Abstract
Transient receptor potential vanilloid 1 (TRPV1) is a cation-permeable ion channel found in the peripheral and central nervous systems. The membrane surface expression of TRPV1 is known to occur in neuronal cell bodies and sensory neuron axons. TRPV1 receptors are also expressed in the hippocampus, the main epileptogenic region in the brain. Although, previous studies implicate TRPV1 channels in the generation of epilepsy, suppression of ongoing seizures by TRPV1 antagonists has not yet been attempted. Here, we evaluate the role of TRPV1 channels in the modulation of epileptiform activity as well as the anti-convulsant properties of capsazepine (CZP), an established TRPV1 competitive antagonist, using in vitro and in vivo models. To this end, we used 4-aminopyridine (4-AP) to trigger seizure-like activity. We found that CZP suppressed 4-AP induced epileptiform activity in vitro (10–100 µM) and in vivo (50 mg/kg s.c.). In contrast, capsaicin enhanced 4-AP induced epileptiform activity in vitro (1–100 µM) and triggered bursting activity in vivo (100 µM dialysis perfusion), which was abolished by the TRPV1 antagonist CZP. To further investigate the mechanisms of TRPV1 modulation, we studied the effect in capcaisin and CPZ on evoked potentials. Capsaicin (1–100 µM) and CZP (10–100 µM) increased and decreased, respectively, the amplitude of extracellular field evoked potentials in a concentration-dependent manner. Additional in vitro studies showed that the effect of the TRPV1 blocker on evoked potentials was similar whether the response was orthodromic or antidromic, suggesting that the effect involves interference with membrane depolarization on cells bodies and axons. The fact that CPZ could act directly on axons was confirmed by decreased amplitude of the compound action potential and by an increased delay of both the antidromic potentials and the axonal response. Histological studies using transgenic mice also show that, in addition to the known neural expression, TRPV1 channels are widely expressed in alvear oligodendrocytes in the hippocampus. Taken together, these results indicate that activation of TRPV1 channels leads to enhanced excitability, while their inhibition can effectively suppress ongoing electrographic seizures. These results support a role for TRPV1 channels in the suppression of convulsive activity, indicating that antagonism of TRPV1 channels particularly in axons may possibly be a novel target for effective acute suppression of seizures.
Keywords: 4-AP, capsazepine, capsaicin, epilepsy, electrophysiology, oligodendrocytes, TRPV1 channels
Introduction
Transient receptor potential (TRP) channels have been identified as the main molecular transducers in sensorial systems. Made of six-transmembrane polypeptide subunits that assemble as tetramers to form cation-permeable pores, they function as polymodal ion channels ubiquitously expressed in the body (Clapham, 2003). TRPV1 is a member of the vanilloid sub-family (TRPV), one of seven subfamilies into which the TRP receptors are divided (Nilius and Owsianik, 2011). TRPV1 is a nonselective cation channel that can be activated by heat (> 42°C), protons, voltage, arachidonic acid metabolites and the naturally occurring vanilloids capsaicin and resiniferatoxin (RTX) (Kaszas et al., 2012). When activated, TRPV1 channels lead to augmented permeability to Na+ and Ca++, increasing neuronal excitability (Van Der Stelt and Di Marzo, 2004). Initially the expression of TRPV1 channels was thought to be confined to sensorial neuron clusters in the dorsal root and trigeminal ganglia, with a critical role in mediating pain and inflammation. Further research demonstrated that TRPV1 channels are widely expressed in the brain, where they may regulate a variety of different physiological process not yet well understood (Messeguer et al., 2006). TRPV1 is expressed in the hippocampus and dentate gyrus (Mezey et al., 2000; Roberts et al., 2004; Tóth et al., 2005; Cristino et al., 2006; Cavanaugh et al., 2011). These areas of the brain are prone to activate recurrent excitatory circuitry that has proven to be critical in epileptogenesis (Bhaskaran and Smith, 2010). Capsazepine (CZP) is the first reported blocker of the TRPV1 ion channel that has been extensively used as a standard competitive antagonist in both in vitro and in vivo pharmacological studies (Maggi et al., 1993; Walpole et al., 1994). As a synthetic compound developed as a structural analog to the capsaicin molecule (Messeguer et al., 2006), capsazepine binds in the channel pore region, interacting with residues from all four monomers of the tetrameric channel.
Evidence that TRPV1 channels may be implicated in epilepsy comes from studies in the pilocarpine and pentylenetetrazol epilepsy models. Using brain slices from mice that developed spontaneously generated seizures after a single injection of pilocarpine, Bhaskaran and Smith (2010) showed that activation of TRPV1 receptors with capsaicin increases both action potential-dependent and -independent firing of dentate gyrus granule cells. This capsaicin-induced effect was prevented by preapplication of the selective TRPV1 antagonist capsazepine (CZP), indicating it was TRPV1 receptor-mediated, while no effect of capsazepine alone was observed. More recently, Manna and Umathe (2012), using intracerebroventricular (ICV) administration of capsaicin and capsazepine before seizure induction with a systemic injection of pentylenetetrazol (PTZ), found that an ICV injection of capsaicin exhibited pro-convulsant activity that was blocked by an ICV CZP pre-treatment. Conversely, ICV CZP was able to prevent PTZ-induced seizures. These studies by Manna and Umathe (2012) offer the first observation in vivo of CZP anti-epileptic action. However, they reported only behavioral observations and CZP was used as a pre-treatment. Thus, the potential effect of CZP following seizure onset remains to be evaluated electrographically in vitro and in vivo. The present study addresses these issues and, in addition, CZP was administered systemically to evaluate whether the drug could act through the blood brain barrier.
The main purpose of the present investigation was twofold: (1) to test the ability of capsazepine to suppress ongoing epileptiform activity in vitro in a concentration-dependent manner and (2) to determine whether systemic administration of capsazepine could acutely suppress ongoing electrographic seizures in vivo.
The effect of the capsazepine on the suppression of epileptiform activity was tested using 4-aminopyridine (4-AP) as a convulsant agent. It has been well documented that 4-AP blocks voltage-activated K+ channels (Rudy 1988; Ulbricht and Wagner, 1976; Stephens et al., 1994). 4-AP induces epileptiform electrical discharges in rat hippocampal (Perrault and Avoli, 1991; Yonekawa et al., 1995; Avoli et al., 1996 Toprani and Durand, 2012) and cortical (Siniscalchi et al., 1997) slices and in vivo produces intense seizure activity in the rat (Gandolfo et al., 1989; Fragoso-Veloz and Tapia, 1992; Morales-Villagrhn et al., 1996), mouse (Yamaguchi and Rogawsh, 1992; Cramer et al., 1994), and human (Spyker et al., 1980). For the in vivo studies we delivered 4-AP using a reverse dialysis procedure. Through this method, 4-AP is delivered locally to the hippocampus in a time-controlled manner. The pharmacokinetic features of the 4-AP delivery by reverse dialysis have been extensively described (See methods Peña and Tapias, 1999).
Here we report that CZP suppressed 4-AP-induced epileptiform activity in vitro and was able to reduce ongoing electrographic seizures in vivo. Several additional findings indicate that CZP is able to suppress seizures by interfering with axonal propagation: (1) Field potential recording demonstrates that CZP suppressed the amplitude of both orthodromic and antidromic evoked potentials to similar extent, (2) CZP suppressed axonal compound action potential amplitude in the alveus, (3) CZP was able to reduce propagation of epileptiform activity through the temporal-septal longitudinal pathway in vivo, and (4) Histological observations show that TRPV1 channels are abundantly expressed in axonal oligodendrocytes in the alveus.
Material and Methods
Animals
Wild-type (C57BL/6J) and the pan-neuronal reporter Thy1-YFP mice were obtained from Jackson Laboratory. Transgenic mice expressing the oligodendrocyte reporter PLP-EGFP were obtained from by Dr. Wendy Macklin (Mallon et al, 2002). Animals were maintained in a SPF room under light (12-h light/12-h dark cycle), temperature and humidity controlled conditions. All animal protocols performed in this study followed NIH guidelines and were reviewed and approved by the Institutional Care and Use Committee at Case Western Reserve University.
In vitro hippocampal slice preparation and 4-AP model
Mice were anesthetized by isoflurane inhalation and euthanized by decapitation. The brains were rapidly removed and immersed in sucrose-rich artificial cerebrospinal fluid (S-aCSF). Transverse hippocampal brain slices (horizontal sections 350 µm thick) were prepared using a vibrating-blade microtome (VT1000S, Leica, Nusslock, Germany) while the tissue was bathed in a refrigerated (3–4 °C) and oxygenated (O2 95%, CO2 5%) S-aCSF buffer consisting of (mM): sucrose 220, KCl 3, NaH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2, dextrose 10 (pH 7.45). The resulting hippocampal transverse slices were immediately transferred to a special maintenance chamber containing oxygenated (O2, 95%, CO2, 5%) artificial cerebral spinal fluid (aCSF) consisting of (mM): NaCl 124, KCl 3.75, KH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2, dextrose 10 (pH 7.4), and incubated at room temperature for at least 60 min before being transferred to an interface-recording chamber (Harvard Apparatus, MA) (aCSF, temperature = 33 ± 2 °C, bubbled with O2 95%, CO2 5%). CA1 pyramidal cell population spikes (PSs) were evoked, using a cathodic stimulus pulse (100 µs, 50–350 µA, 0.05–0.1 Hz) delivered to the Schaffer Collaterals (orthodromic) or the alveus (antidromic) by a tungsten electrode. Extracellular field recordings were obtained with glass microelectrodes (3–10 MΩ) filled with 150 mM NaCl. Antidromic and orthodromic evoked potentials were independently recorded in the CA1 stratum pyramidal of hippocampus slices. Spontaneous epileptiform activity was induced by 4-aminopyridine (4-AP, 100 µM) and recordings were made in the CA1 pyramidal cell layer. The signals were amplified using an Axoclamp-2A microelectrode amplifier (Axon Instruments, Inc., Union City, CA) and filtered (low-pass cutoff 1 kHz). Experimental Controls: washout phases were included in each experiment. In all the cases we observed a complete reversal of the effects. In addition, we performed evoked potential recordings for approximately 2 hours under aCSF only. These recordings were evaluated and showed that there were no significant changes over time on the electrical evoked potentials (data not shown).
In vivo electrophysiological recording and 4-AP-induced status epilepticus
Stereotaxic surgery was performed under 3% isoflurane anesthesia in a 95% 02 / 5% CO2 mixture. Two tungsten electrodes were inserted into the septal hippocampus bilaterally (AP −1.8, ML ±2.0, DV −2.1) to record neural activity. In some experiments, one additional electrode was inserted in temporal hippocampus (AP −3.1, ML ±2.5, DV −4.1) in order to monitor CA3 longitudinal propagation. Two screw electrodes were positioned in the skull and used as reference electrode (parietal location) and ground electrode (frontal location).
Continuous infusion of capsaicin or 4-AP by microdialysis
A microdialysis probe (Basi MBR Probe 1 mm membrane, 5 mm cannula) was implanted in the left dorsal hippocampus (AP −1.7, ML +2.0, DV −2.0) and aCSF (composition described in the in vitro section) was infused at 2 µL/min. After a 1-h equilibration period, baseline neural activity was recorded for 30 minutes. It has been shown through systematic comparison with non-infused controls that the microdialysis procedure is innocuous (Peña and Tapia, 1999). The infusion was then switched to capsaicin (100 µM in aCSF) or 4-AP (20 or 40 mM in aCSF) to induce seizures. The solution containing capsaicin or 4-AP was adjusted to pH 7.4 and continuously infused through the microdialysis system until the end of the experiment.
Chemicals
4-AP (Sigma, St. Louis, MO, U.S.A.) was dissolved in aCSF. Capsaicin and capsazepine (Sigma-Aldrich) were dissolved in DMSO and then in aCSF so that final concentration of either compound were 1, 10 and 100 µM and final concentration of DMSO in aCSF was 1:1000 (Gibson et al., 2008).
Histology
Brains from WT C57BL/6 and reporter mutants were transcardially perfused with 4% paraformaldehyde, post-fixed overnight in the same solution and cryoprotected in 30% sucrose (in PBS) for 2 days. Then brains were flash frozen in 2-methylbutane on dry ice and sectioned using a Leica CM3050 cryostat (40 µm thick). Free-floating sections were stained for TRPV1 using a TRPV1 antibody at 1:1000 concentration (rabbit, Abcam, CA; or goat, Santa Cruz BioTech, Delaware, CA). Incubation time was 24 h at 4°C. Secondary antibodies (donkey FITC 1: 300 and Cy3 1:700, Jackson ImmunoResearch, West Grove, PA) directed against the species of the primary antibody were incubated for 1 h. TOTO-3 (Invitrogen) was used for nuclear staining and added to the secondary antibody solution at 1:2000 concentration. Images were taken using a confocal microscope (Zeiss LSM 510META). For every 5 sections one control section without primary antibody was included. In the control section wells, we replaced the primary antibody with PBS. We did not observe any morphological signal under the confocal microscopy when the TRPV1 primary antibody was absent. Cell counting procedure: For cell type identification we examined a minimum of 5 transgenic animals and sample the area of interest in the hippocampus using 5 sections of 40 µm separated by 160 µm intervals. Total number of positive cells per animal was averaged for the 5 animals×5 sections and the percentage of co-expression was estimated from averages.
Data Analysis and Statistics
Amplitude and delay of field potentials (as illustrated in Fig 1B) as well as root mean square (RMS µV) of 4-AP induced epileptiform discharges were sampled from 30 second epochs every 5 min and calculated as average for 20-min intervals using a Matlab custom program. For RMS calculation, the signals were squared and integrated. Data were normalized, reported as mean ± SEM and groups analyzed by repeated measures ANOVA followed by Tukey HSD post-hoc test (Statistica Software, 2000). Normalization was applied by expressing the effect of the drugs as a percentage of the average of control values for each subject. Asterisks or Greek letters in the graphs refer to significant post-hoc tests and “n” refers to number of brain slices in vitro or number of animals tested in the in vivo studies.
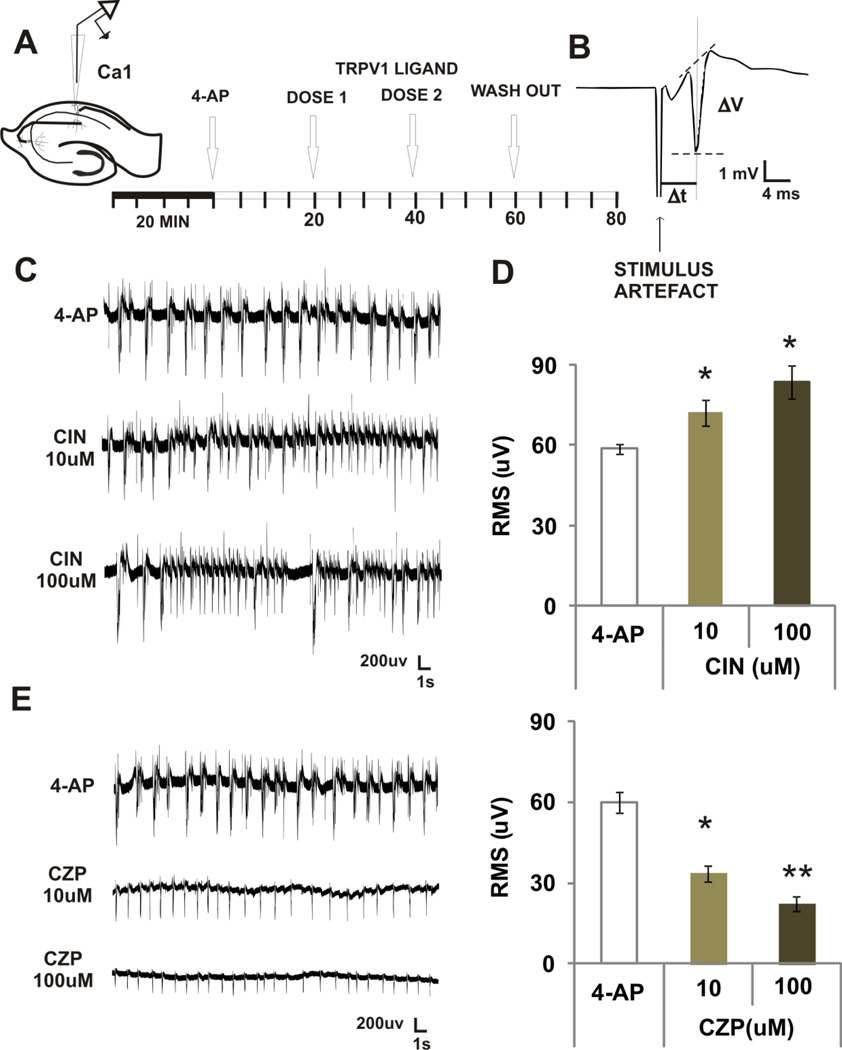
FIGURE 1. EFFECTS OF TWO VANILLOIDS ON 4-AP INDUCED EPIEPTIFORM ACTIVITY IN VITRO.
A. For recording population spikes induced by 4-AP. A recording electrode was positioned in CA1 pyramidal cell layer. The orthodromic response was first evoked to test the integrity of the slice. After 20 min of acclimatization in the interface chamber under 4-AP superfusion, we started recording baseline levels (4-AP). Next, two doses of either drug were administered subsequently at 20 min intervals.
B. Example of an orthodromic evoked potential. The arrow indicates the position of the stimulus artifact and the references used to calculate the evoked potential amplitude (ΔV) and delay (Δt) are also shown.
C. Examples of epileptiform discharges induced by 4-AP and increase in population spikes after superfusion of capsaicin (CIN)
D. Mean ± SEM RMS µV showing a dose related increase in power induce by CIN. * p < 0.05, repeated measures ANOVA followed by post-hoc tests.
E. Examples of epileptiform discharges induced by 4-AP and decrease in population spikes after superfusion of capsazepine (CZP).
F. Mean ± SEM RMS µV showing a dose related decrease in power induced by CZP. ** p < 0.001 * p < 0.05, compared with control or other doses, repeated measures ANOVA followed by post-hoc tests.
Results
IN VITRO STUDIES
Capsaicin and CPZ can modulate 4-AP induced epileptiform activity
To evaluate whether capsaicin or CZP could modulate 4-AP induced epileptiform activity, a recording microelectrode was located to the CA1 region and orthodromic evoked potentials from stimulation of the Schaffer collaterals were elicited at low frequency to assess the integrity of the slice. Once orthodromic evoked potentials were established, stimulation was stopped and 4-AP was superfused for 20 min to induced stable epileptiform activity. The activity was recorded for an additional 20 min period. Next, capsaicin or CZP was superfused in two consecutive doses (10 and 100 µM) in 20 min intervals.
Capsaicin increased the power of the signal as measured by RMS by 23% (10 µM) and 43% (100 µM) (n=6, ANOVA, p=0.003, Fig 1A). In contrast, CZP decreased power by 44% (10 µM) and 63% (100 µM) (n=7, ANOVA, p < 0.0001, Fig 1B). Further analysis of these effects revealed that the capsaicin-induced increase in power was consistent with an increase in burst frequency and duration, while the CZP-induced decrease in power was related to a decrease in burst duration and amplitude (Table 1). Post-hoc analysis confirmed dose-response power relationships (as indicated by Greek letters in the figure). In control experiments, we attempted to elicit spontaneous activity by enhancing TRPV1 activity with capsaicin alone. Under normal aCSF, superfusion of capsaicin (without addition of 4-AP) did not elicit any spontaneous firing as recorded in CA1 pyramidal cell layer (1, 10 or 100 µM concentrations were tested, data not shown).
Table 1.
Effect of TRPV1 ligands on frequency, duration and pick-to-pick amplitude of 4-AP induced bursting.
| Bursts Feature | CAPSAICIN (n=6) | CAPSAZEPINE (n=7) | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 10 µM | 100 µM | WASH | Control | 10 µM | 100 µM | WASH | |
| Frequency (Hz) | 1.3±0.07 | 1.7±0.05* | 2.25±0.2** | 1.2±0.04 | 1.1±0.2 | 1.0±0.01 | 1.0±0.1 | 1.1±0.1 |
| Duration (ms) | 197.1±35.2 | 354.1±72.4** | 347.0±53.3* | 198.8±24.3 | 211.5±18.9 | 145.4±14.3** | 130.4±12.9** | 196.0±15.7 |
| P-P amplitude (mV) | 0.9±0.09 | 0.8±0.07 | 0.8±0.05 | 0.9±0.05 | 0.9±0.08 | 0.5±0.04** | 0.3±0.1*** | 0.8±0.2 |
p < 0.05,
p < 0.001,
p< 0.0001 compared to control, post-hoc test after ANOVA.
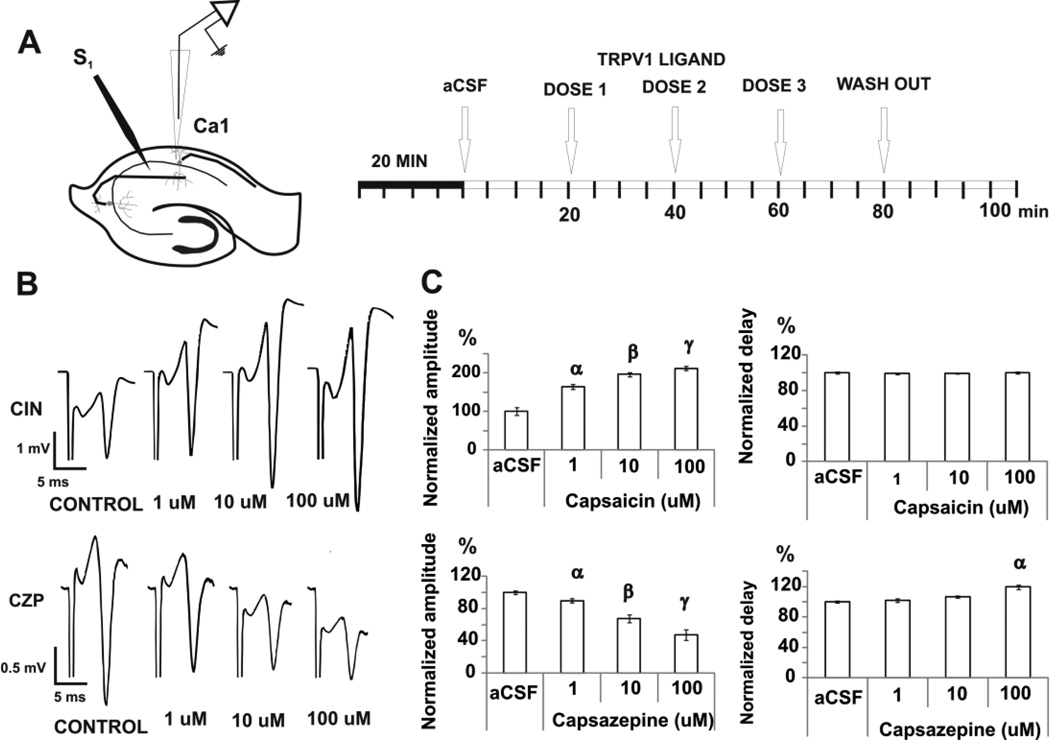
Capsaicin increases the amplitude of the orthodromic evoked potentials
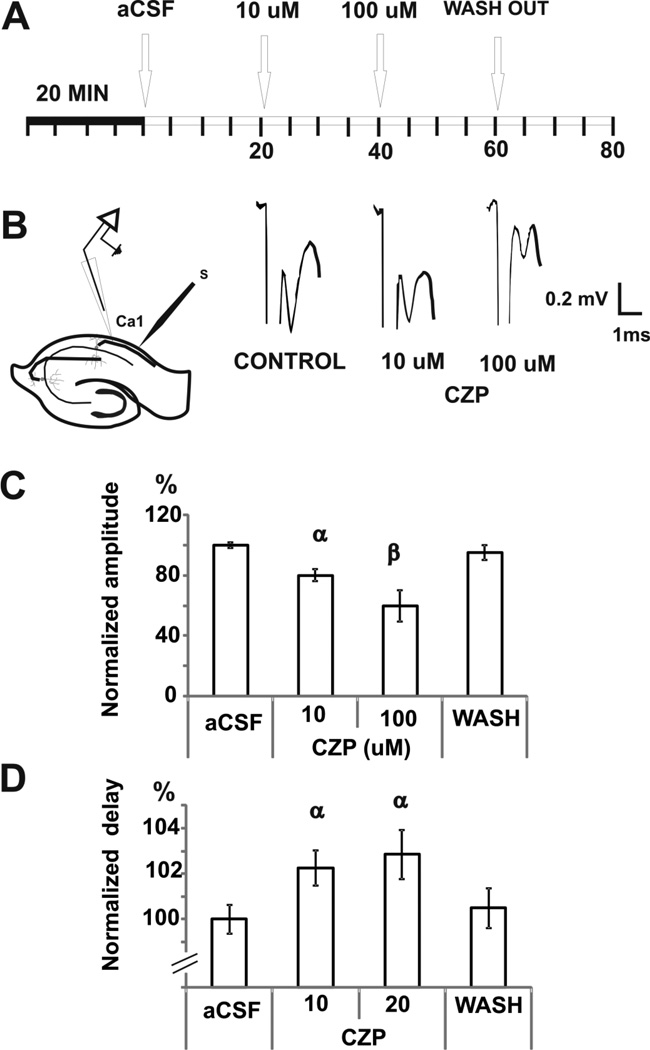
To study the mechanism of action of capsaicin and CZP, we first sought to determine whether these agents could modulate the pyramidal cell response to electrical stimulation. Orthodromic responses were elicited by applying cathodic pulses in the Schaffer collaterals and recorded as extracellular field potentials in CA1 (Fig 2A–B). Capsaicin and CZP were perfused in three consecutive doses (1, 10 and 100uM). Capsaicin increased the amplitude of the orthodromic potential by 64% (1 µM), 96% (10 µM) and 113% (100 µM) [from (mV) 2.3 ± 0.4 (control), n=6, ANOVA, p < 0.0001, Fig 2C], whereas there was no change of the orthodromic delay [control level = 5.4 ± 0.08 mV, n=6, ANOVA, p = 0.8, Fig 2C]. Conversely, CZP reduced the amplitude of the orthodromic potential by 33% (10 µM) and 53% (100 µM) [from (mV) 2.0 ± 0.04 (control); n=7, ANOVA, p < 0.0001, Fig 2C], and increased delay by 19% (100 µM) [from (ms) 4.2 ± 0.02 (control), n=7, ANOVA, p < 0.0001, Fig 2C]. The changes induced by CZP on the amplitude and delay of the orthodromic potential were correlated significantly (see Table 2).
FIGURE 2. EFFECTS OF TRPV1 MODULATION ON THE ORTHODROMIC RESPONSE.
A. After 20 min of acclimatization in the interface chamber, cathodic stimuli pulse (100 µs, 50–350 µA, 0.05–0.1 Hz) were delivered to the Schaffer Collaterals by a tungsten electrode to reach a stable evoked response and recorded in the pyramidal cell layer of CA1 for 20 min. Three doses of either drug were administered subsequently at 20 min intervals.
B. For orthodromic evoked potentials, the stimulating electrode was positioned in the Shaffer collaterals and the recording electrode in the pyramidal cell layer of CA1. Examples of orthodromic potentials in each pharmacologic condition for capsaicin (CIN) and capsazepine (CZP) are shown.
C. Mean ± SEM of normalized amplitude and delay of the evoked response show bidirectional modulation, with capsaicin increasing and capsazepine reducing the amplitude of the response. α p < 0.05 compared with control, β p < 0.05 compared with control or 1 uM group. γ p<0.005 compared with control, 1 uM or 10 uM group; repeated measures ANOVA followed by post-hoc test.
Table 2.
Correlation analysis between changes elicited by TRPV1 ligands on the amplitude and delay of electrically evoked field potentials.
| TRPV1 ligand | Field potential | Amplitude | Delay | Pearson correlation | ||
|---|---|---|---|---|---|---|
| p | r2 | t | ||||
| Capsaicin | antidromic: | (↑) | (↓) | 0.02 | 0.95 | −6.7 |
| orthodromic | (↑) | (-) | 0.8 | 0.003 | −0.3 | |
| Capsazepine | antidromic: | (↓) | (↑) | 0.02 | 0.94 | −5.8 |
| orthodromic | (↓) | (↑) | 0.04 | 0.91 | −4.4 | |
| cAP | (↓) | (↑) | 0.04 | 0.92 | −4.7 | |
Arrows indicate a significant increase or decrease as described in the result section.
The effect of TRPV1 antagonist is non-synaptic
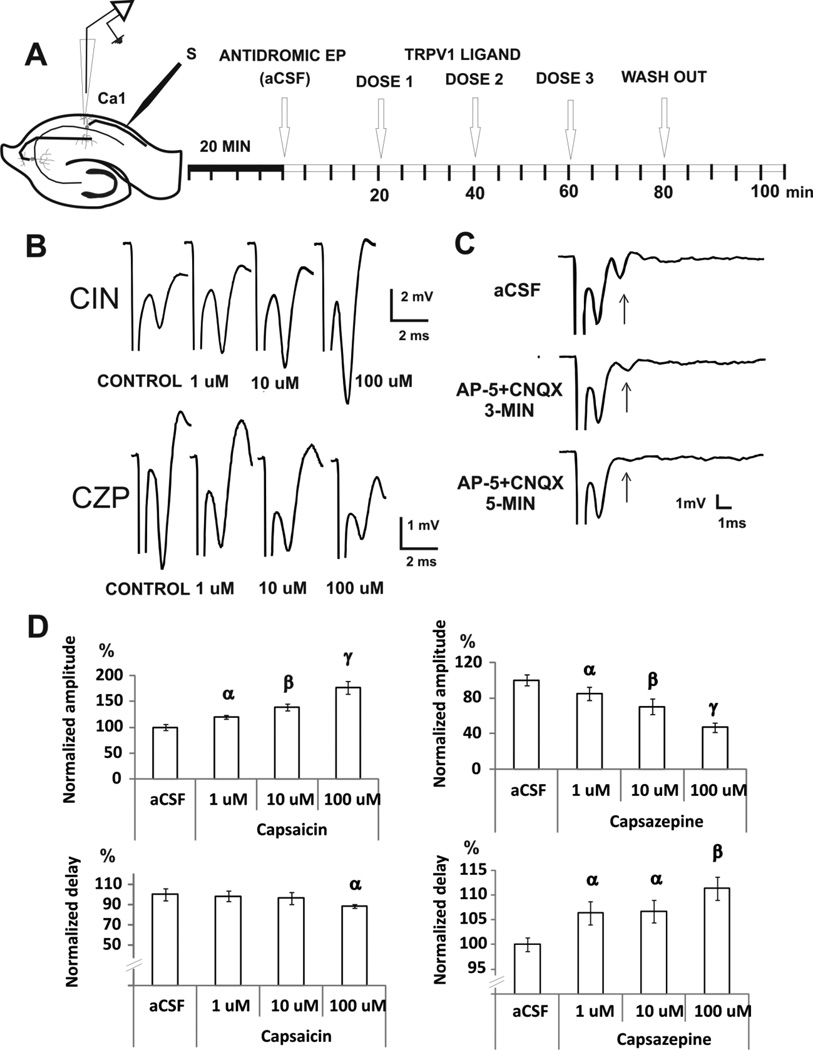
To determine if synaptic transmission is involved in the effects produced by TRPV1 agonism and antagonism in the hippocampus, the amplitude of the antidromic evoked potential was also measured since the antidromic pyramidal cell response results from the direct activation of cells and does require synaptic activity. Antidromic activity was induced by stimulation of the alveus and the response recorded in the CA1 region (Fig 3A–B). To make sure that synaptic transmission was not involved in these recordings, 50µM of APV and 20µM of CNQX were added to the solution (Fig 3C). The amplitude of the antidromic evoked potential was unaffected, while the orthodromic responses were completed abolished after 5 min of the APV/CNQX superfusion. The antidromic potential amplitude was 3.5 ± 0.7 mV in control conditions and 3.4 ± 0.7 mV after glutamatergic block (APV + CNQX, n=5). Orthodromic potential amplitude was significantly reduced from 1.4 ± 0.2 mV (control) to 0.1 ± 0.03 mV (APV+CNQX, n=5, t-test, p = 0.01). Perfusion with capsaicin significantly increased the amplitude of the antidromic response by 39% (10 µM) and 76% (100 µM) [from 1.3 ± 0.07 mV (control), n= 6, ANOVA, p <0.0001, Fig 3D], and significantly reduced antidromic delay by 22% [from 2.3 ± 0.08 ms (control); n=6, ANOVA, p = 0.001 Fig 3D]. The changes induced by CZP on amplitude and delay of the antidromic response were correlated significantly (see Table 2).
FIGURE 3. EFFECTS OF TRPV1 MODULATION ON THE ANTIDROMIC RESPONSE.
A. For the antidromic evoked potentials, the stimulating electrode was positioned in the alveus and the recording electrode in the pyramidal cell layer of CA1. After 20 min of acclimatization in the interface chamber, we start recording antidromic potentials control levels (aCSF). Next, three doses of the drug were administered subsequently at 20 min intervals.
B. Examples of antidromic potential traces are shown for capsaicin (CIN) and capsazepine (CZP) experiments.
C. The antidromic evoked potential was confirmed by blocking excitatory synaptic transmission using AP5 and CNQX, which abolished the orthodromic (arrow) but not the antidromic component of the response.
D. Mean ± SEM of normalized amplitude and delay of the evoked response, show bidirectional modulation with capsaicin increasing and capsazepine reducing the amplitude of the response. α p < 0.05 compared with control, β p < 0.05 compared with control or 1 uM group. γ p<0.005 compared with control, 1 uM or 10 uM group; repeated measures ANOVA followed by post-hoc test.
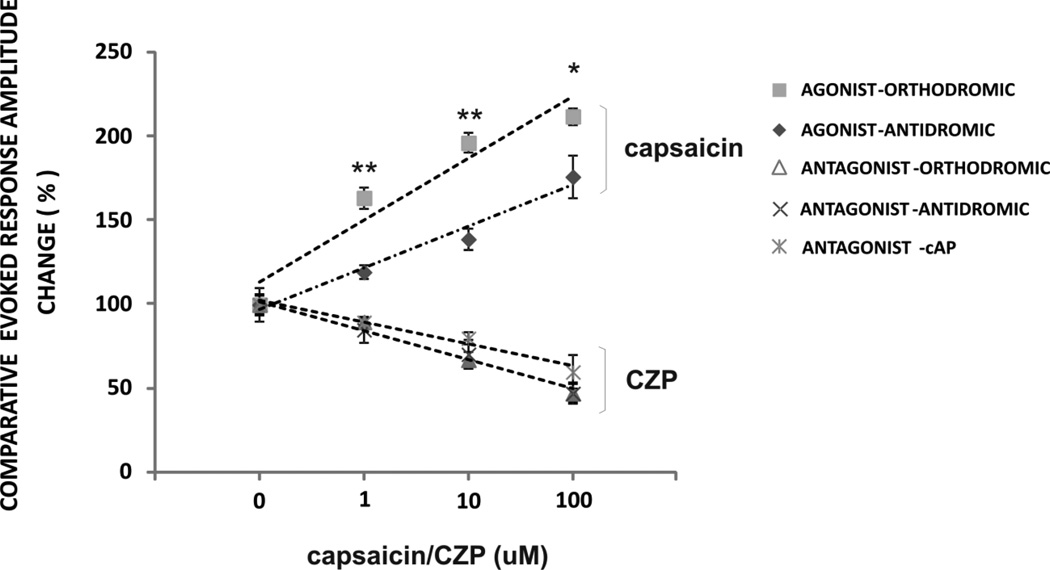
CZP reduced the amplitude of the antidromic potential by 30% (10µM) and 53% (100µM) [from 3.0 ± 0.2 mV (control), n=6, ANOVA, p < 0.0001, Fig 3D] and increased delay by 11% (100 µM) [from 1.9 ± 0.03 ms (control), n=6, ANOVA, p=0.002, Fig 3D]. When relative changes in amplitude of the evoked potential were compared, the effect of capsaicin was greater on the orthodromic response compared to that of the antidromic response, suggesting that the agonist may have some effect of synaptic transmission (two-way ANOVA, drug factor X evoked potential factor, p = 0.05, n=6–7/group, Fig 5). In contrast, the effects of the antagonist CZP on the antidromic and orthodromic responses were not significantly different (two-way ANOVA, drug factor X evoked potential factor, p = 0.3, n=6–7/group, Fig 5). Taken together these results show activation of TRPV1 channels can affect synaptic transmission whereas blockade of endogenous tone on these channels operates independently of synaptic transmission.
FIGURE 5. EVOKED REPONSE COMPARATIVE ANALYSIS.
The effect on mean ± SEM normalized amplitude trend line of evoked potentials was separately analyzed for capsaicin and CZP. Direct comparison between orthodromic and antidromic relative amplitude change shows that the agonist capsaicin increased both the orthodromic and antidromic potentials, but its effect was significantly greater on the orthodromic potential. In contrast, the suppressive effect of CZP was equivalent for the antidromic response, orthodromic response, and compound action potential. This suggests that although activation of TRPV1 channels may modulate synaptic activity, the blockage of the endogenous tone on TRPV1 channels could modulates membrane excitability in cell bodies and axons independently of synaptic transmission. ** p < 0.001 * p < 0.01, between orthodromic and antidromic changes induced by capsaicin, two-way ANOVA interaction evoked potential X drug, followed by post-hoc tests.
CZP affects axonal propagation
We also investigated whether CZP could have a direct effect on antidromic axonal propagation. Thus, a recording microelectrode was positioned in the alveus to measure the compound action potential (cAP) evoked by stimulation in the alveus 2–4 mm from the recording electrode. CZP was added to the perfusion solution (10 and 100 µM, Fig 4A). CZP reduced the amplitude of the cAP by 20% (10 µM) and 40% (100 µM) [from 0.44 ± 0.008 mV (control), n=7, ANOVA, p < 0.0001, Fig 4C], and increased delay by 5% (100 µM) [from 1.65 ± 0.01 ms (control), n=7, ANOVA, p=0.02, Fig 4D]. The post-hoc test analysis is shown in Fig 5. CZP suppressive effects on cAP, antidromic and orthodromic amplitudes were shown to be equivalent after normalization, indicating that these effects are not affected by synaptic transmission (two-way ANOVA, drug X evoked potential, p = 0.3, n=6–7/group, Fig 5). The changes induced by CZP on amplitude and delay of cAP were correlated significantly (see Table 2).
FIGURE 4. EFFECTS OF CZP ON THE cAP.
A. For the cAP, both the stimulating and recording electrodes were positioned in the alveus 2–4 mm away from each other. After 20 min of acclimatization in the interface chamber to reach a stable cAP, we started recording control levels (aCSF). After that, two doses of CZP were administered subsequently at 20 min intervals.
B. Examples of the compound action potential (cAP) are shown that reflect changes in the cAP amplitude elicited by CZP.
C. Mean ± SEM of normalized amplitude of cAP, showing that CZP reduced the amplitude of the evoked response. α p < 0.001, β p < 0.05 compared with control or 10 uM dose; repeated measures ANOVA followed by post-hoc test.
D. Mean ± SEM of normalized delay show that CZP increases the delay of the cAP. α p < 0.05 compared with control, repeated measures ANOVA followed by post-hoc test.
These in vitro results show that CZP can suppress interictal activity by decreasing excitability of cell bodies and axons and does not require synaptic transmission. In order to determine the effect of CZP on ictal activity, similar experiments were repeated in vivo.
IN VIVO STUDIES
CZP as a silent antagonist in normal tissue
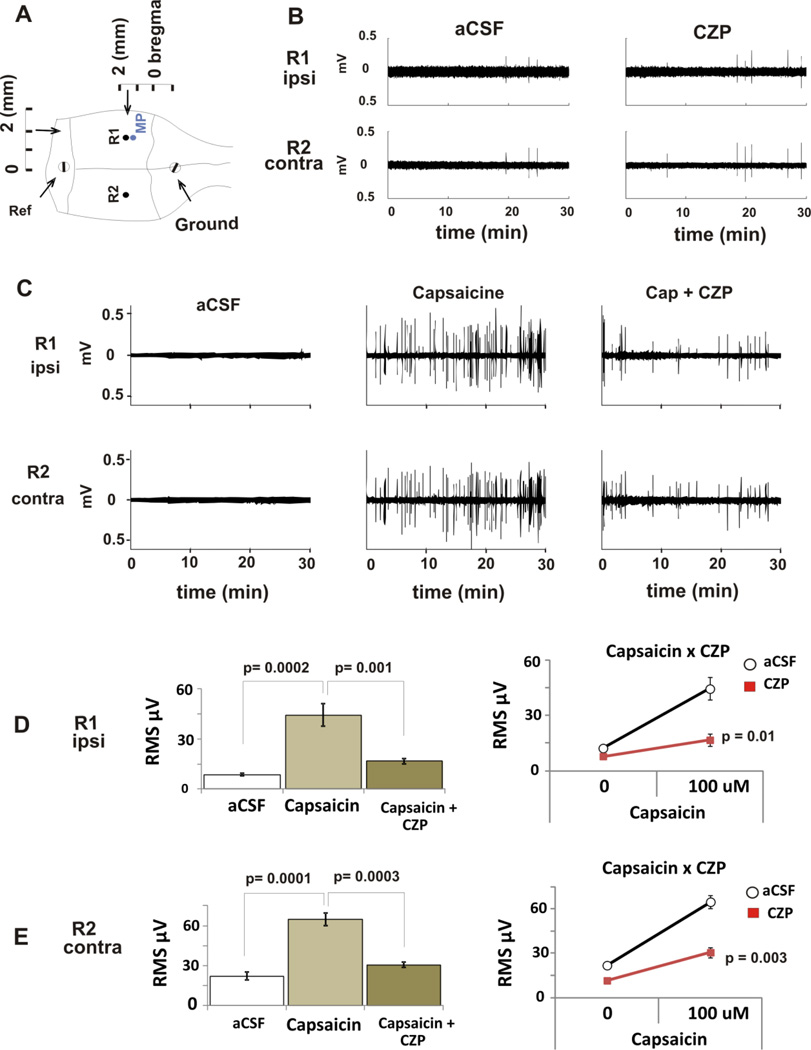
Before attempting the antagonism assay, we first tested whether CZP could elicit effects on baseline EEG. After 30 min of aCSF perfusion, a subcutaneous injection of CZP (50 mg/kg) was made and the hippocampal electrical activity was recorded for further 30 min. As shown in Figure 6B, CZP had no effect on EEG activity following systemic administration (ipsilateral side, n=5, ANOVA CZP factor, p=0.2; contralateral side, n=5/group, ANOVA CZP factor, p =0.2).
FIGURE 6. EFFECTS OF TRPV1 LIGANDS ON BASAL HIPPOCAMPAL EEG RECORDING IN MICE.
A. For in vivo recording of hippocampal activity, two tungsten recording electrodes (R1 and R2) were positioned in the dorsal hippocampus and two screw electrodes served as reference and ground. A microdialysis (MP) probe was positioned near the left (ipsilateral) recording electrode.
B. After a 1-h equilibration period, baseline activity was recorded for 30 min. Next, CZP (50 mg/kg) was perfused for 30 min. There was no different on RMS between both periods (see D and E). Representative traces for 30 min periods are shown.
C. Pharmacological Interaction: after a 1-h equilibration period, baseline activity was recorded for 30 min. Next, capsaicin (100 µM) was perfused continually to the end of the experiment. After 30 min of capsaicin administration, CZP (50 mg/kg) was subcutaneously administered. Representative traces from each of these periods are shown.
D. Mean ± SEM RMS (µV) for consecutive 30 min periods with aCSF (control), capsaicin, and capsaicin + CZP are shown. Capsaicin induced a significant increase in power in the ipsilateral hippocampus that was reversed by CZP * p-values on bars refer to repeated measures ANOVA followed by post-hoc tests, and p-values on the lines chart refer to two-way ANOVA (Capsaicin×CZP) factor showing significant interaction.
E. Mean ± SEM RMS (µV) for consecutive 30 min period with aCSF, capsaicin, and capsaicin + CZP are shown. Capsaicin induced a significant increase in power in the contralateral hippocampus that was reversed by CZP. * p-values on bars refer to repeated measures ANOVA followed by post-hoc tests, and p-values on the lines chart to two-way ANOVA (Capsaicin×CZP) factor showing significant interaction.
Pharmacological interaction of TRPV1 ligands on hippocampal basal neural activity in vivo
We then evaluated the ability of systemic CZP (administered subcutaneously) to reduce the excitatory effects induced by intracerebral infusion of capsaicin. The purpose of this assay was twofold: (1) to evaluate whether capsaicin may trigger spontaneous activity in vivo, and (2) to evaluate specific TRPV1 antagonist properties by CZP after systemic administration. Simultaneous in vivo electrophysiological recording and intracerebral infusion were performed during stereotaxic surgery. After a 1-h equilibration period of the aCSF microdialysis infusion through the probe implanted in the left dorsal hippocampus, baseline neural activity was recorded for 30 minutes before capsaicin (100 µM, concentration based on Afrah et al., 2001) was infused through the microdialysis probe for a subsequent 1-h period. After 30 min of capsaicin infusion, CZP was injected (50 mg/K, s.c., dose based on Walker et al. 2006). EEG was monitored over the next 30 min after this single subcutaneous injection of CZP while capsaicin continued to be delivered via the microdialysis probe (Figure 6). Capsaicin induced excitatory bursting that consisted of ripples of high frequency oscillations (58 ± 7 Hz, 0.3 ± 0.1 mV peak-to-peak amplitude), ripples appeared with a frequency of 0.1 ± 0.05 Hz (n=5, Fig 6C, Supplemental FS1). The power of the signal was measured using RMS and significantly increased (n=5, ANOVA followed by post-hoc Tukey, p=0.0002). The effect was reversed by CZP s.c. (ipsilateral side, n=5/group, two-way ANOVA, CZP X capsaicin interaction, p=0.01; contralateral side, n=5/group, two-way ANOVA, capsaicin X CZP interaction, p =0.003) (Fig 6C, D and E).
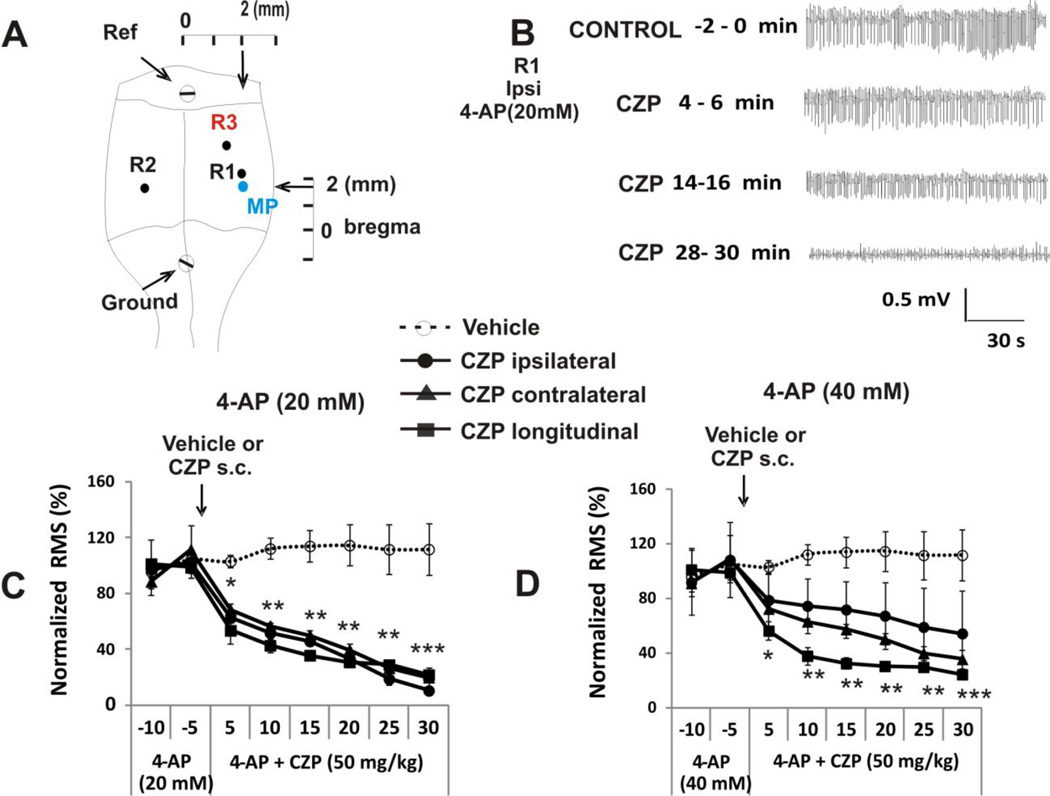
CPZ can suppress 4-AP-induced epileptic status activity
To evaluate the effect of systemic CZP on 4-AP induced epileptiform activity we used in vivo electrophysiological recording techniques and intracerebral dialysis (see methods). Baseline neural activity was recorded for 30 minutes, then microdialysis perfusion was switched to 4-AP (20 or 40 mM in aCSF). Spectrometry has shown that this method delivers an 11.3 ± 0.28 % fraction of the 4-AP concentration in aCSF (Morales and Tapia, 1996). During the 4-AP infusion period, single interictal spikes appeared at increasing frequency to reach a regular synchronized frequency (0.5 to 1Hz) by 10–20 min following the onset of the perfusion. After 30 min of 4-AP infusion, electrographic seizure and status epilepticus had developed. Once continuous ictal activity (> 7 Hz) persisted for at least 10 min, CZP (50 mg/kg, s.c) or vehicle (s.c.) was injected. CZP was able to suppress epileptiform activity in the three recorded locations: ipsilateral [control RMS (-5-0 min) = 114.4±21.8 µV, n=5, ANOVA, p < 0.0001], contralateral [control RMS (-5-0 min) = 117.5±25.1 µV, n=5, ANOVA, p < 0.0001] and temporal hippocampus [control RMS (-5-0 min) = 121.3±17.7 µV, n=5, ANOVA, p < 0.0001), when epileptiform activity was induced using 4-AP at a concentration of 20 mM (Fig 7C). However, CZP failed to reverse epileptiform activity in the ipsilateral side of animals infused with 40 mM 4-AP (control RMS (-5-0 min) = 121.0±20.9 µV, n=5, ANOVA, p = 0.4), but was able to significantly reduce activity in the contralateral side [control RMS (-5-0 min) = 121.6±23.1 µV, n=5, ANOVA, p = 0.0001] and in the distal recording location in the temporal hippocampus [control RMS (-5-0 min) =132.5±22.8 µV, n=5, ANOVA, p < 0.0001] (Fig 7D), a region connected by the longitudinal hippocampal pathway.
FIGURE 7. EFFECT OF TWO VANILLOIDS ON 4-AP INDUCED ELECTROGRAPHIC SEIZURES IN VIVO.
A. For in vivo recording of hippocampal activity, two tungsten recording electrodes were positioned in the right (contralateral) and left (ipsilateral) sides of the septal hippocampus and an additional electrode was positioned in the left temporal hippocampus, which is connected to the septal region by the longitudinal pathway (longitudinal). Two screws served as reference and ground. A microdialysis probe (MP) was positioned near the left (ipsilateral) recording electrode.
B. Representative 2 min traces for the ipsilateral sides during administration of 4-AP (20 mM) and after administration of CZP. After normalization the change in RMS for the three recording electrodes was the same (see C below).
C. Signal RMS after CZP administration normalized to activity induced by 20 mM 4-AP showing ongoing seizure activity for either vehicle or CZP in the three electrode locations. CZP significantly decreased seizures in the three locations. **p < 0.001, *p < 0.01, repeated measures ANOVA followed by post-hoc tests for the ipsi-lateral, contra-lateral and longitudinal recording regions.
D. Normalized RMS data activity induced by 40 mM 4-AP showing ongoing seizure activity for either vehicle or CZP in the three electrode locations. CZP significantly decreased seizures in contralateral and longitudinal electrodes but failed to modify seizure activity in the ipsilateral side. **p < 0.001, *p < 0.01, repeated measures ANOVA followed by post-hoc tests for only contra-lateral and longitudinal groups.
TRPV1 is expressed in neurons and glia in the hippocampus
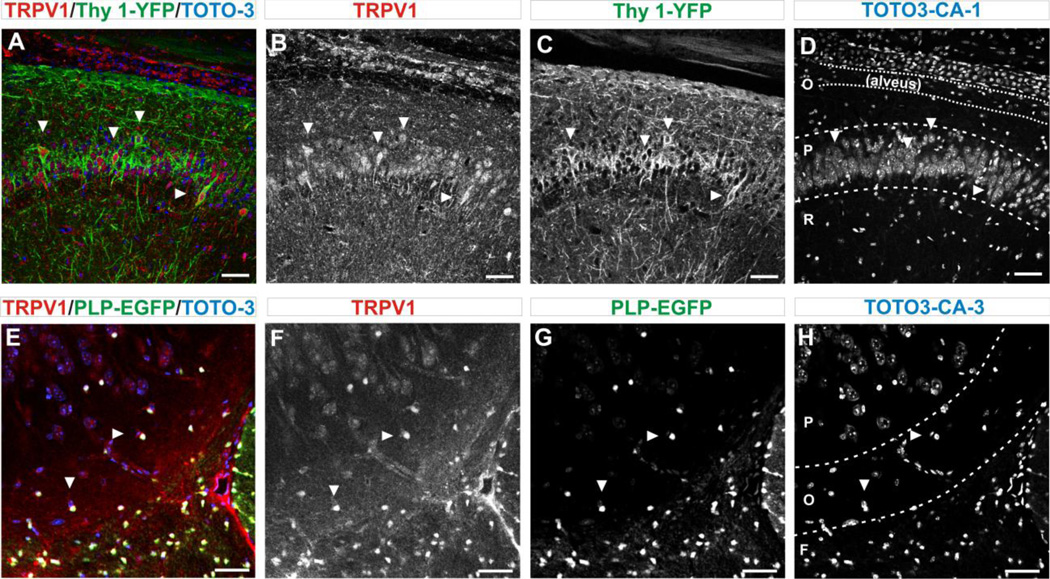
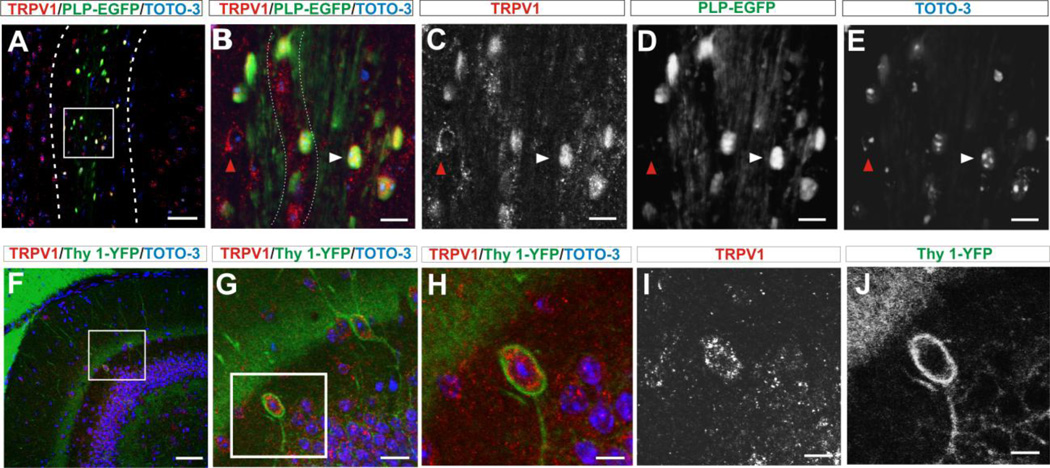
Based on the electrophysiological recordings reported here, one would expect to find TRPV1 channels on cells bodies in the hippocampus but also on axons. Diffuse expression of TRPV1 channels in the hippocampus was indeed found (Fig 8–9). However, with the use of the Thy1-YFP tracer, we observed that 42% of neurons (YFP+ cells) in the CA1 region expressed TRPV1 channels (from 252.4±11.3 YFP+ cell, 104.4±8.8 were TPRPV1+ cells, n=5 slices×5 animals, Fig 8 A–D). Similarly, in the dentate gyrus, there was reliable (100%) co-expression of the Thy1-YFP tracer and TRPV1 channels in small round neurons located off the granular cell layer (of 27.4±2.5 YFP+ cells, 27.4±2.5 were TRPV1+ cells , n = 5 slices×5 animals, Fig 9 F–J). The morphology and localization of these neurons are compatible with that described as Cajal-Retzius cells of the hippocampus (Cavanaugh et al., 2011). Observation of axonal expression of TRPV1 channels was carried in the alveus. Since this sub-region is mostly devoid of neural cell bodies, we investigated whether TRPV1 channels were expressed by alveus oligodendrocytes (ODC). Confocal exploration using anti-TRPV1 antibodies and the ODC tracer PLP-EGFP showed that 87% of EGFP positive cells co-express TRPV1 in CA1 (of 174±5.9 PLP+ cells, 151.8±4.4 were TRPV1+cells (n=5x5), Fig 9 A–D) and 93% of EGFP positive cells co-express TRPV1 in CA3 (of 320±9.1 PLP+ cells, 300.2±7.6 were TRPV1+, n=5x5, Fig 8 E–H) regions of the hippocampus.
FIGURE 8. OVERALL EXPRESSION OF TRPV1 CHANNELS IN NEURONS AND OLIGODENDROCYTES (ODC).
A. Immuno-labelling for TRPV1 in CA1 pyramidal cell layer of the hippocampus in the Thy1-YFP transgenic mouse show co-expression of neural marker Thy-1-YFP (green) and TRPV1 channels (red). Arrows indicate examples of cells in which co-expression is clear. Sub-regions of hippocampus CA1 are demarcated in D. Confocal images are 25X magnification/ 0.7X field. Scale bar = 50 µm.
B. Immuno-labelling for TRPV1 channels with anti-TRPV1 antibody show expression in the pyramidal layer. Arrows indicate examples of cells in which expression is clear. Scale bar = 50 µm.
C. The Thy1-YFP tracer showing positive neurons along the pyramidal cell layer. Scale bar = 50 µm.
D. Staining with the nuclear marker TOTO3 show the pattern of cell distribution. The organization of the hippocampus was demarcated: (O) stratum oriens containing the alveus, (P) stratum pyramidal and (R) stratum radiatum. Arrows indicate nuclei from cells that co-express Thy1-YFP and TRPV1 markers. Scale bar = 50 µm.
E. Co-expression of TRPV1 channels (red) and the ODC tracer PLP-EGFP (green) in the fornix and CA3 region, using a transgenic reporter mouse (Mallon et al., 2002). Arrows indicate examples of cells in which co-expression is clear. Sub-regions of the hippocampus CA3 are delineated in H. Confocal images are 25X magnification/ 0.7X field. Scale bar = 50 µm.
F. Immuno-labeled for TRPV1 channels with anti-TRPV1 antibody show greater channel expression in small round ODC and a large blood vessel. Arrows indicate examples of cells in which expression is clear.
G. Typical pattern of expression of ODC with greater number of ODC in the fornix and with few ODC that can be seen in both the stratum oriens and pyramidale. Arrows indicate examples of cells in which expression is clear.
H. The nuclear marker (TOTO3) for corresponding area of the hippocampus. CA3 structural elements were demarcated: (F) fornix, (O) stratum oriens and (P) stratum pyramidale. Arrows indicate examples of nuclei from cells in which co-expression is indicated in E, F and G.
FIGURE 9. DETAILED EXPRESSION OF TRPV1 CHANNELS IN GLIAL AND NEURAL ELEMENTS.
A. Low magnification view of the CA1 region to indicate the area within the alveus where the magnified images (B-D) were taken. (O) stratum oriens and (P) stratum pyramidale. (Scale bar = 50 µm)
B. Higher magnification view of ODC immuno-labeled for TRPV1 channels with anti-TRPV1 and coexpressing the ODC specific tracer PLP-EGFP. The white arrow shows a cell that co-expresses both markers, while the red arrow shows an example of a cell expressing only TRPV1 but not PLP-EGFP. Fine lines indicate a nerve fiber in the alveus stained by TRPV1. Scale bar = 10µm.
C. Immuno-labeled for TRPV1 channels with anti-TRPV1 antibody show clear expression in the ODC. Arrow shows two cells expressing TRPV1. Scale bar = 10 µm.
D. The PLP-EGFP tracer showed abundant ODCs along the alveus. Red arrow shows spot of cell that expresses the TRPV1 but not the PLP marker, while white arrow show a PLP+ cell. Scale bar = 10µm.
E. Staining for cell nuclei with the nuclear marker TOTO3, show typical nuclear morphology of the ODC nuclei contain finely granular chromatin and small halos. Arrows track nuclei corresponded to cells in B, C and D. Scale bar = 10µm.
F. Panoramic view of the dentate gyrus (DG) to show the area in the granular layer border where closed up images (panels G and H) were taken. Scale bar = 100 µm.
G. Magnified view of two small neurons located off the granular cell layer in the dentate gyrus. Scale bar = 20 µm.
H. Magnified view of one neuron compatible with Cajal-Reitzus neurons (Cavanaugh et al., 2011). Scale bar= 10 µm
I. Immunolabeled for TRPV1 channels with anti-TRPV1 antibody (red) showing abundant expression in the cytoplasm. Scale bar = 10 µm.
J. Immunolabeled for Thy1-YFP confirming neural expression. Scale bar = 10 µm.
Discussion
These experiments show that the TRPV1 antagonist capsazepine (CZP) was able to suppress ongoing epileptiform activity generated by 4-aminopyridine in vitro and in vivo. The in vitro experiments showed that CZP reduced the amplitude of both the antidromic and orthodromic responses. Comparative analysis of the effects on antidromic and orthodromic evoked potential amplitudes show that there was no difference in the amount of suppression induced by CZP (Fig 5). This finding implies that TRPV1 channels can modulate neural excitability independently of synaptic function. We also found that CZP reduced the amplitude of compound action potential (cAP), a field potential produced by membrane depolarization in axons. The effects of several types of anti-epileptic drugs on the cAP have previously been evaluated. Importantly, anti-epileptic drugs that block cationic channels but not other types of anti-epileptics, were able to suppress the cAP (Teriakidis et al., 2006).
Likewise, in the in vivo experiments we found that CZP had no effect on baseline activity but was able to modulate 4-AP or capsaicin-induced epileptiform activity. This result is in line with the observation that CZP did not affect firing of dentate gyrus granule cells but rather antagonized the increase in firing induced by capsaicin (Bhaskaran and Smith, 2010). The suppressive effect of CZP on 4-AP induced epileptiform activity in vivo was dependent on 4-AP local concentrations. At the low 4-AP concentration (20 mM), CZP significantly suppressed ictal activity. However, at the high 4-AP concentration (40 mM), CZP was not able to suppress the epileptiform activity in the side of administration, but clearly reduced the activity recorded in the contralateral side and in the posterior regions of the hippocampus, indicating that CZP could suppress propagation through both the commissural tracts as well as the longitudinal hippocampal pathways. These observations, in combination with our in vitro data, suggest that TRPV1 channels primarily modulate neural activity by modifying membrane excitability, affecting both the antidromic and axonal responses independently of synaptic function.
The electrophysiological results suggest that TRPV1 channels are expressed throughout the hippocampus and this fact was confirmed by histological studies. Thus, using conventional histological techniques in combination with transgenic mice, we found co-expression of TRPV1 channels and YFP in some neurons in the CA1 pyramidal cell layer (the site of the in vitro recordings) using the pan-neuronal tracer Thy1-YFP (Fig 8). Since our electrophysiological results showed that CZP could also suppress the cAP when both stimulation and recording were confined to the alveus (a region mostly occupy by fibers and devoid of neural somas), we analyzed TRPV1 channels expression in this area. We found that the expression was basically restricted to small cells distributed along the alveus, which suggested expression in oligodendrocyte glial cells. This hypothesis was confirmed using the PLP-EGFP mouse line, as TRPV1 channels was highly expressed in oligodendrocytes (ODC) marked with EGFP, a selective tracer for ODCs (see Fig 9).
It is worth noting that the expression of TRPV1 in ODCs could contribute to the effects on alveus cAP. It has been reported that direct depolarization of ODCs shortened the latencies of action potentials evoked by antidromic stimulation in the alveus region (Yamazaki et al., 2007). However, the present study does not rule out that TRPV1 may act directly on the axonal membrane as expression of TRPV1 channels along the axons has been demonstrated (Canetta et al., 2011).
Contrary to CPZ, capsaicin did have a direct effect on neural baseline activity in vivo, in line with previous findings by Manna and Umathe (2012) who showed that capsaicin exhibited pro-convulsant activity. The excitatory bursting activity triggered by capsaicin was completely abolished by a silent dose of CZP, suggesting it was mediated by TRPV1 channels (Fig 6). The ability of capsaicin to elicit extracellular activity in vivo but not in vitro could be a reflection of the fact that TRP channel agonists are enhanced at more depolarized membrane potentials (Voets et al., 2004), and under in vivo conditions, greater basal neural activity may increase the sensitivity of TRPV1 channels. In addition, it has been found that isoflurane and other general anesthetics may sensitize TRPV1 channels in sensory neurons (Cornett et al., 2008). In our study, the presence of isoflurane could have contributed to increase the sensitivity of the TRPV1 channels during the in vivo administration.
Capsaicin also increased the amplitude of both the antidromic and orthodromic responses, which is in line with the observation that capsaicin increases both action-potential-dependent and -independent events (Bhaskaran and Smith, 2010). In sharp contrast to CZP, the comparative analysis showed that capsaicin had a greater effect on the orthodromic potential (Fig 5). Synaptic plasticity could contribute to this effect as long term depression (LTD) has been shown to occur within few minutes of capsaicin superfusion. This capsaicin-induced LTD is selective for inhibitory interneurons (Gibson et al., 2008), which could lead to the enhanced orthodromic amplitude.
In conclusion, the present results show that TRPV1 channels are broadly expressed in the hippocampus and indicate their functional role in the modulation of epileptogenic neural networks, directly implicating both neural and glial elements. Blockade of TRPV1 channels by CZP administration was sufficient to suppress in vivo ongoing ictal activity and, remarkably, CZP interfered with the propagation of seizure activity along the hippocampal pathways. Furthermore, the finding that CZP is effective when peripherally injected demonstrates that the drug crosses the blood brain barrier to reach anti-convulsant concentrations in the brain. However, these studies so far apply to acute seizure suppressing effects, while chronic anti-epileptic effects of TRPV1 antagonism in models of spontaneous seizures remain to be evaluated. In any event, these results encourage the development of anti-epileptic drugs that can prevent the propagation of seizures and decrease general excitability by targeting TRPV1 channels.
Supplementary Material
HIGHLIGHTS.
The TRPV1 antagonist capsazepine suppresses epileptiform activity.
Capsazepine decreases the amplitude of non-synaptic antidromic potentials.
The TRPV1 agonist capsaicin was able to elicit excitatory bursting in vivo.
Capsazepine significantly reduced electrographic seizures in vivo.
Capsazepine was effective after subcutaneous administration
Acknowledgment
This research was funding by EL Lindseth Endowed Chair to DM Durand. PLP-EGFP mice were kindly donated by Dr. Robert Miller (Department of Neuroscience, CWRU)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afrah AW, Stiller CO, Olgart L, Brodin E, Gustafsson H. Involvement of spinal N-methyl-Daspartate receptors in capsaicin-induced in vivo release of substance P in the rat dorsal horn. Neurosci Lett. 2001;316:83–86. doi: 10.1016/s0304-3940(01)02380-1. [DOI] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran MD, Smith BN. Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy. Exp Neurol. 2010;223:529–536. doi: 10.1016/j.expneurol.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta SE, Luca E, Pertot E, Role LW, Talmage DA. Type III Nrg1 back signaling enhances functional TRPV1 along sensory axons contributing to basal and inflammatory thermal pain sensation. PLoS One. 2011;6(9):e25108. doi: 10.1371/journal.pone.0025108. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cornett PM, Matta JA, Ahern GP. General anesthetics sensitize the capsaicin receptor transient receptor potential V1. Mol Pharmacol. 2008;74:1261–1268. doi: 10.1124/mol.108.049684. [DOI] [PubMed] [Google Scholar]
- Cramer CL, Stagnitto ML, Knowles MA, Palmer GC. Kainic acid and 4-aminopyridine seizure models in mice: evaluation of efficacy of anti-epileptic agents and calcium antagonists. Life Sci. 1994;54:PL271–PL275. doi: 10.1016/0024-3205(94)00845-0. [DOI] [PubMed] [Google Scholar]
- Cristino L, De Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Fragoso-Veloz J, Tapia R. NMDA receptor antagonists protect against seizures and wet-dog shakes induced by 4-aminopyridine. Eur J Pharmacol. 1992;221:275–280. doi: 10.1016/0014-2999(92)90713-e. [DOI] [PubMed] [Google Scholar]
- Gandolfo G, Gottesmann C, Bidard JN, Lazdunski M. Subtypes of K+ channels differentiated by the effect of K+ channel openers upon K+ channel blocker-induced seizures. Brain Res. 1989;495:189–192. doi: 10.1016/0006-8993(89)91236-5. [DOI] [PubMed] [Google Scholar]
- Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 Channels Mediate Long-Term Depression at Synapses on Hippocampal Interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszas K, Keller JM, Coddou C, Mishra SK, Hoon MA, Stojilkovic S, Jacobson KA, Ladarola MJ. Small molecule positive allosteric modulation of TRPV1 activation by vanilloids and acidic pH. J Pharmacol Exp Ther. 2012;340:152–160. doi: 10.1124/jpet.111.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Bevan S, Walpole CS, Rang HP, Giuliani S. A comparison of capsazepine and ruthenium red as capsaicin antagonists in the rat isolated urinary bladder and vas deferens. Br J Pharmacol. 1993;108:801–805. doi: 10.1111/j.1476-5381.1993.tb12881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–85. doi: 10.1523/JNEUROSCI.22-03-00876.2002. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol. 2006;4:1–15. doi: 10.2174/157015906775202995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Villagrán A, López-Pérez S, Medina-Ceja L, Tapia R. Cortical catecholamine changes and seizures induced by 4-aminopyridine in awake rats, studied with a dual microdialysis-electrical recording technique. Neurosci Lett. 1999;275:133–136. doi: 10.1016/s0304-3940(99)00759-4. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña F, Tapia R. Seizures and neurodegeneration induced by 4-aminopyridine in rat hippocampus in vivo: role of glutamate- and GABA-mediated neurotransmission and of ion channels. Neuroscience. 2000;101:547–561. doi: 10.1016/s0306-4522(00)00400-0. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Siniscalchi A, Calabresi P, Mercuri NB, Bernardi G. Epileptiform discharge induced by 4-aminopyridine in magnesium-free medium in neocortical neurons: physiological and pharmacological characterization. Neuroscience. 1997;81:189–197. doi: 10.1016/s0306-4522(97)00178-4. [DOI] [PubMed] [Google Scholar]
- Spyker DA, Lynch C, Shabanowitz J, Sinn JA. Poisoning with 4-aminopyridine: report of three cases. Clin Toxicol. 1980;16:487–497. doi: 10.3109/15563658008989978. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Garratt JC, Robertson B, Owen DG. On the mechanism of 4-aminopyridine action on the cloned mouse brain potassium channel mKv1.1. J Physiol. 1994;477:187–196. doi: 10.1113/jphysiol.1994.sp020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teriakidis A, Brown JT, Randall A. Frequency-dependent inhibition of antidromic hippocampal compound action potentials by anti-convulsants. Pharmacol Rep. 2006;58:859–869. [PubMed] [Google Scholar]
- Toprani S, Durand DM. Fiber tract stimulation can reduce epileptiform activity in an in-vitro bilateral hippocampal slice preparation. Exp Neurol. 2012;240C:28–43. doi: 10.1016/j.expneurol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Ulbricht W, Wagner HH. Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflugers Arch. 1976;367:77–87. doi: 10.1007/BF00583659. [DOI] [PubMed] [Google Scholar]
- Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- Walpole CS, Bevan S, Bovermann G, Boelsterli JJ, Breckenridge R, Davies JW. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J. Med. Chem. 1994;37:1942–1954. doi: 10.1021/jm00039a006. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Rogawski MA. Effects of anticonvulsant drugs on 4-aminopyridine-induced seizures in mice. Epilepsy Res. 1992;11:9–16. doi: 10.1016/0920-1211(92)90016-m. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hozumi Y, Kaneko K, Sugihara T, Fujii S, Goto K, Kato H. Modulatory effects of oligodendrocytes on the conduction velocity of action potentials along axons in the alveus of the rat hippocampal CA1 region. Neuron Glia Biol. 2007;3:325–334. doi: 10.1017/S1740925X08000070. [DOI] [PubMed] [Google Scholar]
- Yonekawa WD, Kapetanovic IM, Kupferberg HJ. The effects of anticonvulsant agents on 4-aminopyridine induced epileptiform activity in rat hippocampus in vitro. Epilepsy Res. 1995;20:137–150. doi: 10.1016/0920-1211(94)00077-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.