Abstract
The beta 1-6 structure of N-linked oligosaccharides, formed by beta-1,6-N-acetylglucosaminyltransferase (GnT-V), is associated with metastatic potential. We established a highly metastatic subclone, B16-hm, from low metastatic B16-F1 murine melanoma cells. The gene encoding beta-1,4-N-acetylglucosaminyltransferase (GnT-III) was introduced into the B16-hm cells, and three clones that stably expressed high GnT-III activity were obtained. In these transfectants, the affinity to leukoagglutinating phytohemagglutinin was reduced, whereas the binding to erythroagglutinating phytohemagglutinin was increased, indicating that the level of beta 1-6 structure was decreased due to competition for substrate between intrinsic GnT-V and ectopically expressed GnT-III. Lung metastasis after intravenous injection of the transfectants into syngeneic and nude mice was significantly suppressed, suggesting that the decrease in beta 1-6 structure suppressed metastasis via a mechanism independent of the murine system. These transfectants also displayed decreased invasiveness into Matrigel and inhibited cell attachment to collagen and laminin. Cell growth was not affected. Our results demonstrate a causative role for beta 1-6 branches in invasion and cell attachment in the extravasation stage of metastasis.
Full text
PDF
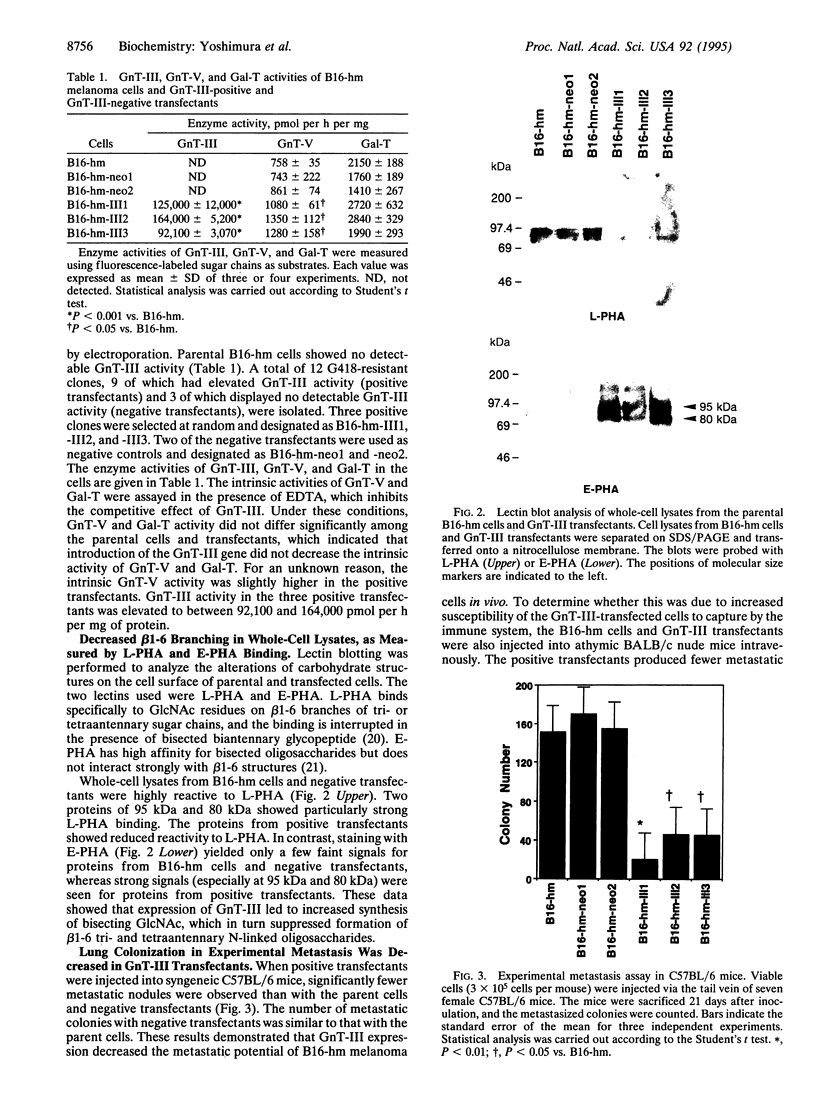
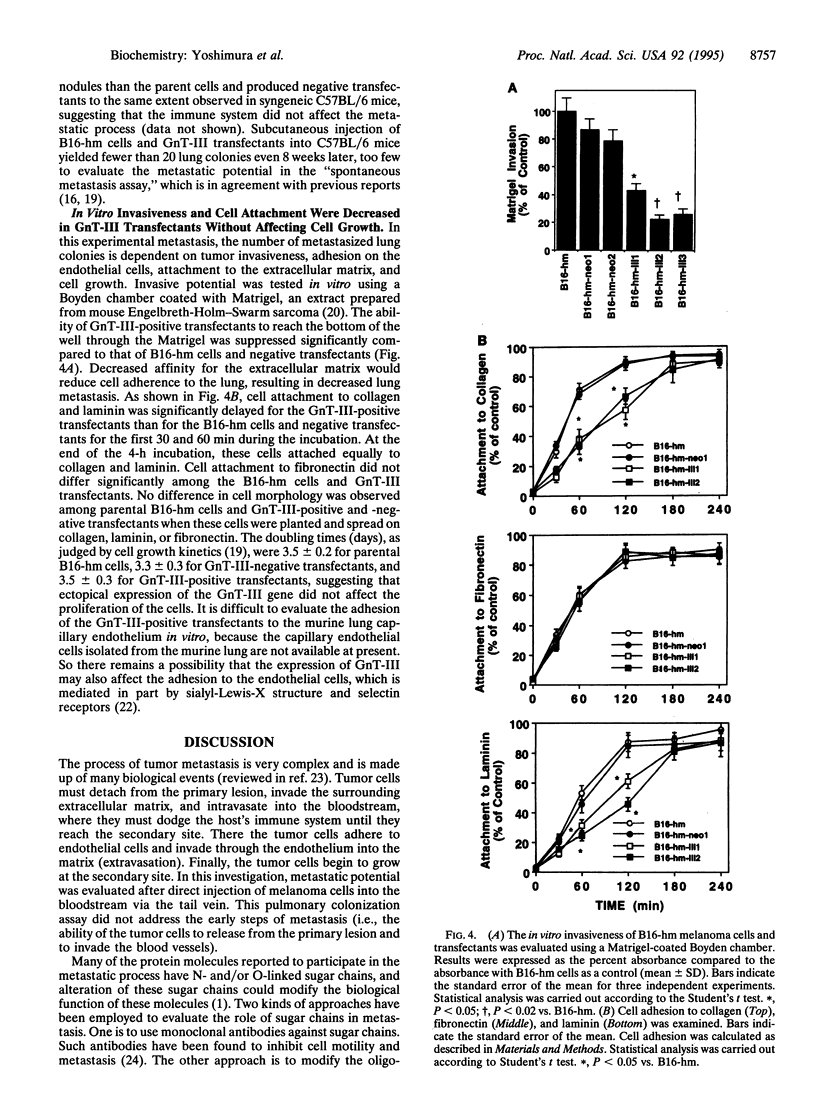

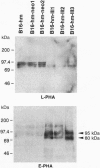
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Yamada K. M. Analysis of the role of glycosylation of the human fibronectin receptor. J Biol Chem. 1989 Oct 25;264(30):18011–18018. [PubMed] [Google Scholar]
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Dennis J. W., Kosh K., Bryce D. M., Breitman M. L. Oncogenes conferring metastatic potential induce increased branching of Asn-linked oligosaccharides in rat2 fibroblasts. Oncogene. 1989 Jul;4(7):853–860. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Fujii S., Nishiura T., Nishikawa A., Miura R., Taniguchi N. Structural heterogeneity of sugar chains in immunoglobulin G. Conformation of immunoglobulin G molecule and substrate specificities of glycosyltransferases. J Biol Chem. 1990 Apr 15;265(11):6009–6018. [PubMed] [Google Scholar]
- Hart I. R., Saini A. Biology of tumour metastasis. Lancet. 1992 Jun 13;339(8807):1453–1457. doi: 10.1016/0140-6736(92)92039-i. [DOI] [PubMed] [Google Scholar]
- Honn K. V., Tang D. G. Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992 Nov;11(3-4):353–375. doi: 10.1007/BF01307187. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Matsumoto K., White S. L., Olden K. Oligosaccharide modification by swainsonine treatment inhibits pulmonary colonization by B16-F10 murine melanoma cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1752–1756. doi: 10.1073/pnas.83.6.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M., Hakomori S. I. A specific cell surface glycoconjugate controlling cell motility: evidence by functional monoclonal antibodies that inhibit cell motility and tumor cell metastasis. Biochemistry. 1991 Apr 2;30(13):3328–3334. doi: 10.1021/bi00227a023. [DOI] [PubMed] [Google Scholar]
- Nishikawa A., Fujii S., Sugiyama T., Taniguchi N. A method for the determination of N-acetylglucosaminyltransferase III activity in rat tissues involving HPLC. Anal Biochem. 1988 May 1;170(2):349–354. doi: 10.1016/0003-2697(88)90641-0. [DOI] [PubMed] [Google Scholar]
- Nishikawa A., Ihara Y., Hatakeyama M., Kangawa K., Taniguchi N. Purification, cDNA cloning, and expression of UDP-N-acetylglucosamine: beta-D-mannoside beta-1,4N-acetylglucosaminyltransferase III from rat kidney. J Biol Chem. 1992 Sep 5;267(25):18199–18204. [PubMed] [Google Scholar]
- Ogata S. I., Muramatsu T., Kobata A. New structural characteristic of the large glycopeptides from transformed cells. Nature. 1976 Feb 19;259(5544):580–582. doi: 10.1038/259580a0. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Qian F., Bajkowski A. S., Steiner D. F., Chan S. J., Frankfater A. Expression of five cathepsins in murine melanomas of varying metastatic potential and normal tissues. Cancer Res. 1989 Sep 1;49(17):4870–4875. [PubMed] [Google Scholar]
- Qian F., Vaux D. L., Weissman I. L. Expression of the integrin alpha 4 beta 1 on melanoma cells can inhibit the invasive stage of metastasis formation. Cell. 1994 May 6;77(3):335–347. doi: 10.1016/0092-8674(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Saitoh O., Wang W. C., Lotan R., Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992 Mar 15;267(8):5700–5711. [PubMed] [Google Scholar]
- Santer U. V., Glick M. C. Partial structure of a membrane glycopeptide from virus-transformed hamster cells. Biochemistry. 1979 Jun 12;18(12):2533–2540. doi: 10.1021/bi00579a016. [DOI] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986 Mar;64(3):163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Taniguchi N., Nishikawa A., Fujii S., Gu J. G. Glycosyltransferase assays using pyridylaminated acceptors: N-acetylglucosaminyltransferase III, IV, and V. Methods Enzymol. 1989;179:397–408. doi: 10.1016/0076-6879(89)79139-4. [DOI] [PubMed] [Google Scholar]
- Warren L., Buck C. A., Tuszynski G. P. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behavior. Biochim Biophys Acta. 1978 Sep 18;516(1):97–127. doi: 10.1016/0304-419x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Hitoi A., Kobata A. Structural determinants of Phaseolus vulgaris erythroagglutinating lectin for oligosaccharides. J Biol Chem. 1983 Dec 25;258(24):14753–14755. [PubMed] [Google Scholar]
- Yousefi S., Higgins E., Daoling Z., Pollex-Krüger A., Hindsgaul O., Dennis J. W. Increased UDP-GlcNAc:Gal beta 1-3GaLNAc-R (GlcNAc to GaLNAc) beta-1, 6-N-acetylglucosaminyltransferase activity in metastatic murine tumor cell lines. Control of polylactosamine synthesis. J Biol Chem. 1991 Jan 25;266(3):1772–1782. [PubMed] [Google Scholar]
- Zheng M., Fang H., Hakomori S. Functional role of N-glycosylation in alpha 5 beta 1 integrin receptor. De-N-glycosylation induces dissociation or altered association of alpha 5 and beta 1 subunits and concomitant loss of fibronectin binding activity. J Biol Chem. 1994 Apr 22;269(16):12325–12331. [PubMed] [Google Scholar]
- van den Eijnden D. H., Koenderman A. H., Schiphorst W. E. Biosynthesis of blood group i-active polylactosaminoglycans. Partial purification and properties of an UDP-GlcNAc:N-acetyllactosaminide beta 1----3-N-acetylglucosaminyltransferase from Novikoff tumor cell ascites fluid. J Biol Chem. 1988 Sep 5;263(25):12461–12471. [PubMed] [Google Scholar]