Abstract
The identification of oncogenic driver mutations in non-small cell lung cancer (NSCLC) has led to a paradigm shift and the development of specific molecular treatments. Tumors harboring a rearranged EML4–ALK fusion oncogene are highly sensitive to therapy with ALK-targeted inhibitors. Crizotinib is the first approved treatment for advanced lung tumors containing this genetic abnormality. In this mini review, we discuss the existing data on crizotinib as well as ongoing trials involving this medication. A brief overview of the known resistance mechanisms to crizotinib will also be presented followed by a summary of the ongoing trials involving next-generation ALK-inhibitors or other targeted therapies in patients with ALK+ NSCLC.
Keywords: non-small cell lung cancer, driver mutations, anaplastic lymphoma kinase, crizotinib, ALK-inhibitors, clinical trials as topic
Introduction
Treatment for non-small cell lung cancer (NSCLC) has historically consisted of cytotoxic chemotherapy. Recent advances in molecular biology had led to the discovery of oncogenic driver mutations with subsequent development of oral agents that target these molecular pathways. In NSCLC, the main two driver mutations, with FDA approved targeted therapies, consist of echinoderm microtubule protein like-4/anaplastic lymphoma kinase (EML-4/ALK) translocations and epidermal growth factor receptor (EGFR) mutations. Crizotinib is the first FDA approved treatment for patients with ALK+ NSCLC. To this date, only the final data from one phase III randomized trial has been published, evaluating the use of crizotinib as a second-line therapy (1). Multiple phase III randomized trials are in progress to assess the efficacy of crizotinib as first-line chemotherapy. Eventually, most patients on treatment with crizotinib develop resistance to this drug within 1 year of treatment. Most clinical trials in progress in the ALK+ patient population involve “new-generation ALK-inhibitors,” or crizotinib in combination with novel drugs to bypass known resistance mechanisms.
The scope of this review article is twofold. First, the existing data on crizotinib will be presented as well ongoing trials involving this medication. Second, a brief overview of the known resistance mechanisms to crizotinib will be presented followed by a summary of the ongoing trials involving newer generation ALK-inhibitors or other targeted therapies in patients with ALK+ NSCLC.
ALK Fusion Gene and Its Target Crizotinib
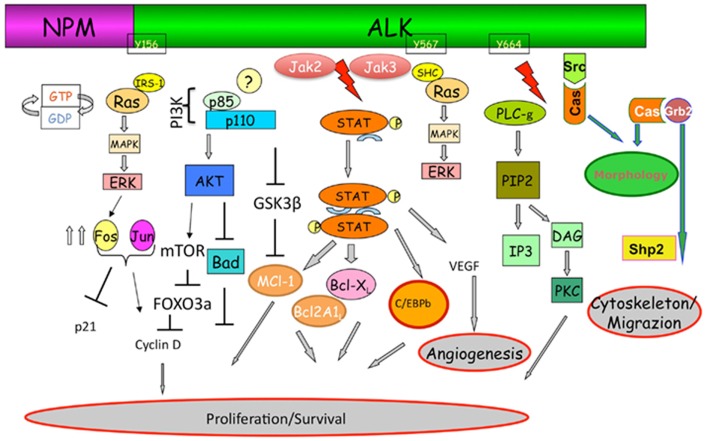
Crizotinib is an orally active inhibitor of multiple tyrosine kinase inhibitors (TKIs), including ALK, c-Met, hepatocyte growth factor receptor (HGFR), and c-ros oncogene 1 (ROS1) (Figure 1) (2). In 2007, the ALK gene rearrangement in which the 5′ end of EML4 gene is fused to the 3′ end of ALK was first identified by Soda et al. in NSCLC cell lines (3). The fusion protein resulting from this translocation has constitutive kinase activity, leading to downstream activation of multiple diverse signaling cascades involved in cell proliferation and carcinogenesis. Currently, multiple EML4–ALK fusion combinations have been identified (4). All these fusion proteins have a similar ALK kinase domain, but differ in the EML4 breakpoint. Pre-clinical data from in vitro studies suggested different crizotinib sensitivity for each variant of the EML4–ALK fusion protein (5). However, a subgroup analysis from the phase I trial of crizotinib failed to demonstrate such correlation between variant fusion proteins and clinical response to therapy (6). In addition, fusions of ALK with other partners including TRK-fused gene TFG and KIF5B have also been described in lung cancer patients, but appear to be much less common than EML4-ALK (7).
Figure 1.
The ALK signaling pathway with its cross-talk with other pathways involved in the resistance to ALK-inhibitors. Adapted from Tabbo et al. (47).
Clinical Trials Involving Crizotinib
In 2013, Shaw et al. published the first phase III randomized trial involving crizotinib in the second-line setting (1). Patients with locally advanced or metastatic ALK+ NSCLC were randomly assigned to receive oral treatment with crizotinib (250 mg) twice daily or intravenous chemotherapy with either pemetrexed or docetaxel. The median progression-free survival was 7.7 months in the crizotinib group and 3.0 months in the chemotherapy group. An interim analysis of overall survival showed no significant improvement with crizotinib as compared with chemotherapy. This analysis was nevertheless immature with a total of 96 deaths (40% of the required events) and censoring of over 70% of patients in either treatment arm. In addition, the analysis was likely confounded by the high crossover rate of patients in the chemotherapy group, with nearly 90% of patients on the chemotherapy arm crossing over to the other arm upon disease progression. The response rates were 65% with crizotinib, as compared with 20% with chemotherapy. Common adverse events associated with crizotinib were visual disorder, gastrointestinal side effects, and elevated liver aminotransferase levels. Given the positive response rates with crizotinib, multiple phase III trials are currently in progress to address the efficacy of crizotinib as first-line therapy. Details of these trials, including patient population and their respective primary endpoints, are summarized in Table S1 in Supplementary Material.
One challenging clinical problem remains the treatment of ALK+ NSCLC patients with brain metastasis. These patients suffer from an adverse impact on quality of life and survival. Although it has been shown that crizotinib is effective for brain metastasis, it is penetration into cerebrospinal fluid (CSF) has been demonstrated to be very poor (8). Costa et al. measured the CSF-to-plasma ratio of crizotinib being only at 0.0026 (9). Past experience with erlotinib and gefitinib in patients with EGFR-mutated lung cancer has uncovered similar challenges: despite good systemic control of disease, a subset of patients would progress in the CNS, without any new acquired resistance mechanism, owing to the poor penetration of these TKIs in the CSF. Although pulse EGFR–TKIs doses have been used in this setting, there is limited data to support its use with crizotinib (10). Newer generation of ALK-inhibitors with better CSF penetration are currently under study.
Resistance Mechanisms of Crizotinib
In order to understand the rationale behind the majority of ongoing clinical trials involving ALK+ NSCLC patients, it is important to survey the currently known mechanism of resistance to crizotinib. ALK-dependant resistance mechanism occurs upon mutations in the tyrosine kinase (TK) domain, and activation of alternative signaling pathways. Alternatively, true ALK-independent resistance may arise through the outgrowth of clones that do not harbor an ALK gene fusion and contain a separate activated oncogene (11). Given that multiple resistance mechanisms are occasionally found within the same biopsy specimen, as well as different resistance mechanisms may be found in separate tumor deposits within the same patient, it is important to consider re-biopsy of the tumor upon progression on treatment, whenever technically feasible, to correctly identify the resistance mechanism accounting for progression of disease (12).
Mutations in target tyrosine kinases
Past experience with the use of TKIs in chronic myelogenous leukemia as well as EGFR-mutated lung cancer teaches us that most common mechanisms of resistance to this class of medications are secondary mutations in the TK domains (13). This also holds true for crizotinib, given that mutations in the TK domains of the different targets of crizotinib is currently the best studied and most prevalent form of resistance to this drug, accounting for up to 25% of all cases resistant to ALK therapy (14).
The first major “gatekeeper” mutation identified in the TK domain of EML4–ALK involves the substitution of leucine for a methionine at position 1196 (L1196M) of the kinase domain of ALK, thus creating a mutant bulky amino-acid side chain in the ATP-binding pocket of the receptor, ultimately interfering with the binding of crizotinib to its receptor (15). This is analogous to the EGFR T790M mutation in the ATP biding pocket of the EGFR, which is the most common mechanism of resistance in EGFR-mutated lung cancer (12).
Tissue analysis of harvested tumor cells from patients resistant to crizotinib has demonstrated other non-gatekeeper secondary mutations in the ALK TK receptor. ALK secondary mutations in NSCLC are distributed throughout the kinase domain, including the solvent front (G1202R, S1206Y), ATP-binding pocket (G1269A), and N-terminal to the C-helix (1151Tins, F1174L, L1152R, and C1156Y) (11, 15–20). The prevalence and clinical significance of these secondary mutations remains to be elucidated. Of interest, a separate secondary ALK mutation, F1174L, has also been identified in inflammatory myofibroblastic tumors (21).
Crizotinib is an inhibitor of multiple TKIs beside ALK, including ROS1 oncogene. A recent report of a G2032R mutation in the ROS1 TK domain leading to crizotinib resistance has also been identified (22). Although this mutation does not lie at the gatekeeper position, it confers resistance to ROS1 kinase inhibition through steric interference with drug binding.
Activation of alternative pathways
Activation of alternative downstream signaling pathways, even in the setting of complete ALK receptor inhibition, is increasingly recognized as mechanisms of resistance to crizotinib. These include activation of the EGFR, heat shock protein 90 (HSP90), and the PI3K/AKT/mTOR pathways. The activation of these alternative pathways is present in up to 20% of patients (23).
Recent data from cell line experiments showed the activation of EGFR to be associated with ALK resistance (18). This was further corroborated with clinical analysis of tumor cells biopsied from patients resistant to ALK therapy (24). In most studies, the activation of EGFR occurred through increased phosphorylation and upregulation of EGFR ligands, such as amphiregulin, rather than being caused by mutations in the EGFR gene itself (18, 25). Although ALK mutations are usually mutually exclusive to other driver mutations such as EGFR, there have been reports of de novo mutation of the EGFR gene in patients and cell lines treated with crizotinib, accounting for resistance to this drug (11, 24, 26, 27).
The PI3K/AKT/mTOR pathway is an intracellular signaling pathway important in cell cycle regulation and apoptosis also implicated in resistance to ALK-targeted therapy. Recent ALK-resistant cell line analysis revealed that the activation of the mTOR pathways was associated with increased autophagy of the ALK receptor thus leading to decreased response to crizotinib treatment (28). The exact mechanism by which the ALK receptor induces activation of the mTOR pathway remains to be elucidated, but inhibition of AKT by phosphorylation seems to play a key factor. One study showed synergistic in vitro growth inhibitory activity of ALK inhibitor when combined with an mTOR inhibitor (29). The clinical significance of mTOR activation in NSCLC patients remains to be elucidated.
HSP90 is a highly abundant and ubiquitous molecular chaperone, which plays an essential role in many cellular processes including cell cycle control, hormone signaling, as well as protein folding, and degradation. The ALK receptor is one of the many client proteins of HSP90. HSP90 inhibition induced loss of EML4-ALK expression and depletion of multiple oncogenic signaling proteins in ALK-driven NSCLC cell lines (30). These results were further corroborated in murine models of NSCLC as well as anecdotal case reports of tissues derived from ALK therapy resistant patients (31, 32).
Ongoing Clinical Trials Involving New-Generation ALK-Inhibitors and Combination Therapy
Most ongoing trials in ALK+ NSCLC patients involve newer generation ALK-inhibitors or combination therapy targeting currently known resistance mechanism to crizotinib. These include agents with activity against NSCLC with the L1196M gatekeeper mutation or the ROS1 mutation, as well as combination therapy targeting the EGFR and HSP90 proteins/pathways. A summary of these trials are presented in Table 1. Current evidence for some of these new-generation ALK-inhibitors are presented here.
Table 1.
Ongoing clinical trials involving novel ALK- and HSP90-inhibitors in NSCLC.
|
ONGOING CLINICAL TRIALS INVOLVING NOVEL ALK-INHIBITORS | ||||||
|---|---|---|---|---|---|---|
| Drug | Company | Activity against | Activity against | Ongoing trials | Study phase | Previous treatment |
| other kinases | L1196M mutation | |||||
| LDK378 (ceritinib) | Novartis | IFG-1R | Yes | NCT01772797 | Phase I | None |
| c-MET | NCT02040870 | Phase I/II | Crizotinib/chemotherapy | |||
| NCT01685138 | Phase II | 0-3 lines of chemotherapy | ||||
| NCT01685060 | Phase II | Crizotinib or 1-3 lines of chemotherapy | ||||
| NCT01947608 | Phase II | Crizotinib | ||||
| NCT01964157 | Phase II | 1 line of chemotherapy | ||||
| NCT01828099 | Phase III | None | ||||
| NCT01828112 | Phase III | Crizotinib | ||||
| CH5424802/RO5424802 (alectinib) | Roche/Chugai | ROS1 | Yes | NCT01588028 | Phase I | |
| NCT01871805 | Phase II | Crizotinib | ||||
| NCT01801111 | Phase II | Crizotinib | ||||
| NCT02075840 | Phase III | |||||
| AP26113 | Ariad | EGFR | Unknown | NCT01449461 | Phase I/II | Refractory to standard therapy |
| ROS1 | NCT02094573 | Phase II | Crizotinib | |||
| ASP3026 | Astellas | ROS1 | Yes | NCT01401504 | Phase I | Refractory to standard therapy |
| NCT01284192 | Phase I | Refractory to standard therapy | ||||
| TSR-001 | Tesaro | Unknown | Yes | NCT02048488 | Phase I | None |
| PF-06463922 | Pfizer | EGFR | Unknown | NCT01970865 | Phase I/II | None |
| ROS1 | ||||||
| X-396 | Xcovery | Unknown | Yes | NCT01625234 | Phase I | None |
| ONGOING CLINICAL TRIALS INVOLVING HSP90 INHIBITORS | ||||||
| Drug | Company | Ongoing trials | Study phase | Combination therapy | Previous treatment | |
| AUY922 | Novartis | NCT01772797 | Phase I | LDK378 | None | |
| NCT01752400 | Phase II | Crizotinib | ||||
| NCT01124864 | Phase II | Two lines of chemotherapy | ||||
| NCT01922583 | Phase II | One line of chemotherapy | ||||
| STA-9090 | Synta | NCT01579994 | Phase I/II | Crizotinib | Standard chemotherapy | |
| NCT01562015 | Phase II | Three lines of therapy | ||||
| IPI-504 | Infinity | NCT01228435 | Phase II | Refractory to standard therapy | ||
| AT13387 | Astex | NCT01712217 | Phase I/II | Crizotinib | Crizotinib | |
| DS-2248 | Daiichi Sankyo | NCT01288430 | Phase I | Crizotinib | ||
Ceritinib (LDK378)
Ceritinib (LDK378, Novartis) is a novel and potent selective TKI targeting ALK. Results from a recent phase I/II study of this drug in both crizotinib-naïve and crizotinib-resistant patients have been published (33). The maximum tolerated dose of ceritinib was 750 mg once daily. Dose-limiting toxic events included diarrhea, vomiting, dehydration, elevated aminotransferase levels, and hypophosphatemia. Among 114 patients with NSCLC who received at least 400 mg of Ceritinib per day, the overall response rate was 58%. For 80 patients previously treated with crizotinib, the response rate was 56%. Responses were observed in patients with various resistance mutations in ALK, including L1196M, and in patients without detectable mutations. The median progression-free survival of patients receiving at least 400 mg of ceritinib was 7 months. Based on these findings, Ceritinib has recently been granted FDA approval for treatment of patients with ALK+ NSCLC in the second-line setting following failure or intolerance to crizotinib.
Multiple trials are nevertheless ongoing for this drug. These include three phase II trials of ceritinib for crizotinib-resistant patients (NCT01685060; NCT02040870) and crizotinib-naïve patients (NCT01685138). One phase II study is looking specifically at patients with ROS1 mutation (NCT01964157). Two phase III trials are comparing ceritinib with standard chemotherapy in patients previously treated with platinum-based chemotherapy (NCT01828112), and chemotherapy-naïve patients (NCT01828099). One phase I study is assessing the combination of ceritinib with a HSP90 inhibitor (NCT01772797).
Alectinib (CH5424802/RO5424802)
Alectinib (Chugai and Roche Pharmaceuticals) is a highly potent selective ALK inhibitor with activity against L1196M gatekeeper mutation as well as other secondary mutations such as F1174L and R1275Q (34, 35). Results from a recent phase I/II study with alectinib in a Japanese population have been published (36). In the phase I study, alectinib was given up to a maximum dose of 300 mg twice daily without dose-limiting toxicity. In the phase II part of the study, 40/46 (87%) of patients achieved a partial response within 6 weeks of treatment. Two patients had a complete response. Median PFS had not been reached at the time of the report. Responses were seen in brain metastases in three patients. Grade 3 adverse events including neutropenia and increase creatine phosphokinase occurred in 12 (26%) of patients.
Three phase II/III trials with alectinib are in progress in crizotinib-naïve (ALEXA trial-NCT02075840) as well as in crizotinib-resistant (NCT01871805; NCT01801111) patients.
AP26113
AP26113 (Ariad Pharmaceuticals) is a novel inhibitor of ALK with activity against L1196M gatekeeper mutation as well as against ROS1 and EGFR (including mutant form with the T790M gatekeeper mutation) (37). In an ongoing phase I/II study (NCT01449461), the established dose was at 180 mg once daily with good antitumor activity in ALK+ NSLC patients (38). Objective response was observed in 15/24 (63%) patients (1 complete response and 14 partial responses), including 12/16 (75%) in patients resistant to crizotinib. Of interest, 4/5 patients with brain metastasis had objective responses as well. The most common treatment-related adverse events were nausea (33%), fatigue (22%), and diarrhea (20%). A confirmatory phase II studies in crizotinib-resistant (NCT02094573) is currently in progress.
Other new-generation ALK agents
Multiple other new-generation ALK agents are currently under phase I study. These include TSR-001, ASP3026, PF-06463922, as well as X-396 (39–42). Details about these studies can be found in Table 1.
HSP90 Inhibitors
Pre-clinical studies involving HSP90 inhibitors in ALK+ NSCLC led to decreased ALK fusion protein levels in vitro, and led to tumor regression in in vivo models (43). Currently, multiple HSP90 inhibitors are in phase I/II clinical trials involving ALK+ NSCLC patients, either as stand-alone drugs or in combination with other ALK-inhibitors such as crizotinib. Details about ongoing studies can be found in Table 1.
Early results from phase II studies involving IPI-504 (Infinity Pharma) in three NSCLC patients resulted in two partial responses and one case of prolonged stable disease (44). In another phase II study of ganetespib (STA-9090), 4/8 patients had partial responses and three patients had stable disease (45). The most common adverse effects were diarrhea, fatigue, nausea, and anorexia. Two patients in this study died of cardiac arrest and renal failure associated with ganetespib. There has also been one case report of a crizotinib-resistant ALK+ NSCLC with an objective response to this drug (31). AUY922 (Novartis) has been studied as a single agent in both crizotinib-resistant and – naïve patients. Objective response was achieved in 6/21 (29%) of patients, 4 of which were crizotinib-naïve and remainder 2 had been previously treated with crizotinib (46).
Summary and Conclusion
The identification of the ALK fusion protein in 2007 and the fast development and approval of a FDA targeted treatment in <5 years constitutes a remarkable feat in the field of targeted therapies. Crizotinib holds many promises from its early debut in treatment-refractory NSCLC patients. Final results from phase III randomized trials using Crizotinib as a first-line therapy are eagerly awaited for. Despite excellent response in the initial stages, most patients develop resistance to crizotinib. Elucidating resistance mechanism and subsequently developing therapeutic strategies to overcome resistance to ALK-inhibitors constitutes a priority, with the vast majority of ongoing clinical trials in this field involving new-generation ALK-inhibitors or combination therapy with other targeted agents. Key questions remain on how to correctly identify the resistance mechanism of a tumor progressing on ALK-targeted therapy given re-biopsy is often technically challenging and resource intensive, as well as on how to correctly identify and stream the correct combination of therapies to the appropriate patient populations as first-line therapy. The ongoing Q-CROC-05 multicenter phase IV clinical trial (NCT02041468) will aim to shed some light on these important clinical issues.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/Journal/10.3389/fonc.2014.00174/abstract
References
- 1.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med (2013) 368(25):2385–94 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 2.Frampton JE. Crizotinib: a review of its use in the treatment of anaplastic lymphoma kinase-positive, advanced non-small cell lung cancer. Drugs (2013) 73(18):2031–51 10.1007/s40265-013-0142-z [DOI] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature (2007) 448(7153):561–6 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 4.Heuckmann JM, Holzel M, Sos ML, Heynck S, Balke-Want H, Koker M, et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin Cancer Res (2011) 17(23):7394–401 10.1158/1078-0432.CCR-11-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuckmann JM, Balke-Want H, Malchers F, Peifer M, Sos ML, Koker M, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res (2012) 18(17):4682–90 10.1158/1078-0432.CCR-11-3260 [DOI] [PubMed] [Google Scholar]
- 6.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med (2010) 363(18):1693–703 10.1056/NEJMoa1006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res (2009) 15(9):3143–9 10.1158/1078-0432.CCR-08-3248 [DOI] [PubMed] [Google Scholar]
- 8.Kaneda H, Okamoto I, Nakagawa K. Rapid response of brain metastasis to crizotinib in a patient with ALK rearrangement-positive non-small-cell lung cancer. J Thorac Oncol (2013) 8(4):e32–3 10.1097/JTO.0b013e3182843771 [DOI] [PubMed] [Google Scholar]
- 9.Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol (2011) 29(15):e443–5 10.1200/JCO.2010.34.1313 [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Ozasa H, Nagai H, Sakamori Y, Yoshida H, Yagi Y, et al. High-dose crizotinib for brain metastases refractory to standard-dose crizotinib. J Thorac Oncol (2013) 8(9):e85–6 10.1097/JTO.0b013e31829cebbb [DOI] [PubMed] [Google Scholar]
- 11.Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res (2012) 18(5):1472–82 10.1158/1078-0432.CCR-11-2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol (2013) 31(31):3987–96 10.1200/JCO.2012.45.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med (2013) 19(11):1389–400 10.1038/nm.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez CA, Velez M, Raez LE, Santos ES. Overcoming the resistance to crizotinib in patients with non-small cell lung cancer harboring EML4/ALK translocation. Lung Cancer (2014) 84(2):110–5 10.1016/j.lungcan.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med (2010) 363(18):1734–9 10.1056/NEJMoa1007478 [DOI] [PubMed] [Google Scholar]
- 16.Huang D, Kim DW, Kotsakis A, Deng S, Lira P, Ho SN, et al. Multiplexed deep sequencing analysis of ALK kinase domain identifies resistance mutations in relapsed patients following crizotinib treatment. Genomics (2013) 102(3):157–62 10.1016/j.ygeno.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Kim TM, Kim DW, Go H, Keam B, Lee SH, et al. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J Thorac Oncol (2013) 8(4):415–22 10.1097/JTO.0b013e318283dcc0 [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res (2011) 71(18):6051–60 10.1158/0008-5472.CAN-11-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Wang F, Keats J, Zhu X, Ning Y, Wardwell SD, et al. Crizotinib-resistant mutants of EML4-ALK identified through an accelerated mutagenesis screen. Chem Biol Drug Des (2011) 78(6):999–1005 10.1111/j.1747-0285.2011.01239.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovly CM, Pao W. Escaping ALK inhibition: mechanisms of and strategies to overcome resistance. Sci Transl Med (2012) 4(120):120–2 10.1126/scitranslmed.3003728 [DOI] [PubMed] [Google Scholar]
- 21.Sasaki T, Okuda K, Zheng W, Butrynski J, Capelletti M, Wang L, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res (2010) 70(24):10038–43 10.1158/0008-5472.CAN-10-2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awad MM, Katayama R, McTigue M, Liu W, Deng YL, Brooun A, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med (2013) 368(25):2395–401 10.1056/NEJMoa1215530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boland JM, Jang JS, Li J, Lee AM, Wampfler JA, Erickson-Johnson MR, et al. MET and EGFR mutations identified in ALK-rearranged pulmonary adenocarcinoma: molecular analysis of 25 ALK-positive cases. J Thorac Oncol (2013) 8(5):574–81 10.1097/JTO.0b013e318287c395 [DOI] [PubMed] [Google Scholar]
- 24.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med (2012) 4(120):120ra17. 10.1126/scitranslmed.3003316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanizaki J, Okamoto I, Okabe T, Sakai K, Tanaka K, Hayashi H, et al. Activation of HER family signaling as a mechanism of acquired resistance to ALK inhibitors in EML4-ALK-positive non-small cell lung cancer. Clin Cancer Res (2012) 18(22):6219–26 10.1158/1078-0432.CCR-12-0392 [DOI] [PubMed] [Google Scholar]
- 26.Rossing HH, Grauslund M, Urbanska EM, Melchior LC, Rask CK, Costa JC, et al. Concomitant occurrence of EGFR (epidermal growth factor receptor) and KRAS (V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) mutations in an ALK (anaplastic lymphoma kinase)-positive lung adenocarcinoma patient with acquired resistance to crizotinib: a case report. BMC Res Notes (2013) 6:489. 10.1186/1756-0500-6-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gainor JF, Varghese AM, Ou SH, Kabraji S, Awad MM, Katayama R, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res (2013) 19(15):4273–81 10.1158/1078-0432.CCR-13-0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi N, Lucena-Araujo AR, Nakayama S, de Figueiredo-Pontes LL, Gonzalez DA, Yasuda H, et al. Dual ALK and EGFR inhibition targets a mechanism of acquired resistance to the tyrosine kinase inhibitor crizotinib in ALK rearranged lung cancer. Lung Cancer (2014) 83(1):37–43 10.1016/j.lungcan.2013.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji C, Zhang L, Cheng Y, Patel R, Wu H, Zhang Y, et al. Induction of autophagy contributes to crizotinib resistance in ALK-positive lung cancer. Cancer Biol Ther (2014) 15:5. 10.4161/cbt.28162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J, Yin M, Zhu Y, Gu L, Zhang Y, Li Q, et al. Prognostic significance and therapeutic potential of the activation of anaplastic lymphoma kinase/protein kinase B/mammalian target of rapamycin signaling pathway in anaplastic large cell lymphoma. BMC Cancer (2013) 13:471. 10.1186/1471-2407-13-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sang J, Acquaviva J, Friedland JC, Smith DL, Sequeira M, Zhang C, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov (2013) 3(4):430–43 10.1158/2159-8290.CD-12-0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Sasaki T, Tan X, Carretero J, Shimamura T, Li D, et al. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res (2010) 70(23):9827–36 10.1158/0008-5472.CAN-10-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med (2014) 370(13):1189–97 10.1056/NEJMoa1311107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell (2011) 19(5):679–90 10.1016/j.ccr.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 35.Kinoshita K, Asoh K, Furuichi N, Ito T, Kawada H, Hara S, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem (2012) 20(3):1271–80 10.1016/j.bmc.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 36.Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol (2013) 14(7):590–8 10.1016/S1470-2045(13)70142-6 [DOI] [PubMed] [Google Scholar]
- 37.Rivera VM, Wang F, Anjum R, Zhang S, Squillace R, Keats J, et al. AP26113 is a dual ALK/EGFR inhibitor: characterization against EGFR T790M in cell and mouse models of NSCLC. Proceedings: AACR 103rd Annual Meeting Chicago: AACR (2012). [Google Scholar]
- 38.Camidge DR, Bazhenova L, Salgia R, Weiss GJ, Langer CJ, Shaw AT, et al. First-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies: updated results. J Clin Oncol (2013) 31(Suppl):Abstr.8031 [Google Scholar]
- 39.Wilcoxen KM, Brake RL, Saffran D, Teffera Y, Choquette D, Whittington D, et al. Proceedings of AACR, 15 April 2012. Cancer Res (2012) 71(Suppl 1):Abstr.1795. 10.1158/1538-7445.AM2012-1795 [DOI] [Google Scholar]
- 40.Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res (2011) 71(14):4920–31 10.1158/0008-5472.CAN-10-3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patnaik APA. Pharmacokinetics and safety of an oral ALK inhibitor, ASP3026, observed in a phase I dose escalation trial. J Clin Oncol (2013) 31(Suppl):Abstr.2602 [Google Scholar]
- 42.Kuromitsu S, Mori M, Shimada I, Kondoh Y, Shindoh N, Soga T, et al. Antitumor activities of ASP3026 against EML4-ALK-dependent tumor models. Mol Cancer Ther (2011) 10(Suppl 11):Abstr.A227 [Google Scholar]
- 43.Pillai RN, Ramalingam SS. Heat shock protein 90 inhibitors in non-small-cell lung cancer. Curr Opin Oncol (2014) 26(2):159–64 10.1097/CCO.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 44.Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol (2010) 28(33):4953–60 10.1200/JCO.2010.30.8338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Socinski MA, Goldman J, El-Hariry I, Koczywas M, Vukovic V, Horn L, et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res (2013) 19(11):3068–77 10.1158/1078-0432.CCR-12-3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felip E, Carcereny E, Barlesi F, Gandhi L, Sequist LV, Kim SW, et al. Phase II activity of the HSP90 inhibitor AUY922 in patients with ALK-rearranged (ALK+) or EGFR-mutated advanced non-small cell lung cancer. Ann Oncol (2012) 23(ix152):Abstr.4380. 10.1093/annonc/mds395 [DOI] [Google Scholar]
- 47.Tabbo F, Barreca A, Piva R, Inghirami G. ALK signaling and target therapy in anaplastic large cell lymphoma. Front Oncol (2012) 2:41. 10.3389/fonc.2012.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.