Abstract
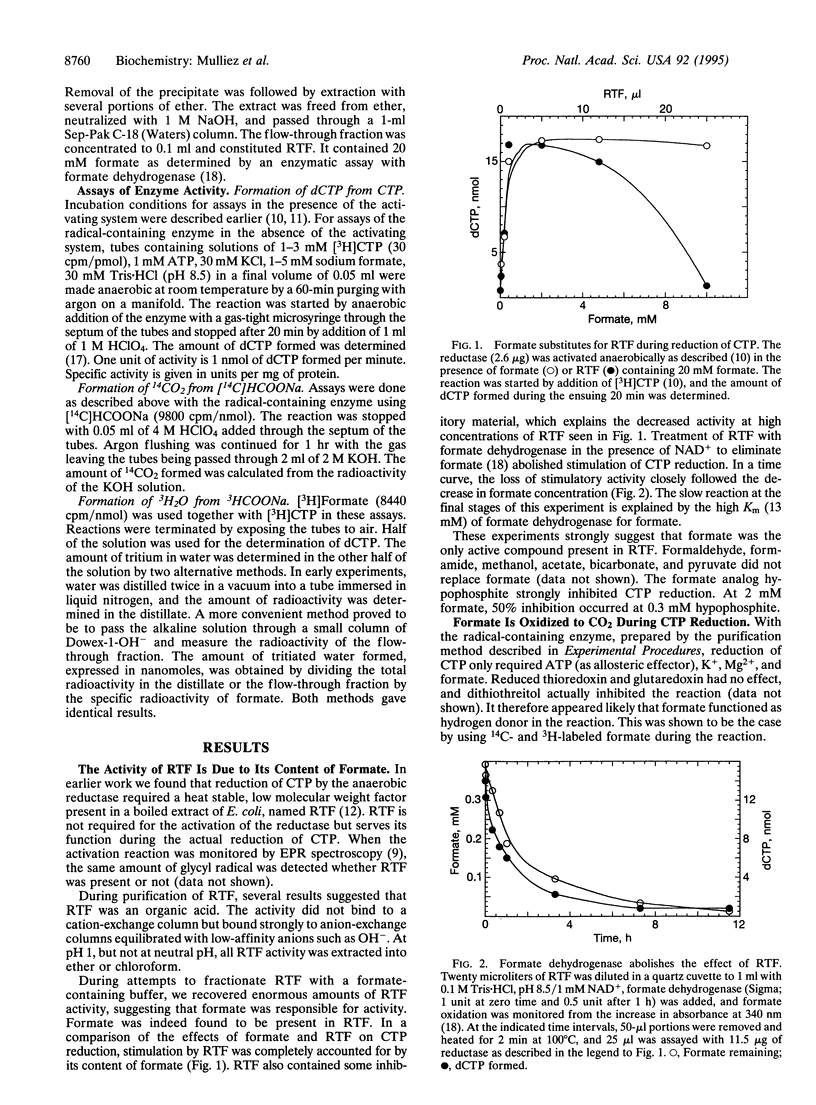
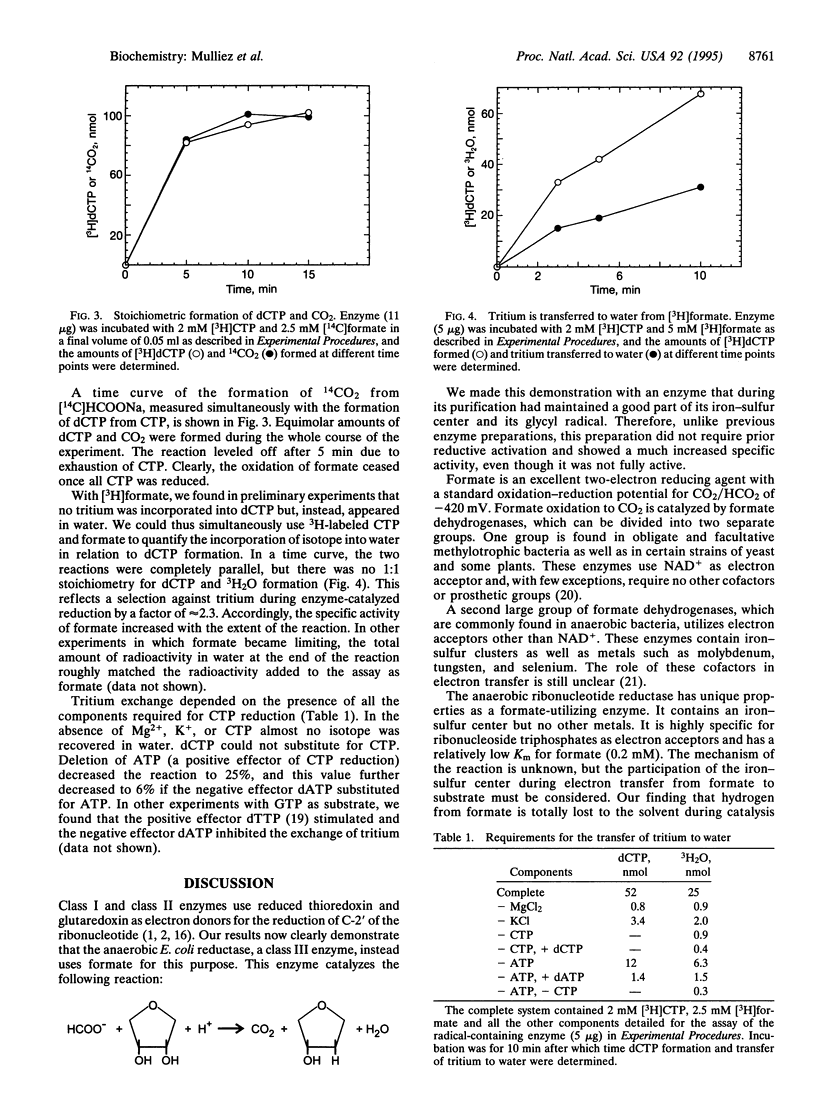
During anaerobic growth Escherichia coli uses a specific ribonucleoside-triphosphate reductase (class III enzyme) for the production of deoxyribonucleoside triphosphates. In its active form, the enzyme contains an iron-sulfur center and an oxygen-sensitive glycyl radical (Gly-681). The radical is generated in the inactive protein from S-adenosylmethionine by an auxiliary enzyme system present in E. coli. By modification of the previous purification procedure, we now prepared a glycyl radical-containing reductase, active in the absence of the auxiliary reducing enzyme system. This reductase uses formate as hydrogen donor in the reaction. During catalysis, formate is stoichiometrically oxidized to CO2, and isotope from [3H]formate appears in water. Thus E. coli uses completely different hydrogen donors for the reduction of ribonucleotides during anaerobic and aerobic growth. The aerobic class I reductase employs redox-active thiols from thioredoxin or glutaredoxin to this purpose. The present results strengthen speculations that class III enzymes arose early during the evolution of DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberg A., Hahne S., Karlsson M., Larsson A., Ormö M., Ahgren A., Sjöberg B. M. Evidence for two different classes of redox-active cysteines in ribonucleotide reductase of Escherichia coli. J Biol Chem. 1989 Jul 25;264(21):12249–12252. [PubMed] [Google Scholar]
- Bianchi V., Eliasson R., Fontecave M., Mulliez E., Hoover D. M., Matthews R. G., Reichard P. Flavodoxin is required for the activation of the anaerobic ribonucleotide reductase. Biochem Biophys Res Commun. 1993 Dec 15;197(2):792–797. doi: 10.1006/bbrc.1993.2548. [DOI] [PubMed] [Google Scholar]
- Bianchi V., Reichard P., Eliasson R., Pontis E., Krook M., Jörnvall H., Haggård-Ljungquist E. Escherichia coli ferredoxin NADP+ reductase: activation of E. coli anaerobic ribonucleotide reduction, cloning of the gene (fpr), and overexpression of the protein. J Bacteriol. 1993 Mar;175(6):1590–1595. doi: 10.1128/jb.175.6.1590-1595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley R. L., Ghambeer R. K., Batterham T. J., Brownson C. Studies with hydrogen isotopes on the mechanism of action of cobamide-dependent ribonucleotide reductase. Biochem Biophys Res Commun. 1966 Aug 12;24(3):418–426. doi: 10.1016/0006-291x(66)90176-8. [DOI] [PubMed] [Google Scholar]
- Booker S., Licht S., Broderick J., Stubbe J. Coenzyme B12-dependent ribonucleotide reductase: evidence for the participation of five cysteine residues in ribonucleotide reduction. Biochemistry. 1994 Oct 25;33(42):12676–12685. doi: 10.1021/bi00208a019. [DOI] [PubMed] [Google Scholar]
- Eliasson R., Pontis E., Eckstein F., Reichard P. Interactions of 2'-modified azido- and haloanalogs of deoxycytidine 5'-triphosphate with the anaerobic ribonucleotide reductase of Escherichia coli. J Biol Chem. 1994 Oct 21;269(42):26116–26120. [PubMed] [Google Scholar]
- Eliasson R., Pontis E., Fontecave M., Gerez C., Harder J., Jörnvall H., Krook M., Reichard P. Characterization of components of the anaerobic ribonucleotide reductase system from Escherichia coli. J Biol Chem. 1992 Dec 15;267(35):25541–25547. [PubMed] [Google Scholar]
- Eliasson R., Pontis E., Sun X., Reichard P. Allosteric control of the substrate specificity of the anaerobic ribonucleotide reductase from Escherichia coli. J Biol Chem. 1994 Oct 21;269(42):26052–26057. [PubMed] [Google Scholar]
- Fontecave M., Nordlund P., Eklund H., Reichard P. The redox centers of ribonucleotide reductase of Escherichia coli. Adv Enzymol Relat Areas Mol Biol. 1992;65:147–183. doi: 10.1002/9780470123119.ch4. [DOI] [PubMed] [Google Scholar]
- Harder J., Eliasson R., Pontis E., Ballinger M. D., Reichard P. Activation of the anaerobic ribonucleotide reductase from Escherichia coli by S-adenosylmethionine. J Biol Chem. 1992 Dec 15;267(35):25548–25552. [PubMed] [Google Scholar]
- Heider J., Böck A. Selenium metabolism in micro-organisms. Adv Microb Physiol. 1993;35:71–109. doi: 10.1016/s0065-2911(08)60097-1. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Knappe J., Elbert S., Frey M., Wagner A. F. Pyruvate formate-lyase mechanism involving the protein-based glycyl radical. Biochem Soc Trans. 1993 Aug;21(3):731–734. doi: 10.1042/bst0210731. [DOI] [PubMed] [Google Scholar]
- Mao S. S., Holler T. P., Yu G. X., Bollinger J. M., Jr, Booker S., Johnston M. I., Stubbe J. A model for the role of multiple cysteine residues involved in ribonucleotide reduction: amazing and still confusing. Biochemistry. 1992 Oct 13;31(40):9733–9743. doi: 10.1021/bi00155a029. [DOI] [PubMed] [Google Scholar]
- Mulliez E., Fontecave M., Gaillard J., Reichard P. An iron-sulfur center and a free radical in the active anaerobic ribonucleotide reductase of Escherichia coli. J Biol Chem. 1993 Feb 5;268(4):2296–2299. [PubMed] [Google Scholar]
- Popov V. O., Lamzin V. S. NAD(+)-dependent formate dehydrogenase. Biochem J. 1994 Aug 1;301(Pt 3):625–643. doi: 10.1042/bj3010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993 Jun 18;260(5115):1773–1777. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- Schüte H., Flossdorf J., Sahm H., Kula M. R. Purification and properties of formaldehyde dehydrogenase and formate dehydrogenase from Candida boidinii. Eur J Biochem. 1976 Feb 2;62(1):151–160. doi: 10.1111/j.1432-1033.1976.tb10108.x. [DOI] [PubMed] [Google Scholar]
- Stubbe J. Ribonucleotide reductases. Adv Enzymol Relat Areas Mol Biol. 1990;63:349–419. doi: 10.1002/9780470123096.ch6. [DOI] [PubMed] [Google Scholar]
- Sun X., Eliasson R., Pontis E., Andersson J., Buist G., Sjöberg B. M., Reichard P. Generation of the glycyl radical of the anaerobic Escherichia coli ribonucleotide reductase requires a specific activating enzyme. J Biol Chem. 1995 Feb 10;270(6):2443–2446. doi: 10.1074/jbc.270.6.2443. [DOI] [PubMed] [Google Scholar]
- Sun X., Harder J., Krook M., Jörnvall H., Sjöberg B. M., Reichard P. A possible glycine radical in anaerobic ribonucleotide reductase from Escherichia coli: nucleotide sequence of the cloned nrdD gene. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):577–581. doi: 10.1073/pnas.90.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L. Reaction mechanism of ribonucleoside diphosphate reductase from Escherichia coli. Oxidation-reduction-active disulfides in the B1 subunit. J Biol Chem. 1974 Aug 10;249(15):4858–4862. [PubMed] [Google Scholar]
- Uhlin U., Eklund H. Structure of ribonucleotide reductase protein R1. Nature. 1994 Aug 18;370(6490):533–539. doi: 10.1038/370533a0. [DOI] [PubMed] [Google Scholar]


