT littermates, shown in Fig. 1B and C. Two way repeated measures ANOVA showed significant main effects of time for both male (P < 0.0001) and female animals (P < 0.0001), with no effect of genotype for either sex (both P >0.05). There was a significant interaction effect for males (P = 0.013), but this accounted for less than 1% of the variance in the data and was therefore was considered to have limited biological relevance.
Fig. 1.
Production and growth of BAC–CHT mice. (A) Illustration of C57BL/6J genomic insert in BAC construct RP24-290K23, showing the span, transcription initiation site (arrow) and exons (black boxes) of the Slc5a7 gene on chromosome 17, including its 3′ untranslated region (grey box). The Armcx3 gene noted lies outside the RP24-290K23 genomic insert. (B) BAC–CHT female (N = 5 each genotype) and male (N = 7 each genotype) mice exhibited normal postnatal weight gain as compared to WT littermates.
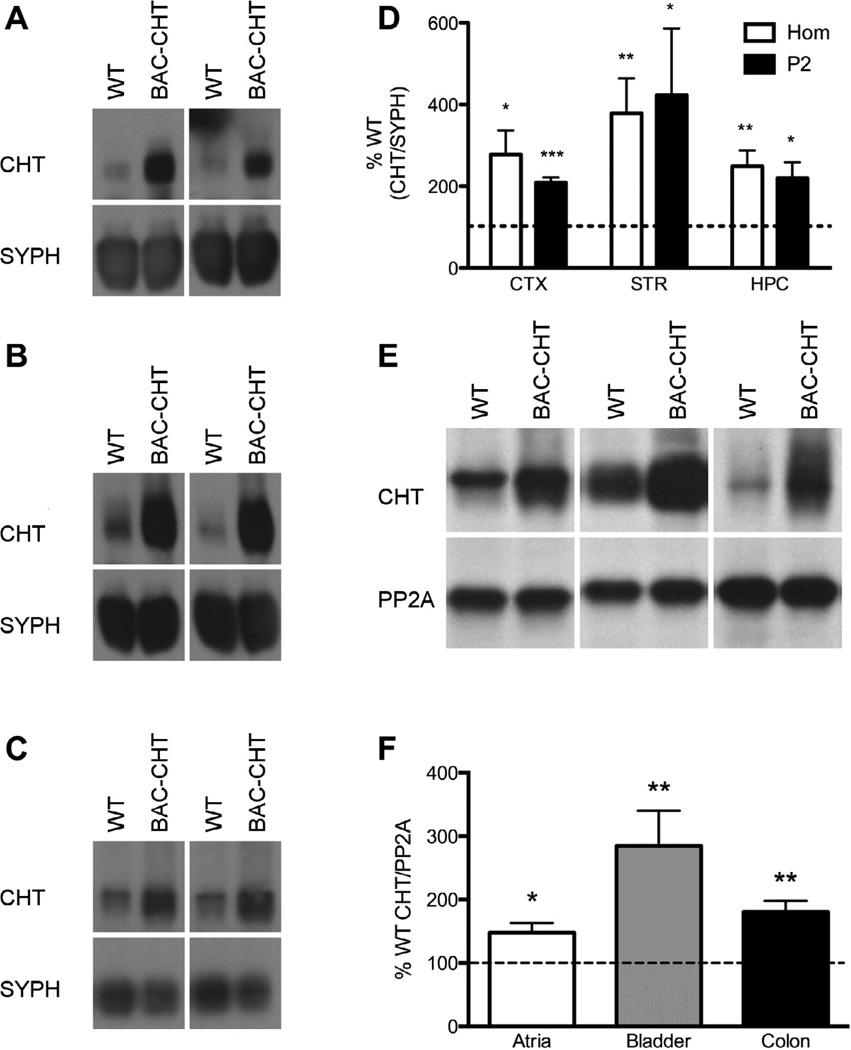
We next evaluated CHT protein expression in brain homogenates and synaptosome-enriched fractions (P2) prepared from cerebral cortex, striatum and hippocampus, using antibodies that have been previously characterized for immunoblotting of CHT (Ferguson et al., 2003). Representative blots are presented in Fig. 2A – C. CHT band density was normalized to that of synaptophysin (SYPH) from the same blot, and normalized BAC–CHT levels were then expressed as a percentage of WT levels from the same experiment. CHT/SYPH values were significantly greater in crude homogenates and P2 synaptosomal fractions from the hippocampus of BAC–CHT animals vs. WT littermates. Expressed as a percentage of the WT levels in the same blots, and illustrated in Fig. 2D, CHT/SYPH ratios of BAC–CHT samples were found to be significantly greater than 100% in homogenate and P2 samples prepared from the cortex ((mean ± SEM) Hom = 318.4 ± 1.3%, P = 0.013; P2 = 224.4 ± 1.1%, P = 0.0008), striatum (Hom = 305.5 ± 1.3%, P = 0.005; P2 = 346.7 ± 1.4%, P = 0.0129), and hippocampus (Hom = 239.3 ± 1.2%, P = 0.004; P2 = 207.5 ± 1.2%, P = 0.014; one tailed t-tests).
Fig. 2.
Elevated CHT protein expression in BAC–CHT mice. (A)–(C) Blots of WT and BAC–CHT extracts of crude homogenates or P2 synaptosomal preparations of mice from (A) cortex, (B) striatum, and (C) hippocampus. Synaptophysin (Syph) was blotted to normalize CHT expression for quantitative comparisons. (D) Quantitative densitometry showed normalized CHT levels in BAC–CHT animals were significantly greater than WT (100%, dotted line) in all regions. (E) Immunoblotting of CHT in extracts prepared from the atria, bladder, and colon of WT and BAC–CHT mice. PP2A immunoreactive bands were used to normalize CHT levels for quantification. (F) Quantitative analysis of CHT band density indicate elevated CHT protein levels in all peripheral tissues sampled. The dotted line represents 100% WT CHT/PP2A level. *P < 0.05, **P < 0.01, ***P < 0.001.
Many peripheral tissues receive parasympathetic cholinergic innervation that prior studies have demonstrated elaborate CHT at presynaptic varicosities and terminals (Guidry et al., 2005; Haberberger et al., 2002; Lips et al., 2003). As shown in Fig. 2E and F, and consistent with our CNS analyses, normalized CHT levels in BAC–CHT tissues were significantly different from WT levels in crude extracts prepared from atria (144.9 ± 1.1%, P = 0.020), bladder (260.6 ± 1.2%, P = 0.004) and colon (177.0 ± 1.1%, P = 0.005). To provide an alternative measure of CHT protein density, we performed [3H] HC-3 binding assays using synaptosomal membranes from cortex and striatum. Consistent with our immunoblotting studies, HC-3 binding levels were significantly elevated in both the cortex (WT: 65.9 ± 11.6 fmoles/mg protein, BAC: 100.3 ± 17.7 fmoles/mg protein, P = 0.026, one tailed paired t-test) and striatum (WT: 291.9 ± 66.5 fmoles/mg protein, BAC: 594.1 ± 89.8 fmoles/mg protein, P = 0.008, one tailed paired t-test).
The vesicular localization of CHT provides a mechanism for effecting activity-dependent increases in choline uptake through synaptic vesicle fusion (Ferguson et al., 2003; Holmstrand et al., 2010; Nakata et al., 2004); however, this mechanism could also sequester excess transporters in BAC–CHT mice, resulting in little to no change in choline uptake activity, despite significant changes in CHT total protein levels. To determine if high affinity choline uptake was upregulated in BAC–CHT mice, we performed kinetic analyses of HC-3 sensitive choline uptake in synaptosomes. Representative kinetic uptake curves, shown in Fig. 3, revealed significantly elevated choline uptake in synaptosomes prepared from both frontal cortex and striatum. Kinetic parameters obtained from Michaelis–Menten fits of uptake data, provided in Table 1, indicated that increased high-affinity choline uptake was driven by increases in choline transport VMAX as opposed to changes in choline KM.
Fig. 3.
BAC–CHT synaptosomes display a higher capacity for high-affinity choline uptake. (A) and (B) Uptake activity from synaptosomes prepared from (A) the right frontal cortex and (B) the striatum of BAC–CHT (closed circles) and WT mice (open circles). WT n = 5, BAC–CHT n = 4. VMAX values (see Table 1) from BAC–CHT synaptosomes were significantly higher than WT for all regions examined with no changes in KM values between genotypes. (P > 0.05).
Table 1.
Kinetic parameters of high-affinity choline uptake in WT and BAC–CHT mice.
|
VMAX (pmol/mg prot/min) |
KM (µM) |
|||
|---|---|---|---|---|
| WT | BAC–CHT | WT | BAC–CHT | |
| Right FC | 15.26 ± 1.21 | 30.82 ± 1.46* | 1.21 ± 0.18 | 1.14 ± 0.16 |
| Left FC | 13.47 ± 1.13 | 41.54 ± 2.06* | 1.05 ± 0.27 | 1.38 ± 0.19 |
| Striatum | 22.45 ± 2.16 | 55.39 ± 1.29* | 0.86 ± 0.26 | 1.17 ± 0.08 |
P < 0.05, unpaired Student’s t-test.
As noted above, CHT activity, as opposed to enzymatic turnover capacity, is believed to be the rate-limiting factor in ACh synthesis. Therefore, the elevated high-affinity choline uptake observed in BAC–CHT mice should lead to higher tissue levels of ACh. To examine this hypothesis, we used HPLC methods to assess ACh and choline levels in multiple CNS tissues. As shown in Fig. 4A, tissue ACh content was significantly elevated (2 to 3-fold) in all brain regions of the BAC–CHT mice vs. WT littermates ((mean ± SEM) Cortex: WT = 0.21 ± 0.05 nmoles/mg protein, BAC = 0.35 ± 0.03, P = 0.0081; Striatum: WT = 0.53 ± 0.11, BAC = 1.74 ± 0.22, P = 0.001; Hippocampus: WT = 0.23 ± 0.04, BAC = 0.47 ± 0.05, P = 0.008; one tailed Mann Whitney U test). Tissue choline levels were also elevated in the striatum (WT = 1.06 ± 0.23, BAC = 2.16 ± 0.32 nmoles/mg protein, P = 0.010, one tailed MWU) but did not differ significantly by genotype in hippocampal or cortical samples (P > 0.05) (Fig. 4B). The higher ACh levels we observed in BAC–CHT mice did not result from an enhancement of ChAT activity (Fig 4C; two way ANOVA, P = 0.676), nor were they associated with changes in AChE activity (Fig 4D; P = 0.684).
Fig. 4.
CHT overexpression leads to elevated tissue levels of ACh. (A) HPLC analyses of cortical, striatal, and hippocampal extracts demonstrate uniform elevations of ACh in BAC–CHT vs. WT mice Cortex, Hippocampus n = 4 (WT), 8 (BAC–CHT). Striatum n = 5 (WT), 9 (BAC–CHT) (B) Tissue choline levels were elevated in the striatum of BAC–CHT mice, but not in the cortex or hippocampus. Sample sizes are the same as in (A). (C) BAC–CHT mice show no evidence of changes in (ChAT activities Cortex: n = 8 (WT), 7 (BAC–CHT); Striatum and Hippocampus: n = 8, each genotype. (D) BAC–CHT mice show no changes in AChE activities (P > 0.05, both enzyme n = 9 each genotype, all regions). **P < 0.01, ***P < 0.001.
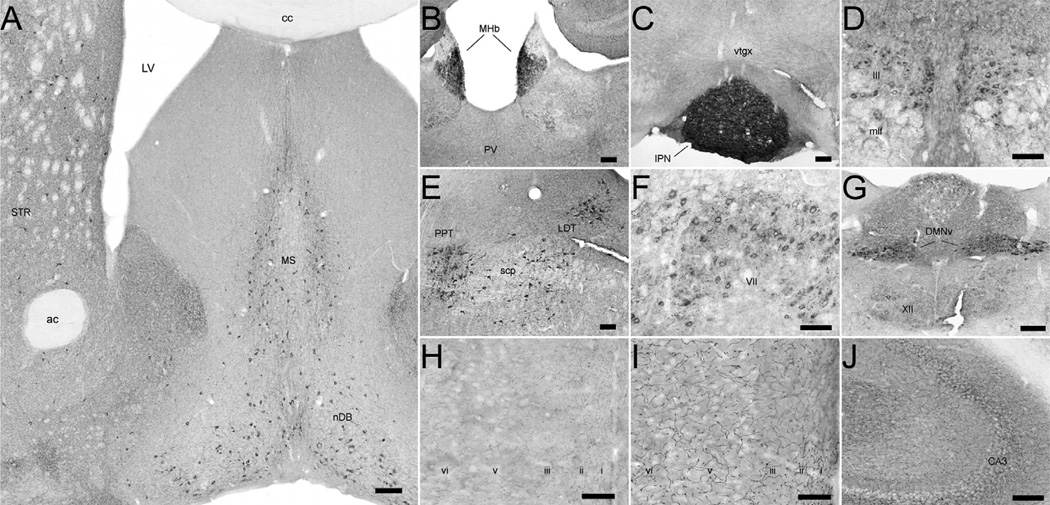
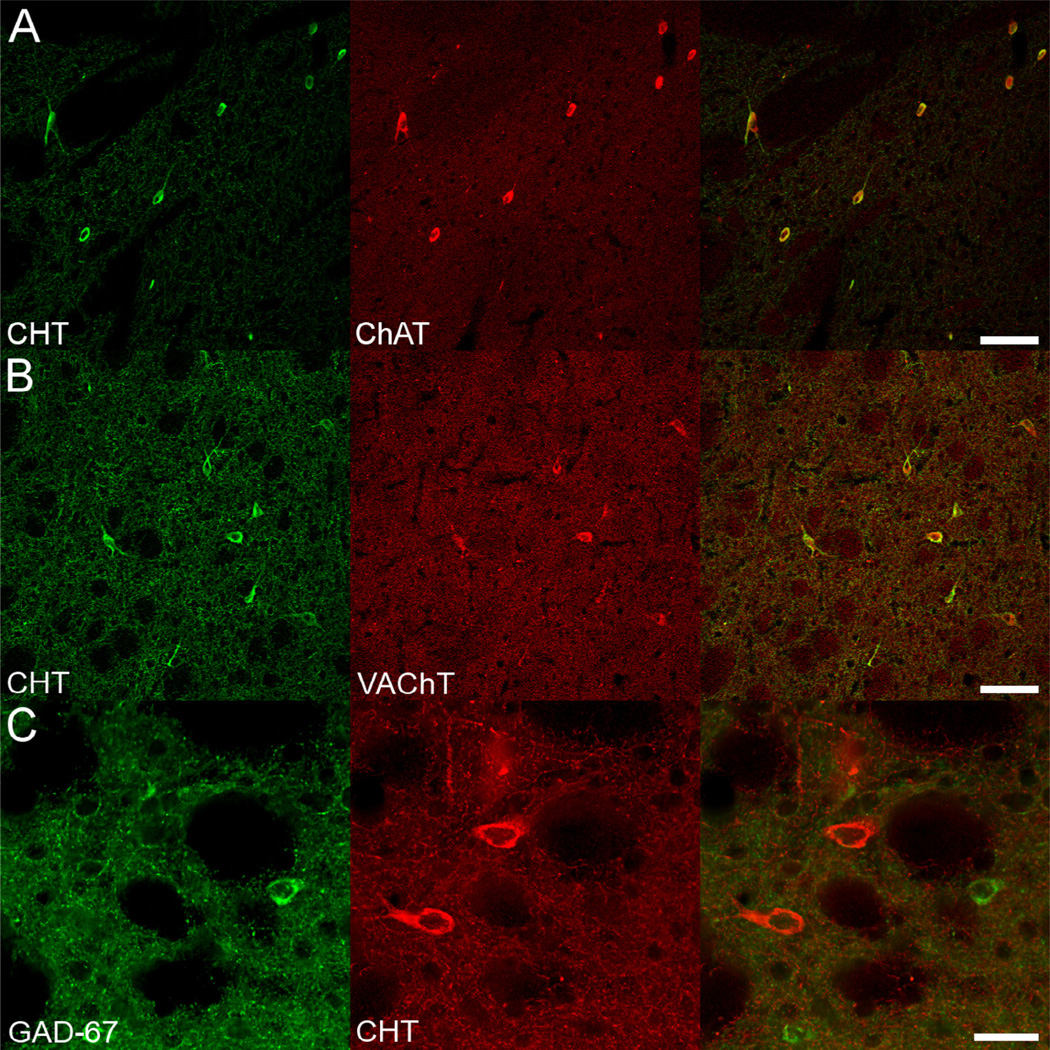
Biochemical measures like those reported above do not assess the critical question as to whether CHT is expressed by known cholinergic neurons and projections versus at ectopic sites. We therefore examined the cellular distribution of CHT in the CNS using immunoperoxidase and immunofluorescence histochemistry. As shown in Fig. 5, CHT immunoreactivity could be readily detected in sections from BAC–CHT mice, at levels higher than that seen with WT mice, in known cholinergic neurons, including striatal interneurons, medial septal/basal forebrain neurons (Fig. 5A), medial habenula neurons (Fig. 5B) and brainstem motoneurons (Fig. 5F and G). Enhanced labeling of neuronal fibers, compared to WT CHT labeling, was also evident in many targets of cholinergic innervation. An example is shown demonstrating intense labeling of CHT-immunoreactive fibers in the retrosplenial granular cortex of WT (Fig. 5H) vs. BAC–CHT (Fig. 5I) mice. To confirm that the expression of CHT in BAC–CHT mice was indeed restricted to cholinergic neurons, we examined the co-localization of CHT with the vesicular ACh transporter (VAChT), the ACh biosynthetic enzyme ChAT, and GAD-67, the enzyme responsible for synthesis of γ-ami-nobutyric acid (GABA). As shown in Fig. 6, CHT immunoreactivity in the dorsal striatum co-localized with labeling for VACHT and ChAT, but not with GAD-67. These qualitative findings support the view that CHT expression in the BAC–CHT mice is limited to cholinergic neurons.
Fig. 5.
CHT-immunoreactivity in the CNS of BAC–CHT mice suggests that CHT protein overexpression is restricted to cholinergic neurons. (A)–(G) Immunoperoxidase labeling for CHT conforms to the known locations of central cholinergic neurons and their axonal arborizations. (A) CHT labeling is present in the cell bodies of basal forebrain cholinergic neurons in the medial septum (MS), diagonal band of Broca (nDB), and in large, aspiny interneurons of the striatum (STR). Punctate immunoreactivity is also detectable in the surrounding neuropil, with regional variations in density consistent with the known distribution of cholinergic axon terminals in the mouse. (B) and (C) The habenulopeduncular cholinergic projection shows intense labeling for CHT. (B) Immunoreactive cell bodies in the medial habenula (MHb) send a dense projection to the (C) interpeduncular nucleus (IPN), where intense CHT labeling is evident. (D)–(G) CHT-immunoreactivity in the caudal midbrain and brainstem is restricted to known cholinergic groups and the relative density of labeling between groups is preserved in BAC–CHT brains. (D) Oculomotor neurons (III) overlying the medial longitudinal fasciculus (mlf) exhibit relatively light labeling for CHT. (E) Heavy labeling evident in the brainstem pedunculopontine (PPT) and laterodorsal (LDT) tegmental nuclei. (F) Moderate cell body labeling and prominent C-bouton-like punctate staining in the facial nucleus (VII). (G) Strong immunoperoxidase labeling in the dorsal motor nucleus of the vagus (DMNv) contrasts with the fainter labeling in the hypoglossal nucleus (XII). (H)–(J) CHT labeling reveals intense axonal labeling in BAC–CHT mice. (H) CHT immunoreactivity in the retrosplenial granular cortex from a WT mouse is predominately punctate. (I) A similar section as (H) from a BAC–CHT mouse demonstrates markedly heavier axonal labeling, with increased intervaricose segment labeling without any apparent change to the laminar distribution of labeled fibers. (J) Prominent axonal fiber labeling evident in the hippocampus of a BAC–CHT mouse, in addition to dense, punctate labeling. Scale bars: (A)–(G), J = 100 µm; H and I = 50 µm.
Fig. 6.
CHT expression is restricted to cholinergic neurons in BAC–CHT brain sections. (A) and (B) In the dorsal striatum, CHT immunoreactivity is observed exclusively in large interneurons that also contain immunoreactivity for (A) ChAT and (B) VAChT. (C) In contrast, small interneurons immunoreactive for GAD-67 are intermingled, but not colocalized with CHT labeled cell bodies. Scale bars equal A and B = 100 µM; C = 50 µM.
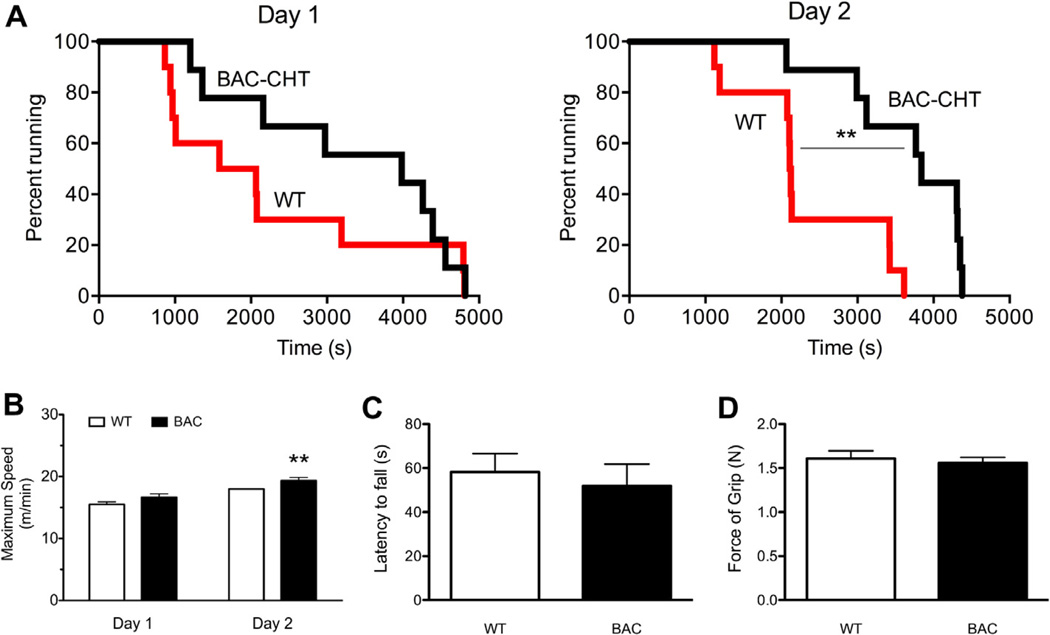
Finally, we sought to determine whether CHT overexpression leads to phenotypes in the BAC–CHT mice that could arise from altered cholinergic signaling. As noted above, when motor fatigue was assessed on the treadmill test, CHT+/− mice displayed reduced endurance and failed to attain the speeds reached by WT littermates (Bazalakova et al., 2007). In evaluation of the BAC–CHT mice on the same test, we found that these animals exhibited increased endurance. Although elevated, the increased endurance on the treadmill test did not reach significance on day 1 of testing (Fig. 7A; Mantel Cox logrank test, P = 0.265). Similarly, mean endurance time approached significance (WT: 2230.3 ± 511.7 s, BAC–CHT: 3299.9 ± 500.5 s, P = 0.067; two tailed t-test). However, by day 2, the endurance of BAC–CHT animals statistically exceeded that of WT animals (Mantel Cox logrank test, P = 0.003). Additionally, we observed a statistically significant increase in mean endurance time (WT: 2230.3 ± 295.1 s, BAC–CHT: 3680.7 ± 284.14 s, P = 0.001; two tailed t-test). Similarly, on the first day of testing, a trend was observed for BAC–CHT mice to attain greater maximum speed than WT mice (Fig. 7C; P = 0.051, one tailed t-test). However, on day 2, BAC–CHT mice attained greater speeds than WT mice (Fig. 7D; one tailed t-test, P = 0.008). Interestingly, as seen with CHT+/− mice, BAC–CHT animals did not differ from WT animals in grip strength (mean ± SEM, WT: 1.61 ± 0.09 N, BAC–CHT: 1.56 ± 0.07 N; P = 0.664, two tailed t-test) or latency to fall on the wire-hang task (mean ± SEM, WT: 58.2 ± 8.4 s, BAC–CHT: 51.9 ± 9.9 s, P = 0.633; two tailed t-test).
Fig. 7.
Treadmill endurance, but not muscular strength, is enhanced in BAC–CHT mice compared to WT. (A) BAC–CHT (n = 9) and WT (n = 10) mice were assessed for motor endurance on a treadmill set at a fixed speed, over two sessions. Kaplan–Meier survival curves demonstrate a trend for BAC–CHT mice to run longer, whereas on Day 2 this difference reached statistical significance. (B) Maximum speed achieved attained by mice in the treadmill test at increasing speeds. No genotype differences were observed on Day 1 of testing, but a statistically significant increase was evident on Day 2 of testing. (C) Latency to fall in the wire hang test demonstrated no significant genotype effects.WT n = 11; BAC–CHT n = 9. (D) Grip strength demonstrated no significant genotype effects. WT n = 11; BAC–CHT n = 8. **P < 0.01.
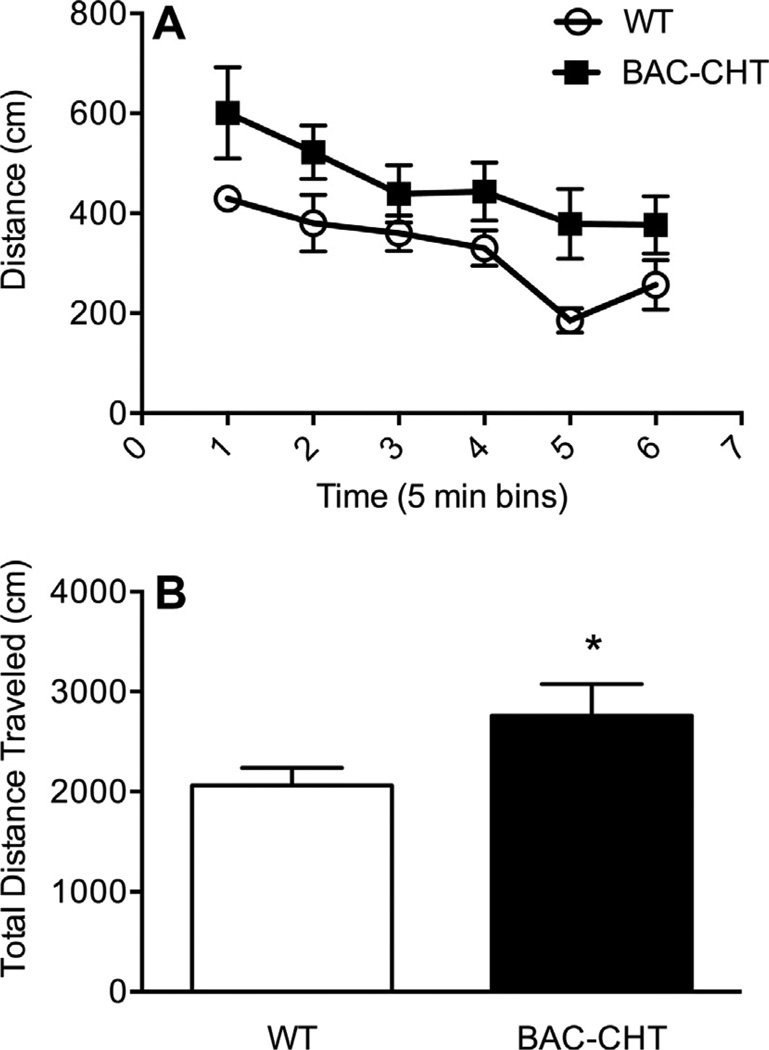
Fig. 8 shows the locomotor activity of BAC–CHT mice in the open-field arena. WT and BAC–CHT mice displayed similar acclimation to the novel environment of the open field test (Fig. 8A), but BAC–CHT mice demonstrated greater horizontal activity (one tailed t-test, P = 0.036) over the 30 min testing period (Fig. 8B). Vertical activity did not differ between groups (two tailed t-test: P = 0.839). No differences were evident between genotypes for center-surround preference (data not shown).
Fig. 8.
BAC–CHT mice are hyperactive in the open field test. (A) Both BAC–CHT(n = 7) and WT (n = 8) mice acclimated at similar rates to the open field test chamber. (B) Cumulative distance traveled during the 30 minute testing period was greater in BAC–CHT vs. WT mice.*P < 0.05.
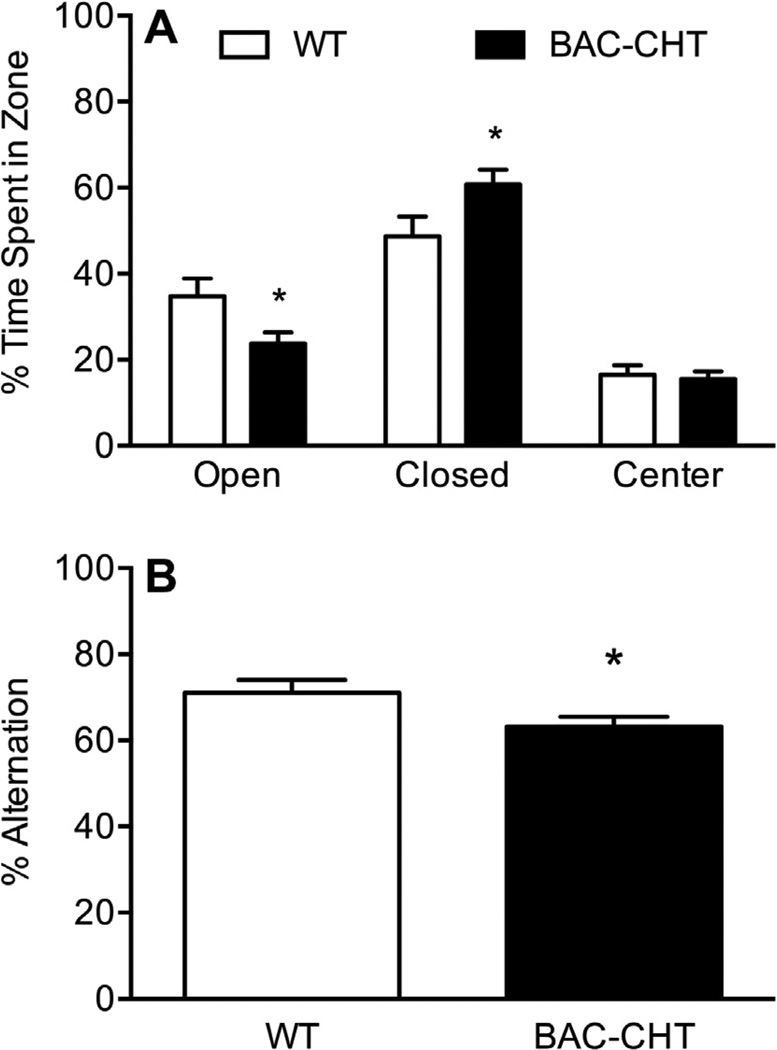
Increased activity in the open-field task could arise from a general locomotor hyperactivity, or an increase in sensitivity to the stress of a novel environment. To determine if BAC–CHT mice are more anxious than their WT littermates, we evaluated their performance in the elevated plus maze. The distribution of time spent in each zone of the maze is shown in Fig. 9A. Analysis of these data revealed that BAC–CHT mice spent significantly less time in the open arms of the apparatus (mean ± SEM: 23.8 ± 2.6%) than WT mice (34.8 ± 4.1%, P = 0.023, two tailed unpaired t-test), as well as more time in the closed arms (BAC: 60.8 ± 3.4%, WT: 48.7 ± 4.6%, P = 0.038, two tailed unpaired t-test). We detected no genotype differences in the percent time spent in the center zone (P = 0.716, two tailed unpaired t-test), nor in the total number of arm entries (P = 0.873, two tailed unpaired t-test). Finally, spontaneous alternations of BAC–CHT mice in the Y-maze, shown in Fig. 9B, demonstrated reduced alternate arm choices compared to WT littermates (BAC: 63.2 ± 2.2%, WT: 71.1 ± 3.0%, P = 0.041, two tailed unpaired t-test). Total number of arm entries (not shown) did not differ between groups (P = 0.091).
Fig. 9.
BAC–CHT mice demonstrate increased anxiety behavior and impaired spontaneous alternation. (A) Performance on the elevated plus maze revealed a genotype difference in arm preference, with BAC–CHT mice spending less time in the open arms of the apparatus and more time in the closed arms. WT n = 15; BAC–CHT n = 21. (B) BAC–CHT mice (n = 20) demonstrate reduced spontaneous alternation in the Y-maze relative to WT controls (n = 12). *P < 0.05.
4. Discussion
These biochemical and immunocytochemical studies confirm that BAC–CHT mice present with significant overexpression of the transporter while maintaining a normal anatomical pattern of expression. Higher CNS–CHT protein expression was accompanied by increased binding of HC-3, elevated high-affinity choline uptake, and an increase in tissue ACh content. These changes occurred in the absence of detectable alterations to ChAT activity, consistent with the well-recognized role of CHT as the rate-limiting step in ACh synthesis (Cooper et al., 2003; Köppen et al., 1997). Furthermore, AChE activity remained unchanged, indicating that the capacity for enzymatic degradation of ACh is not saturated under normal physiological conditions (Giuliano et al., 2008; Sarter et al., 2009). Our present findings also suggest that the cellular mechanisms governing the delivery and retention of CHT at the plasma membrane have sufficient capacity to translate elevated transporter protein levels into increased choline uptake activity, consistent with prior work showing the main driver of surface CHT expression to be total CHT protein expression (Holmstrand et al., 2010). Further studies are warranted in order to examine the downstream effects of increased tissue ACh on tonic and phasic ACh release (Howe et al., 2013), pre- and post-synaptic receptor expression and sensitivity, and synaptic transmission in circuits that are modulated by ACh. The BAC–CHT transgenic line will be an invaluable tool in the pursuit of a more complete understanding of cholinergic contributions to brain function.
We observed diverse behavioral phenotypes that support the overexpression of CHT in all cholinergic neurons, and the functional upregulation of choline transport and ACh synthesis at these synapses. BAC–CHT mice display enhanced endurance on the treadmill test, particularly after prior exposure to the task. While the simplest explanation for this increased endurance is that CHT overexpression at motor end plates augments synaptic reserves of ACh, it is possible that enhanced cardiovascular function may also contribute. Autonomic dysregulation has been reported for both CHT+/− mice (English et al., 2010) and for VAChT knockdown mice (Lara et al., 2010), and CHT+/− shows compromised recovery of heart-rate after strenuous exercise. However, the increased performance of BAC–CHT mice on the treadmill test mirrors our findings with motor-neuron specific CHT overexpressing mice (Lund et al., 2010), indicating that heightened CHT expression at CNS or cardiovascular synapses is not needed to achieve enhanced treadmill endurance. The lack of a change in grip strength or performance on the wire hang task in BAC–CHT mice indicates that the factors assessed in the treadmill test are independent of a change in strength per se. We also note that CHT+/− mice (Bazalakova et al., 2007), VAChT knock-down animals (Martins-Silva et al., 2011), and VAChT overexpressers (Nagy and Aubert, 2013) fail to display any phenotype in this task, indicating that grip strength is not affected by changes to cholinergic signaling capacity. As with studies of sustained attention in CHT+/− mice (Parikh et al., 2013), our findings of sustained motor function in the BAC–CHT mice indicate that phenotypes dependent on CHT are most likely to be revealed under conditions that are taxing to cholinergic transmission.
We found that spontaneous locomotion in a novel environment was significantly enhanced in BAC–CHT mice. Locomotor activity is often taken as an indicator of activity in corticostriatal circuits, particularly as striatal dopamine (DA) levels are correlated with the amount of voluntary movement (Salahpour et al., 2008). The mammalian striatum contains a population of large cholinergic interneurons (Woolf, 1991) that modulate striatal information processing. Both global knock-down and forebrain-specific deletion of VAChT reduce ACh transmission in the striatum, and result in hyperactivity in the open field (Martins-Silva et al., 2011; Martyn et al., 2012). However, CHT+/− mice demonstrated normal locomotion in the open-field test (Bazalakova et al., 2007), possibly due to a compensatory movement of CHT protein to the plasma membrane that preserves ACh transmission despite reduced levels of transporter protein. A simple conceptual model in which ACh and DA are mutually antagonistic might therefore predict that the elevations in striatal ACh present in BAC–CHT mice would lead to decreases in locomotor activity; we observed hyperactivity instead, indicating that the relationship between ACh, DA and locomotor activity is more complex.
Early pharmacological and lesion studies suggested that ACh signaling might counteract the effects of increased DA tone (Sandberg et al., 1984). Furthermore, the muscarinic ACh receptor antagonist scopolamine induces locomotor hyperactivity, and some cholinesterase inhibitors can inhibit locomotion (Shannon and Peters, 1990). However, a prominent effect of ACh released from cholinergic interneurons is the facilitation of DA release through a direct action at presynaptic nicotinic ACh receptors on DA terminals (Threlfell et al., 2012; Wonnacott, 1997), and deficits in choline uptake lead to decreased basal and stimulant-induced striatal DA release (Dong et al., 2013). These more recent findings suggest that the enhancement of cholinergic signaling capacity observed in the BAC–CHT mice could produce DA-dependent increases in locomotion. Furthermore, enhanced ACh transmission in non-striatal regions may underlie the observed hyperactivity, as striatum-specific knockdown of VAChT fails to alter locomotor behavior (Guzman et al., 2011). Models that achieve conditional, region specific changes to expression of CHT are needed to dissect the contributions of individual components of corticostriatal circuitry, as well as to evaluate potential compensatory changes that may arise from constitutive transporter overexpression. Finally, we note that VAChT-overexpressing transgenic mice demonstrate hypoactivity in the open field paradigm (Nagy and Aubert, 2013), in contrast to the present findings. The reason for the difference in locomotor activity between this model of cholinergic hyperfunction and the BAC–CHT model is presently unclear, but may reflect a differential contribution of each transporter to cholinergic signaling capacity. In any case, the change in locomotor activity observed indicates that the elevated tissue ACh content that we detected in BAC–CHT mice is likely to result in elevated release of ACh as well, as we would not expect any phenotypes to arise in the absence of real changes to cholinergic transmission.
An alternative explanation for the observed hyperactivity in BAC–CHT mice is that these mice may have enhanced sensitivity to the stress of a novel and intrinsically anxiogenic environment, as our open-field tests were conducted without prior habituation to the test apparatus. Although we did not detect differences in center vs. surround locomotion in the open field, BAC–CHT mice displayed a decreased time spent exploring open arms of the elevated plus maze, as compared to WT mice, typically interpreted as evidence of heightened anxiety. Increased anxiety could also underlie the deficit detected in spontaneous alternation seen with BAC–CHT mice in the Y-maze, as stress is known to compromise performance on this task (Hughes, 2004; Lalonde, 2002). In support of this idea, a recent study utilizing genetic and pharmacological manipulation of AChE levels suggests that elevated hippocampal cholinergic signaling may contribute to anxiety and depressionlike behaviors (Mineur et al., 2013).
Our characterization of the BAC–CHT mice, along with our prior analyses of CHT+/− animals, provides clear evidence that modulation of CHT activity can be achieved through the manipulation of influence cholinergic signaling. Motivated by this awareness, we recently reported a screening platform for the identification of CHT-targeted inhibitors and enhancers (Ruggiero et al., 2012). Agents that modulate CHT surface expression or function, without changing transporter protein levels, could produce alterations in biochemistry, physiology and behavior that are quite distinct from those seen with our transgenic manipulations. Furthermore, such drugs may act differently in the context of the normal and diseased brain. Comparisons between loss and gain of expression in relation to pharmacological manipulations should provide insights into the synaptic plasticity that attend changes in cholinergic signaling capacities, and help identify treatments that can best ameliorate disorders associated with cholinergic dysfunction.
Acknowledgements
We thank Chris Svitek, Angela Steele, Tracy Moore-Jarrett, Qiao Han and Kathryn Lindler for excellent, general laboratory support. Sarah Whitaker worked tirelessly to maintain and genotype mouse lines used in this study. Tommy Sabarido, Sabata Lund and Lise Harbom provided excellent technical assistance with biochemical and behavioral studies. We also thank Jeremy Veenstra-Vander-Weele for microscopy support and Cassandra Retzlaff for help with western blots. These studies were supported by NIH Awards MH065215 and MH099744 (E.C.H.), MH073159 (R.D.B) and MH086530 (M.S.).
References
- Antonelli T, Beani L, Bianchi C, Pedata F, Pepeu G. Changes in synaptosomal high affinity choline uptake following electrical stimulation of guinea-pig cortical slices: effect of atropine and physostigmine. Br. J. Pharmacol. 1981;74:525–531. doi: 10.1111/j.1476-5381.1981.tb10460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apparsundaram S, Ferguson SM, George AL, Jr, Blakely RD. Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem. Biophys. Res. Commun. 2000;276:862–867. doi: 10.1006/bbrc.2000.3561. [DOI] [PubMed] [Google Scholar]
- Apparsundaram S, Martinez V, Parikh V, Kozak R, Sarter M. Increased capacity and density of choline transporters situated in synaptic membranes of the right medial prefrontal cortex of attentional task-performing rats. J. Neurosci. 2005;25:3851–3856. doi: 10.1523/JNEUROSCI.0205-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, Levey AI, Blakely RD. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes Brain Behav. 2007;6:411–424. doi: 10.1111/j.1601-183X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. Acetylcholine. In: Cooper JR, Bloom FE, Roth RH, editors. The Biochemical Basis of Neuropharmacology. eighth ed. New York: Oxford University Press; 2003. pp. 151–178. [Google Scholar]
- Dong Y, Dani JA, Blakely RD. Choline transporter hemizygosity results in diminished basal extracellular dopamine levels in nucleus accumbens and blunts dopamine elevations following cocaine or nicotine. Biochem. Pharmacol. 2013;86:1084–1088. doi: 10.1016/j.bcp.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English BA, Appalsamy M, Diedrich A, Ruggiero AM, Lund D, Wright J, Keller NR, Louderback KM, Robertson D, Blakely RD. Tachycardia, reduced vagal capacity, and age-dependent ventricular dysfunction arising from diminished expression of the presynaptic choline transporter. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H799–H810. doi: 10.1152/ajpheart.00170.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S, Bazalakova M, Savchenko V, Tapia J, Wright J, Blakely R. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc. Natl. Acad. Sci. USA. 2004;101:8762–8767. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J. Neurosci. 2003;23:9697–9709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JJ, KoshJ W, Parrish JS. Peripheral toxicity of hemicholinium-3 in mice. Br. J. Pharmacol. 1982;77:239–244. doi: 10.1111/j.1476-5381.1982.tb09291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano C, Parikh V, Ward JR, Chiamulera C, Sarter M. Increases in cholinergic neurotransmission measured by using choline-sensitive microelectrodes: enhanced detection by hydrolysis of acetylcholine on recording sites? Neurochem. Int. 2008;52:1343–1350. doi: 10.1016/j.neuint.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry G, Willison BD, Blakely RD, Landis SC, Habecker BA. Developmental expression of the high affinity choline transporter in cholinergic sympathetic neurons. Auton. Neurosci. 2005;123:54–61. doi: 10.1016/j.autneu.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P, Lefresne P, Rossier J, Beaujouan JC, Glowinski J. Effect of sodium, hemicholinium-3 and antiparkinson drugs on (14C)acetylcholine synthesis and (3H)choline uptake in rat striatal synaptosomes. Brain Res. 1973;62:523–529. doi: 10.1016/0006-8993(73)90717-8. [DOI] [PubMed] [Google Scholar]
- Guzman M, De Jaeger X, Raulic S, Souza I, Li A, Schmid S, Menon R, Gainetdinov R, Caron M, Bartha R, Prado V, Prado M. Elimination of the vesicular acetylcholine transporter in the striatum reveals regulation of behaviour by cholinergic-glutamatergic co-transmission. PLoS Biol. 2011;9:e1001194. doi: 10.1371/journal.pbio.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberberger RV, Pfeil U, Lips KS, Kummer W. Expression of the high-affinity choline transporter, CHT1, in the neuronal and non-neuronal cholinergic system of human and rat skin. J. Invest. Dermatol. 2002;119:943–948. doi: 10.1046/j.1523-1747.2002.00182.x. [DOI] [PubMed] [Google Scholar]
- Haga T, Noda H. Choline uptake systems of rat brain synaptosomes. Biochim. Biophys. Acta. 1973;291:564–575. doi: 10.1016/0005-2736(73)90508-7. [DOI] [PubMed] [Google Scholar]
- Holmstrand EC, Asafu-Adjei J, Sampson AR, Blakely RD, Sesack SR. Ultrastructural localization of high-affinity choline transporter in the rat anteroventral thalamus and ventral tegmental area: differences in axon morphology and transporter distribution. J. Comp. Neurol. 2010;518:1908–1924. doi: 10.1002/cne.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance. Converging electrochemical and fMRI evidence from rats and humans. J. Neurosci. 2013;33:8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Iwamoto H, Blakely RD, De Felice LJ. Na+, Cl−, and pH dependence of the human choline transporter (hCHT) in Xenopus oocytes: the proton inactivation hypothesis of hCHT in synaptic vesicles. J. Neurosci. 2006;26:9851–9859. doi: 10.1523/JNEUROSCI.1862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Gonzalez R, Koppen A, Loffelholz K. Free choline and choline metabolites in rat brain and body fluids: sensitive determination and implications for choline supply to the brain. Neurochem. Int. 1993;22:293–300. doi: 10.1016/0197-0186(93)90058-d. [DOI] [PubMed] [Google Scholar]
- Klein J, Köppen A, Löffelholz K. Uptake and storage of choline by rat brain: influence of dietary choline supplementation. J. Neurochem. 1991;57:370–375. doi: 10.1111/j.1471-4159.1991.tb03762.x. [DOI] [PubMed] [Google Scholar]
- Köppen A, Klein J, Erb C, Löffelholz K. Acetylcholine release and choline availability in rat hippocampus: effects of exogenous choline and nicotinamide. J. Pharmacol. Exp. Ther. 1997;282:1139–1145. [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lara A, Damasceno DD, Pires R, Gros R, Gomes ER, Gavioli M, Lima RF, Guimaraes D, Lima P, Bueno CR, Jr, Vasconcelos A, Roman-Campos D, Menezes CA, Sirvente RA, Salemi VM, Mady C, Caron MG, Ferreira AJ, Brum PC, Resende RR, Cruz JS, Gomez MV, Prado VF, de Almeida AP, Prado MA, Guatimosim S. Dysautonomia due to reduced cholinergic neurotransmission causes cardiac remodeling and heart failure. Mol. Cell. Biol. 2010;30:1746–1756. doi: 10.1128/MCB.00996-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips KS, Pfeil U, Reiners K, Rimasch C, Kuchelmeister K, Braun-Dullaeus RC, Haberberger RV, Schmidt R, Kummer W. Expression of the high-affinity choline transporter CHT1 in rat and human arteries. J. Histochem. Cytochem. 2003;51:1645–1654. doi: 10.1177/002215540305101208. [DOI] [PubMed] [Google Scholar]
- Lund D, Ruggiero AM, Ferguson SM, Wright J, English BA, Reisz PA, Whitaker SM, Peltier AC, Blakely RD. Motor neuron-specific overexpression of the presynaptic choline transporter: impact on motor endurance and evoked muscle activity. Neuroscience. 2010;171:1041–1053. doi: 10.1016/j.neuroscience.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Silva C, De Jaeger X, Guzman MS, Lima RD, Santos MS, Kushmerick C, Gomez MV, Caron MG, Prado MA, Prado VF. Novel strains of mice deficient for the vesicular acetylcholine transporter: insights on transcriptional regulation and control of locomotor behavior. PLoS One. 2011;6:e17611. doi: 10.1371/journal.pone.0017611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn AC, De Jaeger X, Magalhaes AC, Kesarwani R, Goncalves DF, Raulic S, Guzman MS, Jackson MF, Izquierdo I, Macdonald JF, Prado MA, Prado VF. Elimination of the vesicular acetylcholine transporter in the forebrain causes hyperactivity and deficits in spatial memory and long-term potentiation. Proc. Natl. Acad. Sci. USA. 2012;109:17651–17656. doi: 10.1073/pnas.1215381109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. USA. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder AH, Yamamura HI, Kuhar MJ, Snyder SH. Release of acetylcholine from hippocampal slices by potassium depolarization: dependence on high affinity choline uptake. Brain Res. 1974;70:372–376. doi: 10.1016/0006-8993(74)90329-1. [DOI] [PubMed] [Google Scholar]
- Nagy PM, Aubert I. B6eGFPChAT mice overexpressing the vesicular acetylcholine transporter exhibit spontaneous hypoactivity and enhanced exploration in novel environments. Brain Behav. 2013;3:367–383. doi: 10.1002/brb3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Okuda T, Misawa H. Ultrastructural localization of high-affinity choline transporter in the rat neuromuscular junction: enrichment on synaptic vesicles. Synapse. 2004;53:53–56. doi: 10.1002/syn.20029. [DOI] [PubMed] [Google Scholar]
- Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I. Identification and characterization of the high-affinity choline transporter. Nat. Neurosci. 2000;3:120–125. doi: 10.1038/72059. [DOI] [PubMed] [Google Scholar]
- Parikh V, St Peters M, Blakely RD, Sarter M. The presynaptic choline transporter imposes limits on sustained cortical acetylcholine release and attention. J. Neurosci. 2013;33:2326–2337. doi: 10.1523/JNEUROSCI.4993-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero AM, Wright J, Ferguson SM, Lewis M, Emerson KS, Iwamoto H, Ivy MT, Holmstrand EC, Ennis EA, Weaver CD, Blakely RD. Nonisotopic assay for presynaptic choline transport reveals capacity for allosteric modulation of choline uptake. ACS Chem. Neurosci. 2012;3:767–781. doi: 10.1021/cn3000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc. Natl. Acad. Sci. USA. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg K, Sanberg PR, Coyle JT. Effects of intrastriatal injections of the cholinergic neurotoxin AF64A on spontaneous nocturnal locomotor behavior in the rat. Brain Res. 1984;299:339–343. doi: 10.1016/0006-8993(84)90715-7. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat. Rev. Neurosci. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon HE, Peters SC. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine-induced hyperactivity in mice. J. Pharmacol. Exp. Ther. 1990;255:549–553. [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog. Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]