Abstract
Inactivation of p53, the master regulator of cellular stress and damage signals, often allows cells that should die or senesce to live. Loss of Dicer, an RNAse III-like enzyme critical in microRNA biogenesis, causes embryonic lethality and activation of the p53 pathway. Several non-hematopoietic cell types that contain inactivated p53 have been shown to survive Dicer deletion, suggesting p53 loss may protect cells from the negative consequences of Dicer deletion. However, here, we report that loss of p53 did not provide a survival advantage to B-cells, as they underwent rapid apoptosis upon Dicer deletion. Moreover, a deficiency in p53 neither rescued the Dicer deletion-induced delay in Myc-driven B-cell lymphomagenesis, nor allowed a single B-cell lymphoma to develop with biallelic deletion of Dicer. A p53 deficiency did, however, restore the pre-B/B-cell phenotype and CD19 surface expression of the lymphomas that emerged in conditional Dicer knockout Eμ-myc transgenic mice. Moreover, p53 loss in transformed B-cells did not confer protection from apoptosis, as Dicer deletion in established p53-null B-cell lymphomas induced apoptosis, and all of the 1,260 B-cell lymphoma clones analyzed that survived Cre-mediated Dicer deletion retained at least one allele of Dicer. Moreover, Dicer deletion in lymphomas in vivo reduced tumor burden and prolonged survival. Therefore, inactivation of p53 is insufficient to allow untransformed B-cells and B-cell lymphomas to survive without Dicer, presenting a potential therapeutic opportunity for the treatment of B-cell lymphomas.
Keywords: B cell lymphoma, Dicer, microRNA, Myc, p53
Introduction
MicroRNA (miRNA) are small non-coding RNA that regulate gene expression post-transcriptionally and have essential roles in development, proliferation, apoptosis, and transformation (1, 2). Alterations in miRNA expression are linked to tumor development, including hematopoietic malignancies (1, 2, 3). Moreover, the oncogene c-Myc, which is frequently overexpressed in many human malignancies and a driver of B-cell lymphomagenesis, transcriptionally regulates the expression of many miRNA (4).
miRNA are transcribed in a precursor form and processed with enzymes, such as Dicer, an RNase III enzyme with critical roles in cell differentiation, proliferation, and survival (5). Loss of one allele of DICER or reduced DICER expression or enzymatic activity is reported in multiple solid organ tumors (6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16). Mouse models revealed Dicer is a haploinsufficient tumor suppressor in soft tissue sarcoma, lung adenocarcinoma, and retinoblastoma (17, 18). In contrast, we showed Dicer heterozygosity had no effect on the rate of B-cell lymphoma development (19). Therefore, differences in the requirements for Dicer and the effects of reduced Dicer expression in different tissues remain unresolved.
The p53 tumor suppressor, which induces apoptosis or cell cycle arrest upon cellular stresses (20), responds to defects in miRNA biogenesis, and therefore, may be required to signal problems in this pathway. Specifically, in untransformed murine embryonic fibroblasts (MEFs), deletion of Dicer leads to p53 activation and premature senescence, which is delayed with loss of p53 (21). We previously detected an increased frequency of p53 inactivation in lymphomas in a mouse model of Myc-induced B-cell lymphoma (Eμ-myc) expressing B-cell-directed Cre and two conditional Dicer alleles, suggesting a connection between p53 activation and Dicer deletion in B-cells (19). Moreover, data from three groups, including our own, showed expression of Cre in Dicerfl/fl mice in B-cell progenitors or mature B-cells results in B-cell apoptosis (19, 22, 23). This apoptosis was partially rescued by overexpressing the anti-apoptotic Bcl-2 protein or reducing the pro-apoptotic Bim protein (22). Although p53-null murine sarcoma cells and p53 inactivated mesenchymal stem cells can survive Dicer deletion (23), p53 deletion was synthetically lethal in Dicer and Rb deficient retinal progenitor cells (24). Therefore, the role of p53 in monitoring defects in miRNA biogenesis and cell survival in the context of a Dicer deficiency remains unclear.
Using mouse models, we determined the contribution of p53 to B-cell survival and lymphoma development with loss of Dicer. A p53 deficiency did not rescue the defect in B-cell development, the reduction in B-cell survival, or the delay in Myc-induced lymphomagenesis upon Dicer deletion. It did restore the B-cell lymphoma phenotype. However, none of the lymphomas that emerged had deleted both alleles of Dicer. Moreover, established B-cell lymphomas lacking p53 underwent apoptosis when Dicer was deleted, significantly extending survival in mouse models. Thus, p53 loss is insufficient to allow survival and growth of B-cells and B-cell lymphomas in the absence of Dicer, and thus, targeting Dicer may have therapeutic potential for treating B-cell lymphomas.
Materials and Methods
Mice
C57Bl/6 Eμ-myc (25) and CD19-cre (26) transgenic mice, Dicerfl/fl mice from Dr. Steve Jones (21), and p53−/− mice from Dr. Guillermina Lozano (27) were intercrossed to obtain the mice needed for this study. Littermates were used in all analyses. For experiments with nude mice, 1.5×106 or 0.5×106 p53 deleted Dicerfl/fl/Eμ-myc lymphoma cells expressing a tamoxifen-inducible form of Cre (CreERT2) were injected (subcutaneous or intravenous, respectively) into 6-week-old Foxn1nu/nu female mice (Harlan labs). Tamoxifen (2 mg) or corn oil (vehicle control) was injected (intraperitoneal) once daily for 3 days starting the day of lymphoma injection for two cohorts (one subcutaneous and one tail vein injected cohort) or after lymphomas were 90–150mm3 for a second subcutaneous cohort. Subcutaneous tumors were measured with calipers and tumor volume calculated. Blood was collected for flow cytometric and microscopic analyses from the mice where lymphoma was injected into the tail vein. Mice were humanely sacrificed prior to lymphoma development or for survival studies, at humane endpoints, and tumors/tissues were harvested and analyzed. Log-rank tests determined statistical significance for survival. All studies were in accordance with state and federal guidelines and were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Western and Southern blotting
Whole cell protein lysates from B-cell lymphomas and pre-B cells were generated and Western blotted as previously described (28). Antibodies against p19Arf (GeneTex), p53 (Ab-7; Calbiochem), Mdm2 (C-18; Santa Cruz), Cre (Novagen), Dicer (Cell Signaling), cleaved Caspase 3 (Cell Signaling), and β-actin (Sigma) were used. As previously described (28, 29), p53 was sequenced and Southern blots for p53 with genomic DNA from lymphomas was performed.
Phenotype analysis
Lymphoma cells and splenocytes from littermates prior to lymphoma development were analyzed by flow cytometry following incubation with fluorochrome-linked antibodies against surface receptors as previously reported (19, 29).
Quantitative real-time PCR
Total RNA was isolated from lymphomas with TRIzol (Invitrogen) according to the manufacturer’s protocol. As previously described, cDNA was generated, and SybrGreen (SABiosciences) and TaqMan MicroRNA Assays (Applied Biosciences) were used to perform qRT-PCR, in triplicate, for mRNA and miRNA analysis, respectively (19, 30). mRNA and miRNA expression were normalized to β-actin and RNU6b expression, respectively, and the data presented as 2−ΔCt.
Dicer gene rearrangement analysis
Genomic DNA was isolated from frozen and cultured lymphomas, pre-B cells, and MEFs using the REDExtract-N-Amp Tissue PCR Kit (Sigma). PCR was performed with primers specific for unrearranged and Cre-lox-deleted Dicer alleles, as previously published (19, 21). PCR conditions allowed for 10–15% contaminating normal tissue without detecting unrearranged floxed Dicer alleles.
Pre-B cell and lymphoma cell survival analyses
Primary pre-B cell cultures from p53−/−/Dicerfl/fl, p53+/−/Dicerfl/fl, and p53−/−/Dicer+/fl mice and primary p53 deleted or Arf deleted Dicer+/fl or Dicerfl/fl Eμ-myc lymphoma cells were generated as previously described (19, 28). Cells were infected with a bicistronic retrovirus (MSCV) encoding CreERT2 (31) and GFP or GFP alone. Cell number and viability were determined by Trypan Blue Dye exclusion assays and proliferation was measured by MTS assays (490 nm; CellTiter 96 AQueous One Solution Cell Proliferation Assay; Promega) after plating equal numbers of cells, in triplicate, and adding 1 μM 4-OHT or vehicle (ethanol) control. Apoptosis was evaluated by Western blotting for cleaved Caspase 3 and by flow cytometry following propidium iodide staining for fragmented (sub-G1) DNA and Annexin V/7-AAD staining after adding 1 μM 4-OHT or vehicle (ethanol) control in triplicate, in vitro, or after administering tamoxifen or vehicle (corn oil) for the nude mouse experiments. For single-cell analyses, GFP-positive lymphoma cells were placed one cell/well into 96-well plates by a flow cytometer and visually inspected. Vehicle (ethanol) control or 4-OHT (1 μM) was added to each well and surviving clones were harvested and Dicer gene rearrangement was determined by PCR.
Results
p53 deficiency does not rescue lymphoma latency in Myc overexpressing Dicerfl/fl mice
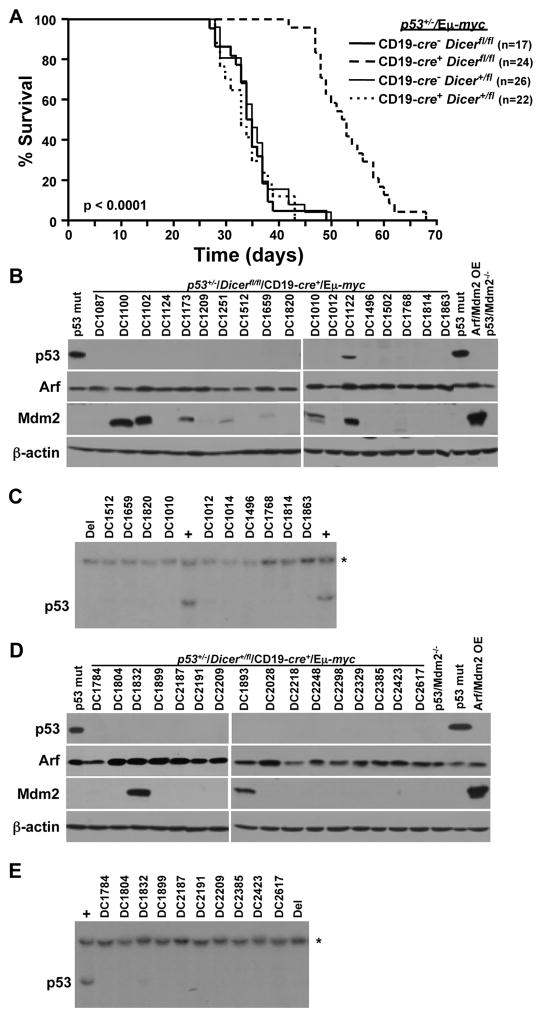
Previously, we reported Dicer deletion in B-cell precursors resulted in delayed Myc-induced B-cell lymphoma development and the inability of a B-cell lymphoma to emerge with biallelic Dicer deletion (19). To determine whether B-cell lymphomas could develop without Dicer in the context of a p53 deficiency, we generated p53+/−/Dicerfl/fl/Eμ-myc mice and littermate controls that were also transgenic for B lineage-restricted CD19-cre recombinase; p53-null Eμ-myc mice cannot be generated (26). c-Myc in Eμ-myc transgenic mice and Cre in CD19-cre transgenic mice are first expressed in B-cell precursors and continue throughout the life of the B-cell (25, 26). There was a pronounced delay in lymphomagenesis and extended survival in CD19-cre+/p53+/−/Dicerfl/fl/Eμ-myc mice compared to their CD19-cre−/p53+/−/Dicerfl/fl/Eμ-myc littermates (53 and 34 days mean survival, respectively; Fig. 1A, p<0.0001, log-rank test). All but one (DC1122) of the 23 lymphomas analyzed lacked p53 protein expression, and all overexpressed p19Arf protein, an indicator of p53 inactivation (subset of those analyzed is shown in Fig. 1B). Sequencing of p53 in DC1122 revealed a mutation (G263R) in its DNA binding domain. Southern blots showed all lymphomas lacking p53 protein had deleted their wild-type allele of p53 (representative data of those analyzed is shown in Fig. 1C). Therefore, all lymphomas were functionally p53-null. In addition, Mdm2, a negative regulator of p53, was overexpressed in 35% of the lymphomas (Fig. 1B). Thus, there was a delay in Myc-induced lymphomagenesis caused by Dicer deletion in p53 heterozygous mice, and a deficiency in Dicer did not alter selection for p53 inactivation in the lymphomas that arose.
Figure 1. Delayed lymphomagenesis in p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc mice.
(A) Kaplan-Meier survival curves of the indicated genotypes of mice (p<0.0001, log-rank test comparing each genotype to CD19-cre+/Dicerfl/fl/p53+/−/Eμ-myc). The number (n) of mice is indicated. (B, D) Western blots of lymphomas for the proteins and genotype indicated. Controls include lymphomas containing mutant (mut) p53 or overexpressing (OE) Arf and Mdm2 and p53−/−/Mdm2−/− MEFs. A subset of lymphomas analyzed shown. (C, E) Representative Southern blots for p53 of lymphomas in B and D. Lymphomas that contain (+) or have deleted (Del) p53 were controls. Asterisk (*) denotes the DNA loading control, the p53 pseudogene.
Dicer is not a haploinsufficient tumor suppressor in Myc-induced B-cell lymphoma (19). To determine whether a p53 deficiency would allow Dicer to function as a haploinsufficient tumor suppressor in B-cells, we evaluated B-cell lymphoma development in the context of Dicer heterozygosity. Cre-positive and Cre-negative p53+/−/Dicer+/fl/Eμ-myc transgenic mice had a similar rate of lymphoma development with mean survivals of 35 and 36 days, respectively (Fig. 1A). Evaluation of lymphomas that developed in CD19-cre+/p53+/−/Dicer+/fl/Eμ-myc mice showed 100% (17 of 17 analyzed) lacked p53 protein, due to deletion of the wild-type allele, and overexpressed Arf (subset of those analyzed is shown in Figs. 1D and 1E). These results indicate a p53 deficiency did not allow Dicer heterozygosity to accelerate B-cell lymphomagenesis.
Loss of p53 rescues the type of B-cell lymphoma that develops
Previously, we determined approximately 40% of the lymphomas that emerged in CD19-cre+/Dicerfl/fl/Eμ-myc mice were of very early precursor B-cell origin, B220+/CD4+/CD43+/Sca1+ (19). We evaluated whether a p53 deficiency would alter the development or frequency of this phenotype by assessing lymphomas from p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc mice. Fourteen of 16 (88%) of the lymphomas analyzed were typical Eμ-myc pre-B and/or B-cell lymphomas (25) and expressed B220 and CD19, and were either IgM− or IgM+; none were B220+/CD4+/CD43+/Sca1+ (Table 1). Unexpectedly, 2 of 16 lymphomas were CD3−/CD4+/CD8+/CD43+ early T-cell lymphomas (Table 1). All lymphomas analyzed from p53+/−/CD19-cre−/Dicerfl/fl/Eμ-myc littermate controls and from Dicer heterozygous p53+/−/CD19-cre+/Eμ-myc mice were typical Eμ-myc lymphomas (Table 1). Thus, a p53 deficiency fully restored development of the characteristic Eμ-myc B-cell lymphoma in CD19-cre+/Dicerfl/fl/Eμ-myc mice, but it also allowed T-cell lymphomas to develop.
Table 1.
Dicerfl/fl Eμ-myc lymphoma phenotypes are rescued with a p53 deficiency
| Phenotype |
Dicer+/fl p53+/− Eμ-myc
|
Dicerfl/fl p53+/− Eμ-myc
|
|
|---|---|---|---|
| CD19-cre+ | CD19-cre− | CD19-cre+ | |
| B220+ CD19+ CD43− IgM− | 4/10 (40%) | 7/10 (70%) | 10/16 (63%) |
| B220+ CD19+ CD43− IgM+ | 6/10 (60%) | 3/10 (30%) | 4/16 (25%) |
| CD3− CD4+ CD8+ CD43+ | 0/10 (0%) | 0/10 (0%) | 2/16 (13%) |
A deficiency in p53 rescues CD19 expression in B-cell lymphomagenesis
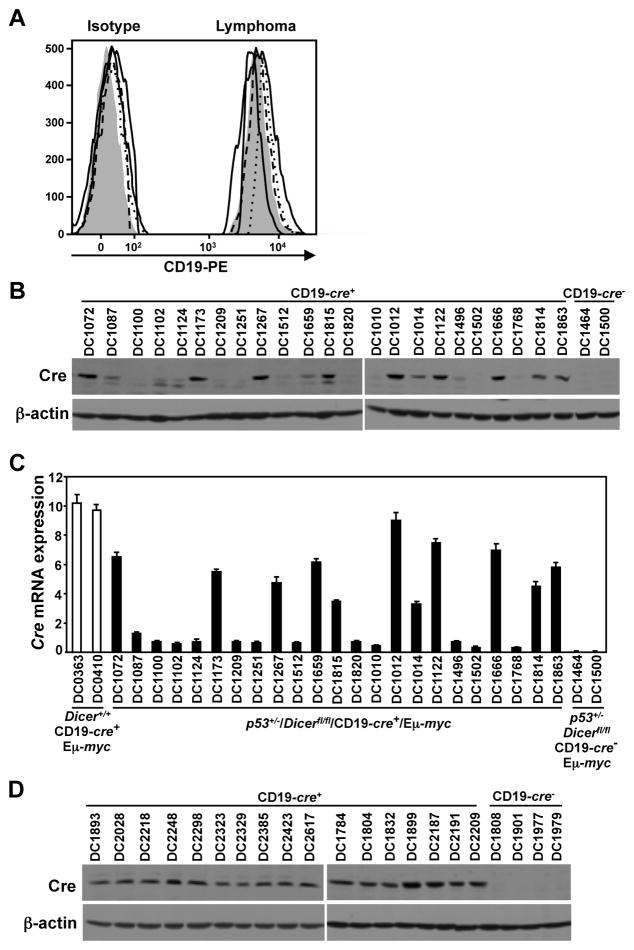
CD19 expression was absent or decreased in 65% of the lymphomas from CD19-cre+/Dicerfl/fl/Eμ-myc mice, resulting in reduced or absent Cre expression (19). Preventing CD19 expression was one mechanism by which lymphomas could avoid Dicer deletion. To assess the consequences of a p53 deficiency on CD19 expression in the lymphomas in this study, we evaluated p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc lymphomas for CD19 cell surface expression. None of the 14 pre-B/B-cell lymphomas analyzed by flow cytometry lacked or had reduced CD19 cell surface expression (Fig. 2A; p<0.0001, Fisher’s exact test). However, 13 of 23 (57%) p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc lymphomas analyzed lacked or had significantly decreased Cre protein (Fig. 2B), and 12 of the 13 (92%) had reduced Cre mRNA (Fig. 2C). This is an unexpected result, since all the lymphomas expressed CD19 and Cre expression is driven by the endogenous CD19 promoter. Of note, Cre expression occurred significantly more frequently in p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc lymphomas (43%) than was previously observed in CD19-cre+/Dicerfl/fl/Eμ-myc lymphomas [12% (19); p=0.022, Fisher’s exact test]. Analysis of 17 heterozygous floxed Dicer p53+/−/CD19-cre+/Eμ-myc lymphomas showed they all expressed Cre protein (Fig. 2D). Therefore, a deficiency in p53 rescued CD19 surface expression and partially restored Cre expression in B-cell lymphomas from CD19-cre+/Dicerfl/fl/Eμ-myc mice.
Figure 2. A deficiency in p53 rescues CD19 and Cre expression during B-cell lymphomagenesis.
(A) Histograms of CD19 surface expression and corresponding isotype controls of lymphomas from four representative p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc mice compared to a control p53+/−/CD19-cre−/Dicerfl/fl/Eμ-myc lymphoma (shaded peaks). (B, D) Western blots for Cre and β-actin from Dicerfl/fl (B) and Dicer+/fl (D) p53+/−/CD19-cre+/Eμ-myc lymphomas. Lysates of lymphomas from a Dicer+/+/CD19-cre+/Eμ-myc mouse (B) and Dicerfl/fl (B) or Dicer+/fl (D) CD19-cre−/p53+/−/Eμ-myc mice were controls. (C) qRT-PCR for Cre expression relative to β-actin in lymphomas from p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc mice. RNA from Dicer+/+/CD19-cre+/Eμ-myc and CD19-cre−/p53+/−/Dicerfl/fl/Eμ-myc lymphomas were positive and negative controls, respectively.
p53 deficiency is insufficient to allow Dicer deletion during B-cell lymphomagenesis
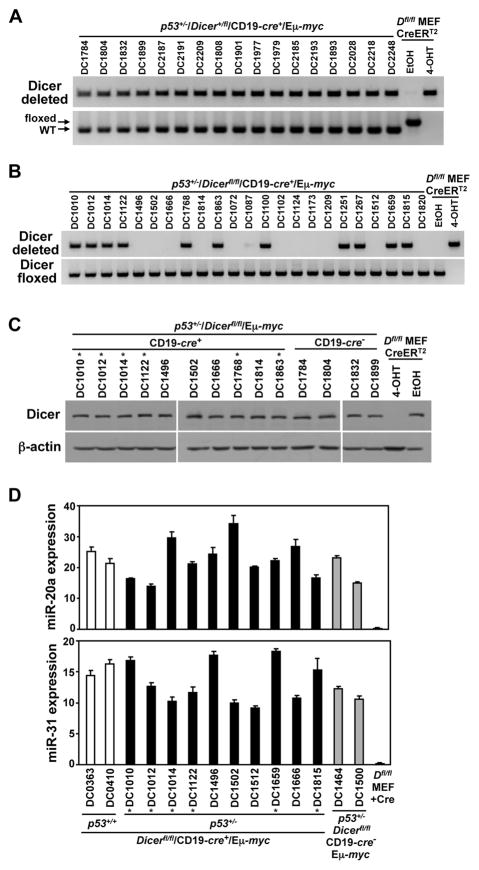
We previously reported that not a single lymphoma from CD19-cre+/Dicerfl/fl/Eμ-myc mice had deleted both Dicer alleles (19). In this study, we assessed whether the exons flanked by loxP sites in the Dicer gene had been deleted. Evaluation of Dicer heterozygous p53+/−/CD19-cre+/Eμ-myc lymphomas showed that all 17 analyzed had deleted their one floxed Dicer allele (Fig. 3A). However, 11 of 23 (48%) lymphomas analyzed from Dicerfl/fl/p53+/−/CD19-cre+/Eμ-myc mice deleted one conditional Dicer allele, whereas the other 12 lymphomas retained both floxed alleles (Fig. 3B). None of the 23 Dicerfl/fl/p53+/−/CD19-cre+/Eμ-myc lymphomas had deleted both floxed Dicer alleles.
Figure 3. Biallelic Dicer deletion is selected against during lymphoma development.
(A, B) PCR analysis for conditional deleted and floxed (not deleted) Dicer alleles from lymphomas of the indicated genotype. DNA from Dicerfl/fl (Dfl/fl) MEFs expressing an inducible CreERT2 treated with 4-OHT or vehicle control (EtOH) were controls. Arrows indicate unrearranged (floxed) and wild-type (WT) Dicer alleles. (C) Representative Western blots for Dicer and β-actin from CD19-cre+ and CD19-cre− p53+/−/Dicerfl/fl/Eμ-myc lymphomas. Lysates from Dicerfl/fl (Dfl/fl) MEFs were controls. (D) qRT-PCR for miR-20a and miR-31 relative to internal RNU6b small RNA in lymphomas from p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc mice. p53+/+/CD19-cre+/Dicerfl/fl/Eμ-myc and p53+/−/Dicerfl/fl/CD19-cre−/Eμ-myc lymphomas and Cre-expressing Dicerfl/fl (Dfl/fl) MEFs served as controls. Asterisks (*) denote lymphomas that deleted one Dicer allele (C and D).
Given that Cre protein expression was lost in half of the p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc lymphomas, we evaluated whether Cre had ever been functional in these tumors. Four of the 13 lymphomas that lacked Cre protein (Fig. 2B) had rearranged one Dicer allele (Fig. 3B), indicating they had active Cre at some point in B-cell development. Because Cre protein was present more frequently in the lymphomas that arose in p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc mice compared to mice that were p53+/+ (43% vs 12% (19), respectively), we expected an increased incidence of Cre-mediated deletion of at least one Dicer allele in the p53+/− lymphomas. However, there was no statistical difference in the frequency of deleting one allele of Dicer between these two groups [48% vs 38% (19), respectively; p=0.57, Fisher’s exact test; Fig. 3B]. Importantly, our data indicate a p53 deficiency is insufficient to allow a lymphoma to emerge when both alleles of Dicer have been deleted.
To determine whether Dicer was functional in the lymphomas that emerged, we first assessed Dicer protein levels. Lymphomas with one allele of Dicer expressed an analogous amount of Dicer protein as lymphomas that retained both alleles of Dicer (Fig. 3C). Moreover, mature miRNA transcript levels of miR-20a and miR-31, Dicer-dependent miRNA, were similar regardless of Dicer status in all lymphomas analyzed (Fig. 3D). These data indicate all lymphomas, including those with only one Dicer allele, expressed wild-type levels of Dicer that was fully functional in miRNA biogenesis.
p53 loss cannot rescue B-cell development following Dicer deletion
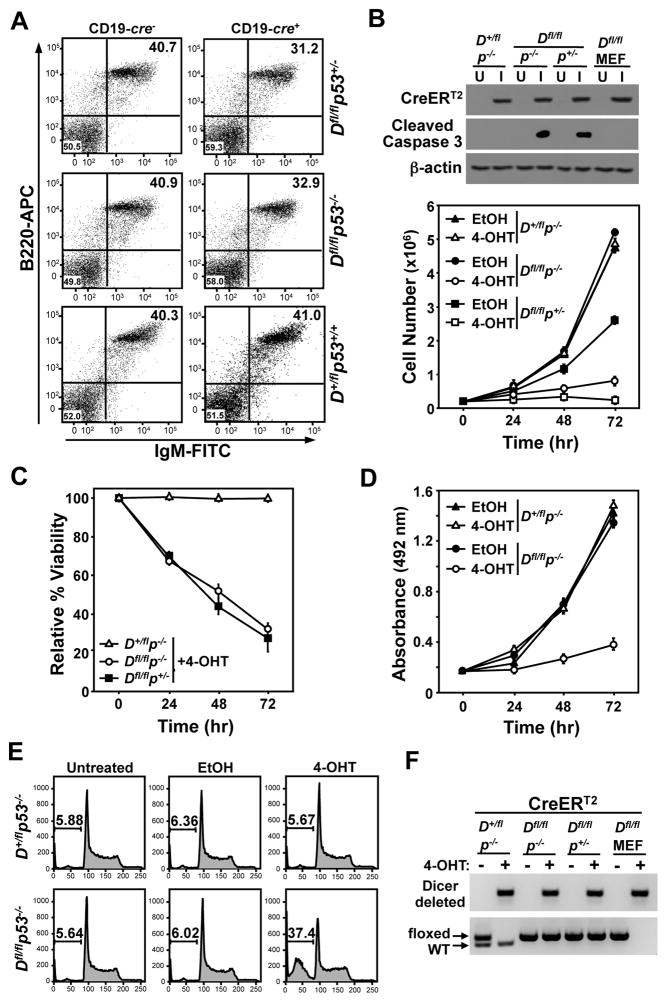
In vivo, biallelic Dicer deletion in developing B-cells with wild-type p53 induces apoptosis, causing a developmental defect, resulting in decreased mature splenic B-cells (19, 22). Protecting B-cells from this apoptosis partially rescues B-cell development (22). Since a p53 deficiency rescued the pre-B/B-cell lymphoma phenotype in CD19-cre+/Dicerfl/fl/Eμ-myc mice, we questioned whether p53 mediates the Dicer deletion-induced B-cell apoptosis. To address this, we evaluated splenic B-cells from pre-cancerous p53−/− and p53+/− CD19-cre+/Dicerfl/fl mice and CD19-cre− littermate controls. There was a modest, but statistically significant, reduction in the percentage of B220+/IgM+ B-cells in CD19-cre+/p53−/−/Dicerfl/fl mice (32.9% ± 1.42) compared to CD19-cre−/p53−/−/Dicerfl/fl littermates (40.9% ± 1.46; p<0.0001, paired t-test; Fig. 4A). A comparable reduction in B-cells was also observed in p53+/− littermates that were either CD19-cre+/Dicerfl/fl or CD19-cre−/Dicerfl/fl (31.2% ± 0.90 and 40.7% ± 0.51, respectively; p<0.0001, paired t-test; Fig. 4A). As an additional control, we assessed B-cells in p53+/+/Dicer+/fl mice with or without CD19-cre and the percentages of B-cells were similar in both, demonstrating B-cell expression of Cre did not alter B-cell numbers in the mice (Fig. 4A). Thus, deletion of one or two alleles of p53 could not rescue the decrease in B-cell numbers induced by Dicer deletion, in vivo.
Figure 4. Loss of p53 is insufficient for B-cell survival when Dicer is deleted.
(A) Representative dot plots of littermate-matched splenic B-cells from CD19-cre+ or CD19-cre− Dicer+/fl and Dicerfl/fl mice that were p53+/+, p53+/−, or p53−/−. Total lymphocytes were gated and B220-APC versus IgM-FITC was assessed. (B–F) Primary pre-B cells from p53−/−/Dicerfl/fl, p53+/−/Dicerfl/fl, and p53−/−/Dicer+/fl littermates were infected (I) with a retrovirus encoding CreERT2 or left uninfected (U). 4-OHT (+) or vehicle control (EtOH, −) was added to pre-B cell cultures at time 0 and cell number (B), viability (C), proliferation (MTS assay; D), apoptosis (cleaved Caspase 3 protein, B; sub-G1 DNA, E), and Dicer gene rearrangement (F) were evaluated. Western blots shown in B. Arrows indicate unrearranged (floxed) and wild-type (WT) Dicer alleles in F. CreERT2 expressing Dicerfl/fl (Dfl/fl) MEFs treated with 4-OHT (+) or ethanol (−) were controls in B and F.
To further test the requirement for p53 in B-cell survival in the absence of Dicer, we derived primary pre-B cells from bone marrow of p53−/−/Dicer+/fl, p53−/−/Dicerfl/fl, and p53+/−/Dicerfl/fl littermates. Pre-B cells were infected with a bicistronic retrovirus encoding GFP and a 4-hydroxytamoxifen (4-OHT)-inducible CreERT2 (31), and GFP-positive cells were sorted by flow cytometry. All three genotypes of pre-B cells expressed equal levels of CreERT2 protein (Fig. 4B). To delete Dicer, pre-B cells were treated with 4-OHT to activate CreERT2. As expected for primary pre-B cells with functional p53, the Dicerfl/fl/p53+/− cells grew at a slower rate and were sensitive to Dicer loss, as indicated by decreased cell numbers and viability (Figs. 4B and 4C). Similarly, following 4-OHT treatment, Dicerfl/fl/p53−/− pre-B cells experienced a dramatic decrease in total number, viability, and growth, and an increased percentage of cells containing fragmented DNA (sub-G1) and appearance of cleaved Caspase 3, compared to vehicle-treated cells, which were unaffected (Figs. 4B–E). When 4-OHT was administered to CreERT2 expressing Dicer+/fl/p53−/− pre-B cells, no change in cell number, viability, growth, fragmented DNA, or cleaved Caspase 3 was observed (Figs. 4B–E), as would be expected for pre-B cells with one wild-type Dicer allele. Dicer gene rearrangement was assessed in the surviving pre-B cells and showed that, regardless of genotype, only one Dicer allele was rearranged in the CreERT2 activated pre-B cells (Fig. 4F). Notably, Dicerfl/fl fibroblasts containing similar levels of CreERT2 protein (Fig. 4B) deleted both floxed Dicer alleles (Fig. 4F). Therefore, loss of p53 could not rescue the rapid apoptosis induced by biallelic Dicer deletion in primary untransformed pre-B cells, and only pre-B cells that had retained one allele of Dicer could survive.
Dicer is required for B-cell lymphoma survival
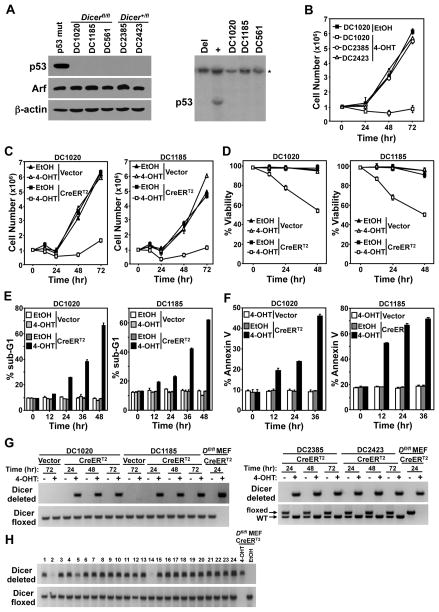
Recently, Dr. Sharp and colleagues reported that a p53-null murine sarcoma cell line could survive and proliferate without Dicer (23), suggesting cellular transformation may alter the requirements for Dicer. We tested whether transformed B-cells could survive loss of Dicer if they also lacked p53. B-cell lymphomas were isolated from two p53+/−/Dicerfl/fl/Eμ-myc mice (DC1020 and DC1185) and as controls, two p53+/−/Dicer+/fl/Eμ-myc mice (DC2385 and DC2423). p53 protein was not detected by Western blot, and Southern blot showed deletion of the remaining wild-type allele of p53 in all four lymphomas (Figs. 5A, 1D, and 1E). The lymphomas were infected with a bicistronic retrovirus encoding CreERT2 and GFP or GFP alone. CreERT2 activation with 4-OHT in the p53 deleted Dicer+/fl/Eμ-myc lymphomas had no effect on cell number compared to a p53 deleted Dicerfl/fl/Eμ-myc lymphoma, which showed a significant decrease in cell number after CreERT2 activation (Fig. 5B and Supplemental Figure S1). CreERT2 activation in both p53 deleted Dicerfl/fl/Eμ-myc lymphomas resulted in apoptosis, whereas there was little effect following addition of vehicle control or 4-OHT to lymphomas infected with empty retrovirus (Figs. 5C–F). Specifically, the total number and viability of CreERT2 p53 deleted Dicerfl/fl/Eμ-myc lymphoma cells decreased, while the percentage of apoptotic cells (cells with fragmented, sub-G1 DNA or that were Annexin V+) increased after addition of 4-OHT (Figs. 5C–F). PCR analysis revealed the p53 deleted Dicer+/fl/Eμ-myc lymphoma cells deleted their one floxed Dicer allele, while the p53 deleted Dicerfl/fl/Eμ-myc lymphoma cells surviving CreERT2 activation had only deleted one of the conditional Dicer alleles (Fig. 5G). Analogous results were obtained with Dicerfl/fl/Eμ-myc B cell lymphomas that had deleted Arf and retained p53 (Supplemental Figure S2).
Figure 5. A deficiency in p53 does not allow B-cell lymphomas to survive without Dicer.
(A) p53+/−/Dicerfl/fl/Eμ-myc (DC1020 and DC1185), p53+/−/Dicer+/fl/Eμ-myc (DC2385 and DC2423) lymphoma cell lines and the Dicerfl/fl/Eμ-myc lymphoma cell line (DC561) from our previous study (19) were subjected to Western blot (left) for the proteins indicated and Southern blot (right and Fig. 1E) for p53. A lymphoma containing mutant p53 was a control for the Western blot. Lymphomas that contain (+) or have deleted (Del) p53 were controls for the Southern blot. Asterisk (*) denotes the DNA loading control, the p53 pseudogene. (B–F) DC2385, DC2423, DC1020, and/or DC1185 lymphoma cells were infected with a CreERT2-encoding retrovirus or empty retrovirus (Vector). 4-OHT or vehicle control (EtOH) was added to the cultures at time 0 and cell number (B, C), viability (D), and apoptosis (sub-G1 DNA, E; Annexin V, F) were measured. (G) Dicer gene rearrangement was evaluated at the indicated intervals by PCR. (H) Representative PCR product analysis of Dicer gene rearrangement of GFP-positive single cell-sorted lymphoma clones that survived CreERT2 activation of the 328 analyzed. Conditional deleted and floxed (not deleted) Dicer alleles shown (G and H). CreERT2 expressing Dicerfl/fl (Dfl/fl) MEFs treated with 4-OHT or ethanol (EtOH) were controls (G and H).
We postulated it was possible for preferential outgrowth of lymphoma cells possessing one allele of Dicer, masking the presence of a small population of lymphoma cells that had deleted both alleles of Dicer. To evaluate this possibility, we performed single-cell sorting for GFP-positive cells of two independent CreERT2 expressing p53 deleted Dicerfl/fl/Eμ-myc lymphoma lines into 96-well plates. After visually confirming the presence of a single cell per well, CreERT2 was activated with 4-OHT, and the surviving clones were assessed. Only 26% (328 of 1,260) of the clones survived CreERT2 activation, whereas 98.5% (394 of 400) of the vehicle-treated clones grew out. Analysis of all 328 lymphoma clones that survived CreERT2 activation revealed none had deleted both Dicer alleles (a subset of those analyzed is shown in Fig. 5H). Instead, 306 (93.3%) had deleted one Dicer allele, whereas the other 22 (6.7%) maintained both floxed alleles. Moreover, analysis of the Dicerfl/fl/Eμ-myc lymphoma used in the single-cell analysis in our previous study [DC561, (19)] where we obtained analogous results, revealed that it had biallelic p53 deletion (Fig. 5A). Collectively, these data illustrate that B-cell lymphomas cannot survive without Dicer, even when p53 is deleted. Therefore, at least one allele of Dicer is required for B-cell lymphoma survival.
In vivo Dicer deletion inhibits lymphoma growth and extends survival
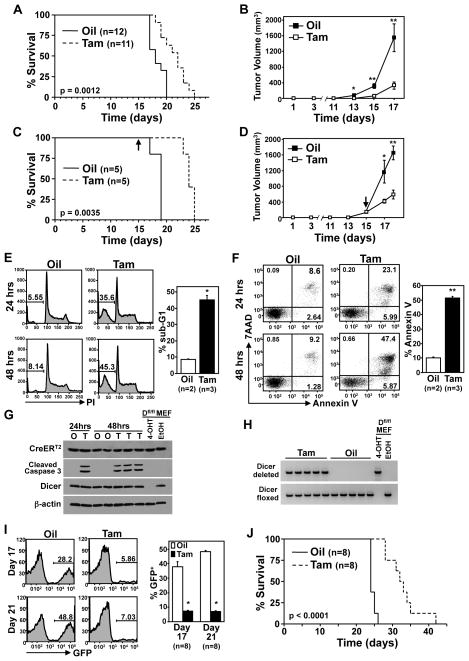
Given that B-cell lymphomas require Dicer for survival, in vitro, we tested whether inactivating Dicer would alter lymphoma growth in vivo with three different mouse experiments. Firstly, p53 deleted Dicerfl/fl/Eμ-myc lymphoma cells (DC1020) expressing CreERT2 were subcutaneously injected into nude mice and CreERT2 was activated by tamoxifen the same day. There was a significant delay in lymphoma progression and extended survival in the mice that received tamoxifen compared to the vehicle-treated mice (Fig. 6A; p=0.0012, log-rank test). Tumors from vehicle-treated mice grew significantly larger more quickly compared to tumors from mice that received tamoxifen to activate CreERT2 (Fig. 6B; *p=0.0051, **p<0.003).
Figure 6. Dicer inactivation impedes tumor growth, in vivo.
(A, C) Kaplan-Meier survival curves of nude mice injected (subcutaneously) with CreERT2 expressing p53 deleted Dicerfl/fl/Eμ-myc lymphoma cells (DC1020) and administered tamoxifen or vehicle (corn oil) control starting the day of injection (A; p=0.0012, log-rank test) or once lymphomas were 90–150mm3 (C; p=0.0035, log-rank test). Arrow indicates the day tamoxifen administration began for C. The number (n) of mice is indicated. (B, D) Tumor volumes for mice in A and C, respectively, were measured at the indicated intervals (for B: *p=0.0051, **p<0.003; for D: *p=0.0288, **p=0.0005). In D, the arrow indicates the day tamoxifen administration began. (E–G) Apoptosis was measured at intervals following tamoxifen or vehicle control administration in matched tumor pairs by propidium iodide staining of fragmented (sub-G1) DNA (E), Annexin V/7AAD staining (F), and cleaved Caspase 3 protein detection (G). Representative data (left) and mean values at 48 hours (right) are shown for E and F; *p=0.0008, **p<0.0001, t-tests. Western blots of whole cell lysates for the proteins indicated (G). (H) PCR product analysis of Dicer gene rearrangement of the mice from C. Controls for G and H include protein lysates or DNA from Dicerfl/fl MEFs treated with 4-OHT or ethanol. (I, J) Nude mice were injected intravenously with CreERT2 expressing p53 deleted Dicerfl/fl/Eμ-myc lymphoma cells (DC1020) and administered tamoxifen (Tam) or corn oil (Oil) vehicle control starting the same day. Blood was assessed for GFP-positivity by flow cytometry at intervals post lymphoma injection. Representative data (left) and mean values for the indicated number of mice are shown (I; *p<0.0001, t-test). Kaplan-Meier survival curves (J; p<0.0001, log-rank test).
To determine whether loss of Dicer would impact established lymphomas, we allowed a cohort of mice to grow subcutaneous lymphomas of 90–150mm3 and then administered tamoxifen or vehicle control (tumor sizes were matched between groups) (Figs. 6C and 6D). While the rapid rate of tumor growth continued in the vehicle-treated mice, tumor expansion in the mice that received tamoxifen to activate CreERT2 to delete Dicer slowed dramatically (Fig. 6D; *p=0.0288, **p=0.0005). Analysis of tumors that were equivalent in size prior to tamoxifen addition, showed significant and increasing apoptosis over time following tamoxifen, as indicated by increased sub-G1 DNA content (Fig. 6E, *p=0.008), Annexin V positivity (Fig. 6F, **p<0.0001), and cleaved Caspase 3 protein (Fig. 6G). The consequence of this apoptosis was that the survival of the CreERT2-activated (tamoxifen) mice was significantly increased (Fig. 6C; p=0.0035, log-rank test).
To assess whether the delayed tumor growth in both experiments and the apoptosis detected was a result of CreERT2-mediated Dicer deletion, PCR analysis of Dicer gene rearrangement was performed. Surviving lymphoma cells in the mice administered tamoxifen all retained at least one Dicer allele (Fig. 6H and Supplemental Figure S3), and expressed Dicer protein (Fig. 6G). Therefore, targeting Dicer deletion, in vivo, induced apoptosis, delaying lymphoma progression and extending survival regardless of when Dicer was deleted.
As a third approach to test the effects of Dicer deletion in lymphomas in vivo, we also injected p53 deleted Dicerfl/fl/Eμ-myc lymphoma cells expressing CreERT2 and GFP into the blood stream of nude mice; tamoxifen or vehicle control administration began on the same day. By day 17 and certainly by day 21, vehicle control-treated mice had more lymphoma cells present in their blood compared to mice that received tamoxifen to activate CreERT2 and delete Dicer (Fig. 6I, *p<0.0001 and Supplemental Figure S4). Furthermore, mice that had activated CreERT2 (tamoxifen) lived significantly longer than control mice (Fig. 6J; p<0.0001, log-rank test). Collectively, all three in vivo experiments show that deleting Dicer in B-cell lymphomas leads to apoptosis and decreased lymphoma cell expansion, providing evidence that targeting Dicer in B-cell lymphomas may have therapeutic potential even when lymphomas lack a functional p53 pathway.
Discussion
Previously, we detected an increase in p53 inactivation in B-cell lymphomas from CD19-cre+/Dicerfl/fl/Eμ-myc mice (19), suggesting a connection between p53 activation and Dicer deletion. Moreover, we also observed Dicer deletion in untransformed MEFs increased p53 levels and induced a premature senescent phenotype that could be delayed by deleting either the Ink4a/Arf or p53 locus (21). Others reported a fraction of a murine p53-null, mutant K-Ras expressing sarcoma cell line and SV40-immortalized, and thus p53 and Rb inactivated, mesenchymal stem cells could survive Dicer deletion (23). Although the data pointed to p53 being a critical mediator of the deleterious effects of Dicer deletion, we show here loss of p53 could not rescue the profound apoptosis that occurs in primary B-cells and B-cell lymphomas upon Dicer deletion. All approaches to obtain p53-null B-cells or B-cell lymphomas that had biallelic Dicer deletion resulted in one Dicer allele being retained in any surviving cells, whereas Dicer-null fibroblasts could be easily generated. These results indicate Dicer, and consequently miRNA, have essential functions in B-cell survival for both untransformed and malignant B-cells that cannot be overcome by loss of p53. Also, lymphomas that lacked Arf could not survive Dicer deletion, indicating inactivation of the p53 pathway is insufficient to allow B cell lymphoma survival. Moreover, the data show all stages of B-cell transformation from immortalized (p53-null) to transformed (lymphoma) require Dicer. Additionally, a deficiency in Dicer and Rb combined with p53 inactivation resulted in synthetic lethality in retinal progenitors (24). Therefore, although p53 inactivation may provide protection from the deadly effects of Dicer deletion in some cellular contexts when specific genetic alterations are present, Dicer loss is lethal for B-cells and B-cell lymphomas regardless of p53 status.
Our results did show a deficiency in p53 was able to rescue several aspects of Myc-induced B-cell lymphoma development in the Dicerfl/fl background. Firstly, the early precursor B-cell lymphomas previously observed in ~40% of CD19-cre+/Dicerfl/fl/Eμ-myc mice did not occur in the p53-deficient mice; instead, only typical pre-B/B-cell lymphomas developed. Secondly, CD19 cell surface expression, which was significantly reduced or absent in 65% of the lymphomas in CD19-cre+/Dicerfl/fl/Eμ-myc mice, was fully restored in lymphomas from p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc mice. Unexpectedly, a p53 deficiency also allowed T-cell lymphomas to emerge, albeit at a low frequency. The explanations for changes in B-cell lymphoma phenotype and the rare development of T-cell lymphomas are currently unclear, but likely involve protection from apoptosis of a lymphoid progenitor, allowing differentiation to continue along B- and T-cell lineages. In addition, although CD19 surface expression was restored in the pre-B/B-cell lymphomas that emerged, 57% of the lymphomas lacked or had reduced Cre protein expression. This was unexpected, as all lymphomas expressed CD19 and Cre is driven from the CD19 promoter. Although Cre expression was downregulated in half of the lymphomas, the frequency of its expression (43%) was significantly higher than that of 12% in the CD19-cre+/Dicerfl/fl/Eμ-myc lymphomas (19), indicating the p53 deficiency partially rescued Cre expression. However, although Cre protein expression occurred more frequently in lymphomas in p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc transgenic mice, the number of lymphomas that underwent Cre-mediated deletion of at least one Dicer allele was not statistically different than the number that deleted one Dicer allele in CD19-cre+/Dicerfl/fl/Eμ-myc mice (19). These results indicate that while more lymphomas expressed Cre, the lymphomas still prevented it from deleting both Dicer alleles. Our data show a p53 deficiency still resulted in a delay in lymphoma development and did not allow biallelic Dicer deletion, but it did restore the lymphoma phenotype and CD19 surface expression and partially restored Cre expression in the B-cell lymphomas.
Protection from apoptosis is a critical step in B-cell development and lymphomagenesis (28, 32, 33, 34). Expression of Cre in Dicerfl/fl mice results in early B-cell progenitor (Mb1-Cre) or mature B-cell (Aicda-Cre) apoptosis and a developmental block or a lack of germinal centers, respectively (22, 35). Suppressing apoptosis by overexpressing the anti-apoptotic Bcl-2 protein and/or deleting the pro-apoptotic gene Bim or by expressing an immunoglobulin transgene, which provides survival signals, partially rescued B-cells from apoptosis in these systems. Since neither study confirmed biallelic deletion of Dicer had indeed occurred in the surviving B-cells, and since our data show B-cells do not survive Dicer deletion, it is likely the B-cells that survived in their studies only deleted one allele of Dicer. Moreover, the reduction in apoptosis that allowed more B-cells to survive and differentiate likely reflects effects on the B-cell compartment rather than on the survival of Dicer-deleted B-cells. In addition, it is unlikely that Bcl-2 overexpression alone would protect an untransformed B-cell from apoptosis induced by Dicer deletion, as the B-cell lymphomas we evaluated overexpressed Bcl-2 (unpublished observations) and rapidly died when Dicer was deleted. However, these results could also indicate transformed B-cells rely on Dicer more than untransformed B-cells. Certainly, further studies are needed to determine the conditions, if any, under which B-cells at any maturation stage would survive complete Dicer ablation.
Dicer is reported to function as a haploinsufficient tumor suppressor and promote tumorigenesis in retinal, lung epithelial, and muscle cells (17, 18). However, there is a conflicting report on muscle cells (36). In contrast, the rate of Myc-induced B-cell lymphomagenesis was similar in mice that had one or two alleles of Dicer (19), regardless of p53 status, indicating Dicer was not a haploinsufficient tumor suppressor in B-cells. Moreover, evaluation of Dicer protein and function in p53+/−/CD19-cre+/Dicerfl/fl/Eμ-myc lymphomas with one or two Dicer alleles revealed analogous levels of protein and mature miRNA. Therefore, loss of one allele of Dicer did not change the levels of Dicer protein or function in the B-cell lymphomas. Although our results reveal Dicer inhibition as a potential therapeutic opportunity for treatment of B-cell lymphomas, which are sensitive to Dicer loss, due to its haploinsufficient tumor suppressor functions in other cell types, this may not be possible. Therefore, it will be important in future studies to determine the cell types where Dicer functions as a haploinsufficient tumor suppressor, and whether transient inactivation of Dicer could be therapeutic for lymphoma treatment without being tumor-inducing.
Supplementary Material
Acknowledgments
We thank Maria Pia Arrate, Brandon Metge, and Chris Porter for technical assistance; Dr. Steve Jones for conditional Dicerfl/fl mice; Eischen lab members for discussions and critically reading the manuscript. This study was supported by F31CA165728 (CMA), T32GM008554 (CMA), R01CA148950 (CME), and by the NCI Cancer Center Support Grant P30CA068485 utilizing the Flow Cytometry and Translational Pathology Shared Resources.
Footnotes
The authors declare no conflict of interest
References
- 1.Almeida MI, Reis RM, Calin GA. MicroRNA history: discovery, recent applications, and next frontiers. Mutat Res. 2011;717:1–8. doi: 10.1016/j.mrfmmm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–87. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–33. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui TV, Mendell JT. Myc: Maestro of MicroRNAs. Genes Cancer. 2010;1:568–75. doi: 10.1177/1947601910377491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnegan EF, Pasquinelli AE. MicroRNA biogenesis: regulating the regulators. Crit Rev Biochem Mol Biol. 2013;48:51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, et al. The Catalogue of Somatic Mutations in Cancer (COSMIC) Curr Protoc Hum Genet. 2008;Chapter 10(Unit 10):1. doi: 10.1002/0471142905.hg1011s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:p11. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heravi-Moussavi A, Anglesio MS, Cheng SW, Senz J, Yang W, Prentice L, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med. 2012;366:234–42. doi: 10.1056/NEJMoa1102903. [DOI] [PubMed] [Google Scholar]
- 10.Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karube Y, Tanaka H, Osada H, Tomida S, Tatematsu Y, Yanagisawa K, et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–5. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin RJ, Lin YC, Chen J, Kuo HH, Chen YY, Diccianni MB, et al. microRNA signature and expression of Dicer and Drosha can predict prognosis and delineate risk groups in neuroblastoma. Cancer Res. 2010;70:7841–50. doi: 10.1158/0008-5472.CAN-10-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 14.Melo SA, Ropero S, Moutinho C, Aaltonen LA, Yamamoto H, Calin GA, et al. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nat Genet. 2009;41:365–70. doi: 10.1038/ng.317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Pampalakis G, Diamandis EP, Katsaros D, Sotiropoulou G. Down-regulation of dicer expression in ovarian cancer tissues. Clin Biochem. 2009;43:324–7. doi: 10.1016/j.clinbiochem.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Torres A, Torres K, Paszkowski T, Jodlowska-Jedrych B, Radomanski T, Ksiazek A, et al. Major regulators of microRNAs biogenesis Dicer and Drosha are down-regulated in endometrial cancer. Tumour Biol. 2011;32:769–76. doi: 10.1007/s13277-011-0179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J, et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 2009;23:2700–4. doi: 10.1101/gad.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambertz I, Nittner D, Mestdagh P, Denecker G, Vandesompele J, Dyer MA, et al. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ. 2010;17:633–41. doi: 10.1038/cdd.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arrate MP, Vincent T, Odvody J, Kar R, Jones SN, Eischen CM. MicroRNA biogenesis is required for Myc-induced B-cell lymphoma development and survival. Cancer Res. 2010;70:6083–92. doi: 10.1158/0008-5472.CAN-09-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 21.Mudhasani R, Zhu Z, Hutvagner G, Eischen CM, Lyle S, Hall LL, et al. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. J Cell Biol. 2008;181:1055–63. doi: 10.1083/jcb.200802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860–74. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Ravi A, Gurtan AM, Kumar MS, Bhutkar A, Chin C, Lu V, et al. Proliferation and tumorigenesis of a murine sarcoma cell line in the absence of DICER1. Cancer Cell. 2012;21:848–55. doi: 10.1016/j.ccr.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nittner D, Lambertz I, Clermont F, Mestdagh P, Kohler C, Nielsen SJ, et al. Synthetic lethality between Rb, p53 and Dicer or miR-17-92 in retinal progenitors suppresses retinoblastoma formation. Nat Cell Biol. 2012;14:958–65. doi: 10.1038/ncb2556. [DOI] [PubMed] [Google Scholar]
- 25.Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–8. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 26.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–8. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–6. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 28.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–69. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alt JR, Greiner TC, Cleveland JL, Eischen CM. Mdm2 haplo-insufficiency profoundly inhibits Myc-induced lymphomagenesis. EMBO J. 2003;22:1442–50. doi: 10.1093/emboj/cdg133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, Lushnikova T, Odvody J, Greiner TC, Jones SN, Eischen CM. Elevated Mdm2 expression induces chromosomal instability and confers a survival and growth advantage to B cells. Oncogene. 2008;27:1590–8. doi: 10.1038/sj.onc.1210788. [DOI] [PubMed] [Google Scholar]
- 31.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–7. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 32.Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol. 2001;21:7653–62. doi: 10.1128/MCB.21.22.7653-7662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–70. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 35.Xu S, Guo K, Zeng Q, Huo J, Lam KP. The RNase III enzyme Dicer is essential for germinal center B-cell formation. Blood. 2012;119:767–76. doi: 10.1182/blood-2011-05-355412. [DOI] [PubMed] [Google Scholar]
- 36.Mito JK, Min HD, Ma Y, Carter JE, Brigman BE, Dodd L, et al. Oncogene-dependent control of miRNA biogenesis and metastatic progression in a model of undifferentiated pleomorphic sarcoma. J Pathol. 2013;229:132–40. doi: 10.1002/path.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.