Abstract
Midlife decline in cognition, specifically in areas of executive functioning, is a frequent concern for which menopausal women seek clinical intervention. The dependence of executive processes on prefrontal cortex function suggests estrogen effects on this brain region may be key in identifying the sources of this decline. Recent evidence from rodent, nonhuman primate, and human subject studies indicates the importance of considering interactions of estrogen with neurotransmitter systems, stress, genotype, and individual life events when determining the cognitive effects of menopause and estrogen therapy. Hum Brain Mapp 35:847–865, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: hormone, dopamine, serotonin, genotype, stress, menopause, estrogen replacement therapy
INTRODUCTION
The menopause transition is marked initially by fluctuating levels of ovarian hormones and ends with an overall dearth of estrogen in the postmenopausal period [Burger et al., 2007; Henderson and Popat, 2011]. During this transition, many women report experiencing a decline in cognitive function [Greendale et al., 2009; Mitchell and Woods, 2011], specifically in areas of memory and attention [Halbreich et al., 1995; Mitchell and Woods, 2011; Schaafsma et al., 2010; Weber and Mapstone, 2009]. Evidence as to whether this reduction in cognitive ability is due to the decline in estrogen experienced during menopause is suggestive but not conclusive [Henderson and Popat, 2011]. To make matters more confusing, some studies have found that the objective decline in cognitive function some women experience during menopause may be domain specific [Fuh et al., 2006], while others have suggested that if women do experience such a decline, the decline is either temporary [Greendale et al., 2009] or does not reach clinical significance when performance on neuropsychological tests is considered [Henderson et al., 2003]. Others have suggested that subjective symptoms can be attributed to non‐hormonal factors such as stress, aging, and poor health [Mitchell and Woods, 2011]. Proposed explanations for these discrepancies include lack of consistency in the stages of menopause examined and covariates considered as well as the use of cognitive measures with varying degrees of ecological validity [Luetters et al., 2007].
Estrogen therapy (ET) has been used as a treatment for menopausal symptoms such as hot flashes and vaginal dryness, but its efficacy in alleviating cognitive symptoms has not been consistent [Hogervorst and Bandelow, 2010]. ET has been found to have a negative effect [Maki et al., 2007; Shumaker et al., 2003], positive effect [Voytko et al., 2009; Keenan et al., 2001; Krug et al., 2006; Wegesin and Stern, 2007], or no effect at all [Kocoska‐Maras et al., 2011] on cognition in clinical studies. Again, such inconsistencies in behavioral performance data may be due to length of time between last menstrual period and initiation of ET, the effects of brain aging in women over 65 years [Sherwin and Henry, 2008], and variations in ET compounds, formulations, doses, regimens, and routes of administration [Stevens et al., 2005].
Though the hippocampus is clearly an important site of estrogen's effects on cognition [Dumas et al., 2008; Maki et al., 2011, 2001; Resnick et al., 2009], the effects of estrogen on the structure and function of the prefrontal cortex (PFC) are of growing interest and are particularly relevant to the kinds of cognitive symptoms reported by women undergoing natural, medical, or surgical menopause. The PFC is necessary for tasks requiring executive functions such as sustained attention, working memory, organization, and planning. Functional magnetic resonance imaging (fMRI) studies in humans [Joffe et al., 2006; Keenan et al., 2001; Stevens et al., 2005] as well as rodent and nonhuman primate studies [Bohacek and Daniel, 2010; Daniel et al., 2006; Rapp et al., 2003] together implicate the PFC as the site of estrogen's modulation of executive function. ET and menstrual cycle fluctuation in estrogen have been shown to increase blood oxygen level dependent (BOLD) signal, a proxy for neuronal activity, in the PFC of postmenopausal and premenopausal women, respectively [Amin et al., 2006a; Joffe et al., 2006; Keenan et al., 2001]. Studies in preclinical models have demonstrated the effects of estrogen on neurotransmitter levels and on structural morphology in the PFC. More recently, preclinical and clinical studies have examined estrogen's effects in the dorsolateral prefrontal cortex (DLPFC), an area of the PFC critical in focused attention and working memory [Bailey et al., 2011; Duff and Hampson, 2000; Hao et al., 2007; Kritzer and Kohama, 1999; Sawaguchi and Goldman‐Rakic, 1994; Shaywitz et al., 1999; Wang et al., 2010]. Loss of estrogen modulation of prefrontal systems may be one explanation for the difficulties menopausal women experience in these cognitive domains.
Though there have been several reviews addressing the evidence for or against a decline in cognition specific to menopause and the efficacy of estrogen in alleviating cognitive dysfunction [Bayer and Hausmann, 2011; Henderson and Popat, 2011; Hogervorst and Bandelow, 2009, 2010; Maki, 2005; Rocca et al., 2011; Sherwin, 2012], most have highlighted the contradictory and inconclusive nature of the findings. Each has referenced the above‐mentioned differences in study design as possible reasons for the lack of clarity in the data. Few publications have included a comprehensive review of both the preclinical and clinical evidence, though none have done so with an added focus on a specific brain region and the cognitive processes relevant to that region of interest.
We assert that estrogen's effects on the PFC are critical to the manifestation of the cognitive difficulties experienced by some menopausal women. The proverbial “Now, what did I come in here for?” can be seen as a common example of failure of working memory, reflective of PFC dysfunction. Clearly, men also experience a decline in cognition with aging, and one could argue for a role of declining estrogen as aromatization of androgens to estrogen decreases [Cherrier et al., 2005; Sherwin, 2003]. An important topic in its own right given androgens' direct and indirect effects on brain chemistry and function, the impact of androgens on cognition warrants a separate review. In addition, individual differences that may affect response to ET such as genotype, stress exposure, and early life experiences are usually not considered. By examining the literature surrounding estrogen's effects on executive function and the PFC in the context of such individual considerations, this review seeks to elucidate the possible mechanisms by which the cognitive decline concurrent with menopause occurs and propose a mechanistic explanation for the seemingly inconclusive nature of the behavioral data. After a review of the preclinical literature discussing estrogen's effects in the PFC, a discussion of their significance in clinical populations and directions for future research will be provided.
The Prefrontal Cortex: Location and Function
Before launching into the effects of estrogen on the PFC at the molecular, chemical, and functional level, it is important to review the anatomical and working nature of the PFC. The PFC is located at the anterior of the frontal lobes of the brain and includes Brodmann's areas 8, 9, 10, 11, 44, 45, 46, and 47 (Fig. 1). The PFC is important for executive processes such as inhibiting distracting information and stimuli, planning, evaluating consequences when making decisions, and working memory. Working memory is often defined as the ability to keep desired or target information “online” and accessible as well as the ability to manipulate this information while being presented with new information that could serve as a distraction. Several clinical neuroimaging studies and preclinical studies in rodents and nonhuman primates have established the importance of the PFC in such behaviors. Rodents and monkeys with PFC lesions exhibit impaired performance on tasks requiring working memory [Baeg et al., 2003; Brito and Brito, 1990; Butters and Pandya, 1969; Carlson et al., 1997; Chafee and Goldman‐Rakic, 1998; Chang et al., 2002; Funahashi and Inoue, 2000; Lauwereyns et al., 2001], temporal order memory [Barker et al., 2007; Chiba et al., 1997, 1994; de Saint Blanquat et al., 2010; Lauwereyns et al., 2001; Petrides, 1995; Sakagami et al., 2001], reversal learning and flexibility [Chudasama and Robbins, 2003; Dias and Aggleton, 2000; Floresco et al., 2008; Kim and Ragozzino, 2005; Meunier et al., 1997; Rolls et al., 1996; Rygula et al., 2010], and decision‐making [Churchwell et al., 2009; Endepols et al., 2010; Floresco and Ghods‐Sharifi, 2007; Hauber and Sommer, 2009; Kim et al., 2008; Mobini et al., 2002; O'Neill and Schultz, 2010]. In healthy human subjects, performing tasks requiring these processes, particularly working memory, increases BOLD signal in the PFC [Barch et al., 1997; Braver et al., 1997; Callicott et al., 1999; Clark et al., 2000; D'Esposito et al., 1998; Diwadkar et al., 2000]. The results of this research suggest that certain portions of the PFC are important for particular executive functions. For example, the dorsal and lateral portions of the PFC are key in the regulation of attention, whereas the ventral and medial portions are important for emotional regulation [Arnsten, 2009]. The region of the PFC investigated in the studies included in this review will be given if a specific region is noted by the authors. Optimal executive functioning and PFC regulation of subcortical brain structures are dependent upon prefrontal concentrations of catecholamines, specifically dopamine (DA) and norepinephrine (NE) [Arnsten, 2011]. Serotonin (5‐HT) and acetylcholine (ACh) are also important in PFC neurochemical modulation of attention [Boulougouris and Tsaltas, 2005].
Figure 1.
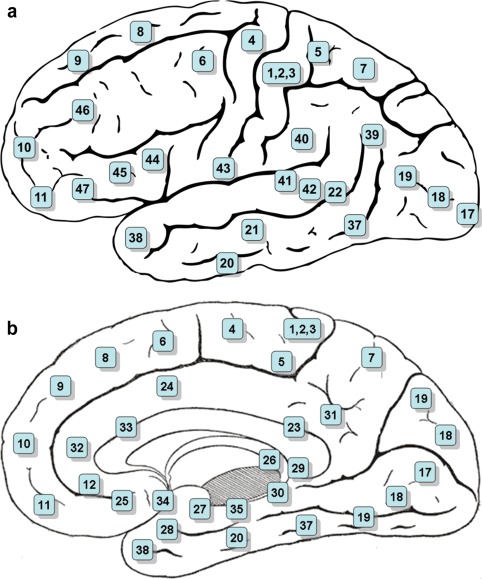
The PFC is located at the anterior of the frontal lobes of the brain and includes Brodmann's areas 8, 9, 10, 11, 44, 45, 46, and 47. Lateral view (a) and medial view (b) as depicted in Gray's Anatomy of the Human Body. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
As suggested by Arnsten and colleagues, DA and NE exhibit an inverted U‐shaped dose–response curve (Fig. 2) on PFC top‐down regulation of behavior in which, when present at ideal levels, NE maintains network connectivity through the stimulation of postsynaptic α2A‐receptors and DA inhibits inappropriate stimuli through stimulation of D1 receptors [Arnsten, 2011, 2009]. Insufficient stimulation of these receptors at lower concentrations can lead to symptoms of executive dysfunction such as fatigue, distractibility, and impulsivity while the excessive D1 stimulation and inappropriate NE α1 and β1 stimulation that occur at higher concentrations mimic symptoms of stress such as mental inflexibility [Arnsten, 2009]. Evidence supporting the withdrawal of estrogen modulation of prefrontal catecholamine systems as one mechanism leading to decline in executive functions will be discussed further below.
Figure 2.
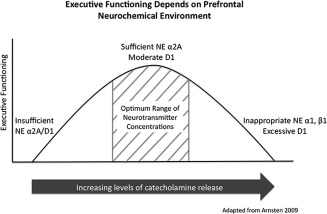
Optimal executive functioning is dependent on the neurochemical environment of the PFC. When present at ideal levels, NE maintains network connectivity through the stimulation of postsynaptic α2A‐receptors and DA inhibits inappropriate stimuli through activation of D1 receptors. Insufficient stimulation of these receptors at lower concentrations can lead to symptoms of executive dysfunction such as fatigue, distractibility, and impulsivity, while the excessive D1 stimulation and inappropriate NE α1 and β1stimulation that occur at higher concentrations mimic symptoms of stress such as mental inflexibility. Withdrawal of estrogen modulation of prefrontal catecholamine systems may be one mechanism leading to a decline in executive functions concurrent with menopause.
Estrogen Effects on the PFC: Animal Studies
Behavioral data: Estrogen effects on behavior
Several rodent and non‐human primate studies have investigated the effects of estrogen on PFC dependent cognitive tasks. Estrogen, alone or in combination with progesterone, has been shown to be successful in correcting ovariectomy (OVX)‐induced impairments in spatial working memory and recognition memory consolidation, preclinical proxies for executive function [Inagaki et al., 2010; Rapp et al., 2003]. In a delayed response test of spatial working memory in intact aged female rhesus monkeys, estrogen replacement reversed cognitive impairments associated with cognitive aging, while OVX worsened the same symptoms [Rapp et al., 2003]. Other studies suggest that the level of estrogen may play a role in the behavioral effects seen [Galea et al., 2001; Wide et al., 2004]. While low levels of estradiol enhanced working memory in cycling female rats [Wide et al., 2004], high levels of estradiol did not [Galea et al., 2001; Wide et al., 2004]. Similarly, a study investigating the efficacy of 17β‐estradiol and 17α‐estradiol in improving recognition memory consolidation in female OVX rats aged 55 to 60 days found that the effects of each isomer were dose, time, and cognitive domain dependent in which each estradiol isomer exhibited an inverted U‐shaped dose–response curve [Inagaki et al., 2010].
In addition, minimizing the time between OVX and initiation of estradiol appears to be important in middle‐aged rats. Estradiol initiated immediately after OVX improved working memory performance in rats OVXed at 12 months and 17 months, though no effect of estrogen was seen after long‐term hormone deprivation [Daniel et al., 2006]. Additionally, the beneficial effects of estradiol on tasks requiring attention appear to attenuate over time in rats OVXed at these ages [Bohacek and Daniel, 2010]. Hence, time of initiation and duration of ET in menopausal women are likely to impact the efficacy of ET. Though these preclinical behavioral studies implicate the PFC as the site of estrogen modulation of executive processes, differences in study designs are a probable source of the apparent contradictions in findings, making the interpretation of the behavioral data elusive, and necessitating the examination of the neural mechanisms underlying these findings.
Monoamine and catecholamine systems
DA and NE, the catecholamines crucial for executive functioning [Arnsten, 2011], are modulated by estrogen in the PFC. Treatment with 20 μg/kg of 17β‐estradiol both improved memory consolidation and affected dopaminergic, noradrenergic, and serotonergic neurotransmission in the rodent PFC. Estrogen significantly raised concentrations of 5‐HT, MHPG (metabolite of NE), and DOPAC (metabolite of DA) in the PFC of rats OVXed at 55 to 60 days as well as the turnover rate of NE to MHPG shortly after treatment [Inagaki et al., 2010]. Estrogen benzoate or ERβ agonist‐induced elevations in prefrontal DA, NE, and 5‐HT metabolites were accompanied by improvements in recognition memory in rats OVXed at 2 months [Jacome et al., 2010]. However, treatment with significantly higher or lower doses of 17β‐estradiol or higher doses of 17α‐estradiol were found to be ineffective in improving spatial and non‐spatial memory consolidation in OVX rats despite the increase in neurotransmitter levels that occurred at the higher doses, indicating that estrogen's beneficial effects on cognition are dose dependent [Inagaki et al., 2010].
Estrogen levels also affect catecholaminergic systems in cycling rats. Rodent mesolimbic dopaminergic activity, including DA release, uptake, and transport, are dependent on estrous cycle phase [Thompson and Moss, 1997]. Basal prefrontal DA concentrations were found to be dependent on the phase of the estrous cycle, where DA concentrations were highest during estrus, reduced during diestrus, and lowest during proestrus, the phase at which estrogen is typically at its peak [Dazzi et al., 2007]. Sensitivity of prefrontal dopaminergic neurons to ethanol administration was also dependent on estrus cycle phase, the consequences of which may play a role in differential drug and alcohol sensitivity between the sexes. Ethanol treatment resulted in increased PFC DA release during estrus, the phase at which estrogen is at its nadir, but had no effect on basal DA concentration during diestrus or proestrus. OVX, treatment with finasteride, a specific 5α‐reductase inhibitor, and treatment with clomiphene, an estrogen receptor antagonist, each blocked the increase in DA output due to ethanol administration seen during estrus. Pretreatment with ethynyl estradiol before acute ethanol administration, however, allowed for the restoration of the ethanol‐induced DA output. Interestingly, treatment with estradiol 30 days following OVX did not have the same restorative effects [Dazzi et al., 2007], lending further support to the importance of timing of estrogen replacement.
Estrogen also affects these neurotransmitter systems through enzymatic regulation of transmitter metabolism. OVX elevated catechol‐o‐methyl transferase (COMT), an enzyme involved in DA metabolism, protein expression and enzyme activity in the PFC of female rats, as did treatment with tamoxifen, an estrogen antagonist. In contrast, decreases in COMT protein expression and enzyme activity were observed in male rats receiving estrogen, suggesting sex differences in the effects of estrogen on DA metabolism [Schendzielorz et al., 2011]. Though many human subjects studies have investigated the effects of tamoxifen on cognition, none of these studies have examined whether COMT genotype may serve as a predictor of women who may benefit from or have adverse effects to tamoxifen treatment or ET.
One study found that estradiol treatment initiated 5 months after OVX increased choline acetyltransferase (ChAT) protein levels in the PFC while the same treatment administered immediately following OVX did not, leading the authors to suggest that the period of hormone deprivation sensitized PFC cholinergic systems to the effects of estrogen [Bohacek et al., 2008]. This delayed sensitization appears contradictory to the critical period hypothesis, which posits that ET's efficacy in alleviating cognitive difficulties due to hormone deprivation is dependent on minimizing the length of time between hypogonadism and initiation of ET. Though the cognitive effects of this delay in PFC sensitization to estradiol are not clear, increased ChAT activity has been shown to alleviate cognitive dysfunctions associated with Alzheimer's disease in rodent models [Park et al., 2012] and may modulate neurotransmission of gamma‐aminobutyric acid (GABA), the primary inhibitory neurotransmitter, through stimulation of nicotinic ACh receptors in the PFC [Aracri et al., 2010].
Glutamate, the primary excitatory neurotransmitter, modulates cognitive process such as long term potentiation (LTP) by binding to N‐methyl‐d‐aspartic acid (NMDA) and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors. Though most studies investigating the interactions of estrogen and glutamate in LTP have focused on the hippocampus [Kullmann et al., 1996], estradiol has recently been shown to modulate glutamate transmission and AMPA receptor binding in the PFC. Estradiol modulation of AMPA receptors in the rodent PFC appears to selectively involve ERα, but not ERβ, receptors. While ET can reverse the increase in [3H]AMPA receptor binding in the PFC caused by OVX, one study found that administration of an ERα receptor agonist was able to cause the same decrease while an ERβ receptor agonist had no effect [Le Saux et al., 2006]. The same selectivity of ER agonists was seen on GluR2 mRNA levels. Estradiol and treatment with an ERα agonist decreased GluR2 mRNA levels in the rodent PFC, while treatment with an ERβ agonist had no effect, and oddly neither did OVX. This estradiol modulation of AMPA receptor binding via the GluR2 subunit, which in turn modulates Ca2+ permeability, may be one mechanism by which estradiol exerts its neuroprotective effects. Increasing Ca2+ permeability, as a result of decreasing GluR2 expression, is correlated with increases in expression of transcription factors such as brain‐derived neurotrophic factor (BDNF), a neurotrophin important for learning and memory [Le Saux et al., 2006]. Therefore, estrogen regulation of AMPA receptors and glutamate transmission may have effects on synaptic plasticity and LTP.
Transcription factors
Regulation of transcription factors is a possible mechanism by which estrogen modulates cognition. In nonhuman primates, short‐term E2 treatment altered DLPFC concentrations of transcription factor mRNA and protein important for cognitive processes. Estrogen treatment increased expression of c‐Fos mRNA, a transcription factor involved in estrogenic neuronal activation and plasticity, regulation of gene transcription, and possibly promotion of insulin‐like growth factor‐1 (IGF‐1) gene expression in the DLPFC of cycling non‐human primates aged 5 to 7 years [Wang et al., 2007] and in the ventromedial PFC of cycling rodents [Zeidan et al., 2001]. Levels of c‐Fos were decreased in a mouse model of fragile X syndrome exhibiting impairments in attention, inhibition, and cognitive flexibility [Braudeau et al., 2011]. Increases in c‐Fos expression were associated with the improvements in memory consolidation seen in rats receiving hippocampal injections of a GABAA agonist used to decrease GABA neurotransmission [Braudeau et al., 2011]. Improved performance on spatial learning tasks observed in rodents after treatment with an acetylcholinesterase inhibitor or phosphodiesterase inhibitor was attributed to the observed increases in IGF‐1 [Narimatsu et al., 2009; Zhao et al., 2010]. Thus, increased c‐Fos expression, and therefore IGF‐1 expression, may be a mechanism by which estrogen supports executive function.
ET was also found to suppress gene and protein expression of E2F transcription factor‐1 (E2F1), a transcription factor involved in activating cell cycle progression, repressing gene expression, and antiproliferative processes. The suppression of E2F1 expression and promotion of IGF‐1 expression are other possible mechanisms for estrogen's neuroprotective effects. ET also decreased expression of general transcription factor IIB mRNA and protein, but the implications of this effect on neuronal function are not clear [Wang et al., 2007].
Brain‐derived neurotrophic factor
BDNF, a neurotrophin involved in synaptic plasticity, neuronal survival, learning, and memory, is important in estrogen's modulation of PFC dependent cognitive processes. BDNF mRNA levels have been found to vary across the rodent estrous cycle. While one study suggested an inverse relationship between estradiol levels and medial PFC BDNF mRNA levels in cycling rodents [Cavus and Duman, 2003], several studies indicate that the estrogen‐induced increases in BDNF levels in other brain regions [Gibbs, 1998; Haraguchi et al., 2012; Kiss et al., 2012; Numakawa et al., 2010] may be key in estrogen's effects on dendritic growth, synaptogenisis [Haraguchi et al., 2012], and spatial reference memory [Kiss et al., 2012]. Estrogen regulation of BDNF mRNA and protein levels in other regions of the brain such as the hippocampus have been shown to affect glutamate and GABA transmission in these neurons [Caldeira et al., 2007a, 2007b]. One mechanism by which estrogen regulates BDNF expression is via the estrogen response element located in the promoter region of the BDNF gene. Therefore, regulation of BDNF mRNA transcription may be a potential pathway by which estrogen modulates PFC function [Sohrabji et al., 1995].
In addition, experiments utilizing a knock‐in mouse model for the BDNF Val66Met polymorphism in which the single nucleotide polymorphism occurring in the prodomain of the BDNF gene at position 66 results in reduced neuronal BDNF release suggest that BDNF genotype indicates an individual's vulnerability to affective disorders during periods of significant hormone fluctuation such as puberty and menopause [Bath et al., 2012; Epperson and Bale, 2012]. Homozygous Met mice demonstrated an increase in anxiety‐like behaviors during estrus, the point at which estrogen levels decline the most rapidly, while no effect of estrous stage on these behaviors was observed in homozygous Val mice. In addition, that a correlation between age and anxiety‐like behaviors were observed in Met mice, but not Val mice, suggests a role for BDNF polymorphism genotype on the impact of reproductive stage in the manifestation of anxiety risk [Bath et al., 2012; Epperson and Bale, 2012].
Development
Developmental effects of estradiol on PFC function are also important to consider. During puberty, IGF‐I receptor signaling in the PFC becomes more sensitive to estrogen regulation [Sanz et al., 2008]. Higher IGF‐1 levels are associated with better performance on tasks of working memory and sustained attention in healthy human subjects [Bellar et al., 2011]. In addition, interactive effects of estradiol and IGF‐1 on neuron development, survival, and plasticity have been observed. One mechanism by which estrogen modulates PFC dependent cognitive process may be through alteration of IGF‐1 signaling in the PFC via the GSK3β/Atk pathway. Acute estradiol administration to prepubertal OVX rats did not induce the same sustained phosphorylation of alpha serine/threonine‐protein kinase (Akt) and glycogen synthase kinase 3β (GSK3β) seen in the PFC of mature OVX rats treated with estrogen [Sanz et al., 2008]. In one positron emission tomography (PET) study in elderly human subjects, subjects with high IGF‐1 levels had shorter reaction times and showed increased regional signal in the left DLPFC during a working memory task [Arwert et al., 2005]. Thus, PFC exposure to estrogen during this period may be critical to the development of pathways used in executive functioning in later life.
Dendritic spine morphology
Changes in hippocampal dendritic spine density have been associated with enhanced performance on tasks of learning and memory as well as increases in LTP. Estrogen treatment has been shown to reverse both the decreases in PFC dendritic spine density and number as well as the impairments in PFC dependent functions caused by OVX in both rodents and nonhuman primates [Chen et al., 2009; Hao et al., 2007; Rapp et al., 2003; Tang et al., 2004]. A significant increase in spine number was seen in layer I of PFC area 46 pyramidal cells of OVX female rhesus monkeys after estrogen replacement [Tang et al., 2004] and in layers III and V of the rodent PFC [Chen et al., 2009]. In layer III, long‐term 17β‐estradiol administration following OVX increased apical and basal dendritic spine density and the number of smaller, more motile spines thought to be important for synaptic plasticity [Hao et al., 2007], while no effect was seen on measurements of dendritic arbor such as total dendritic length and branching [Chen et al., 2009; Hao et al., 2007]. The enhanced performance on a delayed response task probing working memory in OVX nonhuman primates receiving estradiol is thought to be at least in part due to these changes in spine density [Hao et al., 2007; Rapp et al., 2003]. In addition, one study found that the decrease in spine density in the medial PFC following OVX was accompanied by a decline in performance on object recognition and object placement memory tasks [Wallace et al., 2006].
Similarly, dendritic spine morphology varies across the estrous cycle in rodents, and these variations parallel the effects seen with OVX and estrogen administration. Dendritic spines are more numerous and have the greatest density during proestrus than estrus and diestrus, though no effect of estrous cycle phase has been seen on dendritic arbor [Chen et al., 2009]. Increased dendritic spine density in prefrontal pyramidal neurons was also observed in OVX rats maintained on a long‐term diet containing high levels of phytoestrogens [Luine et al., 2006]. The beneficial effect of estrogen on prefrontal dendritic morphology does not appear to be limited to females. Estradiol administration also appears to increase the number of spine synapses in the medial PFC of male gonadectomized rats, though to a lesser extent than increases observed with testosterone treatment [Hajszan et al., 2007].
This correlation between estrogen level and PFC dendritic spine density and number provides further support for estrogen's effect on executive functions through modulation of glutamatergic activity. Increases in the number of smaller, rather than larger, spines were primarily observed [Hao et al., 2007]. These smaller spines have a greater number of NMDA receptors and are more motile, plastic, and sensitive to Ca2+ dependent signaling than larger spines. Small spines have been suggested to be important in learning, while large spines, which have a greater number of AMPA receptors and are therefore also mediated by glutamatergic activity, are more stable and thought to be important in memory [Hao et al., 2007; Kasai et al., 2004]. Because executive functioning is dependent on the number of prefrontal small spines, while memory performance depends on the number of large spines in the hippocampus [Hara et al., 2012], ET may alleviate symptoms of executive dysfunction in menopausal women in part through a selective increase in smaller dendritic spines in the PFC.
Immunoreactivity
The effects of estrogen on prefrontal morphology are not limited to those seen on dendritic spines. Estradiol differentially affects prefrontal region morphology during pubertal maturation. Decreases in region volume and neuron number were observed in the ventromedial PFC, while increases in glial number were seen in the dorsal medial PFC [Markham et al., 2007]. In addition, OVX and subsequent hormone administration have been shown to differentially affect the four extrathalamic afferent systems of the PFC. OVX caused decreases in the density of tyrosine‐hydroxylase immunoreactive neurons in the DLPFC of female rhesus monkeys that could be partially restored by treatment with estrogen or fully restored by treatment with estrogen plus progesterone [Kritzer and Kohama, 1998]. A similar decrease in immunoreactivity for prefrontal ChAT followed OVX, while increases in axon density and innervation were seen for dopamine β‐hydroxylase (DBH) and 5‐HT. Estrogen treatment successfully normalized prefrontal DBH and ChAT immunoreactivity [Kritzer and Kohama, 1999]. Though one study found that estrogen plus progesterone had a greater effect than estrogen alone in attenuating the effects of OVX on 5‐HT immunoreactivity, both treatments remained insufficient in normalizing axon density [Kritzer and Kohama, 1999]. Another study found that long term ET successfully prevented the significant decreases in cholinergic fiber length and density caused by OVX in layer II of the PFC [Tinkler et al., 2004].
Stress
Many studies have shown that acute and chronic stress negatively impact PFC‐dependent cognitive functions in rodents, nonhuman primates, and human subjects [Graybeal et al., 2012; Zahrt et al., 1997] through mechanisms such as modulation of noradrenergic [Birnbaum et al., 1999], dopaminergic [Zahrt et al., 1997], glutamatergic [Graybeal et al., 2012; Yuen et al., 2012], and serotonergic activity [Raftogianni et al., 2012]. The interactions of estrogen and stress in the PFC can be seen in their effects on morphology and neurotransmitter signaling. While the majority of studies examining these interactions demonstrate that estrogen accentuation of the stress response negatively impacts cognition, few studies have shown that estrogen may attenuate the negative cognitive effects of stress; whether the nature of the mechanisms behind such interactions are synergistic, additive, or antagonistic appear to depend on the outcome variable in question. Estrogen sensitizes the neurons in the PFC‐amygdala pathway to the morphological effects of stress. While OVX rats receiving estrogen experienced increases in dendritic branching, length, and spine density expected in neurons in the PFC‐amygdala pathway after stress exposure, OVX rats not receiving estrogen did not, though estrogen did cause an increase in spine density in neurons in this pathway in unstressed rats [Shansky et al., 2010]. In addition, the effects of stress on apical dendritic length in the medial PFC of female rats are dependent on estrogen level. In OVX rats treated with 17β‐estradiol and control rats, restraint stress treatment caused an increase in apical dendritic length in layers II and III of the medial PFC, while this increase was not seen in OVX rats not receiving estradiol [Garrett and Wellman, 2009]. That stress‐induced changes in dendritic morphology were dependent on estrogen level suggests that estrogen must amplify the stress response before these changes in morphology can be observed. Alternatively, these changes in morphology could be due to estrogen exposure alone and independent of stress.
Estrogen not only accentuates the effects of stress, but may also lower the threshold level of stress necessary to cause prefrontal dysfunction [Arnsten and Shansky, 2004]. During proestrus, the length of time in restraint stress needed to induce PFC dysfunction, as measured by performance during a spatial delayed alternation task, in female rats was half that needed to impair PFC function during estrus [Shansky et al., 2006]. Similarly, lower does of FG7142, a benzodiazepine inverse agonist that triggers the stress response, were needed during proestrus than estrus to impair working memory function [Shansky et al., 2004]. That FG7142 was also able to block the effects of guanfacine in protecting working memory in OVX rats receiving estrogen but not in vehicle treated rats, suggests the interactive effects of estrogen and stress on working memory may be in part modulated by NE α‐2a receptor stimulation and cAMP signaling in the PFC [Shansky et al., 2006].
In contrast, two studies showed that estrogen may protect against the effects of chronic stress. One study demonstrated that estradiol treatment decreased anxious behaviors in stressed, OVX rats [Bowman et al., 2002]. Another study examined the interactive effects of estrogen and stress on prefrontal c‐Fos immunoreactivity and extracellular regulated signaling kinase 1 and 2 (ERK 1/2) phosphorylation. Though increases in c‐Fos expression have been associated with improvements in memory consolidation in rodent models deficient in its expression [Braudeau et al., 2011], over expression of hippocampal c‐Fos and ERK 1/2 have been observed in rodents with spatial learning and memory impairments [Schneider et al., 2011]. Cyclic estradiol treatment prevented the unfavorable increases in c‐Fos and pERK 1/2 immunoreactivity seen in the medial PFC of OVX rats after exposure to chronic stress [Gerrits et al., 2006a]. This estrogen‐induced decrease in c‐Fos expression in the presence of stress is interesting, as estrogen alone increases c‐Fos expression, possibly to enhance executive functions. This differential effect of estrogen on c‐Fos expression, a transcription factor involved in executive pathways, highlights the importance of considering stress level in evaluating the efficacy of ET.
Estrogen Effects ON THE PFC and Cognition: Human Subjects Studies
Menopause and cognition
Subjective cognitive complaints, particularly in areas of memory and attention, are commonly reported by women transitioning through menopause [Epperson et al., 2011; Halbreich et al., 1995; Mitchell and Woods, 2011; Schaafsma et al., 2010; Weber and Mapstone, 2009]. Mitchell and Woods 2011 found that 60% of their sample of menopausal women reported experiencing increasing memory problems while 79% of menopausal women in another sample reported experiencing memory loss [Weber and Mapstone, 2009]. Evidence as to whether this midlife onset in cognitive decline can be attributed to the menopause transition is not conclusive. Many studies that use neuropsychological tests as measures of cognitive functioning, as opposed to subjective reports, have suggested that cognitive decline during naturally occurring menopause may be domain specific [Fuh et al., 2006; Luetters et al., 2007]. Greendale et al. 2009 reported that the decline in processing speed and verbal episodic memory is temporary and performance returns to normal in the postmenopausal period, while working memory is unaffected by the menopause transition. Similarly, while some studies found that estrogen in the form of ET [Krug et al., 2006] or HT [Duff and Hampson, 2000; Keenan et al., 2001] improved performance in executive functioning domains such as working memory [Duff and Hampson, 2000; Keenan et al., 2001; Krug et al., 2006] and sustained attention [Krug et al., 2006], large randomized controlled trials including the Women's Health Initiative Study of Cognitive Aging [Resnick et al., 2006] and Cognitive Complaints in Early Menopause Trial [Maki et al., 2007] found that HT did not significantly affect cognitive performance in these areas [Maki et al., 2007; Resnick et al., 2006]. However, Weber and Mapstone 2009 found that, though it initially appeared that there was no correlation between subjective memory complaints and retentive memory, once divided into separate groups, menopausal women with greater memory complaints performed significantly worse on tasks of executive function than those with fewer memory complaints. In addition, women with shorter lengths of time between menopause and initiation of estrogen therapy showed a greater positive change in performance on neuropsychological tests measuring executive function than women who started ET at a later time [Dunkin et al., 2005].
Variations in study design such as length of time since menopause and type or dose of ET appear to contribute to these conflicting results. However, the inverted U‐shaped dose effect curves of DA [Arnsten, 2009] and estrogen [Inagaki et al., 2010] on PFC function, interactions between estrogen and 5‐HT [Amin et al., 2005, 2006a], as well as estrogen's role in modulating the effects of stress should be considered as additional biological explanations for the seemingly contradictory results regarding the occurrence of a cognitive decline concurrent with menopause and the efficacy of ET.
Neuroimaging
Though behavioral data alone regarding the efficacy of estrogen appears inconclusive, several functional imaging studies have found that ET reliably increases BOLD signal in the PFC of postmenopausal women during tasks of working memory, even in the absence of significant behavioral differences [Dumas et al., 2010; Joffe et al., 2006; Shaywitz et al., 1999; Stevens et al., 2005], as does HT [Berent‐Spillson et al., 2010; Smith et al., 2006]. According to a recent fMRI study, estrogen‐induced increases in BOLD signal are dependent on cognitive load, as differences in brain activation were only observed during more difficult trials [Dumas et al., 2010]. The number of years of endogenous estrogen exposure was correlated with increased cerebral metabolism in the superior frontal gyrus and inferior frontal gyrus of postmenopausal women receiving ET in a PET imaging study [Silverman et al., 2011]. In addition, the extent of PFC activation in premenopausal women is correlated with menstrual cycle phase [Amin et al., 2006a; Dreher et al., 2007; Jacobs and D'Esposito, 2011; Konrad et al., 2008] and estradiol levels [Craig et al., 2000; Jacobs and D'Esposito, 2011; Zeidan et al., 2001]. Though these studies examine the effect of estrogen on neural activity and metabolism, few consider estrogen by neurotransmitter interactions.
ET and HT were shown to protect against age related grey matter degradation in the PFC. Postmenopausal women who had used HT had significantly greater prefrontal grey matter volumes than postmenopausal women who had never used HT [Erickson et al., 2007, 2005]. This protective effect on neuronal integrity, as well as the beneficial effect of estrogen in preserving executive functions, was found be time limited. Women receiving HT lasting longer than 10 years showed increases in the extent of deterioration of both prefrontal grey matter and executive functioning processes [Erickson et al., 2007]. In addition, the Women's Health Initiative Memory Study‐Magnetic Resonance Imaging (WHIMS‐MRI) study found that HT resulted in decreased frontal lobe volumes in women ages 71 to 89 years who had been postmenopausal for an average of 28.7 years [Resnick et al., 2009]. Thus, the timing of initiation and duration of ET appears to be important in both animal and human studies. Estrogen's effects on brain structure are not limited to menopause. During development, higher estradiol levels were associated with decreased PFC grey matter volume in pubertal girls [Peper et al., 2009], indicating that the effect of estrogen on cortical grey matter volumes is dependent on developmental and reproductive stage.
Neurotransmitters and Genotype
Dopamine and COMT
Jacobs and D'Esposito 2011 recently showed that the correlation between BOLD signal in the medial frontal gyrus (MFG) during performance of a working memory task (N‐back task) and estradiol levels are dependent on dopaminergic tone, as determined by COMT Val158Met genotype and enzyme activity. Premenopausal women with the genotype Val/Val had greater COMT enzyme activity, and therefore lower baseline DA levels, compared to women who were homozygous for the Met allele. Performance on the N‐back task as well as MFG activation exhibited an inverted U‐shaped curve dependent on both genotype and estradiol level. Women who were homozygous for the Val allele showed better working memory performance and increased MFG activation when estradiol levels were high compared with when estradiol levels were low, because the increase in estradiol increased DA to optimum levels. The opposite was true of women with the genotype Met/Met. In their case, the increase in estradiol resulted in overly heightened DA levels, pushing them to the right side of the inverted U‐shaped curve and impairing executive functioning [Jacobs and D'Esposito, 2011]. Perhaps the COMT Val158Met genotype, among others affecting catecholamine transmission in the PFC, may serve as an indicator of women who will experience a cognitive decline during menopause or benefit from ET.
Serotonin and 5‐HTTLPR
Using the tryptophan depletion (TD) paradigm, Epperson et al. 2012 provided novel evidence for estrogen by 5‐HT interactions in the DLPFC during a working memory task. In this study, the TD paradigm was used to lower central 5‐HT levels in healthy menopausal women before and after treatment with transdermal estradiol. During the 2‐back task, TD caused a decrease in BOLD signal in the right and left DLPFC and middle frontal/cingulate gyrus prior to ET. This attenuation, however, was prevented by estradiol treatment, suggesting that estrogen's effects on brain activation during working memory are in part directly mediated by 5‐HT [Epperson et al., 2012]. When considering estrogen by 5‐HT interactions on PFC function or activation, it is important to consider individual differences in 5‐HT function, as several studies suggest that behavioral and neural responses to TD are dependent on baseline serotonergic function. Genotype for monoamine oxidase‐A (MAO‐A), an enzyme involved in 5‐HT and NE metabolism, predicts the extent of BOLD response in the right ventrolateral PFC during tasks of working memory [Cerasa et al., 2008] and response inhibition [Passamonti et al., 2006]. Genotype for the serotonin transporter polymorphism (5‐HTTLPR) also predicts BOLD activation in the right and left ventrolateral PFC and inferior frontal gyrus during the N‐back task. Individuals homozygous for the short allele showed inferior task performance and increased activation in these areas [Jonassen et al., 2012]. In contrast, another study found that individuals homozygous for the short allele of the serotonin transporter show improved memory and attention in comparison to individuals homozygous for the long allele during TD [Roiser et al., 2007]. Thus, serotonergic modulation of estrogen's effects on PFC function may depend on an individual's genotype for genes involved in 5‐HT metabolism and transport.
Stress sensitivity
In addition to the COMT and 5‐HTTLPR polymorphisms, neurotransmitter by estrogen interactions in the PFC are influenced by an individual's sensitivity to stress and the effects of stress on the interactions between neurotrophins and neurotransmitter systems. Human subjects studies are in accordance with the animal literature in showing that estrogen, while improving executive functioning in many healthy individuals, enhances the negative effects of stress on PFC dependent cognitive functioning. Postmenopausal women receiving ET performed worse on tests of executive function after undergoing the Trier Social Stress Test (TSST), a task used to induce moderate psychological stress, compared with women receiving placebo treatment. On a task measuring attention, tryptophan depletion and tyrosine/phenylalanine depletion further impaired performance after the TSST in women receiving ET, with tyrosine/phenylalanine showing a greater negative effect [Newhouse et al., 2010]. The mechanisms underlying interactions between stress, estrogen, and neurotransmitters in the PFC are not yet completely understood.
History of severe trauma has been linked with reduced 5‐HT1B expression in the anterior cingulate cortex of individuals with and without post‐traumatic stress disorder (PTSD), with the greatest effect observed in those with severe PTSD or early childhood trauma [Murrough et al., 2011]. Hence, early adverse experiences may exert enduring affects on 5‐HT neurotransmission and stress sensitivity. In nonhuman primates early and life‐long stress in the form of social subordination results in decreased D2 receptor function accompanying stressed behaviors in female monkeys [Shively, 1998]. Stress response, as determined by stress‐related changes in salivary cortisol, in humans has been shown to be influenced by numerous factors such as age, hormonal and reproductive status, smoking, coffee and alcohol consumption, caloric intake, genetics, exposure to prenatal stress, birth weight, gestational age, level of early family adversity, and position in social hierarchy indicating individual lifestyle choices and adverse childhood experiences may impact stress sensitivity and therefore response to ET [reviewed by Kudielka et al., 2009]. In addition, exposure to chronic stress, acute psychosocial stress, and various medications, even for short‐term use, such as glucocorticoids or psychoactive drugs may permanently alter stress response and HPA axis sensitivity [Kudielka et al., 2009]. Given the evidence for stress by estrogen interactions in the PFC, any of the above environmental or genetic factors or combination of factors may differentially influence an individual's response to ET in the peri‐ and postmenopausal period.
For instance, the interactive effect of 5‐HT and BDNF genotype on stress sensitivity may contribute to individual differences in response to ET. The BDNF Val66Met polymorphism affects the secretion of mature BDNF protein [Rybakowski, 2008]. One study in a preschool population suggested an epistatic relationship between 5‐HT and BDNF genotype on stress reactivity. Individuals with the long 5‐HTTLPR allele were unreactive to stressors, as measured by changes in salivary cortisol levels, regardless of BDNF genotype. In contrast, cortisol response of individuals homozygous for the short 5‐HTTLPR allele varied according to BDNF genotype. Met carriers had lower initial cortisol levels than Val/Val homozygotes but showed a larger increase in cortisol levels with a laboratory stress [Dougherty et al., 2010]. Animal and human studies have suggested that, in addition to BDNF genotype, stress level of a given situation also plays a role in cortisol response in these short 5‐HTTLPR homozygous individuals; cortisol response of Met carriers is lower than that of Val/Val individuals during low stress situations and higher during high stress situations [Belsky et al., 2009; Boyce and Ellis, 2005; Dougherty et al., 2010]. In contrast, Yu et al. 2012 did not observe epistatic interactions between BDNF and 5‐HTTLPR genotype on the magnitude of BOLD response in the DLPFC and dorsal medial PFC of healthy males during an emotional oddball task, though BDNF genotype alone predicted BOLD signal changes in these regions.
Evidence from animal studies regarding the interactions between estrogen and BDNF at the molecular level suggests that genotype for BDNF secretion is important in predicting an individual's response to ET and stress [Bath et al., 2012; Epperson and Bale, 2012]. Although the majority of studies have shown that elevated estrogen levels are associated with increased BDNF expression [Gibbs, 1998; Haraguchi et al., 2012; Kiss et al., 2012] these data are not completely consistent [Cavus and Duman, 2003] and BDNF genotype is not always considered. As mentioned, rodents homozygous for the Met allele have normal concentrations of central BDNF but reduced stimulated neuronal BDNF release and are phenotypically “anxious” when placed in mildly stressful behavioral paradigms [Yu et al., 2012]. In humans, gender and hormonal status effects on the behavioral and/or physiologic responses to stress vary according to BNDF Val66Met genotype [Colzato et al., 2011; Shaley et al., 2009] with the preponderance of the evidence suggesting sub‐optimal response to ET and stress in Met allele carriers. Familial history of affective disorders appears to be an additional risk factor for Met allele carriers with respect to HPA axis regulation [Vinberg et al 2009]. Although BDNF response to ET diminishes with longer versus shorter periods of hypogonadism [Kiss et al., 2012] whether BDNF Val66Met genotype contributes to this effect is not yet known.
ApoE and cognitive aging
Apolipoprotein E (ApoE) genotype is associated with cognitive aging and determines which of the three major ApoE isoforms, ApoE2, ApoE3, or ApoE4, an individual produces. Single amino acid substitutions that occur at two sites vary the ability of the protein to bind to its receptors and modulate neuroprotective processes [Davignon et al., 1988; Hallman et al., 1991; Menzel et al., 1984; Weisgraber, 1994] and cholinergic neurotransmission [Siegel et al., 2011]. Numerous studies have shown that the ApoE4 allele is associated with an increased risk of developing dementia. Though estrogen has been shown to increase levels of ApoE [McAsey et al., 2006; Srivastava et al., 1996; Struble et al., 2003], the ApoE4 allele appears to inhibit [Yaffe et al., 2000] or prevent [Burkhardt et al., 2004] the beneficial effects of estrogen replacement on cognitive functioning.
In addition to their individual effects, COMT, 5‐HTTLPR, BDNF, and ApoE genes may exert interactive effects on cognition. Older individuals who were homozygous for the COMT Val allele and also heterogyzous for the BDNF Met allele demonstrated slower reaction times on tests of executive function and spatial working memory than individuals with any other combination of age and genotype [Nagel et al., 2008]. In contrast, COMT genotype has been shown to predict executive functioning performance on the Trail‐Making Test, even after covarying for the effects of ApoE and BDNF genotypes [Wishart et al., 2011]. Hence, COMT genotype may serve as a stronger predictor of response to ET than either BDNF or ApoE genotype.
Interactions of estrogen and progesterone
The interactive effects of estrogen and progesterone and the role of progesterone itself on the PFC must also be considered. Just as in animal studies, estrogen in combination with progesterone has been shown to affect executive functions. Performance on tasks requiring attention and planning was shown to depend on estrogen and progesterone levels in premenopausal women [Solis‐Ortiz et al., 2004]. In contrast, another study assessing the effects of hormone suppression in premenopausal women found that an induced decline in estrogen, but not progesterone, was sufficient to induce working memory impairments [Grigorova et al., 2006]. PET imaging studies demonstrate significant increases in 5‐HT2A receptor binding in multiple brain regions in postmenopausal women receiving ET [Kugaya et al., 2003], although there is some evidence that progesterone in addition to estrogen is needed to significantly increase 5‐HT2A binding in the DLPFC [Moses et al., 2000]. In contrast, postmenopausal women receiving estrogen plus progesterone exhibited lower cerebral metabolism in the right inferior frontal gyrus than women receiving estrogen alone [Silverman et al., 2011]. Findings in rodents concur with human subject studies indicating that the CNS effects of estrogen alone can be different from those when estrogen is combined with progesterone [Flores et al., 1999]. The results of progesterone's independent contribution to prefrontal modulation of executive functions have not been as extensively studied as that of estrogen's, but its impact on cognition must be considered in the context of these individual factors to determine its effects.
CONCLUSIONS
Though behavioral data regarding the occurrence of a cognitive decline concurrent with menopause and the efficacy of ET in enhancing cognition appear inconclusive, examination of the preclinical and clinical literature indicates plausible biological mechanisms that, if considered, may yield more definitive answers. The accordance of results between rodent, non‐human primate, and human subject studies investigating the effects of task performance, timing and duration of treatment, estradiol level, and early life experiences on estrogen's modulation of cognition as well as those examining the interactive effects of estrogen, neurotransmitters, and genotype on executive functions highlights the importance of considering individual differences in these areas when considering role of estrogen in human cognition.
Behavioral animal and human subject studies show that the effects of estrogen on cognition are task, time, and dose dependent. That estrogen has been shown to significantly improve performance on PFC dependent cognitive tasks such as the N‐back task [Keenan et al., 2001], Stroop task [Krug et al., 2006], and digit‐span forward task [Gorenstein et al., 2011] in human subjects and the delayed response task in rodents [Inagaki et al., 2010] and non‐human primates [Rapp et al., 2003], suggests estrogen may play a significant role in mediating executive domains such as working memory and sustained attention. The cognitive load required by a particular task also appears to play a role in both the extent to which ET enhances cognitive function and brain activation with more obvious effects of estrogen at higher cognitive loads [Dumas et al., 2010]. The timing of initiation and duration of ET also influence estrogen's effects on cognition and brain morphology. Minimizing the length of time between menopause or OVX and initiation of ET is associated with positive effects of ET on both executive functions and PFC morphology. However, these positive effects of ET on cognition have been shown to be time limited in both rodents and human subjects [Bohacek and Daniel, 2010; Erickson et al., 2007]. The optimal duration of ET in peri and postmenopausal women requires further investigation. Similarly, the dose and/or isomer of estradiol administered and blood level obtained by the individual may play a role in whether cognitive effects are observed. Insufficient or excessive levels of estradiol do not have the same beneficial effects on cognition and can even impair executive functions [Inagaki et al., 2010; Jacobs and D'Esposito, 2011]. Rodent and human subjects studies have proposed an inverted U‐shaped model to explain this response of executive functioning to estrogen [Inagaki et al., 2010; Jacobs and D'Esposito, 2011]. In this type of model, natural or induced alterations in estrogen level correspond to varying degrees of changes in executive functioning abilities.
There is growing evidence from both preclinical and clinical research that where an individual initially falls on this curve and her response to ET is unique to both the neurochemical environment of her PFC and her early life experiences. As DA, NE, 5‐HT, and ACh concentrations are important for executive functioning [Arnsten, 2011; Boulougouris and Tsaltas, 2005], examining estrogen's interactions with these neurotransmitters in the PFC may indicate which women would benefit from ET during menopause. Perhaps estrogen and DA interact in the PFC to produce an inverted U‐shaped curve of executive functioning abilities, as was shown in a neuroimaging study in a premenopausal population [Jacobs and D'Esposito, 2011]. This study suggested that women fall into three rather distinct groups: those who exhibit optimum executive functioning during periods of elevated estrogen, those who exhibit optimum executive functioning during periods of depressed estrogen, and those whose executive functioning capabilities are unaffected by estrogen level. That COMT genotype determines to which group women belong [Jacobs and D'Esposito, 2011], highlights the importance of hormone by gene interactions in PFC neurochemical milieu and function. If these data are replicated, COMT genotype may serve as a predictor of which women will experience a decline in executive functions during menopause and who will benefit from ET. In addition to COMT, studies examining other genotypes known to be involved in DA transmission in the context of estrogen's effects on cognition would be useful in determining the factors affecting the efficacy of ET in individuals, though no such studies have been performed.
Tryptophan depletion studies have illustrated the importance of considering estrogen by 5‐HT interactions in the PFC in the context of executive functioning and brain activation [Epperson et al., 2012; Newhouse et al., 2010]. Therefore, genotypes involved in 5‐HT transport and metabolism such as 5‐HTTLPR [Jonassen et al., 2012] and MAO‐A [Cerasa et al., 2008] may also serve as predictors of response to ET. Genotype for BDNF secretion and ApoE isoform may also affect response to ET given their roles in cognitive aging and impact on serotonergic and cholinergic neurotransmission, respectively. Though a few clinical and preclinical studies have begun to explore estrogen by DA and estrogen by 5‐HT interactions in the PFC, the paucity of studies investigating these areas leaves many questions for future research to answer regarding estrogen by neurotransmitter and genotype interactions. Whether similar interactions between estrogen and other neurotransmitters subserving cognition and mood, such as NE and ACh, would predict response to hypogonadism and ET should be explored.
While genotype for neurotransmitter transport and metabolism could serve as potential predictors of response to ET, individual life events, especially those affecting estrogen or stress levels such as years of endogenous estrogen exposure, adverse childhood experiences, and risk for affective disorders, are equally important to consider. That alterations in sensitivity of IFG‐1 signaling, a pathway involved in mediating executive functions, to estrogen regulation are dependent on pubertal estradiol levels indicates the importance of considering the developmental effects of estrogen on midlife PFC functioning as well. In addition, the effect of ET on prefrontal cerebral metabolism during the postmenopause is affected by the years of endogenous estrogen exposure [Silverman et al.,, 2011]. Thus, reproductive life events may affect PFC estrogen sensitivity.
Because estrogen appears to enhance the negative effects of stress on cognition, individual life events involving acute or chronic stress are likely to affect PFC response to estrogen. Age, prenatal stress, and individual life style choices such as nicotine, coffee, and alcohol consumption affect the stress response and perhaps response to ET. In addition, early life trauma and social subordination stress may influence response to ET via serotonergic and dopaminergic pathways, respectively. Further, an individual's stress response and HPA axis sensitivity may be permanently altered by exposure to chronic stress or certain pharmacologic treatments. Because of the extensive interactions of estrogen and stress, each of these factors affecting the stress response may affect efficacy of ET in a particular individual. In addition, estrogen by stress interactions appear to differ in the extent to which they affect neurotransmitter systems, in part due to the nature of the tasks performed. Additionally, hypersensitivity or hyposensitivity of the HPA axis to the effects of stress resulting from any of these factors may shift an individual to the right or left, respectively, on the inverted U‐shaped curve for executive functions. A proposed model for factors determining estrogen's effects on executive functions is depicted in Figure 3.
Figure 3.
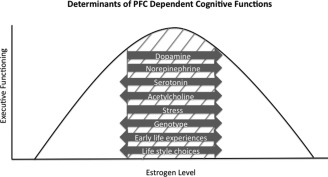
Cognitive response to menopause and ET must be considered in the context of the individual. Factors affecting direction and magnitude of ET's effects include circulating estrogen level, neurotransmitter concentrations, stress level, HPA axis sensitivity, and early life experience. Taken together, preclinical and clinical studies suggest that interactions between estrogen and these factors in the PFC determine where on the inverted U‐shaped curve of executive functioning abilities an individual may fall. The grey cross‐hatching indicates the approximate range of optimum executive functioning. The relative magnitude to which each factor affects executive function is not yet known and may vary between individuals. Increases in DA or NE concentrations, estrogen level, and stress can shift PFC function to the right. Whether significant behavioral effects result from such increases and the direction of these effects may be determined by genotype. HPA axis sensitivity, early life experiences affecting stress or estrogen sensitivity, and individual life style choices may also affect an individual's cognitive response to ET. In addition, interactions between each of these factors must also be considered.
Incorporating genetics testing into the design of neuroimaging studies and considering the effects of estrogen as a function of genes involved in neurotransmission would shed light on the relationship between estrogen and these neurotransmitters. This information may also explain why ET has been shown to have a negative, positive, or no effect on cognitive function in menopausal women. In addition, multimodal imaging studies combing fMRI with proton magnetic resonance spectroscopy (1H‐MRS) would provide information regarding estrogen's effects at the neurotransmitter level in regions of the brain activated by particular tasks. Quantification of estrogen's effects on GABA and glutamate levels in the PFC would be important in revealing the extent to which estrogen‐induced changes in cognition can be attributed to estrogen's effects on neurotransmission.
Further investigation of the interactions between estrogen and various neurotransmitter systems could potentially lead to the development of alternatives to ET. Many perimenopausal and postmenopausal women are not candidates for ET due to medical contraindications or family history [Shifren and Schiff, 2010]. Recently, Epperson et al. 2011 conducted a double‐blinded, placebo‐controlled, crossover study testing whether menopausal women with cognitive symptoms demonstrated alleviation of their subjective attentional and working memory impairments when treated with atomoxetine (ATX), a selective NE reuptake inhibitor used in the treatment of ADHD. Women reported lower scores on a measure of executive dysfunction during ATX treatment than during placebo treatment, and significant changes were found in two of the five cluster scores: those corresponding to sustained attention and working memory [Epperson et al., 2011]. Perhaps other medications targeting catecholamine systems in the PFC would be useful in alleviating the midlife cognitive decline reported by a large portion of this population.
By reviewing the preclinical and clinical literature surrounding estrogen's effects on the PFC, it is clear that estrogen by neurotransmitter interactions play a critical but under explored role in mediating estrogenic effects on PFC function. Examining estrogen response by genotype for genes involved in neurotransmission regulation has the potential to reveal genetic predictors of women who may experience a cognitive decline in executive functions concurrent with menopause and women who will benefit from ET. The inconsistencies in the literature regarding the effect of ET on executive functions may be due to individual variations in genotype for 5‐HT, DA, NE, or ACh transmission or life history events and/or life style choices that affect stress response. Controlling for these factors in future studies investigating the efficacy of ET in alleviating executive dysfunction will likely yield more informative results regarding estrogen's role in PFC dependent cognitive processes.
REFERENCES
- Amin Z, Canli T, Epperson CN (2005): Effect of estrogen‐serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev 4:43–58. [DOI] [PubMed] [Google Scholar]
- Amin Z, Epperson CN, Constable RT, Canli T (2006a): Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage 32:457–464. [DOI] [PubMed] [Google Scholar]
- Amin Z, Gueorguieva R, Cappiello A, Czarkowski KA, Stiklus S, Anderson GM, Naftolin F, Epperson CN (2006b): Estradiol and tryptophan depletion interact to modulate cognition in menopausal women. Neuropsychopharmacology 31:2489–2497. [DOI] [PubMed] [Google Scholar]
- Aracri P, Consonni S, Morini R, Perrella M, Rodighiero S, Amadeo A, Becchetti A (2010): Tonic modulation of GABA release by nicotinic acetylcholine receptors in layer V of the murine prefrontal cortex. Cereb Cortex 20:1539–1555. [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2011): Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry 69:e89–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (2009): Toward a new understanding of attention‐deficit hyperactivity disorder pathophysiology: An important role for prefrontal cortex dysfunction. CNS Drugs 23 ( Suppl 1):33–41. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Shansky RM (2004): Adolescence: Vulnerable period for stress‐induced prefrontal cortical function? Introduction to part IV. Ann NY Acad Sci 1021:143–147. [DOI] [PubMed] [Google Scholar]
- Arwert LI, Veltman DJ, Deijen JB, Lammertsma AA, Jonker C, Drent ML (2005): Memory performance and the growth hormone/insulin‐like growth factor axis in elderly: A positron emission tomography study. Neuroendocrinology 81:31–40. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Huh K, Mook‐Jung I, Kim HT, Jung MW (2003): Dynamics of population code for working memory in the prefrontal cortex. Neuron 40:177–188. [DOI] [PubMed] [Google Scholar]
- Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH (2011): Interactive effects of age and estrogen on cortical neurons: Implications for cognitive aging. Neuroscience 191:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD (1997): Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 35:1373–1380. [DOI] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC (2007): Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27:2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Chuang J, Spencer‐Segal JL, Amso D, Altemus M, McEwen BS, Lee FS (2012): Variant brain‐derived neurotrophic factor (valine66methionine) polymorphism contributes to developmental and estrous stage‐specific expression of anxiety‐like behavior in female mice. Biol Psychiatry 72:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer U, Hausmann M (2011): Estrogen treatment affects brain functioning after menopause. Menopause Int 17:148–152. [DOI] [PubMed] [Google Scholar]
- Bellar D, Glickman EL, Juvancic‐Heltzel J, Gunstad J (2011): Serum insulin like growth factor‐1 is associated with working memory, executive function and selective attention in a sample of healthy, fit older adults. Neuroscience 178:133–137. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R (2009): Vulnerability genes or plasticity genes?Mol Psychiatry 14:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent‐Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, Frey KA, Zubieta JK, Smith YR (2010): Early menopausal hormone use influences brain regions used for visual working memory. Menopause 17:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF (1999): A role for norepinephrine in stress‐induced cognitive deficits: Alpha‐1‐adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry 46:1266–1274. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM (2010): The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle‐aged rats. Psychoneuroendocrinology 35:694–705. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM (2008): Long‐term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle‐aged rats. J Neuroendocrinol 20:1023–1027. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Tsaltas E (2008): Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res 172:517–542. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN (2002): Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience 113:401–410. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ (2005): Biological sensitivity to context: I. An evolutionary‐developmental theory of the origins and functions of stress reactivity. Dev Psychopathol 17:271–301. [DOI] [PubMed] [Google Scholar]
- Braudeau J, Dauphinot L, Duchon A, Loistron A, Dodd RH, Herault Y, Delatour B, Potier MC (2011): Chronic treatment with a promnesiant GABA‐A alpha5‐selective inverse agonist increases immediate early genes expression during memory processing in mice and rectifies their expression levels in a down syndrome mouse model. Adv Pharmacol Sci 2011:153218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC (1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5:49–62. [DOI] [PubMed] [Google Scholar]
- Brito GN, Brito LS (1990): Septohippocampal system and the prelimbic sector of frontal cortex: A neuropsychological battery analysis in the rat. Behav Brain Res 36:127–146. [DOI] [PubMed] [Google Scholar]
- Burkhardt MS, Foster JK, Laws SM, Baker LD, Craft S, Gandy SE, Stuckey BG, Clarnette R, Nolan D, Hewson‐Bower B, Martins RN (2004): Oestrogen replacement therapy may improve memory functioning in the absence of APOE epsilon4. J Alzheimers Dis 6:221–228. [DOI] [PubMed] [Google Scholar]
- Burger HG, Hale GE, Robertson DM, Dennerstein L (2007): A review of hormonal changes during the menopausal transition: Focus on findings from the melbourne women's midlife health project. Hum Reprod Update 13:559–565. [DOI] [PubMed] [Google Scholar]
- Butters N, Pandya D (1969): Retention of delayed‐alternation: Effect of selective lesions of sulcus principalis. Science 165:1271–1273. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, Carvalho AL, Esteban JA, Duarte CB (2007a): Brain‐derived neurotrophic factor regulates the expression and synaptic delivery of alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem 282:12619–12628. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB (2007b): BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci 35:208–219. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR (1999): Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex 9:20–26. [DOI] [PubMed] [Google Scholar]
- Carlson S, Rama P, Tanila H, Linnankoski I, Mansikka H (1997): Dissociation of mnemonic coding and other functional neuronal processing in the monkey prefrontal cortex. J Neurophysiol 77:761–774. [DOI] [PubMed] [Google Scholar]
- Cavus I, Duman RS (2003): Influence of estradiol, stress, and 5‐HT2A agonist treatment on brain‐derived neurotrophic factor expression in female rats. Biol Psychiatry 54:59–69. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Fera F, Passamonti L, Liguori M, Lanza P, Muglia M, Magariello A, Quattrone A (2008): Ventro‐lateral prefrontal activity during working memory is modulated by MAO A genetic variation. Brain Res 1201:114–121. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman‐Rakic PS (1998): Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 79:2919–2940. [DOI] [PubMed] [Google Scholar]
- Chang JY, Chen L, Luo F, Shi LH, Woodward DJ (2002): Neuronal responses in the frontal cortico‐basal ganglia system during delayed matching‐to‐sample task: Ensemble recording in freely moving rats. Exp Brain Res 142:67–80. [DOI] [PubMed] [Google Scholar]
- Chen JR, Yan YT, Wang TJ, Chen LJ, Wang YJ, Tseng GF (2009): Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cereb Cortex 19:2719–2727. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, Raskind MA, Johnson M, Craft S (2005): The role of aromatization in testosterone supplementation: Effects on cognition in older men. Neurology 64:290–296. [DOI] [PubMed] [Google Scholar]
- Chiba AA, Kesner RP, Gibson CJ (1997): Memory for temporal order of new and familiar spatial location sequences: Role of the medial prefrontal cortex. Learn Mem 4:311–317. [DOI] [PubMed] [Google Scholar]
- Chiba AA, Kesner RP, Reynolds AM (1994): Memory for spatial location as a function of temporal lag in rats: Role of hippocampus and medial prefrontal cortex. Behav Neural Biol 61:123–131. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW (2003): Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci 23:8771–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP (2009): Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci 23:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CR, Egan GF, McFarlane AC, Morris P, Weber D, Sonkkilla C, Marcina J, Tochon‐Danguy HJ (2000): Updating working memory for words: A PET activation study. Hum Brain Mapp 9:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Van der Does AJ, Kouwenhoven C, Elzinga BM, Hommel B (2011): BDNF Val66Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology 36:1562–1569. [DOI] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Murphy DG (2008): Physiological variation in estradiol and brain function: A functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Horm Behav 53:503–508. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL (2006): Estradiol replacement enhances working memory in middle‐aged rats when initiated immediately after ovariectomy but not after a long‐term period of ovarian hormone deprivation. Endocrinology 147:607–614. [DOI] [PubMed] [Google Scholar]
- Davignon J, Gregg RE, Sing CF (1988): Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 8:1–21. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G (2007): Estrous cycle‐dependent changes in basal and ethanol‐induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology 32:892–901. [DOI] [PubMed] [Google Scholar]
- de Saint Blanquat P, Hok V, Alvernhe A, Save E, Poucet B (2010): Tagging items in spatial working memory: A unit‐recording study in the rat medial prefrontal cortex. Behav Brain Res 209:267–273. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J (1998): Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res 7:1–13. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP (2000): Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching‐ and matching‐to‐place in the T‐maze in the rat: Differential involvement of the prelimbic‐infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci 12:4457–4466. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Carpenter PA, Just MA (2000): Collaborative activity between parietal and dorso‐lateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. Neuroimage 12:85–99. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Congdon E, Canli T, Hayden EP (2010): Interaction between 5‐HTTLPR and BDNF Val66Met polymorphisms on HPA axis reactivity in preschoolers. Biol Psychol 83:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF (2007): Menstrual cycle phase modulates reward‐related neural function in women. Proc Natl Acad Sci USA 104:2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SJ, Hampson E (2000): A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav 38:262–276. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur‐Bucci C, Naylor M, Sites C, Newhouse P (2008): Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav 53:159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA (2010): Increased memory load‐related frontal activation after estradiol treatment in postmenopausal women. Horm Behav 58:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkin J, Rasgon N, Wagner‐Steh K, David S, Altshuler L, Rapkin A (2005): Reproductive events modify the effects of estrogen replacement therapy on cognition in healthy postmenopausal women. Psychoneuroendocrinology 30:284–296. [DOI] [PubMed] [Google Scholar]
- Endepols H, Sommer S, Backes H, Wiedermann D, Graf R, Hauber W (2010): Effort‐based decision making in the rat: An [18F]fluorodeoxyglucose micro positron emission tomography study. J Neurosci 30:9708–9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J (2012): Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology 37:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Bale TL (2012): BDNF Val66Met polymorphism and brain‐derived neurotrophic factor levels across the female life span: implications for the sex bias in affective disorders. Biol Psychiatry 6:434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Pittman B, Czarkowski KA, Bradley J, Quinlan DM, Brown TE (2011): Impact of atomoxetine on subjective attention and memory difficulties in perimenopausal and postmenopausal women. Menopause 18:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF (2007): Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging 28:179–185. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Raz N, Korol DL, Scalf P, Webb A, Cohen NJ, McAuley E, Kramer AF (2005): Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiol Aging 26:1205–1213. [DOI] [PubMed] [Google Scholar]
- Flores C, Salmaso N, Cain S, Rodaros D, Stewart J (1999): Ovariectomy of adult rats leads to increased expression of astrocytic basic fibroblast growth factor in the ventral tegmental area and in dopaminergic projection regions of the entorhinal and prefrontal cortex. J Neurosci 19:8665–8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Ghods‐Sharifi S (2007): Amygdala‐prefrontal cortical circuitry regulates effort‐based decision making. Cereb Cortex 17:251–260. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT (2008): Inactivation of the medial prefrontal cortex of the rat impairs strategy set‐shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res 190:85–96. [DOI] [PubMed] [Google Scholar]
- Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD (2006): A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas 53:447–453. [DOI] [PubMed] [Google Scholar]
- Fuh JL, Wang SJ, Lu SR, Juang KD, Lee SJ (2003): Alterations in cognitive function during the menopausal transition. J Am Geriatr Soc 51:431–432. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Inoue M (2000): Neuronal interactions related to working memory processes in the primate prefrontal cortex revealed by cross‐correlation analysis. Cereb Cortex 10:535–551. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB (2001): High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res 126:115–126. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL (2009): Chronic stress effects on dendritic morphology in medial prefrontal cortex: Sex differences and estrogen dependence. Neuroscience 162:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits M, Bakker PL, Koch T, Ter Horst GJ (2006a): Stress‐induced sensitization of the limbic system in ovariectomized rats is partly restored by cyclic 17beta‐estradiol administration. Eur J Neurosci 23:1747–1756. [DOI] [PubMed] [Google Scholar]
- Gerrits M, Westenbroek C, Koch T, Grootkarzijn A, Ter Horst GJ (2006b): Increased limbic phosphorylated extracellular‐regulated kinase 1 and 2 expression after chronic stress is reduced by cyclic 17beta‐estradiol administration. Neuroscience 142:1293–1302. [DOI] [PubMed] [Google Scholar]
- Gibbs RB (1998): Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res 787:259–268. [DOI] [PubMed] [Google Scholar]
- Gorenstein C, Renno JJr, Vieira Filho AH, Gianfaldoni A, Goncalves MA, Halbe HW, Fernandes CE, Demetrio FN (2011): Estrogen replacement therapy and cognitive functions in healthy postmenopausal women: A randomized trial. Arch Womens Ment Health 14:367–373. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Kiselycznyk C, Holmes A (2012): Stress‐induced impairments in prefrontal‐mediated behaviors and the role of the N‐methyl‐d‐aspartate receptor. Neuroscience 211:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS (2009): Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72:1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova M, Sherwin BB, Tulandi T (2006): Effects of treatment with leuprolide acetate depot on working memory and executive functions in young premenopausal women. Psychoneuroendocrinology 31:935–947. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C (2007): Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology 148:1963–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH (1995): Possible acceleration of age effects on cognition following menopause. J Psychiatr Res 29:153–163. [DOI] [PubMed] [Google Scholar]
- Hallman DM, Boerwinkle E, Saha N, Sandholzer C, Menzel HJ, Csazar A, Utermann G (1991): The apolipoprotein E polymorphism: a comparison of allele frequencies and effects in nine populations. Am J Hum Genet 49:338–349. [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH (2007): Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA 104:11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, Morrison JH (2012): Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age (Dordr) 34:1051–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi S, Sasahara K, Shikimi H, Honda S, Harada N, Tsutsui K (2012): Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF. Cerebellum 11:416–417. [DOI] [PubMed] [Google Scholar]
- Hauber W, Sommer S (2009): Prefrontostriatal circuitry regulates effort‐related decision making. Cereb Cortex 19:2240–2247. [DOI] [PubMed] [Google Scholar]
- Henderson VW (2011): Gonadal hormones and cognitive aging: A midlife perspective. Womens Health (Lond Engl) 7:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Popat RA (2011): Effects of endogenous and exogenous estrogen exposures in midlife and late‐life women on episodic memory and executive functions. Neuroscience 191:129–138. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L (2003): Estrogen exposures and memory at midlife: A population‐based study of women. Neurology 60:1369–1371. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S (2009): Brain and cognition. Is there any case for improving cognitive function in menopausal women using estrogen treatment?Minerva Ginecol 61:499–515. [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S (2010): Sex steroids to maintain cognitive function in women after the menopause: A meta‐analyses of treatment trials. Maturitas 66:56–71. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V (2010): Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory‐related brain areas. Horm Behav 58:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E, D'Esposito M (2011): Estrogen shapes dopamine‐dependent cognitive processes: Implications for women's health. J Neurosci 31:5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V (2010): Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol Learn Mem 94:488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun‐Todd D, Martin KA (2006): Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double‐blind, placebo‐controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 13:411–422. [DOI] [PubMed] [Google Scholar]
- Jonassen R, Endestad T, Neumeister A, Foss Haug KB, Berg JP, Landro NI (2012): Serotonin transporter polymorphism modulates N‐back task performance and fMRI BOLD signal intensity in healthy women. PLoS One 7:e30564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Honkura N (2004): Structure‐stability‐function relationships of dendritic spines. Tanpakushitsu Kakusan Koso (Japan) 49:276–281. [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ (2001): Prefrontal cortex as the site of estrogen's effect on cognition. Psychoneuroendocrinology 26:577–590. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME (2005): The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem 83:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Hwang J, Lee D (2008): Prefrontal coding of temporally discounted values during intertemporal choice. Neuron 59:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo‐Franci JA, Zanata SM, Ferraz AC (2012): 17beta‐estradiol replacement in young, adult and middle‐aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory‐ and depression‐related brain areas. Behav Brain Res 227:100–108. [DOI] [PubMed] [Google Scholar]
- Kocoska‐Maras L, Zethraeus N, Radestad AF, Ellingsen T, von Schoultz B, Johannesson M, Hirschberg AL (2011): A randomized trial of the effect of testosterone and estrogen on verbal fluency, verbal memory, and spatial ability in healthy postmenopausal women. Fertil Steril 95:152–157. [DOI] [PubMed] [Google Scholar]
- Konrad C, Engelien A, Schoning S, Zwitserlood P, Jansen A, Pletziger E, Beizai P, Kersting A, Ohrmann P, Luders E, Greb RR, Heindel W, Arolt V, Kugel H (2008): The functional anatomy of semantic retrieval is influenced by gender, menstrual cycle, and sex hormones. J Neural Transm 115:1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG (1999): Ovarian hormones differentially influence immunoreactivity for dopamine beta‐ hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 409:438–451. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG (1998): Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 395:1–17. [PubMed] [Google Scholar]
- Krug R, Born J, Rasch B (2006): A 3‐day estrogen treatment improves prefrontal cortex‐dependent cognitive function in postmenopausal women. Psychoneuroendocrinology 31:965–975. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S (2009): Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34:2–18. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB (2003): Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry 160:1522–1524. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Erdemli G, Asztely F (1996): LTP of AMPA and NMDA receptor‐mediated signals: Evidence for presynaptic expression and extrasynaptic glutamate spill‐over. Neuron 17:461–474. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, Sakagami M, Tsutsui K, Kobayashi S, Koizumi M, Hikosaka O (2001): Responses to task‐irrelevant visual features by primate prefrontal neurons. J Neurophysiol 86:2001–2010. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Estrada‐Camarena E, Di Paolo T (2006): Selective estrogen receptor‐alpha but not ‐beta agonist treatment modulates brain alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptors. J Neurosci Res 84:1076–1084. [DOI] [PubMed] [Google Scholar]
- Luetters C, Huang MH, Seeman T, Buckwalter G, Meyer PM, Avis NE, Sternfeld B, Johnston JM, Greendale GA (2007): Menopause transition stage and endogenous estradiol and follicle‐stimulating hormone levels are not related to cognitive performance: Cross‐sectional results from the study of women's health across the nation (SWAN). J Womens Health (Larchmt) 16:331–344. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M (2006): Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res 1126:183–187. [DOI] [PubMed] [Google Scholar]
- Maki PM (2005): A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann NY Acad Sci 1052:182–197. [DOI] [PubMed] [Google Scholar]
- Maki PM, Dennerstein L, Clark M, Guthrie J, LaMontagne P, Fornelli D, Little D, Henderson VW, Resnick SM (2011): Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res 1379:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K (2007): Hormone therapy in menopausal women with cognitive complaints: A randomized, double‐blind trial. Neurology 69:1322–1330. [DOI] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM (2001): Enhanced verbal memory in nondemented elderly women receiving hormone‐replacement therapy. Am J Psychiatry 158:227–233. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM (2007): Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience 144:961–968. [DOI] [PubMed] [Google Scholar]
- McAsey ME, Cady C, Jackson LM, Li M, Randall S, Nathan BP, Struble RG (2006): Time course of response to estradiol replacement in ovariectomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Exp Neurol 197:197–205. [DOI] [PubMed] [Google Scholar]
- Menzel HJ, Assmann G, Rall SCJr, Weisgraber KH, Mahley RW (1984): Human apolipoprotein A‐I polymorphism. Identification of amino acid substitutions in three electrophoretic variants of the Munster‐3 type. J Biol Chem 259:3070–3076. [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M (1997): Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia 35:999–1015. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Woods NF (2011): Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric 14:252–261. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM (2002): Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 160:290–298. [DOI] [PubMed] [Google Scholar]
- Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, Greer PJ, Lopresti B, Loucks TL, Berga SL (2000): Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: A PET study. Biol Psychiatry 48:854–860. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot JD, Gueorguieva R, Planeta‐Wilson B, Krystal JH, Neumaier JF, Huang Y, Ding YS, Carson RE, Neumeister A (2011): The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Arch Gen Psychiatry 68:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A, Heekeren HR, Backman L, Lindenberger U (2008): Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu N, Harada N, Kurihara H, Nakagata N, Sobue K, Okajima K (2009): Donepezil improves cognitive function in mice by increasing the production of insulin‐like growth factor‐I in the hippocampus. J Pharmacol Exp Ther 330:2–12. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Dumas J, Wilkins H, Coderre E, Sites CK, Naylor M, Benkelfat C, Young SN (2010): Estrogen treatment impairs cognitive performance after psychosocial stress and monoamine depletion in postmenopausal women. Menopause 17:860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Yokomaku D, Richards M, Hori H, Adachi N, Kunugi H (2010): Functional interactions between steroid hormones and neurotrophin BDNF. World J Biol Chem 1:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M, Schultz W (2010): Coding of reward risk by orbitofrontal neurons is mostly distinct from coding of reward value. Neuron 68:789–800. [DOI] [PubMed] [Google Scholar]
- Park D, Joo SS, Kim TK, Lee SH, Kang H, Lee HJ, Lim I, Matsuo A, Tooyama I, Kim YB, Kim SU (2012): Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid‐induced learning and memory deficit animals. Cell Transplant 21:365–371. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fera F, Magariello A, Cerasa A, Gioia MC, Muglia M, Nicoletti G, Gallo O, Provinciali L, Quattrone A (2006): Monoamine oxidase‐a genetic variations influence brain activity associated with inhibitory control: New insight into the neural correlates of impulsivity. Biol Psychiatry 59:334–340. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre‐Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE (2009): Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology 34:332–342. [DOI] [PubMed] [Google Scholar]
- Petrides M (1995): Impairments on nonspatial self‐ordered and externally ordered working memory tasks after lesions of the mid‐dorsal part of the lateral frontal cortex in the monkey. J Neurosci 15:359–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftogianni A, Diamantopoulou A, Alikaridis F, Stamatakis A, Stylianopoulou F (2012): Effects of interaction of an early experience of reward through maternal contact or its denial with social stress during adolescence on the serotonergic system and the stress responsiveness of adult female rats. Neuroscience 209:84–96. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA (2003): Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci 23:5708–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C (2009): Postmenopausal hormone therapy and regional brain volumes: The WHIMS‐MRI study. Neurology 72:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA, Women's Health Initiative Study of Cognitive Aging Investigators (2006): Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab 91:1802–1810. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT (2011): Oophorectomy, menopause, estrogen treatment, and cognitive aging: Clinical evidence for a window of opportunity. Brain Res 1379:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Muller U, Clark L, Sahakian BJ (2007): The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. Int J Neuropsychopharmacol 10:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Mason R, Wakeman EA (1996): Orbitofrontal cortex neurons: Role in olfactory and visual association learning. J Neurophysiol 75:1970–1981. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK (2008): BDNF gene: Functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics 9:1589–1593. [DOI] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC (2010): Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci 30:14552–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Tsutsui K, Lauwereyns J, Koizumi M, Kobayashi S, Hikosaka O (2001): A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. J Neurosci 21:4801–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Carrero P, Pernia O, Garcia‐Segura LM (2008): Pubertal maturation modifies the regulation of insulin‐like growth factor‐I receptor signaling by estradiol in the rat prefrontal cortex. Dev Neurobiol 68:1018–1028. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman‐Rakic PS (1994): The role of D1‐dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed‐response task. J Neurophysiol 71:515–528. [DOI] [PubMed] [Google Scholar]
- Schaafsma M, Homewood J, Taylor A (2010): Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric 13:84–98. [DOI] [PubMed] [Google Scholar]
- Schendzielorz N, Rysa A, Reenila I, Raasmaja A, Mannisto PT (2011): Complex estrogenic regulation of catechol‐O‐methyltransferase (COMT) in rats. J Physiol Pharmacol 62:483–490. [PubMed] [Google Scholar]
- Schneider A, Mehmood T, Pannetier S, Hanauer A (2011): Altered ERK/MAPK signaling in the hippocampus of the mrsk2[lowem]KO mouse model of coffin‐lowry syndrome. J Neurochem 119:447–459. [DOI] [PubMed] [Google Scholar]
- Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Ebstein RP, Kaitz M (2009): BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology 34:382–388. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF (2006): The effects of sex and hormonal status on restraint‐stress‐induced working memory impairment. Behav Brain Funct 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH (2010): Estrogen promotes stress sensitivity in a prefrontal cortex‐amygdala pathway. Cereb Cortex 20:2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Glavis‐Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AF (2004): Estrogen mediates sex differences in stress‐induced prefrontal cortex dysfunction. Mol Psychiatry 9:531–538. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC (1999): Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA 281:1197–1202. [DOI] [PubMed] [Google Scholar]
- Sherwin BB (2003): Steroid hormones and cognitive functioning in aging men: A mini‐review. J Mol Neurosci 20:385–393. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF (2008): Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: A critical review. Front Neuroendocrinol 29:88–113. [DOI] [PubMed] [Google Scholar]
- Sherwin BB (2012): Estrogen and cognitive functioning in women: lessons we have learned. Behav Neurosci 126:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifren JL, Schiff I (2010): Role of hormone therapy in the management of menopause. Obstet Gynecol 115:839–855. [DOI] [PubMed] [Google Scholar]
- Shively CA (1998): Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry 44:882–891. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil‐Smoller S, Wactawski‐Wende J, WHIMS Investigators (2003): Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The women's health initiative memory study: A randomized controlled trial. JAMA 289:2651–2662. [DOI] [PubMed] [Google Scholar]
- Siegel JA, Benice TS, Van Meer P, Park BS, Raber J (2011): Acetylcholine receptor and behavioral deficits in mice lacking apolipoprotein E. Neurobiol Aging 32:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DH, Geist CL, Kenna HA, Williams K, Wroolie T, Powers B, Brooks J, Rasgon NL (2011): Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology 36:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK (2006): Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab 91:4476–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran‐Allerand CD (1995): Identification of a putative estrogen response element in the gene encoding brain‐derived neurotrophic factor. Proc Natl Acad Sci USA 92:11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis‐Ortiz S, Guevara MA, Corsi‐Cabrera M (2004): Performance in a test demanding prefrontal functions is favored by early luteal phase progesterone: An electroencephalographic study. Psychoneuroendocrinology 29:1047–1057. [DOI] [PubMed] [Google Scholar]
- Srivastava RA, Bhasin N, Srivastava N (1996): Apolipoprotein E gene expression in various tissues of mouse and regulation by estrogen. Biochem Mol Biol Int 38:91–101. [PubMed] [Google Scholar]
- Stevens MC, Clark VP, Prestwood KM (2005): Low‐dose estradiol alters brain activity. Psychiatry Res 139:199–217. [DOI] [PubMed] [Google Scholar]
- Struble RG, Rosario ER, Kircher ML, Ludwig SM, McAdamis PJ, Watabe K, McAsey ME, Cady C, Nathan BP (2003): Regionally specific modulation of brain apolipoprotein E in the mouse during the estrous cycle and by exogenous 17‐beta estradiol. Exp Neurol 183:638–644. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH (2004): Estrogen replacement increases spinophilin‐immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex 14:215–223. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL (1997): Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett 229:145–148. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML (2004): Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol 469:507–521. [DOI] [PubMed] [Google Scholar]
- Vinberg M, Trajkovska V, Bennike B, Knorr U, Knudsen GM, Kessing LV (2009): The BDNF Val66Met polymorphism: relation to familiar risk of affective disorder, BDNF levels and salivary cortisol. Psychoneuroendocrinology 34:1380–1389. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ (2009): Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci 29:10362–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M (2006): Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126:176–182. [DOI] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH (2010): Synaptic estrogen receptor‐alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci 30:12770–12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng CM, Zhou J, Smith A, Weickert CS, Perlman WR, Becker KG, Powell D, Bondy CA (2004): Estradiol alters transcription factor gene expression in primate prefrontal cortex. J Neurosci Res 76:306–314. [DOI] [PubMed] [Google Scholar]
- Wang L, Ashley‐Koch A, Steffens DC, Krishnan KR, Taylor WD (2012): Impact of BDNF Val66Met and 5‐HTTLPR polymorphism variants on neural substrates related to sadness and executive function. Genes Brain Behav 11:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Mapstone M (2009): Memory complaints and memory performance in the menopausal transition. Menopause 16:694–700. [DOI] [PubMed] [Google Scholar]
- Wegesin DJ, Stern Y (2007): Effects of hormone replacement therapy and aging on cognition: Evidence for executive dysfunction. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14:301–328. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH (1994): Apolipoprotein E: Structure‐function relationships. Adv Protein Chem 45:249–302. [DOI] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA (2004): High level estradiol impairs and low level estradiol facilitates non‐spatial working memory. Behav Brain Res 155:45–53. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Roth RM, Saykin AJ, Rhodes CH, Tsongalis GJ, Pattin KA, Moore JH, McAllister TW (2011): COMT Val158Met genotype and individual differences in executive function in healthy adults. J Int Neuropsychol Soc 17:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L (2000): Estrogen use, APOE, and cognitive decline: evidence of gene‐environment interaction. Neurology 54:1949–1954. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY (2012): Variant brain‐derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 32:4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z (2012): Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73:962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997): Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17:8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR (2011): Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Harada N, Kurihara H, Nakagata N, Okajima K (2010): Cilostazol improves cognitive function in mice by increasing the production of insulin‐like growth factor‐I in the hippocampus. Neuropharmacology 58:774–783. [DOI] [PubMed] [Google Scholar]


