Abstract
Purpose
Diabetes mellitus (DM) causes diabetic bladder dysfunction (DBD). We aimed to identify the pathogenic roles of polyuria and hyperglycemia on DBD in rats.
Materials and Methods
Seventy-two female Sprague-Dawley rats were divided: age-matched controls (control), sham urinary diversion (sham), urinary diversion (UD), streptozotocin-induced diabetes after sham UD (DM), streptozotocin-induced diabetes after UD (UD+DM), and 5% sucrose-induced diuresis after sham UD (DIU). UD was performed by ureterovaginostomy 10d before DM induction. Animals were evaluated 20 wks after DM or diuresis induction. We measured 24-hr drinking and voiding volumes and cystometry (CMG). Bladders were harvested for quantification of smooth muscle, urothelium, and collagen. We measured nitrotyrosine and manganese superoxide dismutase (MnSOD) in bladder.
Results
Diabetes and diuresis caused increases in drinking volume, voiding volume and bladder weight. Bladder weights decreased in the UD and UD+DM groups. Intercontractile intervals, voided volume, and compliance increased in the DIU and DM groups, decreased in the UD, and further decreased in the UD+DM group. The total cross-sectional tissue, smooth muscle and urothelium areas increased in the DIU and DM groups, and decreased in the UD and UD+DM groups. As percentages of total tissue area, collagen decreased in the DIU and DM groups, and increased in the UD and UD+DM groups, and smooth muscle and urothelium decreased in the UD and UD+DM groups. Nitrotyrosine and MnSOD increased in DM and UD+DM rats.
Conclusions
Polyuria induced bladder hypertrophy, while hyperglycemia induced substantial oxidative stress in the bladder, which may play a pathogenic role in late stage DBD.
Keywords: Urinary diversion, Cystometry, Histology, Animal model
INTRODUCTION
Diabetic bladder dysfunction (DBD), a collective description of clinical symptoms including decreased sensation, increased capacity, poor emptying1, and detrusor overactivity2, is among the most common and costly complications of diabetes mellitus (DM). It is estimated that DBD occurs in approximately 87% of individuals diagnosed with DM, a higher rate than that of widely recognized complications such as neuropathy (60%) and nephropathy (50%)1,3. DBD substantially affects quality of life4. In murine models we have shown that DM induces time-dependent alterations in bladder morphometry5, function6, contractility7, innervation and vasculature8. Yet, the pathogenic mechanisms of DBD are poorly understood.
The bladder, unlike most organs affected by DM, experiences both hyperglycemia and an increased volume of urine, or polyuria. Hyperglycemia-induced pathophysiological changes have been studied extensively, and oxidative stress (OS) has been shown to play an important role in multiple diabetic complications9,10. We have previously shown that polyuria plays an important role in the temporal changes of DBD and its manifestations5–8. However, to date, the independent contributions of hyperglycemia and polyuria to the development and progression of DBD are not known. Separating those two precursors of DBD may help us clarify their roles and identify the appropriate targets for alleviating or reversing DBD.
In the current study we used rat models of diuresis, urinary diversion (UD), type I DM to determine the independent and relative roles of polyuria and hyperglycemia in DBD pathogenesis.
MATERIALS AND METHODS
Experimental animals and design
Seventy-two female Sprague-Dawley rats (10 wks-old, Harlan Laboratories, Inc., Indianapolis, IN), were used in this study. Rats were randomly allocated to one of six groups: age-matched controls (control), sham urinary diversion (sham), urinary diversion (UD), streptozotocin (STZ)-induced diabetes after sham urinary diversion (DM), STZ-induced diabetes after urinary diversion (UD+DM), and 5% sucrose-induced diuresis after sham urinary diversion (DIU). DM was induced by a single intraperitoneal injection of STZ dissolved in 0.1 M citrate buffer, 35mg/kg. Animals with blood glucose >300mg/dl 72hr after STZ injection were classified as diabetic. Diuresis was induced by adding 5% sucrose to the rats’ normal drinking water. UD or sham procedures were performed 10d prior to administering STZ injection, 5% sucrose water, or STZ vehicle (citrate buffer). UD was performed by surgical ureterovaginostomy as described recently by us11. The sham procedure included laparotomies and the identification of ureters. No surgery was performed in age-matched controls. To prevent excessive hyperglycemia, weight loss and ketonuria, DM rats received one-sixth (or more when needed) of Linplant sustained-release insulin implants (LinShin, Toronto, Canada) every 2 months. Our previous experience demonstrated that this protocol allows us to maintain healthy DM rats. Blood glucose was measured weekly, and glycated hemoglobin (HbA1c) (in2it™ analyzer, Bio-Rad, Hercules, CA) every 4 wks.
Each group was evaluated for 24-hr drinking and voiding volumes or conscious cystometry (CMG) 20 wks after DM or diuresis induction. The bladders from half of the animals (n=6 per group) were removed at the level of the bladder neck, weighed, and sectioned at the equatorial midline. The top half of the bladder was allowed to equilibrate for 15 min at room temperature in physiological buffer and then fixed in 10% formalin for morphological examination and quantification of smooth muscle, urothelium, and collagen. The bladders from the remaining animals (n=6 per group) were harvested for evaluation of OS indirectly by measuring the levels of nitrotyrosine and manganese superoxide dismutase (MnSOD) in bladder. All of the animals were sacrificed by injection of pentobarbital (200 mg/kg, ip). The experimental protocol was approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Urinary diversion
UD was performed by surgical disconnection of the ureters from the bladder and implantation to the uterine cervix as described previously by us11. The ureters were identified and ligated distally. Two small orifices were made using a drill (Dremel, Model 770, Racine, WI) in the uterus cervix and 1–2 mm of ureter was brought through the orifice and secured to the cervix with one suture close to the medial side (3 or 9 o’clock point). The abdomen was closed by simple suturing.
24-hour drinking and voiding volume measurement
Twenty-four hour drinking and voiding volumes were measured as described previously5. Briefly, rats were placed in individual metabolic cages (Nalgene, Nalge Company, Rochester, NY). After 24-hr acclimation, a known volume of water or 5% sucrose was placed in the drinking bottles. Clean plastic beakers were used to collect urine. At the end of 24hr, the remaining volume in the drinking bottles and voiding volumes were measured. The consumed volume was calculated.
Suprapubic bladder catheter implantation and conscious CMG measurement
Catheter implantation was performed as described previously11. The bladder was exposed and a circular purse string suture of 7-0 silk was placed on the bladder wall. A small incision was made in the bladder wall and the catheter (PE-50 tubing with a flared tip) was implanted; a purse string suture was tightened around the catheter. The catheter was tunneled subcutaneously and externalized at the back of the neck. The distal end of the tubing was sealed, and the incisions were closed.
CMG was performed 2d later. The bladder was filled via catheter with 0.9% saline (10ml/hr for DM and DIU groups, 5 ml/hr for other groups12) while bladder pressure was recorded. The data on at least 5 representative micturition cycles were collected and means were calculated for analyzing the CMG parameters including intercontraction interval, peak pressure, and voided volume. In addition, functional capacity (multiply intercontraction interval by infusion rate) and bladder compliance (functional capacity divided by the difference between basal and threshold pressures) were calculated.
Bladder fixation, staining and image analysis
The bladder cross-sections at the equatorial midline were sectioned and stained with Masson’s trichrome. The sections were analyzed with Image-Pro Plus 5.1 image analysis software (Media Cybernetics, Bethesda, MD)5. This software can distinguish regions stained with different colors and can accurately measure such areas by counting the pixels and converting pixels to square millimeters. This color segmentation method was employed to determine the whole cross-sectional area and the tissue areas that were stained “pink” (urothelium), “blue” (collagen), and “red” (smooth muscle). The percentages of each component in the total tissue area were calculated.
Immunoblotting
Proteins were separated by SDS-PAGE, then transferred to nitrocellulose membranes, probed with a primary antibody (anti-nitrotyrosine [sc-65385, 1:200], rabbit anti-MnSOD [sc-30080, 1:500], Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and then incubated with the secondary antibodies. The membrane was also incubated with mouse anti β-actin antibody (A1978, 1:5000, Sigma-Aldrich, St Louis, MO). Band intensities were evaluated using Scion Image Beta 4.02 software (Scion Corporation, Frederick, MD). The intensity of each target band was divided by that of the β-actin band of the same sample, and then expressed relative to the age-matched control group.
Statistical analysis
All data are expressed as the mean ± standard error of the mean. Comparisons of intercontraction interval in CMG measurements were made using two-tailed t test because of the different infusion rate. All other comparisons among 6 groups were performed using one-way analysis of variance followed by multiple pair-wise post hoc comparisons using Prism 4 (GraphPad, La Jolla, CA). P<0.05 was established as statistically significant.
RESULTS
General characteristics
The initial body weight was similar among the six groups (Table 1). Twenty wks after induction, the DM and UD+DM groups weighed less than the control, sham, and DIU groups. The mean blood glucose levels of the DM and UD+DM rats were approximately 4 times higher than control, sham, and DIU rats.
Table 1.
General characteristics of experimental animals.
| Initial weight (g) |
Final weight (g) |
Blood glucose (mg/dl) |
HbA1C (%) |
Bladder weight (mg) |
|
|---|---|---|---|---|---|
| Control | 245.8±4.6 | 310.8±8.0 | 138.3±12.4 | 5.0±0.1 | 86.8±3.0 |
| Sham | 249.1±4.5 | 328.3±13.3 | 131.6±10.8 | 4.9±0.2 | 110.2±8.4 |
| DIU | 249.6±4.3 | 340.2±10.9 | 137.3±13.1 | 5.1±0.1 | 203.2±6.0c |
| DM | 246.7±2.4 | 260.3±3.7a | 486.6±25.4a | 11.9±0.3a | 245.5±11.3c |
| UD | 251.5±4.2 | 304.0±6.5 | 120.5±8.0 | 5.0±0.2 | 56.0±4.4d |
| UD+DM | 251.7±3.5 | 282.3±4.6b | 546.0±41.8a | 12.3±0.8a | 50.5±3.1d |
Values are expressed as means plus or minus standard error of the mean of 6 individual rats.
significantly different from corresponding value in control, sham, DIU and UD groups (P <0.01).
significantly different from corresponding value in sham and DIU groups (P <0.05).
significantly different from corresponding value in control, sham, UD and UD+DM groups (P <0.01).
significantly different from corresponding value in control, sham, DIU, and DM groups (P <0.01).
Abbreviations: DIU, 5% sucrose-induced diuretics after sham urinary diversion; DM, diabetes mellitus; UD, urinary diversion.
24-hour drinking and voiding volume
There were no significant differences in 24-hr drinking and voiding volumes among control, sham, and UD rats (Table 2). Those animals drank between 34–55 ml and voided between 16–28 ml within a 24-hr period. However, DIU, DM, and UD+DM rats showed significantly increased 24-hr drinking volumes (about 5 times more) and urine output (about 10 times more) compared with control, sham, and UD rats. In addition, drinking and voiding volumes were higher in the UD+DM group compared to the DM group, possibly due to better glucose control in the latter group (Table 1).
Table 2.
24-hour drinking and voiding volumes.
| Drinking volume (ml) |
Voiding volume (ml) |
|
|---|---|---|
| Control | 34.6±3.5 | 16.3±2.1 |
| Sham | 35.2±2.1 | 19.2±2.2 |
| DIU | 130.4±15.5a | 110.1±12.5a |
| DM | 181.0±25.0a | 171.3±28.1a |
| UD | 55.8±7.9 | 28.0±5.0 |
| UD+DM | 257.5±30.4a | 224.0±28.1a |
Values are expressed as means plus or minus standard error of the mean of 6 individual rats.
significantly different from corresponding value in control, sham, and UD groups (P <0.01).
Abbreviations: DIU, 5% sucrose-induced diuretics after sham urinary diversion; DM, diabetes mellitus; UD, urinary diversion.
CMG measurement
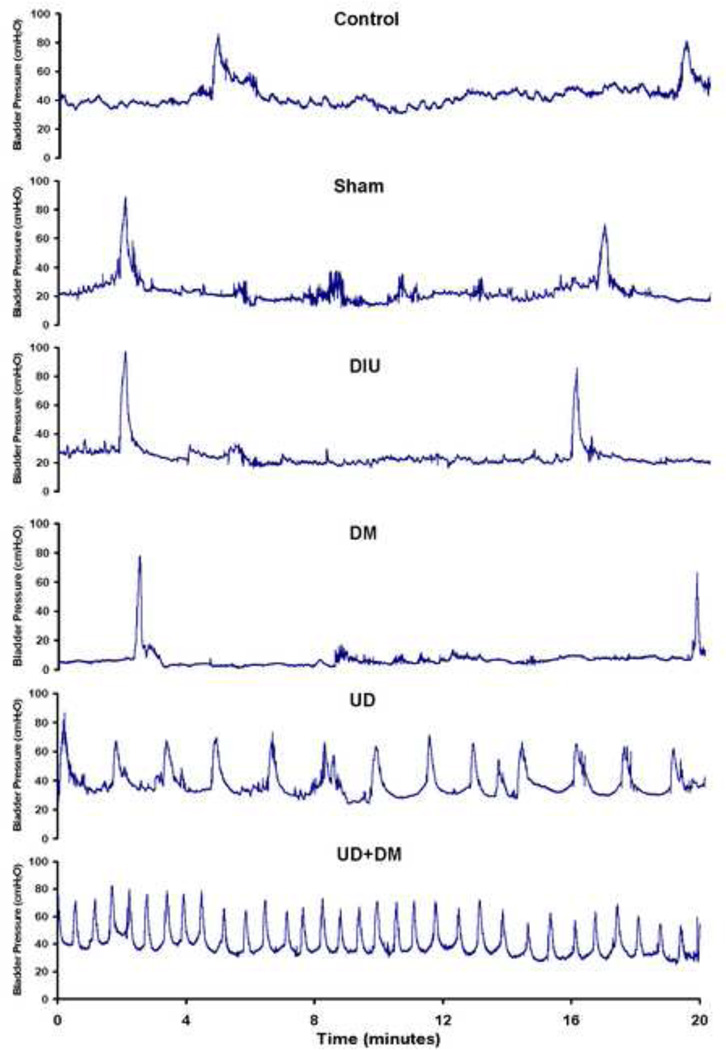
All rats showed a regular and periodic emptying of the bladder assessed by concious CMG (Figure 1). DM and DIU rats had significantly increased voided volumes and compliance compared with the control, sham, UD, and UD+DM groups, whereas no significant differences were found between the DM and DIU groups (Table 3). UD rats had a decreased intercontraction interval, voided volume and compliance compared with the control and sham groups, furthermore the UD+DM rats showed a significant decrease compared with UD group. There were no significant differences in peak pressure among the six groups.
Figure 1.
Representive tracings of conscious cystometrogram (CMG) from control, sham, DIU, DM, UD, and UD + DM rats 20 wk after DM or diuresis induction (from top to bottom). The infusion rate is 5 ml/hr for control, sham, UD, and UD + DM rats; 10 ml/hr for DIU and DM rats. Abbreviations: DIU, 5% sucrose-induced diuretics after sham urinary diversion; DM, diabetes mellitus; UD, urinary diversion.
Table 3.
Parameters of bladder activity during CMG.
| Intercontraction Interval (sec) |
Functional Capacity (ml) |
Peak Pressure (cmH2O) |
Voided Volume (ml) |
Compliance (ml/cmH2O) |
|
|---|---|---|---|---|---|
| Control | 711.3±51.9* | 0.99±0.07 | 67.8±10.9 | 0.93±0.08 | 0.054±0.011 |
| Sham | 907.7±112.4* | 1.26±0.16 | 70.3±8.9 | 1.24±0.16 | 0.091±0.022 |
| DIU | 955.6±99.6# | 2.65±0.28c | 80.4±7.1 | 2.67±0.37c | 0.166±0.023c |
| DM | 1022.2±123.5# | 2.84±0.34c | 72.8±9.9 | 2.91±0.28c | 0.195±0.027c |
| UD | 103.4±9.3*a | 0.14±0.01d | 59.0±6.1 | 0.15±0.02d | 0.012±0.001d |
| UD+DM | 50.9±5.1*b | 0.07±0.01e | 65.5±3.4 | 0.10±0.01e | 0.006±0.001e |
infusion rate is 5 ml/hr;
infusion rate is 10 ml/hr.
Values are expressed as means plus or minus standard error of the mean of 6 individual rats.
significantly different from corresponding value in control, sham and UD+DM groups (P <0.01).
significantly different from corresponding value in control, sham and UD groups (P <0.01).
significantly different from corresponding value in control, sham, UD and UD+DM groups (P <0.01).
significantly different from corresponding value in control, sham, DIU, DM and UD+DM groups (P <0.01).
significantly different from corresponding value in control, sham, DIU, DM and UD groups (P <0.01).
Abbreviations: DIU, 5% sucrose-induced diuretics after sham urinary diversion; DM, diabetes mellitus; UD, urinary diversion.
Morphometric analysis
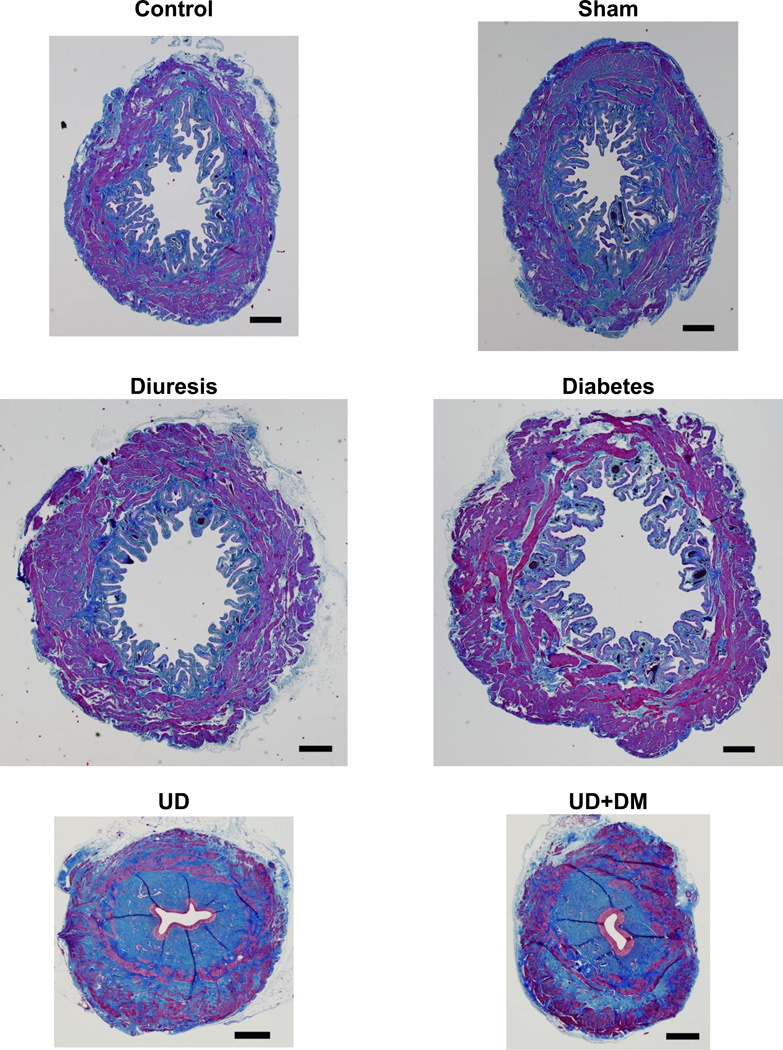
Morphologically, the bladders in the control and sham groups were similar (Figure 2). Bladder hypertrophy, lumen dilation, and lumenal folds were noted in the DM and DIU groups. In contrast, the UD and UD+DM rat bladders had smaller lumens with conspicuously inapparent folds, and showed obvious bladder atrophy. The total cross-sectional bladder wall areas at the equatorial midline were significantly increased in the DM and DIU groups and decreased in UD and UD+DM groups (Table 4). When expressed as percentage of the total tissue area, the percentages of urothelium and detrusor muscle decreased in the UD and UD+DM groups.
Figure 2.
Representative images of Masson’s trichrome staining of equatorial sections of urinary bladders from control, sham, DIU, DM, UD, and UD + DM rats 20 wk after DM or diuresis induction, showing smooth muscle (outer magenta), collagen (blue), and urothelium (inner light magenta). Scale bar, 500 µm. Abbreviations: DIU, 5% sucrose-induced diuretics after sham urinary diversion; DM, diabetes mellitus; UD, urinary diversion.
Table 4.
Changes of urothelium, collagen, and smooth muscle areas in the bladder.
| Tissue cross-sectional area (mm2) | Percentage of the total tissue cross-sectional area (%) |
||||||
|---|---|---|---|---|---|---|---|
| Total | Collagen | Muscle | Urothelium | Collagen | Muscle | Urothelium | |
| Control | 9.83±0.50 | 3.55±0.13 | 4.10±0.17 | 0.65±0.06 | 36.4±2.3 | 41.9±1.7 | 6.7±0.6 |
| Sham | 8.64±0.91 | 3.45±0.25 | 3.63±0.26 | 0.52±0.06 | 40.3±1.3 | 42.5±1.9 | 6.1±0.3 |
| DIU | 13.10±1.00a | 3.96±0.23 | 6.30±0.59a | 0.96±0.09a | 30.6±1.2c | 48.0±1.6 | 7.4±0.7 |
| DM | 14.34±0.52a | 4.07±0.24 | 6.75±0.29a | 1.10±0.13a | 28.4±1.6c | 47.1±1.7 | 7.6±0.7 |
| UD | 6.46±0.40b | 3.18±0.27 | 1.92±0.11b | 0.22±0.03b | 49.2±2.9b | 30.0±1.7b | 3.3±0.4b |
| UD+DM | 6.10±0.28b | 3.14±0.17 | 1.93±0.07b | 0.18±0.01b | 51.5±1.8b | 31.8±0.9b | 2.9±0.2b |
Values are expressed as means plus or minus standard error of the mean of 6 individual rats.
significantly different from corresponding value in control, sham, UD and UD+DM groups (P <0.01).
significantly different from corresponding value in control, sham, DM, and DIU groups (P <0.01)
significantly different from corresponding value in sham, UD and UD+DM groups (P <0.01).
Abbreviations: DIU, 5% sucrose-induced diuretics after sham urinary diversion; DM, diabetes mellitus; UD, urinary diversion.
Immunoblotting
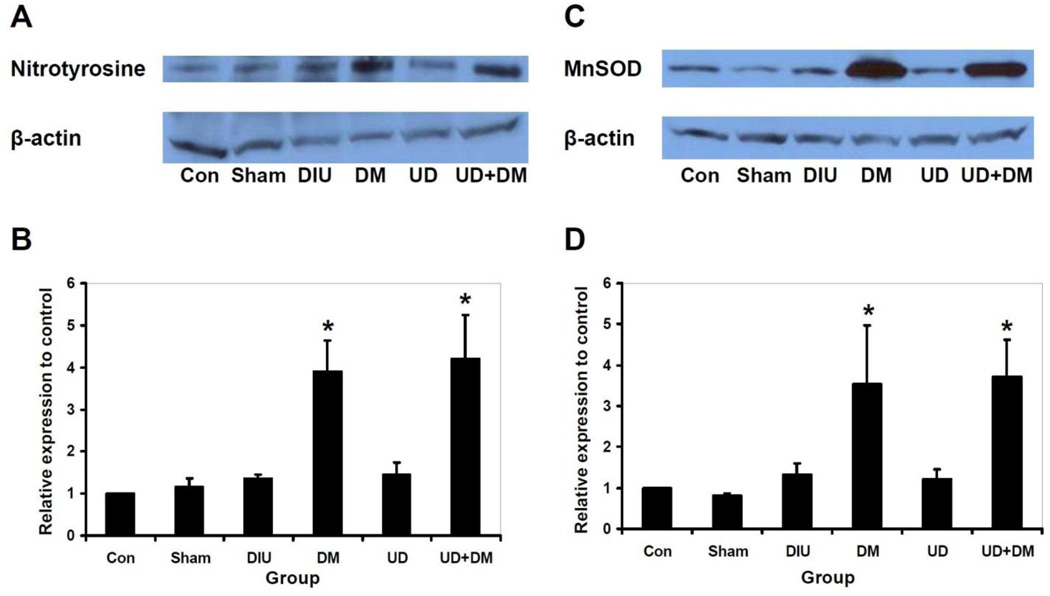
Immunoblotting revealed no significant differences in nitrotyrosine and MnSOD among control, sham, DIU, and UD groups (Figure 3). However, nitrotyrosine and MnSOD levels increased in the DM and UD+DM groups compared with other groups.
Figure 3.
Representative immunoblotting results and quantitative analysis of nitrotyrosine (A, B) and MnSOD (C, D) levels in the bladder in control, sham, DIU, DM, UD and UD + DM rats 20 wk after DM or diuresis induction (n=4 per group). Each lane was from a single rat. Quantitative data are presented as mean plus standard error of the mean. Asterisks indicate significant differences compared with the value of control, sham, DIU and UD groups (P<0.05). Abbreviations: DIU, 5% sucrose-induced diuretics after sham urinary diversion; DM, diabetes mellitus; UD, urinary diversion.
DISCUSSION
The current data supports earlier work indicating that polyuria can induce bladder hypertrophy independently. The bladder weight increased in the DIU and DM groups compared with control and sham groups, but not in the UD+DM group compared with UD group, suggesting that hyperglycemia-induced polyuria is the only reason for bladder hypertrophy in DM. The details of the pathways leading to the increase in bladder mass in response to polyuria need further investigation, but it is clear that the bladder remodels in response to alterations of urine volume and rate of filling of the bladder, and perhaps to the osmolality of the urine. Regardless of the initiating step, changes induced by polyuria can stimulate DNA synthesis, which in turn results in increased cell mass and hyperplasia13.
CMG results showed that the functional bladder capacity, voided volume, and compliance increased in both the DIU and DM groups. These similarities between the DM and DIU group suggest that the altered bladder function in diabetic animals resulted mostly from polyuria. However, the similarities in the above CMG parameters between the DM and DIU groups do not preclude additional effects of hyperglycemia. Unfortunately, we did not collect residual volume data on these animals and do not know their true bladder capacities. Therefore, we cannot comment on decreased voiding efficiency in DM rats compared with DIU rats. The data from UD versus UD+DM provided some clues about the effects of hyperglycemia on bladder function. We found significant decreases in intercontraction intervals, functional bladder capacity, voided volume, and compliance in UD+DM group compared with UD group, suggesting that these differences resulted only from hyperglycemia in the UD+DM rats.
The altered bladder function can be explained partially by the morphological findings and changes in the tissue components. In control animals, collagen accounted for approximately one third of the bladder wall mass, and the detrusor muscle was more than 40%. An appropriate ratio of smooth muscle and connective tissues maintains appropriate bladder compliance14. The observed reduced detrusor muscle mass and resulting increased collagen percentage likely contributed to the reduced compliance in UD and UD+DM rats, whereas the disproportionately increased muscle mass contributed to increased compliance in the DIU and DM groups. In the functional study, we found that UD+DM rats had significantly decreased voided volume and compliance compared to UD group, illustrating the effects of hyperglycemia. However, we did not find evidence of morphological differences in UD versus UD+DM animals, except the trend of reduced urothelium folds and urothelium tissue area in UD+DM compared with UD rats. The molecular constituents of the extracellular matrix (ECM) were likely altered in the bladders of UD+DM rats, which led to the observed functional differences between the UD+DM and UD rats. The impact of DM on the ECM of vascular walls has been well studied with changes including shifted collagen subtype, increased collagen, and decreased elastin15–17. The ECM in the bladder is made up primarily of types I and III collagen18. A shift from type I to type III collagens was found in patients with neurogenic bladders and obstructed human bladders19. In addition, cellular- and molecular-level changes in neurogenic (nerve innervation), myogenic (contraction related proteins), and urogenic (sensory function related molecules) components may result specifically from hyperglycemia. Further molecular level investigations of the changes in ECM and other molecular constituents in the above animal models are needed to clarify the effects of polyuria and hyperglycemia on the remodeling and function of the bladder in diabetes.
The effects of long-term DM-associated OS have been recognized as important pathogenic components of diabetic complications9,10. Diabetes has been associated with altered mitochondrial morphology20, followed by uncoupled mitochondria, with increased electron leakage and generation of reactive oxygen species (ROS)21. Several previous studies have reported a plausible role of OS in the pathogenesis of DBD4,22,23. However, it is not clear if the OS is induced by hyperglycemia alone or if polyuria also plays a role in this process. Nitration of tyrosine residues on proteins is mediated by reactive nitrogen species such as peroxynitrite anion and nitrogen dioxide. Nitrotyrosine has been detected in a large number of pathological conditions and is considered a marker of NO-dependent, reactive nitrogen species-induced nitrative stress24. On the other hand, the extent of damage due to oxygen radicals is largely determined by the activity of free radical scavengers, such as superoxide dismutases (SOD). MnSOD is the most important isozyme in DM because mitochondrial respiration is the major source of hyperglycemia-induced intracellular ROS production25,26. We found increases in nitrotyrosine and MnSOD in DM and UD+DM rats but no significant changes among control, sham, DIU, and UD groups. These data suggest that hyperglycemia alone leads to OS-induction in bladder. The increase in MnSOD levels is probably due to the compensatory response of tissues to excessive OS products. Antioxidant enzymes are frequently upregulated with increased OS. A previous study using microarray analysis also showed upregulation of mitochondrial enzymes involved in antioxidation (MnSOD, chloramphenicol acetyl transferase and glutathione reductase) in the bladder of DM animals27. Hyperglycemia-induced OS may play an important role in the later stage of DBD. OS causes alterations of proteins, lipids and DNA, leading to organ dysfunction. Using a rabbit model of alloxan-induced diabetes, the decrease in the contractility of the detrusor smooth muscle has been shown to be associated with increased lipid peroxidation products22.
A limitation of using the UD model to observe the effects of hyperglycemia on the bladder is that the atrophied state of the bladder in UD could mask or enhance the specific effects of hyperglycemia. Masking could occur as a result of the significant decreases in detrusor muscle and urothelium masses, which may weaken the effects of hyperglycemia. Enhancement could result from the probable inability of the atrophied bladder to defend against the insults of hyperglycemia as efficiently as a normal or hypertrophied bladder.
CONCLUSIONS
Our findings suggest that polyuria and hyperglycemia independently contribute to the pathogenesis of DBD. Polyuria causes significant bladder hypertrophy, which is also seen in induced diuresis, whereas chronic hyperglycemia induces OS in the bladder, which may play an important role in the failure of bladder function seen in late stage of DBD.
ACKNOWLEDGMENTS
We thank Kerry O. Grimberg, PhD and C. Thomas Powell, PhD for their medical editorial assistance. Nan Xiao was partially supported by The Postgraduate Study Abroad Scholarship Program from the China Scholarship Council.
Grant: This study was supported by the American Diabetes Association (ADA) (Grant #1-10-JF-29).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Frimodt-Moller C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med. 1980;92:318. doi: 10.7326/0003-4819-92-2-318. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995;153:342. doi: 10.1097/00005392-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Overcoming Bladder Disease: A Strategic Plan for Research. A report of the bladder research progress review group, National Institute of Diabetes and Digestive and Kidney Diseases. Chapter 10. National Institues of Health; 2002. Bladder Research Progress Review Group: Urologic complications of diabetes mellitus. [Google Scholar]
- 4.Daneshgari F, Liu G, Birder L, Hanna-Mitcell AT, Chacko S. Diabetic bladder dysfunction: Current translational knowledge. J Urol. 2009;182:S18. doi: 10.1016/j.juro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R837. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- 6.Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006;176:380. doi: 10.1016/S0022-5347(06)00582-9. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Daneshgari F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol. 2005;288:F1220. doi: 10.1152/ajprenal.00449.2004. [DOI] [PubMed] [Google Scholar]
- 8.Liu G, Li M, Vasanji A, Daneshgari F. Temporal diabetes and diuresis-induced alteration of nerves and vasculature of the urinary bladder in the rat. BJU Int. 2011;107:1988. doi: 10.1111/j.1464-410X.2010.09840.x. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 10.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Lin YH, Li M, Xiao N, Daneshgari F. Temporal morphological and functional impact of complete urinary diversion on the bladder: a model of bladder disuse in rats. J Urol. 2010;184:2179. doi: 10.1016/j.juro.2010.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G, Lin YH, Yamada Y, Daneshgari F. External urethral sphincter activity in diabetic rats. Neurourol Urodyn. 2008;27:429. doi: 10.1002/nau.20543. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 14.Levin RM, Haugaard N, Levin SS, Buttyan R, Chen MW, Monson FC, et al. Bladder function in experimental outlet obstruction: pharmacologic responses to alterations in innervation, energetics, calcium mobilization, and genetics. Adv Exp Med Biol. 1995;385:7. doi: 10.1007/978-1-4899-1585-6_3. [DOI] [PubMed] [Google Scholar]
- 15.McDonald TO, Gerrity RG, Jen C, Chen HJ, Wark K, Wight TN, et al. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. J Histochem Cytochem. 2007;55:1149. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sainio A, Jokela T, Tammi MI, Jarvelainen H. Hyperglycemic conditions modulate connective tissue reorganization by human vascular smooth muscle cells through stimulation of hyaluronan synthesis. Glycobiology. 2010;20:1117. doi: 10.1093/glycob/cwq076. [DOI] [PubMed] [Google Scholar]
- 17.Searls Y, Smirnova IV, Vanhoose L, Fegley B, Loganathan R, Stehno-Bittel L. Time-dependent alterations in rat macrovessels with type 1 diabetes. Exp Diabetes Res. 2012;2012:278620. doi: 10.1155/2012/278620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson K, Kucich U, Whitbeck C, Levin RM, Howard PS. Functional changes in bladder tissue from type III collagen-deficient mice. Mol Cell Biochem. 2006;283:107. doi: 10.1007/s11010-006-2388-1. [DOI] [PubMed] [Google Scholar]
- 19.Deveaud CM, Macarak EJ, Kucich U, Ewalt DH, Abrams WR, Howard PS. Molecular analysis of collagens in bladder fibrosis. J Urol. 1998;160:1518. [PubMed] [Google Scholar]
- 20.Searls Y, Smirnova IV, Vanhoose L, Fegley B, Loganathan R, Stehno-Bittel L. Time-dependent alterations in rat macrovessels with type 1 diabetes. Exp Diabetes Res. 2012;2012:278620. doi: 10.1155/2012/278620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokini Z, Marcovecchio ML, Chiarelli F. Molecular pathology of oxidative stress in diabetic angiopathy: role of mitochondrial and cellular pathways. Diabetes Res Clin Pract. 2010;87:313. doi: 10.1016/j.diabres.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Changolkar AK, Hypolite JA, DiSanto M, Oates PJ, Wein AJ, Chacko S. Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J Urol. 2005;173:309. doi: 10.1097/01.ju.0000141583.31183.7a. [DOI] [PubMed] [Google Scholar]
- 23.Beshay E, Carrier S. Oxidative stress plays a role in diabetes-induced bladder dysfunction in a rat model. Urology. 2004;64:1062. doi: 10.1016/j.urology.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 26.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Kanika ND, Chang J, Tong Y, Tiplitsky S, Lin J, Yohannes E, et al. Oxidative stress status accompanying diabetic bladder cystopathy results in the activation of protein degradation pathways. BJU Int. 2011;107:1676. doi: 10.1111/j.1464-410X.2010.09655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]