Abstract
Purpose
To examine a relationship between serum transforming growth factor beta-1 (TGF-β1) values and radiation induced fibrosis (RIF).
Methods
We conducted a prospective analysis of development of RIF in 38 women with AJCC Stage 0-I breast cancer treated with lumpectomy and accelerated partial breast irradiation via interstitial brachytherapy (IBAPBI). An ELISA (Quantikine ®, R&D, MN) immunoassay was used to measure serum TGF-β1 pre-surgery, pre- IBAPBI, and during IBAPBI. Blood samples for TGF-β1 were also collected from 15 healthy, non-treated women (controls). The previously validated tissue compliance meter (TCM) was used to objectively assess radiation-induced fibrosis (RIF).
Results
Median time to follow-up for 38 patients is 44 months (range, 5 – 59 months). RIF is graded by the TCM scale as 0, 1, 2 and 3 in 5/20 (25%), 6/20 (30%), 5/20 (25%) and 4/20 (20%) patients, respectively. ΔTCM ≥6mm (moderate-to-severe RIF) is statistically different from ΔTCM ≤3mm (none-to-mild RIF) (p<0.05). Mean serum TGF-β1 values are significantly higher in patients pre-surgery than disease-free controls in: a) all cancer patients (30,201 ± 5,889 pg/ml, p=0.02), b) patients with any type of RIF (32,273 ± 5,016 pg/ml, p<0.0001) and c) women with moderate-to-severe RIF (34,462 ± 4,713 pg/ml, p<0.0001). The post-IBAPBI mean serum TGF-β1 is 21,915pg/ml in patients with ΔTCM ≥6mm (moderate-severe RIF). This is significantly higher than the mean serum TGF-β1, 14,940pg/ml, in patients with ΔTCM ≤3mm (p = 0.036). In patients who develop moderate-to-severe RIF, pre-IAPBI mean TGF-β1 values are also predictive of this sequela (17,885 ± 3,952 pg/ml, p=0.007).
Conclusions
TGF-β1 levels correlate with development of moderate-to-severe RIF. The pre-IBAPBI mean TGF-β1 levels can serve as an early biomarker for development of moderate-to-severe RIF after IBAPBI
Keywords: transforming growth factor beta-1 (TGF-β1), radiation induced fibrosis (RIF), tissue compliance meter (TCM), breast cancer, accelerated partial breast irradiation (APBI), radiation side effects
INTRODUCTION
Accelerated hypofractionated partial breast irradiation (APBI) condenses the standard 6 weeks of radiation treatment into 5 days, offering logistical and quality of life benefits (1, 2). A mode of APBI that has become increasingly popular is interstitial brachythereapy (IBAPBI) (3). Results from studies evaluating various interstitial brachytherapy systems have proven to yield local failure rates as low as 0–5.7% at up to 65 months, as well as good-to-excellent cosmetic results in 67 – 97% of patients (4, 5). Early and late normal tissue complications are thought to be comparable to other modes of APBI (6). In particular, a recent study evaluating rates of normal tissue toxicity for those treated with IBAPBI report grade I, II, and III radiation induced fibrosis (RIF) to occur at a rate of 26%, 2%, and 0%, respectively (7).
RIF is an unwanted normal tissue complication that occurs months to years following initial radiation exposure (8). RIF can be a painful and cosmetically undesirable condition seen clinically as skin retraction, atrophy, and decreased tissue compliance (9). Within tissue affected by RIF, there is an overproduction of extracellular matrix and a replacement of normal tissue by mesenchymal cells (10). These changes are the result of pro-inflammatory and pro-fibrotic cytokines such as transforming growth factor (TGF)-β1 released during the initiating phase of RIF development (11, 12). During the chronic phase of RIF there is actually deregulation of fibrinolysis with diminished ECM break-down, resulting in the maintenance of the overabundant fibrous tissue (13). However, there is great variability in whether or not a patient experiences RIF following breast irradiation (14).
Although differences in radiation delivery can influence RIF rates, increasing evidence suggests variation in patient response can be understood through genetic polymorphisms and gene expression (15). In particular, the TGF-β1 gene has gained increased attention as a potential determinant of RIF occurrence (16). TGF-β1 is a fibrosis promoting cytokine that stimulates fibroblast proliferation, while up-regulating ECM production (17). TGF-β1 further contributes to fibrosis by diminishing epithelial repair, causing vascular damage that perpetuates tissue hypoxia (18). The effect of TGF-β1 following tissue radiation is likely modulated by multiple factors including extracellular signaling, apoptosis, and inhibition of proliferation in response to DNA damage (19). In lung cancer, elevated TGF-β1 levels were shown to positively associate with an elevated risk of radiation-induced pulmonary fibrosis (20). Among breast cancer patients, multiple SNPs associated with the TGF-β1 gene have been shown to correlate with RIF, though a recent meta-analysis has attempted to disprove this correlation (16, 21).
Previously, studies evaluating RIF in patients treated with external beam radiation have found a positive correlation between pre-treatment TGF-β1 and radiation fibrosis (22). In this study, we prospectively assess TGF-β1 as a serum marker of developing RIF in women treated with IBAPBI.
MATERIALS AND METHODS
Patients
Following approval by the institutional review board, patients scheduled to receive IBAPBI following lumpectomy were enrolled in a prospective trial assessing the relationship between TGF- β1 levels with radiation-induced fibrosis (RIF). In total, 38 women with AJCC early-stage 0–1 breast cancer were enrolled. Prior to enrollment, all patients were informed of the risks and benefits of trial participation. All study participants signed informed consent. Enrollment criteria were based on publications by the American Brachytherapy Society (23), while consensus guidelines were used for the selection of patients for IBAPBI (24, 25). Patient, tumor, and dosimetric characteristics are described in Table 1. 15 healthy women were also recruited as control subjects for study participation. The following exclusion criteria were applied: any present or past history of breast or any other type of cancer, prior breast surgery, fibrocystic breast disease, current pregnancy or coagulopathy. Patients were assessed for RIF pre-treatment (baseline), 1 month following IBAPBI, and every subsequent 6 months.
Table 1.
Baseline Patient, Tumor, and Dosimetric Characteristics (n = 39)
| Characteristics | n (%) |
|---|---|
| Age | |
| Median | 69 |
| Range | 47 – 82 |
| Breast treated | |
| Left | 25 (64) |
| Right | 14 (36) |
| Breast (cup) size | |
| A | 0 (0) |
| B | 8 (20) |
| C | 17 (44) |
| D+ | 14 (36) |
| T stage | |
| Tis | 9 (23) |
| T1a | 5 (13) |
| T1b | 16 (41) |
| T1c | 9 (23) |
| Stage | |
| 0 | 5 (13) |
| I | 26 (67) |
| II | 8 (20) |
| Device | |
| Mammosite | 33 (85) |
| Contura | 6 (15) |
| Chemotherapy | |
| Yes | 2 (5) |
| No | 33 (85) |
| Unknown | 4 (10) |
| Median balloon size (range), cc | 41.2 (37.6 – 50.0) |
| Median SBD (range), cm | 1.4 (0.2 – 5.5) |
Abbreviations: SBD = skin-to-balloon distance
Treatment
The MammoSite breast brachytherapy system (Hologic Inc, Bedford, Massachusetts) and Contura MLB (SenoRx, Inc., Aliso Viejo, California) balloons were used for the IBAPBI treatment. We used a prescribed dose of 34.0 Gy in 10 fractions of 3.4 Gy, given two times per day. A three-dimensional 1.0-cm expansion of the balloon cavity was used for the planning target volume (PTV), which was subsequently decreased by a 5 mm skin-to-balloon distance (SBD) to protect the skin from excess dose. Dose volume histogram (DVH) analysis confirmed ≥ 95% of prescribed dose covering ≥ 95% of the evaluable PTV (PTV-EVAL). The area of trapped air or fluid was accounted for by contouring the volume at each level, and subtracting the percentage of the PTV-EVAL displaced from the proposed dose coverage to ensure 95% dose coverage. Maximum skin and rib doses were limited to less than 125% and 145%, respectively. The V150 for breast tissue could not exceed 50 cc, while the V200 was reduced to not exceed 10 cc. Both volumes were set as low as possible while satisfying all other dose parameters.
Immunodetection of TGF- β1
To quantify serum TGF- β1 at pre-surgery, pre-treatment and post-treatment time points, an ELISA (Quantikine ®, R&D, MN) immunoassay was used. All serum samples were harvested with a serum separator tube, allowing the sample to clot at room temperature for 30 minutes. Samples were incubated overnight at 5 °C. The samples were then centrifuged at 1000 × g for 15 minutes before serum was removed and stored at ≤ 70 °C. The assay procedure was then carried out according to Quantikine ® complete assay guidelines. Plates were read using a microplate reader set to 450 nm and measured values were converted into TGF- β1 concentrations by reference to the standard curve.
Tissue compliance meter (TCM)
To assess compliance of the irradiated breast we employed the tissue compliance meter (TCM), a previously validated hand-held mechanical device (9, 16). Tissue compliance is a measurement of the device’s penetration in relation to the force applied (Figure 1). The tissue compliance is measured by a millimeter (mm) scale. The detailed protocol and validation of the TCM as a superior objective quantitative assessment of RIF has been published by Wernicke et al previously (26). ΔTCM (mm) is the difference between the values of the index and control areas. The TCM classification of RIF is graded as follows: 0=none, 1=mild, 2=moderate, and 3=severe, corresponding to a change in TCM (ΔTCM) between the index and control breasts of ≤2.9, 3.0–5.9, 6.0–8.9, ≥9.0mm, respectively.
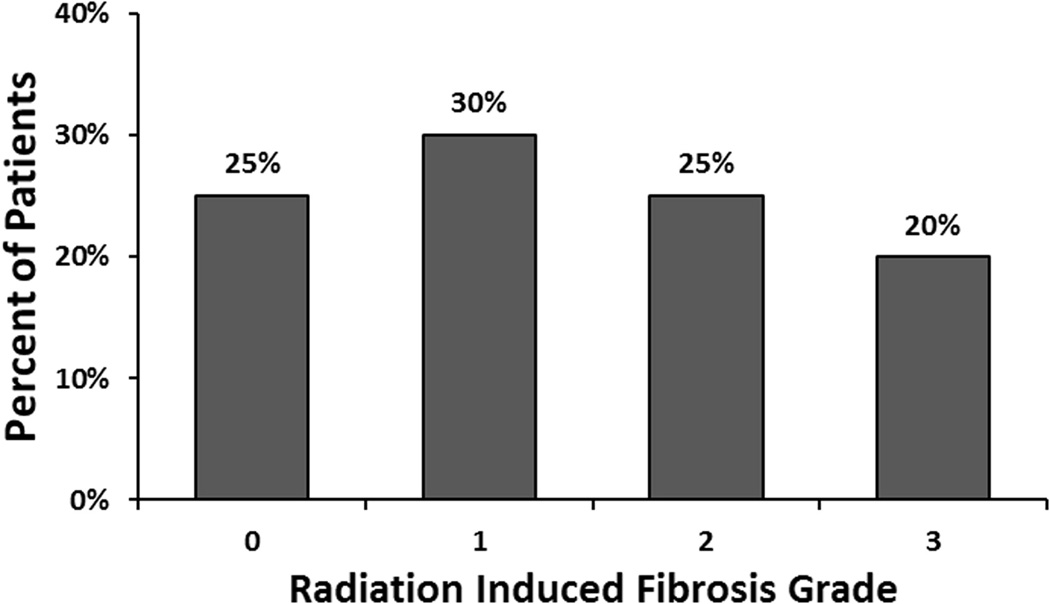
Figure 1.
Distribution of radiation-induced fibrosis among patients.
Statistical analysis
Descriptive statistics (including mean, median, range, proportion) were calculated for demographic and clinical variables of interest. Mean TGF- β1 (pg/ml) and ΔTCM (mm) values reflected the mean difference between the index and control breasts and none-to-mild versus moderate-to-severe RIF. The paired t test and Pearson correlation coefficient (r) were used to assess TGF- β1 and RIF. All p values were two-sided and statistical significance was set at the 0.05 alpha level. All analyses were performed using SPSS version 17 (SPSS Inc., Chicago, IL).
RESULTS
A total of 38 women were evaluated in the study. Median age was 69 years (range, 47 – 82 years). Median follow-up time after completion of IBAPBI was 44 months (range, 5 – 59 months). The majority of patients had tumors that were well or moderately differentiated (90%), and estrogen receptor positive (78%). Most tumors (90%) were situated in the outer quadrants of the breast. Most patients (85%) were treated with Mammosite, and the remaining (15%) received Contura. Hormonal therapy was administered to (51%) of the patients. Complete patient and tumor characteristics can be found in table 1.
RIF, graded by TCM scale as 0, 1, 2, and 3, occurred in 5/20(25%), 6/20 (30%), 5/20(25%), and 4/20 (20%) of patients (Figure 2). A ΔTCM of >6mm (moderate-to-severe RIF) was statistically different from a ΔTCM ≤3mm (none-to-mild RIF) (p<0.05) (figure 3). Post-IBAPBI, the mean serum TGF-β1 was 21,915pg/ml in patients with ΔTCM ≥6mm (moderate-severe RIF). This is significantly higher than the mean serum TGF-β1 of 14,940pg/ml in patients with ΔTCM ≤3mm (none-mild RIF) (p=0.036) (Figure 4). Furthermore, Pearson’s correlation analysis demonstrated that TGF-β1 is positively correlated with occurrence of breast RIF (r=0.61, p=0.0001).
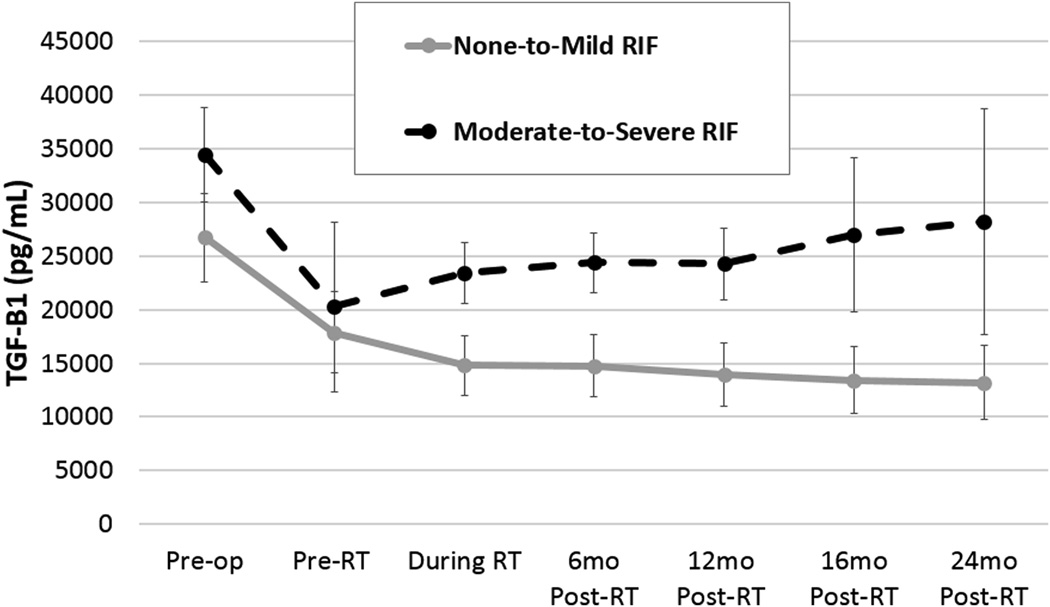
Figure 2.
Comparison of TGF-β1 levels in none to mild and in moderate to severe radiation-induced fibrosis (RIF). RT = radiation therapy; TGF-β1 = transforming growth factor.
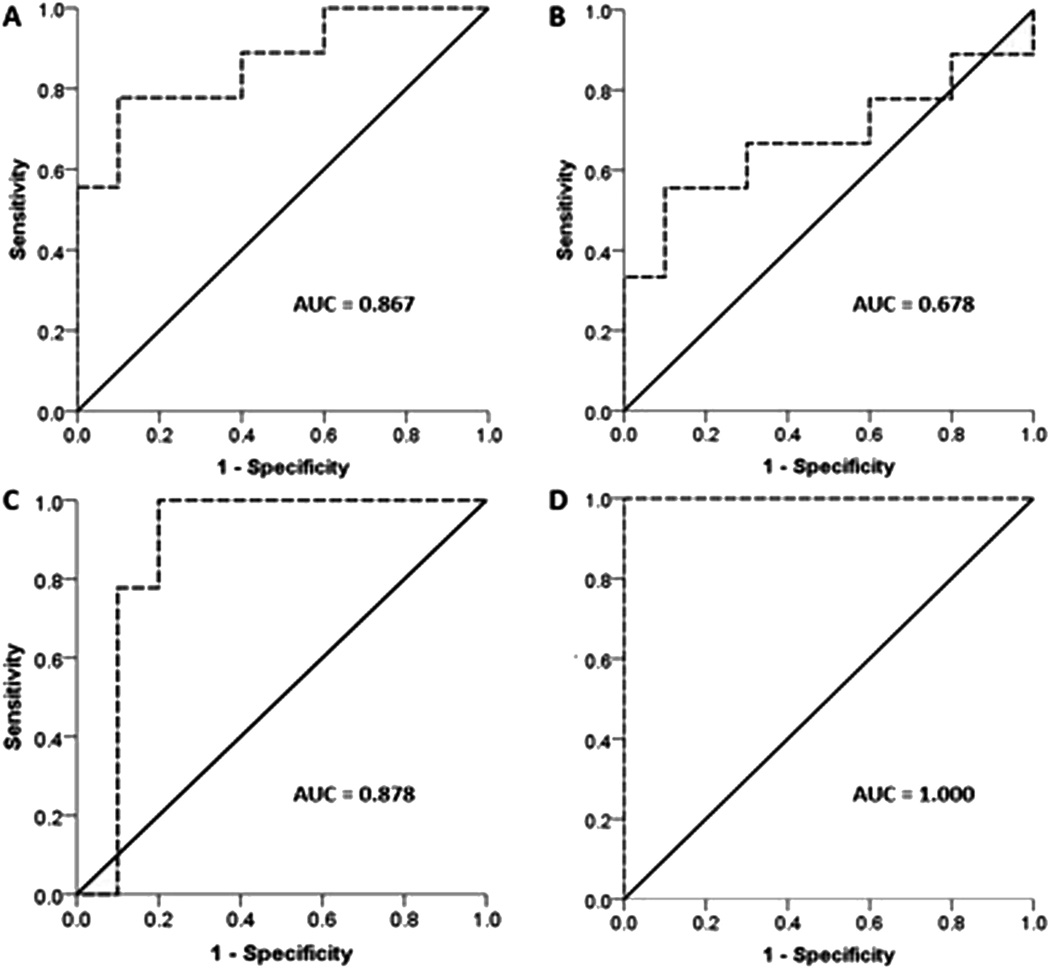
Figure 3.
Receiver operator characteristic (ROC) curve for serum TGF-β1 levels *ROC curves were generated using the state variable of moderate to severe radiation-induced fibrosis. AUC = area under the curve
The mean TGF-β1 value of the post-menopausal controls was 23,692 ± 3,956 pg/ml, significantly lower than values prior to lumpectomy in all cancer patients (30,201 ± 5,889 pg/ml, p=0.02). TGF-β1 levels drawn prior to surgery were predictive of any type of RIF (32,273 ± 5,016 pg/ml, p<0.0001) and moderate-to-severe RIF (34,462 ± 4,713 pg/ml, p<0.0001). TGF-β1 values were also predictive of moderate-to-severe RIF prior to IBAPBI (17,886 ± 3,953 pg/ml, p<0.007). Patients with none-to-mild RIF were not significantly associated with TGF-β1 values prior-to-surgery (p=0.14) or prior-to-IBAPBI (p=0.77). In addition, no TGF-β1 values drawn during IBAPBI were significantly different from controls (Table 2).
Table 2.
TGF-B1 levels before and during radiation treatment
| RIF | Time | TGF-B1 Value | P-value |
|---|---|---|---|
| Any | Prior to surgery | 32,273 ± 5,016 | < 0.0001 |
| Prior to APBI | 20,339 ± 6,775 | 0.22 | |
| During APBI | 29,178 ± 5,113 | 0.52 | |
| None-to-Mild RIF | Prior to surgery | 26,715 + 4,291 | 0.14 |
| Prior to APBI | 20,261 ± 8,415 | 0.30 | |
| During APBI | 27,638 ± 4,059 | 0.77 | |
| Severe RIF | Prior to surgery | 34,468 ± 4,713 | < 0.0001 |
| Prior to APBI | 17,886 ± 3,953 | 0.007 | |
| During APBI | 23,411 ± 3,030 | 0.87 | |
Abbreviations: TGF-β1 = transforming growth factor; RIF = radiation induced fibrosis; APBI: acceleration partial breast irradiation
DISCUSSION
Radiation treatment planning is guided by the known dose tolerances of normal tissues (27). By minimizing dose to normal tissue, the theoretical risk of early or late complications or treatment are decreased (28). A potential normal tissue complication of breast irradiation is RIF. Knowing clinical risk factors for RIF could be a valuable tool in determining the intensity of treatment regimen, thereby individualizing treatment and preventing unnecessary complications.
Given the large doses per fraction and accelerated treatment time with APBI, there is a theoretically elevated risk of RIF with this mode of treatment (29). However, the effects of IBAPBI on acute and late toxicity are still being investigated. Wallace et al. conducted a Phase I/II trial evaluating long-term toxicity in patients treated with IBAPBI for early-stage breast cancer. Among the patients with short-term follow-up (<6 months), Grade I, II and III toxicities occurred at a RIF frequency of 25%, 2%, and 0%, respectively, while long-term follow-up patients (>/= 6 months) had Grade I, II and III RIF frequency of 26%, 2%, and 0%, respectively. However, median follow-up was only 11.4 months (range, 5.4–48 months) (7). Unfortunately, there are no published studies comparing IBAPBI versus other forms of ABPI, such as external beam ABPI. However, the rates of fibrosis determined by other fractionation schedules and modes of ABPI do appear to be comparable (30).
TGF-β1 is a pro-fibrotic cytokine that has been proposed as a genetic and serum marker for radiation fibrosis (17). TGF-β1 is known to stimulate fibroblast differentiation and ECM production, while contributing to endothelial dysfunction thought to underlie fibrosis development (18). The clinical evidence for TGF-β1 in radiation fibrosis pathogenesis is most reliably shown in radiation pneumonitis in lung patients. One of the early studies by Anscher et al revealed that among the 73 patients treated for lung cancer, a return of plasma TGF-β1 to normal following radiation had a sensitivity and positive predictive value of 90% for not developing symptomatic radiation pneumonitis (20). Further studies revealed that normal post-radiation TGF-β1 levels could be used to determine eligibility for dose escalation, potentially improving local control and survival in patients with non-small cell lung cancer (31, 32). Unfortunately, not all subsequent studies supported these conclusions and the relative small patient number and differences in toxicity definitions limit conclusions made (10).
Researchers have also begun to investigate the role of TGF-β1 in RIF among breast cancer patients. Li et al evaluated pre-radiotherapy serum of 91 patients with early stage breast cancer, finding TGF-β1 levels to be significantly elevated in patients with moderate-to-severe RIF of the breast when compared those with none-to-mild RFI. Using 96.0 pg/ml as a cut-off value, an elevated TGF-β1level had a 76% sensitivity and 74% specificity for predicting moderate-to-severe RIF. Further studies have focused on genetic and molecular expression (33). Quarmby et al studied multiple TGF-β1gene polymorphisms within DNA collected from 103 breast cancer patients post-radiation treatment. In particular alleles −509T and +869C were found to be significantly associated with patients who developed severe RIF, contributing up to a 15-fold increase in risk of breast RIF development (16). Multiple subsequent studies supported these findings (32). However, a recent meta-analysis evaluated 2782 patients from 11 different cohorts and concluded that patients with the −509T allele had an odds ratio (OR) of 0.95% (Confidence Interval (CI) 0.85–1.11) (21). Interestingly, Reuther et al. discovered that the potential elevated risk of carrying −509T allele had no effect on gene expression, protein secretion, or cellular radiosensitivity (34).
In this study, we attempted to investigate the impact of serum TGF-β1 values pre-surgery, post-surgery and pre-IBAPBI, and during-IBAPBI and after IBAPBI treatment. Like Li et al (22), we found pre-radiation therapy levels of TGF-β1 to be significantly elevated in breast cancer patients who developed RIF. We were also able to determine that elevated serum TGF-β1 levels are a significant biomarker for severe-to-moderate prior to both IBAPBI and surgery. Therefore, serum TGF-β1 levels could theoretically be drawn soon after diagnosis to assist in risk assessment for treatment planning. Those with low pre-surgery TGF-β1 levels, and therefore a decreased risk of RIF, could be eligible for dose escalation, while those with elevated levels could be treated more conservatively. Should there be a viable treatment to prevent RIF, TGF-β1 levels could be the determinant of eligibility for such treatment. Also, given the lack of consensus on the utility of genes predictive of RIF, using serum TGF-β1 levels could be a simple alternative for RIF risk-assessment.
Our study emphasizes the importance in timing TGF-β1 level assessment. Interestingly, TGF-β1 levels during IBAPBI were statistically insignificant. Also, TGF-β1 levels drawn between radiation and surgery were actually lower than the comparative controls. This may appear to be contradictory; however, it is known that TGF-β1 levels undergo a regulated decrease in cancer patients post-operatively (35). Although post-treatment TGF-β1 levels are also predictive of RIF, we suggest drawing pre-treatment TGF-β1 levels as the superior approach. By doing so, one may not only inform the patient of the inherent risk, but modify treatment accordingly.
CONCLUSION
The current data suggest that pre-surgery and pre-IAPBI mean TGF-β1 levels can serve as an early biomarker for development of moderate-to-severe RIF after IAPBI. Furthermore, a simple TGF-β1 blood test may render a potentially easy and accurate pre-treatment diagnosis of RIF, much earlier than its detection with TCM or TGF-β1 in follow-up. In this way, we can potentially predict which patients are at increased risk for developing RIF after IBAPBI. This will allow customizing radiation treatments accordingly and may render standard fractionation regimens as an option to patients at-risk of moderate-to severe RIF.
SUMMARY.
Radiation induced fibrosis (RIF) is a painful and cosmetically undesirable complication of radiation treatment to the breast. This study evaluates TGF-β1 as a serum biomarker for predicting RIF in patients treated with accelerated partial breast irradiation (IBAPBI). Our data reveals pre-surgery and pre-IBAPBI TGF-β1 levels positively correlate with the development of moderate-to-severe RIF.
Acknowledgments
Dr. Wernicke supported by the funds from the Agency for Healthcare Research and Quality (CERTs grant U18 HSO16075). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Conflict of interest: none
presented at the American Society of Therapeutic Radiation Oncology in 2011, Miami
REFERENCES
- 1.Arthur DW, Vicini FA. Accelerated partial breast irradiation as a part of breast conservation therapy. J Clin Oncol. 2005;23:1726–1735. doi: 10.1200/JCO.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 2.Vicini FA, Kestin L, Chen P, et al. Limited-field radiation therapy in the management of early-stage breast cancer. J Natl Cancer Inst. 2003;95:1205–1210. doi: 10.1093/jnci/djg023. [DOI] [PubMed] [Google Scholar]
- 3.Strauss JB, Dickler A. Accelerated partial breast irradiation utilizing balloon brachytherapy techniques. Radiother Oncol. 2009;91:157–165. doi: 10.1016/j.radonc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Benitez PR, Keisch ME, Vicini F, et al. Five-year results: the initial clinical trial of MammoSite balloon brachytherapy for partial breast irradiation in early-stage breast cancer. Am J Surg. 2007;194:456–462. doi: 10.1016/j.amjsurg.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Brown S, McLaughlin M, Pope K, et al. Initial radiation experience evaluating early tolerance and toxicities in patients undergoing accelerated partial breast irradiation using the Contura Multi-Lumen Balloon breast brachytherapy catheter. Bracytherapy. 2009;8:227–233. doi: 10.1016/j.brachy.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Chao KK, Vicini FA, Wallace M, Mitchell C, Chen P, Ghilezan M, Gilbert S, Kunzman J, Benitez P, Martinez A. Analysis of treatment efficacy, cosmesis, and toxicity using the MammoSite breast brachytherapy catheter to deliver accelerated partial-breast irradiation: the william beaumont hospital experience. Int J Radiat Oncol Biol Phys. 2007 Sep 1;69(1):32–40. doi: 10.1016/j.ijrobp.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Wallace M, Martinez A, Mitchell C, et al. Phase I/II Study Evaluating Early Tolerance in Breast Cancer Patients Undergoing Accelerated Partial Breast Irradiation Treated With the MammoSite Balloon Breast Brachytherapy Catheter Using a 2-Day Dose Schedule. Int J Radiat Oncol Biol Phys. 2010;77(2):531–536. doi: 10.1016/j.ijrobp.2009.05.043. 2010. [DOI] [PubMed] [Google Scholar]
- 8.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, Rabbani Z, Anscher M, Vujaskovic Z. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol. 2007 Apr;17(2):89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Wernicke AG, Coplowitz S, Kulidzhanov F, et al. Tissue Compliance Meter is a More Reproducible Method of Measuring Radiation-induced Fibrosis than LENT-SOMA in Patients Treated with Intracavitary Brachytherapy Accelerated Partial Breast Irradiaton: Results of a Prospective Trial. doi: 10.1111/tbj.12102. (In press). [DOI] [PubMed] [Google Scholar]
- 10.Anscher MS. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. Oncologist. 2010;15(4):350–359. doi: 10.1634/theoncologist.2009-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentzen SM. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 12.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis:A master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 13.Landmark-Høyvik H, Dumeaux V, Reinertsen KV, Edvardsen H, Fosså SD, Børresen-Dale AL. Blood gene expression profiling of breast cancer survivors experiencing fibrosis. Int J Radiat Oncol Biol Phys. 2011 Mar 1;79(3):875–883. doi: 10.1016/j.ijrobp.2010.09.052. [DOI] [PubMed] [Google Scholar]
- 14.Turesson I, Nyman J, Holmberg E, Odén A. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys. 1996 Dec 1;36(5):1065–1075. doi: 10.1016/s0360-3016(96)00426-9. [DOI] [PubMed] [Google Scholar]
- 15.Alsner J, Andreassen CN, Overgaard J. Genetic markers for prediction of normal tissue toxicity after radiotherapy. Semin Radiat Oncol. 2008 Apr;18(2):126–135. doi: 10.1016/j.semradonc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Quarmby S, Fakhoury H, Levine E, Barber J, Wylie J, Hajeer AH, West C, Stewart A, Magee B, Kumar S. Association of transforming growth factor beta-1 single nucleotide polymorphisms with radiation-induced damage to normal tissues in breast cancer patients. Int J Radiat Biol. 2003 Feb;79(2):137–143. [PubMed] [Google Scholar]
- 17.Barcellos-Hoff MH. How do tissues respond to damage at the cellular level? The role of cytokines in irradiated tissues. Radiat Res. 1998 Nov;150(5 Suppl):S109–S120. [PubMed] [Google Scholar]
- 18.Boyd F, Massagué J. Transforming growth factor inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989;264:2272–2278. [PubMed] [Google Scholar]
- 19.Bentzen SM, Parliament M, Deasy JO, Dicker A, Curran WJ, Williams JP, Rosenstein BS. Biomarkers and surrogate endpoints for normal-tissue effects of radiation therapy: the importance of dose-volume effects. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S145–S150. doi: 10.1016/j.ijrobp.2009.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G, Jirtle RL. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998 Jul 15;41(5):1029–1035. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 21.Barnett GC, Elliott RM, Alsner J, Andreassen CN, Abdelhay O, Burnet NG, Chang-Claude J, Coles CE, Gutiérrez-Enríquez S, Fuentes-Raspall MJ, Alonso-Muñoz MC, Kerns S, Raabe A, Symonds RP, Seibold P, Talbot CJ, Wenz F, Wilkinson J, Yarnold J, Dunning AM, Rosenstein BS, West CM, Bentzen SM. Individual patient data meta-analysis shows no association between the SNP rs1800469 in TGFB and late radiotherapy toxicity. Radiother Oncol. 2012 Dec;105(3):289–295. doi: 10.1016/j.radonc.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Wilson PB, Levine E, Barber J, Stewart AL, Kumar S. TGF-beta1 levels in pre-treatment plasma identify breast cancer patients at risk of developing post-radiotherapy fibrosis. Int J Cancer. 1999 Apr 20;84(2):155–159. doi: 10.1002/(sici)1097-0215(19990420)84:2<155::aid-ijc11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.Bentzen SM, Dorr W, Anscher MS, et al. Normal tissue effects: Reporting and analysis. Sem Radiat Oncol. 2003;13:189–202. doi: 10.1016/S1053-4296(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 24.Keish M, Arthur D, Patel R, et al. American Brachytherapy Society Breast Brachytherapy Task Group. American Brachytherapy Society. 2007 Available from: http://www.americanbrachytherapy.org/resources/abs_breast_brachytherapy_taskgroup.pdf. [Google Scholar]
- 25.Benjamin DS, Douglas WA, Thomas AB, et al. Accelerated Partial Breast Irradiation Consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:787–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Wernicke AG, Parashar B, Kulidzhanov F, Riley L, Christos PJ, Fischer A, Nori D, Chao KS. Prospective study validating inter- and intraobserver variability of tissue compliance meter in breast tissue of healthy volunteers: potential implications for patients with radiation-induced fibrosis of the breast. Int J Radiat Oncol Biol Phys. 2011 May 1;80(1):39–46. doi: 10.1016/j.ijrobp.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milano MT, Constine LS, Okunieff P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin Radiat Oncol. 2007;17:131–140. doi: 10.1016/j.semradonc.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Kong FM, Pan C, Eisbruch A, et al. Physical models and simpler dosimetric descriptors of radiation late toxicity. Semin Radiat Oncol. 2007;17:108–120. doi: 10.1016/j.semradonc.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Rosenstein BS, Lymberis SC, Formenti SC. Biologic comparison of partial breast irradiation protocols. Int J Radiat Oncol Biol Phys. 2004;60:1393–1404. doi: 10.1016/j.ijrobp.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 30.Vicini F, Beitsch PD, Quiet CA, Keleher AJ, Garcia D, Snider HC, Jr, Gittleman MA, Zannis VJ, Kuerer HM, Lyden M. Three-year analysis of treatment efficacy,cosmesis, and toxicity by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation (APBI) Cancer. 2008 Feb 15;112(4):758–766. doi: 10.1002/cncr.23227. [DOI] [PubMed] [Google Scholar]
- 31.Anscher MS, Marks LB, Shafman TD, Clough R, Huang H, Tisch A, Munley M, Herndon JE, Garst J, Crawford J, Jirtle RL. Using plasma transforming growth factor beta-1 during radiotherapy to select patients for dose escalation. Journal of Clinical Oncology. 2001;17:3758–3765. doi: 10.1200/JCO.2001.19.17.3758. [DOI] [PubMed] [Google Scholar]
- 32.Kong FM, Ao X, Wang L, et al. The use of blood biomarkers to predict radiation lung toxicity: A potential strategy to individualize thoracic radiation therapy. Cancer Control. 2008;15:140–150. doi: 10.1177/107327480801500206. [DOI] [PubMed] [Google Scholar]
- 33.Andreassen CN, Alsner J, Overgaard M, Overgaard J. Prediction of normal tissue radiosensitivity from polymorphisms in candidate genes. Radiother Oncol. 2003 Nov;69(2):127–135. doi: 10.1016/j.radonc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Reuther S, Metzke E, Bonin M, Petersen C, Dikomey E, Raabe A. No Effect of the Transforming Growth Factor β1 Promoter Polymorphism C-509T on TGFB1 Gene Expression, Protein Secretion, or Cellular Radiosensitivity. Int J Radiat Oncol Biol Phys. 2012 May 15; doi: 10.1016/j.ijrobp.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 35.Kong FM, Anscher MS, Murase T, Abbott BD, Iglehart JD, Jirtle RL. Elevated plasma transforming growth factor-beta 1 levels in breast cancer patients decrease after surgical removal of the tumor. Ann Surg. 1995 Aug;222(2):155–162. doi: 10.1097/00000658-199508000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]