Abstract
We studied the respiratory responses to an increase in airway temperature in patients with allergic rhinitis (AR). Responses to isocapnic hyperventilation (40% of maximal voluntary ventilation) for 4 minutes of humidified hot air (HA; 49 °C) and room air (RA: 21 °C) were compared between AR patients (n=7) and healthy subjects (n=6). In AR patients, cough frequency increased pronouncedly from 0.10±0.07 before to 2.37±0.73 during, and 1.80±0.79 coughs/min for the first 8 minutes after the HA challenge, but not during the RA challenge. In contrast, neither HA nor RA had any significant tussive effect in healthy subjects. The HA challenge also caused respiratory discomfort (mainly throat irritation) measured by the handgrip dynamometry in AR patients, but not in healthy subjects. Bronchoconstriction was not detected after the HA challenge in either group of subjects. In conclusion, hyperventilation of HA triggered vigorous cough response and throat irritation in AR patients, indicating the involvement of sensory nerves innervating upper airways.
Keywords: cough, allergic rhinitis, airway irritation, TRPV1, laryngeal
1. Introduction
Allergic rhinitis (AR) is an inflammatory disease of upper airways characterized by nasal congestion and rhinorrhea, intermittent or persistent sneezing, pruritus in nose, eyes and throat, and coughing. The inflammatory reaction is characterized by early-phase and late-phase allergic responses similar to that in allergic asthma (Bousquet et al., 2012; Wallace et al., 2008). Repeated exposures to environmental allergens result in an IgE mediated type I allergic response that induces a type-2 helper T cell (TH2) inflammation. Cross-linking of IgE antibodies present on the surface of primed mast cells by an antigen activates them and results in degranulation and release of inflammatory mediators such as histamines, tryptase, and leukotrienes, which in turn leads to vasodilatation and increased vascular permeability. The recruitment of TH2 cells and secretion of IL-5 give rise to tissue eosinophilia that characterizes the late phase response (Middleton et al., 2009). Eosinophilic inflammation in turns can result in further tissue damage and sensitization of the afferent nerves innervating the nose, throat and upper airways due to release of additional inflammatory mediators.
Our laboratory has recently reported that an increase in airway temperature by hyperventilation of hot humid air for 4 minutes triggered an immediate and transient bronchoconstriction in patients with mild asthma, but not in healthy individuals (Hayes et al., 2012). The bronchoconstriction was accompanied by cough and prevented by pretreatment with ipratropium, a muscarinic receptor antagonist, suggesting an involvement of activation of airway sensory nerves and the cholinergic reflex pathway. Although direct evidence could not be established in that study, our results suggested activation of a temperature sensors expressed in the vagal bronchopulmonary sensory nerves is probably involved in eliciting these reflex responses. One possible candidate is the transient receptor potential vanilloid type 1 (TRPV1). Indeed, chronic allergic inflammation is known to enhance both the sensitivity and the expression of TRPV1 in airway sensory nerves (Lee and Gu, 2009; Zhang et al., 2008).
TRPV1 is also abundantly expressed in the sensory nerve fibers innervating the pharynx, larynx and upper airways (Hamamoto et al., 2008; Hamamoto et al., 2009; Sasaki et al., 2013; Yamamoto and Taniguchi, 2005). However, whether the sensitivity of these TRPV1-expressing sensory nerves is elevated resulting from the chronic inflammation of upper airways in AR patients is not yet known, and the reflex responses elicited by an increase in airway temperature in these patients have not been previously studied. This study was therefore carried out to answer these questions.
2. Methods
2.1 Subjects
Adult AR patients and healthy subjects were recruited by public advertisement. A screening interview and a spirometry test were performed in each subject after informed consent was obtained. The diagnosis of AR was confirmed according to the standard clinical guidelines in each patient and a documented positive allergy skin test (Wallace et al., 2008). The American Academy of Allergy, Asthma, and Immunology Joint Task Force on Practice Parameters questionnaire was used to assess and compare symptom severity and global impact of AR in all subjects (Spector et al., 2003). Due to the need to stop therapeutic medications for 2 weeks prior to beginning of the study, patients on steroids and/or have poor AR control were excluded. The study protocol was approved by the Institutional Review Board at the University of Kentucky.
2.2 Isocapnic Hyperventilation Challenge
A device designed to deliver air of desired temperature and humidity constructed by the University of Kentucky Center for Manufacturing was used as previously described (Hayes et al., 2012). Briefly, a humidified gas mixture of 4.5% CO2 balanced with air at either hot (HA; 49°C and 75-80% relative humidity measured by an Extech Hygro-Thermometer, model RH101; Nashua, NH) or room temperature (RA; 20-22°C and 65-75% relative humidity) was delivered at 300 liters per minute through a large-bore (7.62-cm) stainless-steel conduit. During the hyperventilation challenge, the subject, while wearing a nose clip, breathed via a mouthpiece into this free stream of humidified gas mixture at ~40% of maximal voluntary ventilation (MVV), determined in each subject in a pre-test, for four minutes; CO2 was added to maintain an isocapnic condition during hyperventilation. Humidity was generated from sterile isotonic saline by an ultrasonic atomizer (Sonaer Ultrasonics; Farmingdale, NY). The amounts of isotonic saline delivered in RA and HA were 12-14 and 56-60 μl/liter of air, respectively. Humidity and hyperventilation at 40% of MVV were used to facilitate the heat transfer from air to the airway tissue. Levels of end-tidal temperature (model IT-18, Physitemp, Clifton, NJ; time constant: 0.1 sec) and CO2 concentration (Novametrix 1260; Murrysville, PA) were measured before and after 2 minutes of hyperventilation when these changes reached steady state; and they were measured again at 8 and 16 minutes after the hyperventilation challenges.
2.3 Pulmonary Function Measurements
Airway resistance (Raw) was measured by a whole-body constant-volume plethysmography (SensorMedics, Yorba Linda, CA) for 6 minutes before and 16 minutes immediately after the hyperventilation challenge. During each measurement, the subject was asked to pant at a frequency of ~2 Hz for ~8 sec; Raw was determined by computer, using the center-fit method for the slope measurement within the flow range of ±0.5 liters/sec. Spirometry test was also performed along with the measurements of other physiological variables (body temperature, heart rate, arterial blood pressure, and oxygen saturation) before and after the challenge.
2.4 Measurement of Cough Frequency
The number of coughs was recorded manually by listening to and counting the number of explosive cough sounds before, during and after each hyperventilation challenge. A VitaloJAK cough monitor [developed by Vitalograph Ltd (Lenexa, KS) and the Respiratory Research Group, University of Manchester, UK] was also used in the second half (61%) of the study for a more objective and quantitative measurement of the cough frequency (Smith et al., 2006). The device used a contact microphone placed on the chest wall, a second free field microphone and a custom-made digital recording device to record cough sounds. Cough signals recorded by the cough monitor were played back, and the cough numbers were counted by an individual not familiar with the protocol. Cough frequency measured as number of coughs per minute was then compared with those obtained from manual counting during the experiment; the difference between the data obtained from these two methods was generally less than 10%.
2.5 Measurement of Respiratory Sensation
Subjects were instructed to indicate the presence and express the degree of respiratory discomfort by squeezing an isometric handgrip dynamometer (model MLT003, ADInstruments; Colorado Springs, CO) with a magnitude of force proportional to the intensity of the sensation felt (Burki et al., 2005; Muza and Zechman, 1984) at intervals of ~2 minutes following both HA and RA hyperventilation challenges. The resultant voltage generated from the dynamometer transducer was recorded continuously in conjunction with the measurements of Raw and cough responses. To compare the response between subjects, the level of discomfort in each subject was quantified by calculating each response signal as a percentage of the maximum handgrip signal (as 100%) that was determined in each subject before each experiment. After the experiment, the subject was asked to describe verbally if there was any type of respiratory discomfort, and if so, the location of the evoked sensation.
2.6 Study Design
HA and RA hyperventilation challenges were given at a random sequence in each subject, usually on two different days. When both challenges were given in the same day, at least two hours elapsed for recovery. The responses to HA and RA hyperventilation challenges were compared in both AR patients and healthy subjects.
2.7 Statistical Analysis
A two-way analysis of variance (ANOVA) was used for the statistical evaluation of the results. When the ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher’s least significant difference). Comparisons between the two groups (AR patients vs. healthy subjects) were made using the one-way ANOVA. Data are reported as means ± SEM. P values of <0.05 were considered significant.
3. Results
Seven AR patients between 21-43 (35 ± 3) year of age and six healthy subjects between 25-48 (32 ± 3) year of age were enrolled in the study; the subject characteristics are shown in Table 1. The AR symptom severity assessment data (Table 1) show that several symptoms with mean scores exceeding 3.5 (out of a total score of 7.0), including sneezing (3.57 ± 0.53; n=7), nasal congestion (5.0 ± 0.58), itchy nose (3.93 ± 0.74), postnasal drip (4.0 ± 0.68), chronic cough (3.57 ± 0.65), eye (3.57 ± 0.53) and ear symptoms (3.57 ± 0.43), were found in AR patients, but none in healthy subjects. A comparison between the two groups of subjects showed that there was no significant difference between AR patients and healthy subjects in any of the base-line measurements of physiological variables (Tables 2, 3 and 4).
Table 1.
Subject Characteristics and AR Symptom Severity Assessments
| AR patients | Healthy subjects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #1 | #2 | #3 | #4 | #5 | #6 | ||
| Physical data | Age (year) | 37 | 40 | 21 | 43 | 41 | 30 | 35 | 25 | 28 | 29 | 32 | 29 | 48 |
| Sex | M | M | F | F | F | F | F | F | M | F | M | M | F | |
| Height (cm) | 180 | 175 | 155 | 150 | 160 | 160 | 165 | 170 | 185 | 157 | 173 | 170 | 160 | |
| Weight (kg) | 122 | 104 | 59 | 73 | 73 | 53 | 61 | 58 | 86 | 54 | 61 | 84 | 60 | |
| A: Nasal | Sneezing | 3 | 2 | 5 | 5 | 2 | 5 | 3 | 1 | 1 | 1 | 1 | 1 | 1 |
| Runny Nose | 3 | 3 | 4 | 4 | 2 | 5 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | |
| Congestion (stuffiness) |
6 | 3 | 6 | 7 | 3 | 5 | 5 | 1 | 1 | 1 | 1 | 2 | 1 | |
| Itchy Nose | 6 | 3 | 6 | 3 | 2 | 6 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Postnasal Drip | 2 | 4 | 6 | 3 | NA | 6 | 3 | 1 | 2 | 1 | 1 | 1 | 1 | |
| Total Nasal Symptoms |
5 | 5 | 6 | NA | 2 | 6 | 3 | 1 | 2 | 1 | 1 | 1 | 1 | |
| B: Non-nasal | Eye Symptoms | 6 | 3 | 4 | 3 | 3 | 3 | 3 | 1 | 2 | 1 | 1 | 1 | 1 |
| Throat Symptoms | 2 | 3 | 6 | 5 | 1 | NA | 3 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Chronic Cough | 4 | 1 | 5 | 4 | 2 | 6 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Ear Symptoms | 3 | 2 | 6 | 3 | 3 | 3 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Headache | 5 | 1 | 2 | 5 | 1 | 4 | 4 | 1 | 2 | 1 | 1 | 1 | 1 | |
| Mental Function | 1 | 1 | 1 | 5 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| C: Q.O.L. | Quality of Life | 2 | 5 | 3 | 2 | 5 | 2 | 4 | 7 | 7 | 7 | 7 | 7 | 7 |
AR: allergic rhinitis; Categories A and B: 1 - None; 7 - Unbearably severe; Category C: 1 - None; 7 -Excellent; Q.O.L.: Quality of life; NA: Not assessed.
Table 2.
Changes in end-tidal (E.T.) temperature and CO2 concentration caused by hyperventilation of humidified air at room (RA) and high temperature (HA).
| AR patients (n=7) | ||||
|---|---|---|---|---|
|
| ||||
| E.T. Temperature (°C) | E.T. CO2 (%) | |||
|
| ||||
| Before | During | Before | During | |
| RA | 33.59 ± 0.29 | 32.79 ± 0.19* | 4.96 ± 0.26 | 4.94 ± 0.17 |
| HA | 33.50 ± 0.31 | 35.00 ± 0.09*† | 5.14 ± 0.43 | 5.18 ± 0.47 |
| Healthy subjects (n=6) | ||||
|---|---|---|---|---|
|
| ||||
| E.T. Temperature (°C) | E.T. CO2 (%) | |||
|
| ||||
| Before | During | Before | During | |
| RA | 33.63 ± 0.35 | 32.56 ± 0.35* | 4.88 ± 0.20 | 5.15 ± 0.33 |
| HA | 33.13 ± 0.27 | 34.49 ± 0.30*† | 4.93 ± 0.20 | 5.11 ± 0.24 |
Measurements were made before and at 2 minutes after the beginning of the 4-minute hyperventilation; the latter was measured immediately after the hyperventilation was interrupted for 3-6 breaths while the subject breathed room air during these measurements.
Significant difference between before and during the hyperventilation challenge.
Significant different from the corresponding RA data.
Table 3.
Changes in forced expiratory volumes caused by hyperventilation of humidified air at hot (HA) and room temperature (RA).
| AR patients (n=7) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| FEV1 (liters) | FVC (liters) | FEV1/FVC | ||||
|
| ||||||
| Before | After | Before | After | Before | After | |
| RA | 3.12 ± 0.28 | 2.99 ± 0.25 | 3.84 ± 0.35 | 3.78 ± 0.32 | 81.26 ± 2.01 | 79.44 ± 1.37 |
| HA | 3.12 ± 0.30 | 3.08 ± 0.30 | 3.82 ± 0.37 | 3.92 ± 0.36† | 82.09 ± 2.12 | 78.31 ± 1.44* |
| Healthy subjects (n=6) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| FEV1 (liters) | FVC (liters) | FEV1/FVC | ||||
|
| ||||||
| Before | After | Before | After | Before | After | |
| RA | 3.67 ± 0.32 | 3.63 ± 0.32 | 4.50 ± 0.44 | 4.54 ± 0.44 | 82.07 ± 2.26 | 80.43 ± 2.56 |
| HA | 3.66 ± 0.33 | 3.63 ± 0.33 | 4.46 ± 0.46 | 4.53 ± 0.49 | 82.52 ± 2.39 | 80.96 ± 2.47 |
Forced expiratory tests were performed before and at ~8 minutes after the hyperventilation challenge in allergic rhinitis (AR) patients and healthy subjects.
Significant difference between before and after the hyperventilation challenge.
Significant different from the corresponding RA data.
Table 4.
Effects of hyperventilation of humidified air at hot (HA) and room temperature (RA) on other physiological variables.
| AR patients (n=7) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Body Temperature (°C) |
Sys. B.P. (mmHg) |
Dia. B.P. (mmHg) |
Heart rate (bpm) |
O2 Saturation (%) |
||||||
|
| ||||||||||
| Before | During | Before | During | Before | During | Before | During | Before | During | |
| RA | 36.5 ± 0.2 | 35.8 ± 0.2* | 124.7 ± 5.9 | 126.4 ± 7.6 | 77.3 ± 5.6 | 78.0 ± 5.6 | 76.3 ± 5.2 | 79.7 ± 5.8 | 97.6 ± 0.5 | 97.7 ± 0.6 |
| HA | 36.3 ± 0.2 | 36.4 ± 0.2† | 124.6 ± 6.9 | 122.1 ± 5.9 | 74.6 ± 4.0 | 80.1 ± 4.6* | 74.6 ± 3.9 | 82.6 ± 4.2* | 97.9 ± 0.6 | 98.0 ± 0.5 |
| Healthy subjects (n=6) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Body Temperature (°C) |
Sys. B.P. (mmHg) |
Dia. B.P. (mmHg) |
Heart rate (bpm) |
O2 Saturation (%) |
||||||
|
| ||||||||||
| Before | During | Before | During | Before | During | Before | During | Before | During | |
| RA | 36.5 ± 0.2 | 35.8 ± 0.2* | 129.3 ± 7.2 | 130.8 ± 7.1 | 76.2 ± 3.9 | 74.8 ± 4.2 | 67.5 ± 5.6 | 69.5 ± 5.2 | 97.8 ± 0.7 | 98.5 ± 0.7* |
| HA | 36.2 ± 0.2† | 36.4 ± 0.1*† | 131.3 ± 6.4 | 129.2 ± 6.0 | 76.2 ± 3.5 | 79.0 ± 3.3† | 66.0 ± 5.3 | 70.3 ± 5.6* | 98.7 ± 0.6† | 98.8 ± 0.5 |
Measurements were made before and at 2 minutes after the beginning of the 4-minute hyperventilation in AR patients and healthy subjects.
Significant difference between before and during the hyperventilation challenge.
Significant different from the corresponding RA data.
In AR patients, hyperventilation of humidified HA generated a significant increase in end-tidal air temperature (Δ=1.5 ± 0.2 °C; P<0.05, Table 2). In contrast, hyperventilation of RA decreased the end-tidal temperature significantly (Δ=0.8 ± 0.24 °C; P<0.05, Table 2) in the same patients. Hyperventilation of either HA or RA did not change the end-tidal CO2 concentration in these patients (Table 2). Similar changes were also recorded in healthy subjects (Table 2).
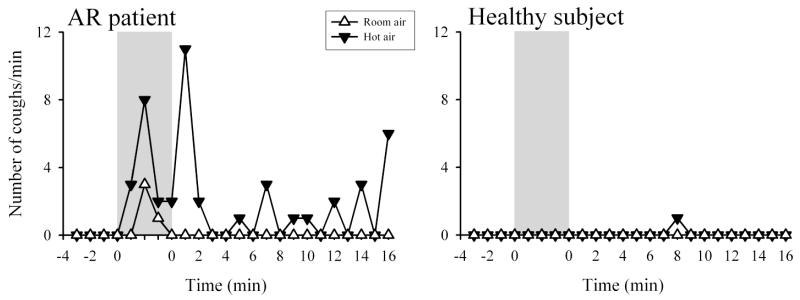
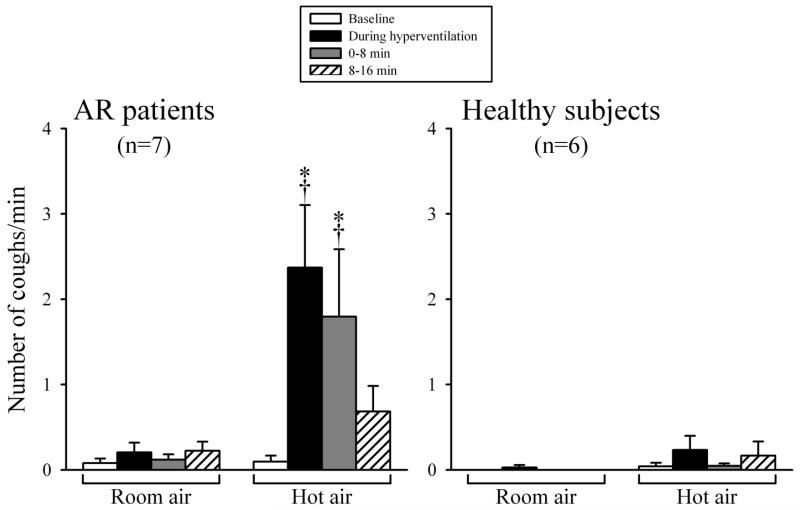
The HA challenge consistently triggered cough in AR patients (e.g., Fig. 1). Number of coughs was 0.10 ± 0.07 coughs/min at baseline, increased to 2.37 ± 0.73 coughs/min (P<0.01, n=7) during the isocapnic hyperventilation of HA, and subsequently to 1.80 ± 0.79 coughs/min (P<0.01, n=7) in the first 8 minutes following the HA challenge (Fig. 2). In contrast, hyperventilation of RA did not cause any significant tussive effect in the same patients (Fig. 2). In two of the AR patients, the cough responses to the HA challenge were compared in separate experiments when the humidity of HA were generated from isotonic saline and distilled water, and similar responses were found; cough frequencies were 4.7 ± 0.5 and 4.1 ± 0.4 coughs/min (n=2) during the HA challenges with the humidity generated from saline and distilled water, respectively.
Fig. 1.
Representative responses of cough frequency (number of coughs per minute) to hyperventilation of humidified room air (open triangles) and hot air (closed triangles) in an AR patient (left panel) and a healthy subject (right panel). During hyperventilation (shaded bars), the subjects breathed at 40% of maximal voluntary ventilation for 4 minutes of a gas mixture of 4.5% CO2 balance air.
Fig. 2.
Comparison of cough responses to hyperventilation of humidified room air and hot air in AR patients (left panel) and healthy subjects (right panel). Cough frequencies were averaged in 8-minute durations before and after hyperventilation challenge in each subject. Data are means ± SEM. *Significantly different from the baseline. †Significant difference between room air and hot air.
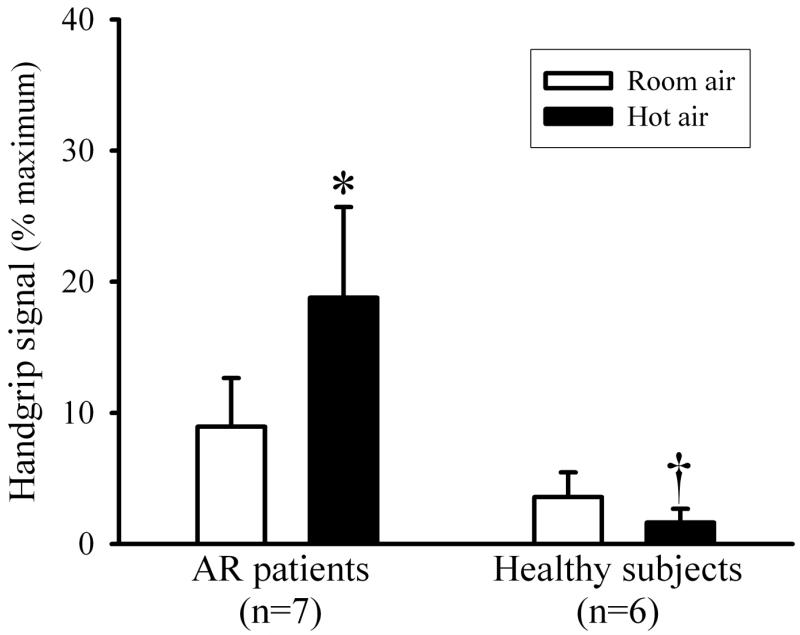
AR patients expressed a significantly higher degree of respiratory discomfort via handgrip dynamometry after the hyperventilation of humid HA (Fig. 3). Their hand grip signal increased to 18.8 ± 6.9% of the maximum hand grip signal after humid HA challenge compared to 8.9 ± 3.7% after RA hyperventilation (P<0.05, n=7). In AR patients, verbal description of respiratory discomfort after the experiment included “throat irritation and tickling”, and “dry and sore throat.” The location of irritation was described by these patients as mainly in or below the larynx area.
Fig. 3.
Airway irritation evoked by hyperventilation of humidified room air (open bars) and hot air (closed bars) in AR patients and healthy subjects. The level of discomfort sensation was expressed by the handgrip dynamometer signal in each individual. *Significant difference between room air and hot air. †Significant difference between AR patients and healthy subjects.
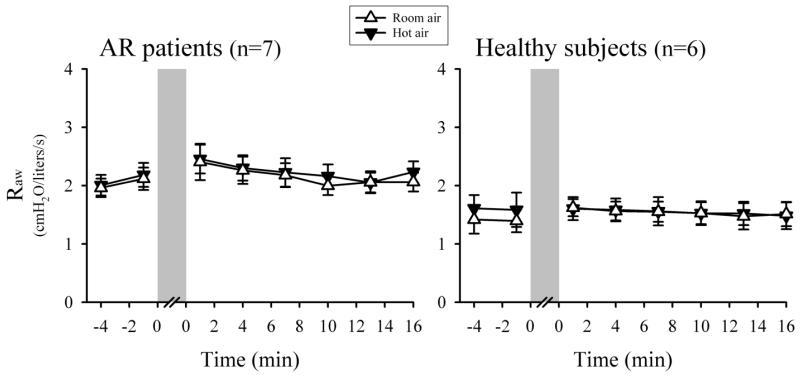
In AR patients, the FEV1/FVC ratio decreased significantly from 82.1 ± 2.1% to 78.3 ± 1.4% at ~ 8 min after the HA challenge (P<0.05, n=7); FEV1 and FVC, however, did not change significantly and remained within normal range (Table 3). The small but significant decrease in the FEV1/FVC ratio seemed to indicate a mild bronchoconstriction, which however was not detected by the measurement of Raw in these patients (Fig. 4). On average, Raw did not significantly change after the HA challenge in AR patients: Raw = 2.08 ± 0.18 cmH2O/L/sec at baseline; the peak Raw = 2.76 ± 0.28 cmH2O/L/sec after the HA challenge (P>0.05, n=7). Heart rate increased slightly but significantly during the HA challenge from 74.6 ± 3.9 to 82.6 ± 4.2 beats/min (P<0.05, n=7) (Table 4). AR patients did not develop any wheezing during and after either HA or RA challenge.
Fig. 4.
Comparison of responses of airway resistance (Raw) to hyperventilation (shaded bars) of humidified room air (open triangles) and hot air (closed triangles) in AR patients (left panel) and healthy subjects (right panel). Each data point represents Raw averaged over 4 consecutive breaths, and data are means ± SEM of all subjects in that group.
Healthy subjects, in a sharp contrast to that in AR patients, did not exhibit significant tussive response during isocapnic HA hyperventilation (Figs. 1 and 2). Healthy individuals also described a subtle feeling of dry throat during the HA hyperventilation challenge (despite the 75-80% relative humidity in the HA). However, none of the healthy subjects expressed throat irritation or respiratory discomfort (Fig. 3). Raw did not change significantly after the HA challenge in healthy subjects, similar to that in AR patients (Fig. 4); FEV1, FVC and FEV1/FVC ratio did not change significantly, either (P>0.05, n=6) (Table 3). Heart rate increased significantly during the HA challenge from 66.0 ± 5.3 to 70.3 ± 5.6 beats/min (P<0.05, n=6), similar to that in AR patients (Table 4). Wheezing was not detected in any of the healthy subjects during and after either HA or RA hyperventilation.
4. Discussion
The results of this study showed that an increase in airway temperature triggered vigorous cough responses and evoked throat irritation in AR patients, but not in healthy individuals. We chose the protocol of hyperventilation of humidified HA to increase the airway temperature because it has been well illustrated that both hyperventilation and the humidity in the HA can facilitate the delivery of “heat load” from the inhaled HA to the airway tissue (Aitken and Marini, 1985). However, we can dismiss hyperventilation as a contributing factor to the tussive effect because hyperventilation of humid RA did not generate cough or throat irritation in the same AR patients. We used isotonic saline to humidify the inspired gas mixture in this study because inhalation of distilled water aerosol is known to trigger bronchoconstriction and cough in patients with asthma, whereas isotonic saline aerosol did not in the same patients (Sheppard et al., 1983). This excitatory effect of distilled water is believed to result from a stimulation of laryngeal sensory nerves and/or rapidly adapting airway receptors due to the low concentration of chloride ion and/or the low osmolarity in distilled water (Anderson et al., 1990; Pisarri et al., 1992). However, when the HA challenges generated from isotonic saline and distilled water were tested separately in two AR patients in this study, their cough responses to saline and distilled water were not different, which may be related to the relatively low water content delivered in the humid HA in our study compared with that delivered in aerosol in the study by Sheppard and coworkers (1983). Furthermore, in a recent study in allergen-sensitized rats, when the same water content as that contained in the humidified HA was delivered by aerosolized saline at room temperature, it failed to generate any significant airway responses (Hsu et al., 2013). In addition, in the present study hyperventilation of humidified RA, despite presence of atomized saline, did not induce any significant cough response in the same AR patients (Fig. 2). Thus, although we cannot dismiss the role of humidity in the delivery of “heat load” (Aitken and Marini, 1985) in this study, the collective evidence suggests that the tussive response and throat irritation were caused primarily by the increase in airway tissue temperature generated by the HA hyperventilation,.
In addition to the tussive effect, hyperventilation of HA also evoked a significantly higher degree of respiratory discomfort, compared to the RA hyperventilation challenge; in this study we used a hand-grip dynamometer for subjects to express the respiratory discomfort (Burki et al., 2005). The device functions on the basis of Stevens’ psychophysical power law that states the perceived magnitude of sensation relates exponentially to the level of the stimulus (Stevens, 1957). It is a cross-modality matching instrument that matches subjects muscle force to their perceived level of discomfort, similar to the magnitude estimation by numerical scale. In fact, the two modalities have been shown to be equivalent in scaling respiratory sensation (Muza and Zechman, 1984). Verbal descriptions of the discomforts in AR patients uniformly pointed to throat irritation and tickling, and the sites of irritation were localized in the larynx area. In contrast, throat irritation was not detected or expressed by any of the healthy individuals despite receiving the same hyperventilation of HA challenge. All these information and evidence seem to suggest the possible involvement of stimulation of sensory nerves innervating the larynx, laryngopharynx and/or upper airways in the AR patients.
The specific types of sensory nerves and temperature sensors that are responsible for evoking the sensation of throat irritation and cough during and after the HA hyperventilation challenge in AR patients cannot be identified in this study. However, some of the potential candidates should be considered. The primary sensors for detecting warm and hot temperature in mammalian species are TRPV channels (Nilius et al., 2007). TRPVs are a family of ion channels containing six trans-membrane domains that form non-selective, non-voltage-gated cationic channels (Nilius et al., 2007). Each of the four subtypes of TRPVs (TRPV1-4) is activated in a different temperature range (Benham et al., 2003; Dhaka et al., 2006). The increase in airway temperature generated by HA challenge in the present study likely activated more than one type of these TRPV channels. Among them, the potential involvement of TRPV1 merits further consideration. TRPV1 is abundantly expressed on the nerve terminals of the vagal and non-vagal C-fiber afferents innervating the entire respiratory tract, including the larynx, pharynx and upper airways (Hamamoto et al., 2008; Hamamoto et al., 2009; Lee and Yu, 2014; Sasaki et al., 2013; Yamamoto and Taniguchi, 2005). Using the whole-cell perforated patch clamping technique, our laboratory has recently demonstrated that an increase in temperature within the normal physiological range (35-41 °C) evoked inward currents (in voltage-clamp mode), and membrane depolarization and action potentials (in current-clamp mode) in isolated vagal pulmonary sensory neurons (Ni et al., 2006). These responses were reduced by >50% after a pretreatment with a selective antagonist of the TRPV1 channel, AMG 9810. This observation is of particular importance because it demonstrated that this effect is mediated primarily through activation of the TRPV1 channel (Ni et al., 2006). More importantly, the bronchopulmonary sensory neurons could be activated by increasing temperature to the levels considerably lower than 43 °C, the temperature that was originally reported as the temperature threshold for activating the heterologously expressed TRPV1 receptor (Caterina et al., 1997).
In this study, the same HA challenge did not evoke cough or throat irritation in healthy subjects. The difference in these responses between AR patients and healthy individuals could probably be related to the fact that the TRPV1 sensitivity can be elevated in the presence of tissue inflammation because endogenous inflammatory mediators such as bradykinin, prostaglandins, and nerve growth factor are known to cause post-translational sensitization of TRPV1 receptor (Shin et al., 2002; Sugiura et al., 2002; Zhang et al., 2005). In addition, our laboratory recently reported that chronic allergic inflammation in Brown-Norway rats actively sensitized with Ova induced a significant increase in the expression of TRPV1 in bronchopulmonary neurons in nodose ganglia, mainly in neurofilament-positive (myelinated) neurons (Zhang et al., 2008). Indeed, the sensitivity to capsaicin, a selective activator of TRPV1, was detected in some of the vagal myelinated (A-fiber) afferents that normally do not exhibit capsaicin sensitivity (Zhang et al., 2008). Our hypothesis is supported by the study of Pecova and coworkers who have reported a significantly higher cough sensitivity to capsaicin in patients with seasonal allergic rhinitis compared to healthy individuals (Pecova et al., 2008). Whether an up-regulation of the TRPV1 expression in the afferent fibers innervating larynx, laryngopharynx and upper airways in the AR patients remains to be investigated, nonetheless,.
In our current study the pronounced tussive effect generated by the HA challenge was not accompanied by any increase in Raw in AR subjects. These results are different from the significant bronchoconstrictive responses to HA challenge that we recently reported in patients with mild asthma (Hayes et al., 2012). It is known that chronic inflammation in asthma results in hypertrophy and hyperplasia of airway smooth muscles, subepithelial fibrosis, increase in mucus glands and vascularity throughout the entire tracheobronchial tree, a process known as airway remodeling (Fahy et al., 2000). The chronic inflammation known to occur in the lower airways of asthmatics and the resultant release of inflammatory mediators and cytokines can lead to the hypersensitivity of bronchopulmonary C-fiber afferents (Lee and Yu, 2014), which may further enhance the bronchoconstrictive response in asthmatic patients by causing additional reflexive contraction of hypertrophic airway smooth muscles through the cholinergic pathway (Hayes et al., 2012). Thus, we postulate that the discrepancy between these two studies is related to the differences in the site and degree of chronic inflammation, and the subsequent post-inflammatory changes between these two patient groups.
In this study we did not have the data to determine the temperature threshold for triggering the cough responses, which precludes us from any speculation regarding the environmental conditions (temperature and humidity) that can cause worsening of symptoms in AR patients. Although the temperature of 49°C used in our HA challenge is relatively high compared to the range of environmental temperature, the HA challenge only generated a small increase in the end-tidal temperature plateau in both AR and healthy subjects in this study (Table 2). Furthermore, it is conceivable that the same increase in airway tissue temperature can be generated by breathing hot humid air at a lower temperature for a longer duration (>4 minutes). More importantly, the hyperventilation at the level of 40% of MVV simulates the breathing during light to moderate levels of exercise when subjects breathe through mouth instead of nose. Hence, based upon our findings in this study, it seems reasonable to postulate that exercise tolerance of AR patients may be adversely affected in hot and humid environments.
In summary, this study clearly demonstrated that hyperventilation of humid HA triggered vigorous cough responses and evoked throat irritation in AR patients, but not in healthy individuals. Furthermore, these tussive and irritating effects were not detected in the same AR patients after hyperventilation of humid RA. Although the mechanisms promoting these responses are not yet fully understood, these findings pointed to a possible involvement of activation of the thermal sensors expressed in the sensory nerves innervating larynx, laryngopharynx and upper airways by an increase in airway temperature.
Acknowledgements
We thank Dr. Tom Henninger for designing and manufacturing the device for regulating temperature and humidity of the inhaled gas mixture; Vitalograph Ltd (Lenexa, KS) for lending us the VitaloJAK cough monitor for this study; Dr. Richard Kryscio for statistical consultation; Robert Morton for technical assistance; and the nursing staff at the University of Kentucky Clinical Research Development and Operations Center for their assistance.
Grants
This study was supported in part by the NIH grants HL-67379 and HL-96914 (to L.Y.L.), Department of Defense DMRDP/ARATD award administered by the U.S. Army Medical Research & Materiel Command (USAMRMC) Telemedicine & Advanced Technology Research Center (TATRC) under Contract Number W81XWH-10-2-0189 (to L.Y.L.), University of Kentucky Clinical Research Development & Operations Center grant UL1TR000117 and Kentucky Pediatric Research Institute support (to D.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitken ML, Marini JJ. Effect of heat delivery and extraction on airway conductance in normal and in asthmatic subjects. The American review of respiratory disease. 1985;131:357–361. doi: 10.1164/arrd.1985.131.3.357. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Sant’Ambrogio FB, Mathew OP, Sant’Ambrogio G. Water-responsive laryngeal receptors in the dog are not specialized endings. Respiration physiology. 1990;79:33–43. doi: 10.1016/0034-5687(90)90058-7. [DOI] [PubMed] [Google Scholar]
- Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell calcium. 2003;33:479–487. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Schunemann HJ, Samolinski B, Demoly P, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. The Journal of allergy and clinical immunology. 2012;130:1049–1062. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- Burki NK, Dale WJ, Lee LY. Intravenous adenosine and dyspnea in humans. Journal of applied physiology (Bethesda, Md.: 1985) 2005;98:180–185. doi: 10.1152/japplphysiol.00913.2004. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annual review of neuroscience. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Corry DB, Boushey HA. Airway inflammation and remodeling in asthma. Current opinion in pulmonary medicine. 2000;6:15–20. doi: 10.1097/00063198-200001000-00004. [DOI] [PubMed] [Google Scholar]
- Hamamoto T, Takumida M, Hirakawa K, Takeno S, Tatsukawa T. Localization of transient receptor potential channel vanilloid subfamilies in the mouse larynx. Acta otolaryngologica. 2008;128:685–693. doi: 10.1080/00016480701669489. [DOI] [PubMed] [Google Scholar]
- Hamamoto T, Takumida M, Hirakawa K, Tatsukawa T, Ishibashi T. Localization of transient receptor potential vanilloid (TRPV) in the human larynx. Acta otolaryngologica. 2009;129:560–568. doi: 10.1080/00016480802273108. [DOI] [PubMed] [Google Scholar]
- Hayes D, Jr., Collins PB, Khosravi M, Lin RL, Lee LY. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. American journal of respiratory and critical care medicine. 2012;185:1190–1196. doi: 10.1164/rccm.201201-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Lin RL, Lin YS, Lee LY. Bronchoconstriction induced by increasing airway temperature in ovalbumin-sensitized rats: role of tachykinins. Journal of applied physiology (Bethesda, Md.: 1985) 2013;115:688–696. doi: 10.1152/japplphysiol.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Current opinion in pharmacology. 2009;9:243–249. doi: 10.1016/j.coph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Yu J. Sensory nerves in lung and airways. Comprehensive Physiology. 2014;4:287–324. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- Middleton E, Reed CE, Ellis EF. Allergy, principles and practice. Mosby; 2009. [Google Scholar]
- Muza SR, Zechman FW. Scaling of added loads to breathing: magnitude estimation vs. handgrip matching. Journal of applied physiology: respiratory, environmental and exercise physiology. 1984;57:888–891. doi: 10.1152/jappl.1984.57.3.888. [DOI] [PubMed] [Google Scholar]
- Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, et al. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;291:R541–550. doi: 10.1152/ajpregu.00016.2006. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiological reviews. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Pecova R, Zucha J, Pec M, Neuschlova M, Hanzel P, et al. Cough reflex sensitivity testing in in seasonal allergic rhinitis patients and healthy volunteers. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2008;59(Suppl 6):557–564. [PubMed] [Google Scholar]
- Pisarri TE, Jonzon A, Coleridge HM, Coleridge JC. Vagal afferent and reflex responses to changes in surface osmolarity in lower airways of dogs. Journal of applied physiology: respiratory, environmental and exercise physiology. 1992;73:2305–2313. doi: 10.1152/jappl.1992.73.6.2305. [DOI] [PubMed] [Google Scholar]
- Sasaki R, Sato T, Yajima T, Kano M, Suzuki T, et al. The distribution of TRPV1 and TRPV2 in the rat pharynx. Cellular and molecular neurobiology. 2013;33:707–714. doi: 10.1007/s10571-013-9938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D, Rizk NW, Boushey HA, Bethel RA. Mechanism of cough and bronchoconstriction induced by distilled water aerosol. The American review of respiratory disease. 1983;127:691–694. doi: 10.1164/arrd.1983.127.6.691. [DOI] [PubMed] [Google Scholar]
- Shin J, Cho H, Hwang SW, Jung J, Shin CY, et al. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Earis JE, Woodcock AA. Establishing a gold standard for manual cough counting: video versus digital audio recordings. Cough (London, England) 2006;2:6. doi: 10.1186/1745-9974-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector SL, Nicklas RA, Chapman JA, Bernstein IL, Berger WE, et al. Symptom severity assessment of allergic rhinitis: part 1. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2003;91:105–114. doi: 10.1016/s1081-1206(10)62160-6. [DOI] [PubMed] [Google Scholar]
- Stevens SS. On the psychophysical law. Psychological review. 1957;64:153–181. doi: 10.1037/h0046162. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. Journal of neurophysiology. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, et al. The diagnosis and management of rhinitis: An updated practice parameter. Journal of Allergy and Clinical Immunology. 2008;122:S1–S84. doi: 10.1016/j.jaci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Taniguchi K. Immunolocalization of VR1 and VRL1 in rat larynx. Autonomic neuroscience: basic & clinical. 2005;117:62–65. doi: 10.1016/j.autneu.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. The Journal of physiology. 2008;586:5771–5786. doi: 10.1113/jphysiol.2008.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. The EMBO journal. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]