Abstract
Background
Obesity-attributable medical expenditures remain high, and interventions that are both effective and cost-effective have not been adequately developed. The Opt-IN study is a theory-guided trial using the Multiphase Optimization Strategy (MOST) to develop an optimized, scalable version of a technology-supported weight loss intervention.
Objective
Opt-IN aims to identify which of 5 treatment components or component levels contribute most meaningfully and cost-efficiently to the improvement of weight loss over a 6 month period.
Study Design
Five hundred and sixty obese adults (BMI 30–40 kg/m2) between 18 and 60 years old will be randomized to one of 16 conditions in a fractional factorial design involving five intervention components: treatment intensity (12 vs. 24 coaching calls), reports sent to primary care physician (No vs. Yes), text messaging (No vs. Yes), meal replacement recommendations (No vs. Yes), and training of a participant’s self-selected support buddy (No vs. Yes). During the 6-month intervention, participants will monitor weight, diet, and physical activity on the Opt-IN smartphone application downloaded to their personal phone. Weight will be assessed at baseline, 3, and 6 months.
Significance
The Opt-IN trial is the first study to use the MOST framework to develop a weight loss treatment that will be optimized to yield the best weight loss outcome attainable for $500 or less.
Keywords: Weight loss, optimization, technology
INTRODUCTION
The Diabetes Prevention Program (DPP) [1], and Action for Health in Diabetes (Look AHEAD) [2] trials have been successful in producing significant and sustained weight losses. These treatment programs have not been extensively adopted, however, because they are considered too burdensome and expensive to be practical [3]. The initial phase of most intensive lifestyle interventions requires at least 16 in-person sessions and costs about $1400/year per participant [4]. The cost of implementing the program can be reduced below $500 per participant by training YMCA staff to deliver treatment [5, 6]. However, this still requires participants to have the access, willingness, and ability to attend multiple in-person sessions. Another approach to reducing the cost is to lower treatment intensity, but that adaptation has yielded mixed results [7, 8]. Reducing treatment intensity decreases accountability and feedback, and usually yields diminished weight loss as frequent coaching contacts are pruned back [9]. The challenge that remains is how to implement a lifestyle intervention that can reduce participant burden, treatment intensity, and costs without eliminating key intervention components necessary for successful weight loss.
Multiphase Optimization Strategy (MOST)
In traditional research methods, lifestyle interventions are typically evaluated as bundled “treatment packages,” making it difficult to assess definitively which aspects of an intervention can be reduced, eliminated, or replaced to improve efficiency. Multiple active intervention components are available; yet it is unknown which have an impact independent of other commonly bundled treatment components. Using traditional research methods (i.e., multi-armed randomized clinical trials (RCTs) to test each new feature one at a time) would require running many studies over decades - - an exorbitantly expensive process that would likely be outpaced by the development of newer technologies. This modern paradox prompted us to apply a new research methodology that may offer a quicker, more efficient way to test the effectiveness of multiple intervention components simultaneously.
The Multiphase Optimization Strategy (MOST) [10] is a comprehensive framework for development, optimization, and evaluation of behavioral interventions. Inspired by engineering methods, MOST employs highly efficient randomized experimentation to assess the effects of individual treatment components, and thereby identify which components and component levels make important contributions to the overall program effect. This information then guides assembly of an optimized treatment package that achieves target outcomes with least resource consumption and participant burden. A factorial experiment is often the most efficient way to examine the effects of individual intervention components. Factorial experimental designs allow the separation of intervention component effects and can be more economical compared to alternative designs because they often require fewer participants, yet achieve the same statistical power for hypothesis tests about intervention component effects [11]. When there are several independent variables, a complete factorial experiment requires a large number of experimental conditions. A more economical variation on the factorial design, the fractional factorial, uses only a fraction of the experimental conditions and therefore can be less costly to implement [12].
MATERIALS AND METHODS
Intervention Component Selection
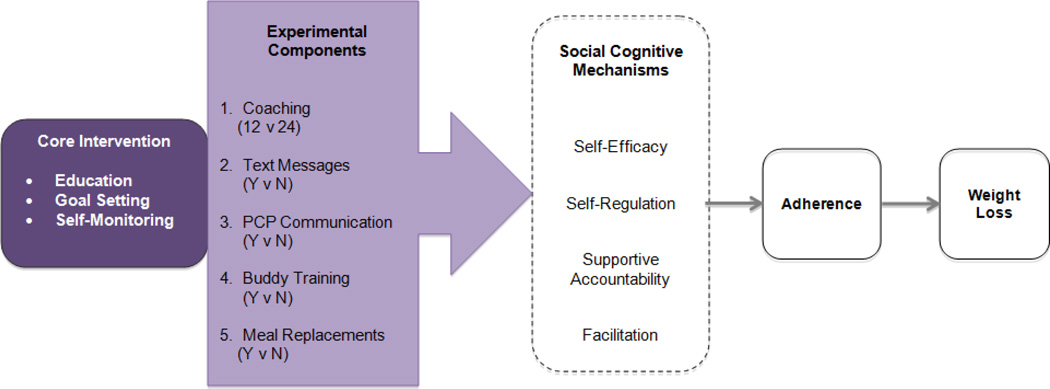
For the current Opt-IN trial, 560 adults will be randomized. A total of five intervention components, each with two possible levels, was chosen for examination based on our conceptual model in Figure 1. The five selected components target social cognitive mechanisms including self-efficacy, self-regulation, supportive accountability, and facilitation, and should enhance treatment adherence, thereby leading to weight loss. The five components are:
Telephone Coaching Intensity. It is well-established that a greater number of treatment sessions results in greater weight loss [13, 14] and behavioral treatment for obesity is usually weekly for 16–26 weeks [15]. Appel et al. [16] has demonstrated the comparable efficacy of telephone and in-person coaching for achieving weight loss. Hence, we chose to implement 12 vs. 24 telephone coaching sessions for the current trial. We expect the supplemental treatment components to bolster the impact of the lower intensity 12 call treatment.
Report to Primary Care Provider (PCP). Weight loss treatment offered in the primary care setting has yielded disappointing results, unless the intervention included medication or food provision [14, 17, 18]. Patients want their PCP to support their weight management progress [19], and supplying progress reports to the PCP has been a component of successful weight loss treatments [16]. Providing a report that engages the PCP with the patient’s weight loss progress is less costly and more scalable than expecting the PCP to provide the weight loss treatment. Half of the participants in the current study will have weight loss progress reports sent to their PCP after the 3 and 6 month assessment.
Text Messages. Text messages show promise as a cost effective method to prompt health behavior change [20, 21]. For the current trial, half of the participants will receive regular text messages.
Meal Replacement Recommendations. Consumption of pre-packaged, portion controlled, calorie-controlled meal replacements to substitute for some meals and snacks has been shown to enhance weight loss outcomes [22–24]. Although meal replacement foods usually are provided at no cost to patients [22–24], supplying products free of charge over an extended period is unsustainable for the health care system. Therefore, we will examine a more scalable variation: we will recommend the use of meal replacements to half of the participants and provide an introductory free one week supply.
Buddy Training. Engaging peers together in weight loss has been found to augment treatment retention and weight loss outcomes [25]. Thus, all participants in the current trial will join with a buddy (friend or family member) and half of those buddies will receive training on how to provide support. Buddy training will occur via telephone and group webinars. We hypothesize that providing training to a buddy will allow him/her to more effectively encourage and assist with the participant’s diet, activity, and weight loss efforts.
Figure 1.
Opt-IN Conceptual Model
Intervention Component Cost Estimation
Table 1 illustrates the estimated cost of implementing the lowest and highest level of the intervention and varying levels of its components. All conditions will receive the core intervention, which includes a website with study materials and the Opt-IN smartphone application. The costs of the core include maintenance and troubleshooting of the website and smartphone application estimated at 20% of a programmer with a salary of $70,000. We estimate the cost per participant to be ($70,000 * .20 effort)/150 participants/year = $93.33/person. A $150 annual fee is also included to access the study’s web infrastructure, server, and food database for a total of $243.33/person. The additional costs associated with each of the intervention components are listed below:
Coaching Calls: Each telephone coaching session was estimated to take approximately 30 minutes including the preparation, call, and conclusion notes, with the salary of a lifestyle coach assumed at $35,000/year or $16.83/hour. The costs would range from $100.98/person for 12 calls ($16.83 * 0.5 * 12 = $100.98/person) or $201.96/person for 24 calls ($16.83 * 0.5 * 24 = $201.96/person).
PCP Reports: The costs associated with sending weight loss progress reports to the individual’s PCP at 3 and 6 months include staff costs ($30/person), postage ($0.49 × 2 = $0.98/person), and printing and paper costs ($2 × 2 = $4/person).
Text Messages: All text messages will be sent through the Opt-IN smartphone application so participants will not be charged per text message or need to have a text messaging service plan. The costs of developing and maintaining the text message component of the smartphone application are estimated at ~5% of a programmers time ($23.33/person). In addition, the staff costs of maintaining and updating the text message database and algorithms is estimated at $20/person.
Meal Replacement Recommendations: One week of meal replacements will be provided and then recommendations will be provided throughout the remainder of the intervention. The cost of a one week supply (14 shakes * $1.50/shake + 14 bars * $0.90/bar) is $33.60/person.
Buddy Training: Lifestyle coaches will provide individual telephone training to each buddy. In addition, buddies will participate in 4 webinars. Estimated staffing cost per person is $33.66/person (45 minutes for phone training and 1.25 hours for webinars). All buddies will also receive up to $40 for participating in the training and webinars. Subscription to a webinar service is estimated at $475/year ($475 * 150 participants/year = $3.17). The total cost is $76.83.
Table 1.
Estimated Cost of Lowest and Highest Level of Intervention Components
| Intervention Component Levels | Lowest Level Cost/Person |
Highest Level Cost/Person |
|---|---|---|
| Core Intervention Cost | $243.33 | $243.33 |
| 1. Phone Sessions: 12 or 24 | $100.98 | $201.96 |
| 2. PCP Reports: no or yes | $0 | $34.98 |
| 3. Text Messages: no or yes | $0 | $43.33 |
| 4. Meal Replacements Recommendations: no or yes | $0 | $33.60 |
| 5. Buddy Training: no or yes | $0 | $76.83 |
| Total | Minimum Cost: $344.31 | Maximum Cost: $634.03 |
Study Design
A complete factorial experiment would have required implementation of 32 experimental conditions. To conserve resources, we selected a fractional factorial design that involves 16 experimental conditions (Table 2) [26].1 It is important to note that although there are 16 experimental conditions, this experiment should not be considered a 16-arm RCT. The purpose of this factorial experiment is to estimate the main effects of the five intervention components and interactions between the components, not to compare the 16 experimental conditions to each other. Each main effect and interaction estimate is based on all of the experimental conditions, and therefore on all of the participants. For example, the main effect of the number of coaching sessions will be estimated by comparing the mean of experimental conditions 1–8 in Table 2 vs. the mean of experimental conditions 9–16. For a more detailed explanation of how a factorial experiment maintains power for estimation of main effects and interactions, see [11].
Table 2.
Experimental Conditions
| Experimental Condition |
CORE | # Coaching Sessions |
Progress Report to PCP |
Text Messages |
Meal Replacement Recommendation |
Buddy Training |
|---|---|---|---|---|---|---|
| 1 | Yes | 12 | Yes | No | No | No |
| 2 | Yes | 12 | Yes | No | Yes | Yes |
| 3 | Yes | 12 | Yes | Yes | No | Yes |
| 4 | Yes | 12 | Yes | Yes | Yes | No |
| 5 | Yes | 12 | No | No | No | Yes |
| 6 | Yes | 12 | No | No | Yes | No |
| 7 | Yes | 12 | No | Yes | No | No |
| 8 | Yes | 12 | No | Yes | Yes | Yes |
| 9 | Yes | 24 | Yes | No | No | No |
| 10 | Yes | 24 | Yes | No | Yes | Yes |
| 11 | Yes | 24 | Yes | Yes | No | Yes |
| 12 | Yes | 24 | Yes | Yes | Yes | No |
| 13 | Yes | 24 | No | No | No | Yes |
| 14 | Yes | 24 | No | No | Yes | No |
| 15 | Yes | 24 | No | Yes | No | No |
| 16 | Yes | 24 | No | Yes | Yes | Yes |
The primary aim of this study is to identify among obese adults over a 6-month period which components or component levels contribute meaningfully to improvement in (a) average weight loss, and (b) percent achieving >7% weight loss. The five components that will be examined are: (1) coaching intensity (12 vs. 24 telephone sessions), (2) text messaging (No vs. Yes), (3) progress report given to participant’s primary care provider (No vs. Yes), (4) recommendation to use meal replacements (No vs. Yes), and (5) training participants’ self-selected buddies to be supportive of weight loss (No vs. Yes).
The secondary aim is to apply the results obtained in Primary Aim 1 to build (A) an intervention made up of only active components and (B) if necessary, a second intervention that is optimized for scalability. Intervention (A) will be made up of the best set of components and component levels, based on the results of the experiment conducted in Primary Aim 1. If Intervention (A) exceeds an estimated cost of $500, we will identify the combination of components and component levels corresponding to the largest treatment effect that can be obtained for implementation costs of no more than $500. (Centers for Medicare and Medicaid covers 20 sessions of face-to-face 15 minute in-person behavioral counseling at $26 per session, totaling $520).
Eligibility
This study will enroll 560 adults between 18 and 60 years old with body mass index (BMI) between 30–39.9 kg/m2. Participants must be weight stable (no loss or gain >25 lbs for the past 6 months), not enrolled in any formal weight loss program or taking anti-obesity medications, but interested in losing weight. Candidates must own an Android or iPhone smartphone and be willing to install the study application on their phone. Participants will be excluded if they have an unstable medical condition (not under treatment by a medical professional), are currently pregnant or planning to become pregnant in the next 6 months, or have any contraindications to exercise. Participants must also obtain physician’s approval to participate (establishing that a physician is agreeable to receiving study reports) and find a “buddy” who is willing to participate. Table 3 lists the full inclusion/exclusion criteria. “Buddy” participants must be at least 18 years old, have access to a computer and internet and be willing to participate in 4 “Buddy Training“ webinars and provide weight loss support and encouragement to the participant. Prior to participating in any study procedure, all potential participants and buddies must voluntarily provide informed consent. All study procedures have been approved by the Northwestern University Institutional Review Board.
Table 3.
Opt-IN inclusion and exclusion criteria
| Inclusion Criteria: |
| BMI between 30.0–39.9 kg/m2 |
| 18–60 years of age |
| Interested in losing weight through diet and physical activity changes |
| Weight stable over the past 6 months (no loss or gain >25 lbs) |
| Not receiving concurrent weight loss treatment |
| Willing to self-monitor on the Opt-IN smartphone application |
| Able to obtain primary care physician approval to participate in the study |
| Have a Buddy who is willing to be trained and support participant throughout the study |
| Owns Android or iPhone smartphone |
| Exclusion Criteria: |
| Has one of the following unstable medical conditions: uncontrolled hypertension or diabetes, angina pectoris, myocardial infarction, plantar fasciitis, transient ischemic attack, cancer undergoing active treatment, uncontrolled hypothyroidism, or cerebrovascular accident within the past 6 months |
| Has diabetes requiring insulin treatment, Crohn’s Disease, obstructive sleep apnea requiring use of CPAP |
| Requires use of an assistive device for mobility |
| Has been hospitalized for a psychiatric disorder in the past 5 years |
| Contraindications for moderate intensity physical activity |
| Currently taking any weight-loss medication, herbal remedy for weight loss, or following an incompatible dietary regimen |
| Currently pregnant, trying to get pregnant, or lactating |
| Current bulimia nervosa or binge eating disorder |
| Current substance or alcohol dependence |
| Current major depressive disorder |
| Currently taking medication(s) known to cause weight gain |
| Expresses active suicidal ideation |
| Unwilling to participate in one or more study conditions |
Recruitment
Participants will be recruited from multiple channels including flyers, online postings, and advertisements in local newspapers and on local public transit buses and trains. Advertisements will briefly list the study description and eligibility criteria and will direct interested participants to the study website. Previous studies with similar recruitment strategies in our urban location have yielded a diverse sample including many minority participants [27].
Online Screening
At the study website, interested candidates may consent to research participation and complete an online screening questionnaire that assesses study entry criteria including height, weight, unstable medical conditions, motivational readiness to change, and exercise contraindications. Within 2–3 days of completing the online screener, eligible candidates will be contacted by staff to undergo telephone screening.
Telephone Screening
Study staff will review study details, confirm candidates’ intent to participate in the study, and obtain verbal assent for telephone screening. Staff will screen for additional eligibility criteria (e.g. BMI, medication use, smartphone ownership). Interested and eligible participants will be scheduled for an in-person orientation session.
Orientation Session
Study staff will invite potential participants to an orientation session that lasts approximately 1–1.5 hours. After thoroughly describing the study, staff will conduct an equipoise induction that encourages candidates to discuss the pros and cons of participating in all possible study conditions, so as to prevent attrition after randomization [28]. After the induction, study staff will answer all questions and interested participants will sign an informed consent form. Participants will be scheduled for a baseline assessment and will be required to obtain approval from their primary care physician. Candidates without a primary care physician or whose physician declines approval to participate will be ineligible to continue. Candidates will be emailed a link to complete baseline questionnaires online as well as a link to direct a potential Buddy to the online screening. Study candidates will then complete a baseline run-in period during which they self-monitor food intake and physical activity for a 7–10 day period.
Baseline Screening and Assessment
After the run-in period, participants will attend a 45 minute in-person baseline session at which they submit all required forms (food and activity self-monitoring diaries and medical approval forms). Staff will collect anthropometric data and conduct brief interviews using the PRIME-MD, PHQ-9 [29], and a modified version of the Mini-International Neuropsychiatric Interview [30] to screen out individuals who meet criteria for bulimia nervosa, current substance abuse or dependence (besides nicotine dependence), major depressive disorder, or active suicidal ideation. Eligible participants will have their personal smartphone assessed for compatibility with the Opt-IN application. Candidates whose phone is not compatible will not be eligible to continue on in the study.
Potential buddies for all candidates will complete an online survey that assesses eligibility. If eligible, study staff will contact potential buddies and obtain verbal consent, which will be necessary before the candidate can be considered for randomization. If a buddy is not eligible, the candidate will be asked to find a new buddy.
Randomization
Eligible participants will return for an in-person randomization session. After staff reassess willingness to participate in any condition, interested candidates will be randomized to one of the 16 intervention conditions. Randomization, stratified by gender, will be computer-generated using the method of randomly permuted blocks. In addition to discussing assigned intervention components, study staff will assign a 7% weight loss goal and calorie and fat gram goals based on initial weight data. Progressive physical activity goals will increase from 100 to 300 minutes of moderate intensity physical activity per week over the 6 month study. Staff will help download the Opt-IN smartphone application onto each participant’s smartphone and will conduct training on the application and portion size estimation. Participants in all conditions will be asked to use the application to record their dietary intake, weight, and physical activity daily throughout the 6-month study.
Core Intervention
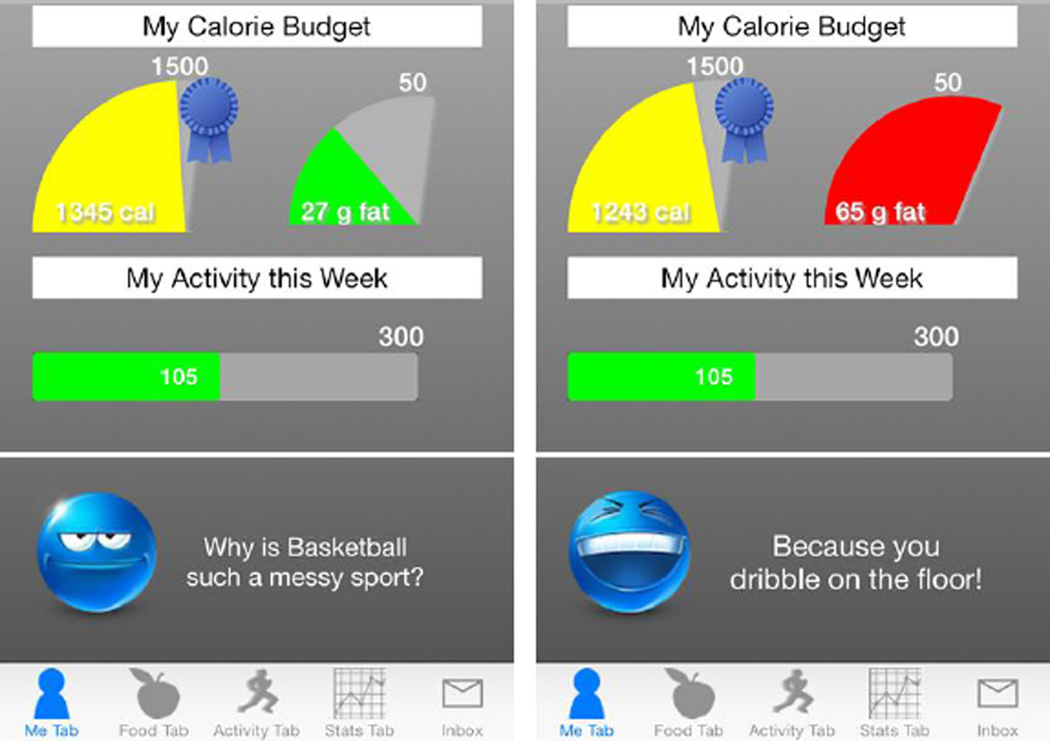
The core intervention, delivered to all participants via the smartphone application and online lessons, builds fundamental competencies needed for weight loss. Lessons available on a secure study website provide knowledge about energy balance and address topics such as portion size estimation, becoming active, and stimulus control. The Opt-IN smartphone application, shown in Figure 2, prompts participants to set goals and provides feedback in response to self-monitoring of dietary intake, physical activity, and body weight. The Opt-IN application is a modified version of our ENGAGED smartphone application [31] that was developed based on Carver and Scheier’s control systems theory [32]. The application was created based on formative work and user experience studies with adults wanting to lose weight.
Figure 2.
Opt-IN Smartphone Application
Intervention Components
Telephone Calls
Participants will receive either 12 (biweekly) or 24 (weekly) coaching calls over 6 months. Each call, lasting approximately 15 minutes, will briefly review a topic that corresponds with the online lesson and the self-monitoring data wirelessly transmitted from the participant’s Opt-IN smartphone application. Participants and coaches will work together to set goals and problem solve about barriers to self-monitoring adherence and goal attainment. Coaches will also inquire about condition-specific components on each call (e.g., discuss buddy support with participants randomized to that condition).
Meal Replacement Recommendation
Participants assigned to receive this condition will be encouraged to replace 2 meals and 2 snacks per day with meal replacement shakes and bars during the 6 month intervention. At the randomization session, participants will receive a one-week supply of 2 meal replacement shakes and 2 snack bars per day. During coaching calls, coaches will continue to recommend the use of meal replacements and will discuss ways to incorporate them into the participants’ daily routine. Meal replacement use will be monitored from daily self-monitoring records on the Opt-IN smartphone application.
Text Messages
Participants randomized to receive text messaging will receive a minimum of 7 text messages per week on a schedule of their own choosing (e.g., 1 text per day over 7 days, 2 texts per day every other day). Texts will target the provision of social support, supportive accountability, and facilitation, as outlined in the study’s conceptual model (Figure 1). Examples of text messages are included in Table 4. Participants may also opt to receive 2 additional texts each week that provide general weight loss information.
Table 4.
Text Message Examples
| Construct | Example |
|---|---|
| Social Support | You've done so well this week with physical activity -awesome job meeting the goal! |
| Supportive Accountability | When recording slips, sometimes other behaviors do too. Remember to log your foods daily to help keep you on track. Start with logging your last meal from today! |
| Facilitation | Adding in vegetables is a good way to stretch a meal and reduce calories per serving. |
| General Weight Loss Information | Fiber satiates us, reduces blood cholesterol, and lowers risk for type 2 diabetes. Whole grains, beans, and produce are all great fiber sources! |
Primary Care Physician Reports
For participants randomized to have reports mailed to their PCP, a personalized letter will be mailed to the physician at the 3- and 6-month assessment points. These reports will remind the physician of their patient’s participation in the study, give a brief overview of the research, and supply a graph depicting the participant’s weight loss from baseline to the respective time point. All PCP report letters also provide tailored behavioral recommendations to aid the physician in encouraging further weight loss (or maintenance) by the participant. Participants also receive a copy of each letter sent to the PCP.
Buddy Training
Buddies assigned to receive training will participate in an initial 30 minute telephone coaching session about how to support the participant in weight loss, and will be scheduled to attend 4 webinars over the course of the study. Each webinar lasts approximately 30–45 minutes and provides additional tips about weight loss, as well as peer discussion of challenges and successes in providing buddies with weight loss support. Buddies will receive a $5 online gift card for attending each webinar. Those Buddies who attend at least 3 of the 4 required webinars will receive an additional $20 online gift card.
Treatment Fidelity
Telephone sessions will be audiotaped and a 15% sample rated for treatment fidelity on a quarterly basis. If fidelity falls below 90%, all coaches will be retrained. Fidelity checklists specify: a) intended session content (e.g.,goal attainment, condition-specific components); b) inappropriate session content (e.g., recommending meal replacement to a participant not assigned to that condition), and c) whether inappropriate session content was initiated by the coach or participant (e.g., if a participant mentioned their Buddy’s positive support when not assigned to the Buddy training condition). Fidelity checklists are specified to the participant’s condition. For example, a coach performing a fidelity check on a Condition 2 call would use a fidelity checklist that mentioned PCP reports, Meal Replacement, and Buddy Training.
Outcome Assessment
Anthropometrics will be assessed at baseline, 3, and 6 months by a staff member who is blinded to randomization. Participants will receive $20 for completing each of the 3 and 6 month assessments.
Height, weight, BMI
Height and weight will be measured with the participant wearing a light-weight gown and no shoes. Height will be measured using a wall-mounted stadiometer to the nearest 0.25 in. Body weight will be measured to the nearest 0.25 lb using a calibrated balance beam scale. BMI will be calculated as weight in pounds/(height in inches)2) × 704.5.
Mediators
Several potential mediators of treatment outcome will be assessed. Treatment adherence will be operationalized as the number of telephone coaching sessions completed, divided by the number of treatment sessions offered (12 or 24). Make-up sessions completed within a week of the target date will count as attended. Self-monitoring adherence will be operationalized as the number of days of recording: a) weight; b) dietary intake (totaling at least 1000 kcal); c. physical activity operationalized as the number of days of recording physical activity.
In addition to adherence, several exploratory mediators will be assessed at baseline, 3 and 6 months. Self-efficacy will be evaluated by examining confidence about changing two categories of behaviors associated with weight loss: diet [33] and physical activity [34]. The Scenario-Based Dieting Self-Efficacy Scale [33] is an 11-item survey that has been found to be a reliable and valid measure of dieting self-efficacy. The Physical Activity Self-Efficacy scale [34] includes 18 items where subjects rate their confidence from 1 (not at all confident) to 5 (completely confident), in their ability to participate in physical activity in several situations. A total self-efficacy score will be calculated with a higher score indicating higher levels of exercise self-efficacy. Self-Regulation will be assessed by the restraint (21 items) and disinhibition (16 items) subscales of the Three Factor Eating Questionnaire (TFEQ) [35]. Responses are summed, with higher scores denoting higher levels of restrained eating or disinhibited eating. Autonomous versus Controlled Motivation for Self-Regulation will be assessed using both subscales of the 15-item Treatment Self-Regulation Questionnaire (TSRQ) [36]. The TSRQ has been found to be a valid assessment tool across various settings and health behaviors [37]. Supportive accountability will be measured by assessing its two defining constructs: therapeutic alliance [38] and perceived autonomy support [39]. The Perceived Autonomy Support Scale for Exercise Settings (PASSES) [39] is a 12-item survey where participants indicate on a 7-point scale ranging from 1 (strongly disagree) to 7 (strongly agree) how much they agree with statements describing how well an individual supports exercise changes. Facilitation will be measured by having participants rate on a 5-point Likert scale (1 = not at all; 5 = very much) how much the tools provided by the study have changed their environment to make it easier to: “eat healthier,” “be more physically active,” and “lose weight.” We will also assess facilitation via the 26-item Weight Management Support Inventory (WMSI) [40] which is a valid measure that assesses the degree to which the participant’s social environment discourages excess eating and encourages exercise. Participants rate on a 5-point scale (ranging from 1 [never] to 5 [daily]) how frequently specific interactions with other people have occurred over the past 4 weeks as well as how helpful each of the event was when it occurred, using a 5-point scale ranging from 1 (not helpful) to 5 (extremely helpful).
Statistical Analysis
The effects of the five individual intervention components will be examined by means of a factorial experiment involving the following factors: 1) Number of telephone coaching calls (12 vs. 24); 2) PCP receives reports (no vs. yes); 3) Text messaging (not provided vs. provided); 4) Meal replacements (not recommended vs. recommended); 5) Buddy training (not provided vs. provided). The primary aim is to test on an intent-to-treat basis using linear mixed models whether each factor has a significant effect on weight change across the time points (baseline, 3- & 6-months). For each of the five components, we will determine whether there is a difference in change across time using baseline as the reference cell (i.e., 3-months vs. baseline and 6-months vs. baseline). From one perspective this is a main effect of each component on the pre-post difference. However, statistically these effects will be modeled as component by time interactions, with the 6-month outcome as the primary endpoint. We will also include two-way interactions between components (e.g., Factor 1 by Factor 2 by time interaction).
The secondary primary aim is to make decisions about intervention components and component levels based on the factorial experiment results. We will use a modified version of a decision making approach frequently used in engineering [41], which emphasizes main effects, using interactions as additional valuable information. This emphasis is consistent with our objective of identifying a set of components and component levels in which each component is making a detectable contribution to the overall effect, and any inactive components have been eliminated.
We will test the mediation effects for significance using the general approach described in MacKinnon [42]. We will be able to fit models specifying which intervention component is mediated by each putative mediator, where the putative mediators are: (a) adherence (treatment attendance; diet, activity, and weight self-monitoring adherence), (b) supportive accountability (therapeutic alliance, autonomy support), (c) self-efficacy (diet, activity), (d) self-regulation (restraint, disinhibition, autonomous motivation), and (e) facilitation.
Sample Size and Power
Power for the study is based on the weight change from baseline to 6 months. Specifically, the study seeks to identify the intervention components that make a more than minimal contribution to average weight loss, or conversely to identify those components that have minimal or no effect on weight loss. Our operational definition of more than minimal is a component effect size of .25; thus, we have powered the study to detect effects of .25 or greater. Using the standard deviation estimate of 4 kg from Amundson et al. [43], this translates to a 1 kg difference in weight loss as a minimal effect. Based on our previous studies [27, 44], we assume an attrition rate of 10% at the 6 month time point. Therefore, we will recruit 560 subjects. Based on these assumptions, we can detect a main effect or interaction effect size of .25 with 80% power under a two-tailed hypothesis test with a sample size of 504.
DISCUSSION
Worldwide, there are over 500 million adults classified as obese according to the World Health Organization [45], but effective intensive lifestyle interventions are costly and burdensome to maintain long-term [3]. Typical interventions provide a bundled treatment package; however this may add unnecessary cost if inactive components are provided or if more than what is necessary is delivered. Alternative methodological frameworks which provide a quicker, more efficient way to simultaneously test the effectiveness of multiple intervention components should be considered. MOST [10] has received increasing attention as a framework for behavioral interventions for health outcomes and behaviors including smoking cessation [46]. MOST has not yet been applied to weight loss interventions.
The current trial is significant in representing the first principled and systematic effort to design an effective and efficient weight loss treatment, such that all of its components (coaching intensity, text messages, meal replacement recommendations, PCP reports, and buddy training) are active, feasible for real world implementation, and make modest and realistic resource demands. The Opt-IN trial also utilizes a fractional factorial experimental design to conserve resources. Although fractional factorial designs enable the examination of several intervention components in a factorial experiment without requiring the full amount of experimental conditions, they do have limitations and are associated with tradeoffs that should be weighed carefully whenever a fractional factorial design is considered [12].
As part of the core intervention, Opt-IN will utilize the smartphone technology that participants already own. Recent estimates suggest that 56% of the total adult population currently owns a smartphone [47]. As usage of smartphones continues to rise, the use of technology will increasingly represent a promising strategy to reduce the cost and burden of weight loss treatment. Although numerous diet and physical activity self-monitoring applications are available commercially, few if any have demonstrated evidence of efficacy for weight loss. In contrast, the Opt-IN smartphone application is modeled after our prior +Mobile application, developed for personal digital assistant, that was shown in a randomized clinical trial to produce significant weight loss sustained for one year [44]. Based on Carver and Scheier’s control systems theory of self-regulation [32], these applications provide decision support for making diet and activity choices, as well as timely feedback that reinforces adherence to self-monitoring - the best established predictor of weight loss success [48]. Having the Opt-IN application continually present on the smartphone and bolstered by texts creates a facilitating environment to keep weight regulation top of mind. By remotely connecting participant and coach, the technology creates a system of supportive accountability that encourages behavior change in a format less costly and more scalable than usual intensive lifestyle intervention for obesity.
The Opt-IN trial is the first study that will use the MOST framework to design a highly effective, efficient, and economical weight loss treatment. Once this study identifies the intervention components and component levels that contribute to weight loss, the Opt-IN intervention will be built to include only active components. More scalable and with greater reach than traditional intensive weight loss interventions, Opt-IN will support significant progress in the fight against obesity.
Acknowledgements
This publication was made possible by Grant Number DK097364 from NIDDK awarded to Drs. Spring and Collins and by NIDPA P50 DA10075 to Dr. Collins. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Fractional factorial designs are chosen strategically to provide a balance of economy and scientific yield. In every fractional factorial design some effect estimates are combined, or aliased. In the design selected for Opt-In, each main effect is aliased with one 4-way interaction, and each 2-way interaction is aliased with one 3-way interaction. The logic behind the choice of this design is that because our model (see Figure 1) does not hypothesize any sizeable 3-way or 4-way interactions, these effects will be considered negligible in size. This suggests that, for example, each combined main effect + 4-way interaction estimate can be attributed primarily to the main effect. For more about managing research resources in selection of experimental designs, see Collins et al. [11].
REFERENCES
- 1.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pi-Sunyer X, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143(4):251–264. doi: 10.7326/0003-4819-143-4-200508160-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hernan WH, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care. 2003;26(1):36–47. doi: 10.2337/diacare.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann RT, et al. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33(1):69, 74–75, 77–78. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 7.Mau MK, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI 'Ohana Pilot project. Prog Community Health Partnersh. 2010;4(1):7–16. doi: 10.1353/cpr.0.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whittemore R, et al. Translating the diabetes prevention program to primary care: a pilot study. Nurs Res. 2009;58(1):2–12. doi: 10.1097/NNR.0b013e31818fcef3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis-Smith YM, et al. Implementing a diabetes prevention program in a rural African- American church. J Natl Med Assoc. 2007;99(4):440–446. [PMC free article] [PubMed] [Google Scholar]
- 10.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5 Suppl):S112–S118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14(3):202–224. doi: 10.1037/a0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins LM, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41(2):208–226. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 14.Wadden TA, et al. Randomized Trial of Lifestyle Modification and Pharmacotherapy for Obesity. New England Journal of Medicine. 2005;353(20):2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 15.Wing RR. Behavioral weight control. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York, NY: Guilford Press; 2002. pp. 301–316. [Google Scholar]
- 16.Appel LJ, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin PD, et al. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity (Silver Spring) 2008;16(11):2462–2467. doi: 10.1038/oby.2008.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24(9):1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract. 2001;50(6):513–518. [PubMed] [Google Scholar]
- 20.Free C, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011;378(9785):49–55. doi: 10.1016/S0140-6736(11)60701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrick K, et al. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res. 2009;11(1):e1. doi: 10.2196/jmir.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heymsfield SB, et al. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27(5):537–549. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 23.Noakes M, et al. Meal replacements are as effective as structured weight-loss diets for treating obesity in adults with features of metabolic syndrome. J Nutr. 2004;134(8):1894–1899. doi: 10.1093/jn/134.8.1894. [DOI] [PubMed] [Google Scholar]
- 24.Wadden TA, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol. 1999;67(1):132–138. doi: 10.1037//0022-006x.67.1.132. [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Hamada M. Experiments, Planning, Analysis, and Optimization. 2nd Edition. Wiley; 2009. [Google Scholar]
- 27.Spring B, et al. Multiple Behavior Changes in Diet and Activity: A Randomized Controlled Trial Using Mobile Technology Behavior Changes in Diet and Activity. Arch Intern Med. 2012;172(10):789–796. doi: 10.1001/archinternmed.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg JH, Kiernan M. Innovative techniques to address retention in a behavioral weight-loss trial. Health Educ Res. 2005;20(4):439–447. doi: 10.1093/her/cyg139. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 31.Pellegrini CA, et al. A smartphone-supported weight loss program: design of the ENGAGED randomized controlled trial. BMC Public Health. 2012;12(1):1041. doi: 10.1186/1471-2458-12-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carver C, Scheier M. Attention and Self-Regulation: A Control Theory Approach to Human Behavior. New York: Springer-Verlag; 1981. [Google Scholar]
- 33.Stich C, Knauper B, Tint A. A scenario-based dieting self-efficacy scale: the DIET-SE. Assessment. 2009;16(1):16–30. doi: 10.1177/1073191108322000. [DOI] [PubMed] [Google Scholar]
- 34.Marcus BH, et al. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63(1):60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 35.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 36.Williams GC, et al. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- 37.Levesque CS, et al. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ-across three different health behaviors. Health Education Research. 2007;22(5):691–702. doi: 10.1093/her/cyl148. [DOI] [PubMed] [Google Scholar]
- 38.Hatcher RL, Barends AW. Patients' view of the alliance of psychotherapy: exploratory factor analysis of three alliance measures. J Consult Clin Psychol. 1996;64(6):1326–1336. doi: 10.1037//0022-006x.64.6.1326. [DOI] [PubMed] [Google Scholar]
- 39.Hagger MS, et al. The perceived autonomy support scale for exercise settings (PASSES): Development, validity, and cross-cultural variance in young people. Psychology of Sport and Exercise. 2007;8:632–653. [Google Scholar]
- 40.Rieder S, Ruderman A. The development and validation of the weight management support inventory. Eat Behav. 2007;8(1):39–47. doi: 10.1016/j.eatbeh.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Wu C, Hamada M. Experiments: Planning, Analysis, and Parameter Design Optimization. Wiley; 2000. [Google Scholar]
- 42.MacKinnon DP, Luecken LJ. How and for whom? Mediation and moderation in health psychology. Health Psychol. 2008;27(2 Suppl):S99–S100. doi: 10.1037/0278-6133.27.2(Suppl.).S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amundson HA, et al. Translating the diabetes prevention program into practice in the general community: findings from the Montana Cardiovascular Disease and Diabetes Prevention Program. Diabetes Educ. 2009;35(2):209–210. 213–214, 216–220. doi: 10.1177/0145721709333269. passim. [DOI] [PubMed] [Google Scholar]
- 44.Spring B, et al. Integrating Technology Into Standard Weight Loss Treatment: A Randomized Controlled Trial. Arch Intern Med. 2012:1–7. doi: 10.1001/jamainternmed.2013.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. Obesity and Overweight Fact Sheet. [cited 2013 September 5];2013 Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 46.Strecher VJ, et al. Web-based smoking-cessation programs: results of a randomized trial. Am J Prev Med. 2008;34(5):373–381. doi: 10.1016/j.amepre.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith A. Pew Internet & American Life Project: Mobile Technology Fact Sheet. [cited 2014 March 26];2014 Available from: http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/. [Google Scholar]
- 48.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatr Clin North Am. 2005;28(1):151–170. ix. doi: 10.1016/j.psc.2004.09.008. [DOI] [PubMed] [Google Scholar]