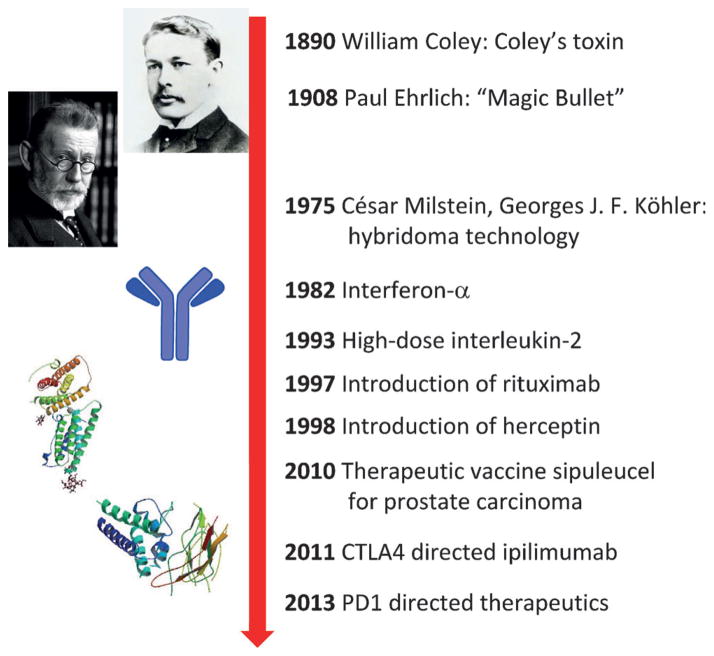
More than 120 years ago William Coley first tested the concept of manipulating the immune system to fight cancer by using bacterial products to treat patients with solid tumors.[1] Low reproducibility and efficacy and serious side effects led to a low acceptance in the clinic. At the beginning of the 20th century Paul Ehrlich, the father of modern immunology, introduced the concept of the use of antibodies to target diseases, nowadays often referred to as the “Magic Bullet”.[2] He proposed that the immune system might control cancer. The introduction of the hybridoma technology for the production of monoclonal antibodies by César Milstein and Georges J. F. Kçhler in 1975 has led to the clinical introduction of multiple antibodies to fight cancer.[3] Truly immune-stimulating approaches included the landmark trials of interferon-α and later high doses of interleukin-2 (IL-2) as well as so-called lymphokine-activated killer cells in patients with multiple tumor types.[4,5] Although antibodies to fight cancer are well-established in the clinic, the use of the patient’s immune system to reject cancer—commonly called cancer immunotherapy—struggled as a means of achieving sustained clinical success by awakening the patient’s own immune system (Figure 1). Recent promising clinical trials of antibodies that target the protein–protein interactions of the receptor for programed death (PD), however, give cause for serious hope and they are considered to be a “game changer” in the area of cancer treatment. The immuno checkpoint drugs that target the programmed death-1 receptor (PD-1) and its ligand (PD-1L) have now been selected as the drug of the year for 2013.[6]
Figure 1.
Milestones in cancer immunotherapy.
An important element of the multilayered immune system is the adaptive immune system, which ultimately leads to immunological memory by signature antigens. According to the current understanding of T-cell activation, two signals are required: 1) a specific peptide epitope of the antigen must be presented on the major histocompatibility complex (MHC) and this must bind to the T-cell receptor to give specificity to the immune response. 2) A faster and stronger attack must occur each time this pathogen is encountered. Although it was mostly believed in the last century that tumors are non-immunogenic, nowadays it is experimentally established that cancer cells produce and correctly display multiple antigens in MHCs and should thus in principle lead to a strong immune response. The dilemma in cancer immunology, however, is that, although patients often initially develop active anti-tumor immune responses, the tumor evades the immune system and grows further. It has been shown that cancer cells submerse the immune system by down-modulating the MHC and co-stimulatory molecules and up-regulating co-inhibitory ligands. Immune checkpoints normally prevent collateral damage to tissues from an ongoing immune response. Immune checkpoint molecules bind to their ligands on the antigen-representing cell (APC), thereby delivering the second signal for T-cell activation. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and PD-1 are two immune checkpoint receptors currently exciting the oncological community (Figure 2). Multiple clinical trials have been performed with different types of molecules and targeting two different immune checkpoint targets (Table 1). The first drug showing therapeutic efficacy of immune checkpoint inhibition introduced into clinical practice in 2011 was ipilumumab. It is an anti-CTLA-4 monoclonal antibody that is active against advanced melanoma. Ipilumumab was selected as the drug of the years 2010–2012 as a result of its additional antitumor activity in advanced renal cell cancer, its capacity to break tolerance, and its paradigm-shifting mechanism.[7] Unfortunately, ipilumumab therapy is associated with frequent immune-mediated adverse events.
Figure 2.
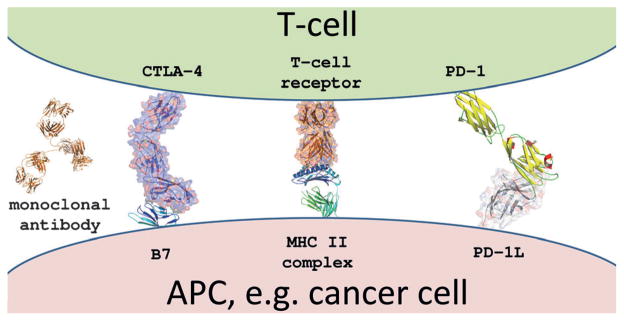
Structural biology model of major cell-surface contacts between an antigen-presenting cell (cancer) and a T-cell: the T-cell receptor major histocompatibility complex located on the T-cell and a cancer cell and several co-stimulatory intracellular protein–ligand interactions. The PD-1/PD-1L interaction model is based on the human NMR structure of PD-1 (3M2D) and the mouse PD-1L (3BIK); TCR-MHC2 complex (1BD2); CTLA-4/B7-2 complex (1I85). The relevant immune checkpoint mAbs targets are CTLA-4, PD-1, and PD-1L.
Table 1.
Biologics targeting co-regulatory receptors currently undergoing clinical trials.
| Name[a] | Type[b] | Originator[c] | Clinical evaluation |
|---|---|---|---|
| ipilimumab (Yervoy) | CTLA-4-directed mAb | Bristol Myers Squibb (BMS) | 2011 FDA approved for the treatment of metastatic melanoma |
| nivolumab | PD-1-directed mAb | Bristol Myers Squibb (BMS) | phase III: renal cell cancer, melanoma, NSCLC[a] |
| lambrolizumab | PD-1-directed mAb | Merck & Co. | phase II: melanoma[a] |
| MPDL3280A (RG7446) | PD-1L-directed mAb | Roche-Genentech | melanoma, solid tumors[b] |
| pidilizumab | CTLA-4-directed mAb | CureTech | phase II: colorectal cancer, melanoma, DLBCL[a] |
| MEDI4736 | PD-1-directed mAb | AstraZeneca | phase II: melanoma, solid tumors[a] |
| tremelimumab | CTLA-4-directed mAb | Pfizer/MedImmune | phase I/II metastatic melanoma |
Data from http://www.clinicaltrials.gov.
mAb =monoclonal antibody.
Data from Roche Pipeline http://www.roche.com.
PD-1, another of these notable “checkpoint” receptors, is a cell surface membrane protein of 268 amino acids that functions to maintain tolerance, abrogates the development of autoimmunity, and minimizes damage to healthy tissue during infection.[8] Several molecules that target the PD-1 pathway are currently in clinical trials (Table 1). The recent clinical results concerning the PD-1 or PD-1 ligand targeting antibodies are now exciting for several reasons. Remarkable antitumor activity was reported for nivolumab and lambrolizumab, both anti PD-1, at the recent ASCO meeting 2013. An overall response rate of 30% and 50% in melanoma was found for nivolumab and lambrolizumab, respectively. Nivolumab-induced responses, for example, showed survival rates of 61% in the first year and 44% in the second year.[9,10] Similar results were obtained with other anti-PD-1 and anti-PD-1L antibodies.
Importantly, PD-1L expression on the surface of melanoma cells appeares to be a predictive biomarker for selecting a responsive patient population. Remarkable activity in other cancers, including lung and renal cell carcinoma, has been reported with various molecules. Investigation of the effect of PD-1-targeting drugs in multiple tumor types is now ongoing and the next decade holds promise for exciting new cancer treatments. For comparison, the recently approved BRAF kinase inhibitor zelboraf selectively targets tumor cells with the BRAFV600E mutation and is used for the treatment of melanoma. The therapy also showed very high initial response rates, but after a few months mutations in the target ATP pocket typically occurred and the corresponding clones were rapidly amplified, thus rendering the patient completely unresponsive to the treatment.[11]
Therapeutics intervening with the function of the immune system are often difficult to control and associated with severe side effects. For example, the CD28-Super mAb TGN1412 was tested a decade ago in an early clinical trial and led to a catastrophic systemic organ failure in all patients through an unforeseen toxic cytokine storm.[12] The so-called immune-related adverse events (irAEs) of immune checkpoint mAbs can involve vitiligo, hepatitis, thyroiditis, hypophysitis, and in severe cases, inflammatory colitis, or pneumonitis. However, these rarely lead to drug-related deaths (1% in the Topalian et al. study;[13] at the date of their analysis 21% patients had died, mostly through disease progression). Multiple clinical trials with molecules directed at the PD-1 pathway indicate their safety. In the cases of severe adverse effects, reduction of the agents dose is mostly sufficient to control the toxicity. Current data clearly indicate that anti-PD-1 molecules outperform CTLA4-directed ipilimumab in terms of their efficacy and safety profile.
Experimental therapies directed at the immune checkpoint interaction of the PD-1 pathway are already celebrated as the “most exciting new melanoma agents”, being a “tsunami” in immune cancer therapy, and have been elected “The drug of the year 2013”.[6,14] Moreover, based on the exciting clinical performance, lambrolizumab was recently awarded a breakthrough designation by the FDA, a mechanism to expedite the development and review of promising new drugs for serious conditions. In addition, the combination of CTLA-4 blockade and PD1 blockade for the treatment of melanoma in a large phase I study was one of the highlights of ASCO2013.[15] The future looks bright for many cancer patients as well as for science and companies bringing such breakthrough drugs to the market.
Footnotes
A.D. thanks the National Institute of Health (1R01GM09708201), Innovative Medicines Initiative (IMI) and the Qatar National Research Foundation (NPRP6-065-3-012) for funding. T.A.H. is funded by a Marie Curie FP7-Reintegration-Grant within the 7th EC Framework Programme and by a Project operated within the Foundation for Polish Science TEAM Programme, cofinanced by the EU European Regional Development Fund.
Contributor Information
Prof. Alexander Dömling, Email: a.s.s.domling@rug.nl, Department for Drug Design, University of Groningen, A. Deusinglaan 1, 9700 AD Groningen (The Netherlands), Homepage: http://www.drugdesign.nl/
Prof. Tad A. Holak, Email: holak@chemia.uj.edu.pl, Department of Organic Chemistry, Jagiellonian University Ingardena 3, 30-060 Cracow (Poland)
References
- 1.Coley WB. Am J Med Sci. 1893;105:487–511. [Google Scholar]
- 2.Ehrlich P. Ned Tijdschr Geneeskd. 1909;53:273–290. [Google Scholar]
- 3.Milstein C. Bioessays. 1999;21:966–973. doi: 10.1002/(SICI)1521-1878(199911)21:11<966::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Sherwin SA, Knost JA, Fein S, Abrams PG, Foon KA, Ochs JJ, Schoenberger C, Maluish AE, Oldham RK. J Am Med Assoc. 1982;248:2461–2466. [PubMed] [Google Scholar]
- 5.Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA, White DE, Steinberg SM. J Natl Cancer Inst. 1993;21:622–632. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Soria JC, Eggermont AMM. Eur J Cancer. 2013;49:2968–2971. doi: 10.1016/j.ejca.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AMM, Robert C. Eur J Cancer. 2011;47:2150–2157. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 8.Hamid O, Carjaval RD. Expert Opin Biol Ther. 2013;13:847–861. doi: 10.1517/14712598.2013.770836. [DOI] [PubMed] [Google Scholar]
- 9.Sznol M, Kluger HM, Hodi FS, et al. J Clin Oncol. 2013;31(Suppl) [abstr. CRA9006] [Google Scholar]
- 10.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton E, Wong B, Tsang G, West BL, Powell B, Shellooe R, Marimuthu A, Nguyen H, Zhang KYJ, Artis DR, Schlessinger J, Su F, Higgins B, Iyer R, D’Andrea K, Koehler A, Stumm M, Lin PS, Lee RJ, Grippo J, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, Chapman PB, Flaherty KT, Xu XW, Nathanson KL, Nolop K. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen LP, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu HY, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullard A. Nat Rev Drug Discovery. 2013;12:489–492. doi: 10.1038/nrd4066. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, Kluger HM, Callahan MK, et al. 2013 ASCO Annual Meeting; 2013; Abstract 9012. [Google Scholar]; Wolchok JD, Kluger HM, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang XL, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]