Summary
Site-specific incorporation of unnatural amino acids into proteins in vivo relies on the genetic reassignment of nonsense or quadruplet codons. Here, we describe a general procedure for the random introduction of these codons into open reading frames resulting in protein libraries that are scanned with unnatural amino acid residues. These libraries can enable large-scale mutagenesis experiments aimed at understanding and improving protein function.
Keywords: Scanning mutagenesis, tRNA, Mu transposon, Codon, Unnatural amino acids
1. Introduction
More than 50 different unnatural amino acids with distinctive properties have been added to the genetic codes of bacteria, yeasts and mammalian cells (1). Central to this methodology is the reassignment of nonsense or quadruplet codons using orthogonal aminoacyl-tRNA synthetase/tRNA pairs. These chemical tools allow one to control protein functions with light (2), detect transient protein-protein interactions (3), perform protein bio-conjugation reactions (4, 5), among other tasks. While the unnatural amino acid mutagenesis methodology is generally efficient, large-scale mutagenesis experiments for scanning unnatural amino acids is hindered by the generation of genes containing random nonsense or quadruplet codons. Indeed some unnatural amino acids that have been genetically encoded would be even more useful if one could randomly distribute them throughout protein sequence space.
We and others have described new approaches to generating collections of proteins that contain single amino acid mutations located in random positions (6–8). These libraries are rationally diversified and the mutation in question can be any genetically encoded amino acid. Here, we provide detailed experimental methods for scanning unnatural amino acid mutagenesis that can be applied to any protein of interest. This is a sequential method that consists of creating a small library of open reading frames in which each member contains a random, single and in-frame amber stop codon TAG (or another nonsense codon). The mutants can be separated and expressed individually with desired unnatural amino acids. Alternatively, the mutant library can be expressed with unnatural amino acid as a mixed population, which can be subsequently used for functional screening or genetic selection. The protocol from start to finish can be expected to take approximately two weeks to complete.
2. Materials
Intein targeting plasmid (pIT) (6).
Entranceposon (M1-CamR) (Finnzymes, Espoo, Finland) for PCR template (see Figure 1 and Note 1).
DNA containing gene of interest (see Note 2).
pInSALect vector (9).
Appropriate expression vector (see Note 3).
MuA transposase and 10× transposon reaction buffer (Finnzymes, Espoo, Finland), stored at −20 °C.
10 mM Tris-HCl buffer, pH 8.5 at 25 °C.
TE buffer, 10 mM Tris-HCl buffer, 0.1 mM EDTA, pH 8.5 at 25 °C.
- Primers (MlyI restriction sites underlined and reverse complement of TAG codon in italic).
- -
-
-Forward and reverse primers for cloning the gene of interest from the pIT vector into a desired expression vector.
-
-MlyI transposon 5'-gcttagatctgactcggcgcacgaaaaacgcgaaag-3’.
-
-Linker FWD 5'Phos-ggatcgactctcctgggtattcgcaataatcttaatactgag-3’.
-
-Linker REV 5'Phos-ctagatctgactcaattaccaatgcttaatcagtgaggcacct-3’ (see Note 6).Dissolve primer stocks in TE buffer at a final concentration of 100 µM and store at −20 °C. Dilute to 10 µM prior to use.
dNTP stock solution, 10 mM total, 2.5 mM each, stored as 20 µL aliquots at −20 °C.
Phusion DNA polymerase (New England Biolabs, Ipswich, MA), stored at −20 °C.
Restriction enzymes Sal I, BamH I, Bgl II, FastDigest Mly I and appropriate buffers (Fermentas, Glen Burnie, MD). Store at −20 °C.
T4 DNA ligase (Fermentas), stored at −20 °C.
0.7% and 1% agarose gels.
QIAquick PCR purification kit (Qiagen, Valencia, CA).
QIAEX II gel extraction kit (Qiagen, Valencia, CA).
Chemically or electro-competent Escherichia coli cells for cloning, such as DH10B. Store at −80 °C.
Chemically or electro-competent Escherichia coli cells for expression, such as BL21 (DE3). Store at −80 °C.
1000× Antibiotic stock solutions: ampicillin (50 mg/mL), kanamycin (50 mg/mL) and chloramphenicol (35 mg/mL).
1000× reagents for protein induction, such as 1 M isopropyl β-D-1-thiogalactopyranoside (IPTG) or 20% arabinose).
Unnatural amino acid, sufficient to make media at 2 mM final concentration.
Luria Bertani (LB) medium (1 L): 10 g tryptone, 5 g yeast extract and 10 g sodium chloride.
SOC medium (1 L): 20 g tryptone, 5 g yeast extract, 10 mM sodium chloride, 25 mM potassium chloride. Add magnesium chloride to 10 mM and glucose to 20 mM after autoclaving.
2×YT medium (1 L): 16 g tryptone, 10 g yeast extract and 5 g sodium chloride, pH 7.2.
LB agar plates with appropriate antibiotics.
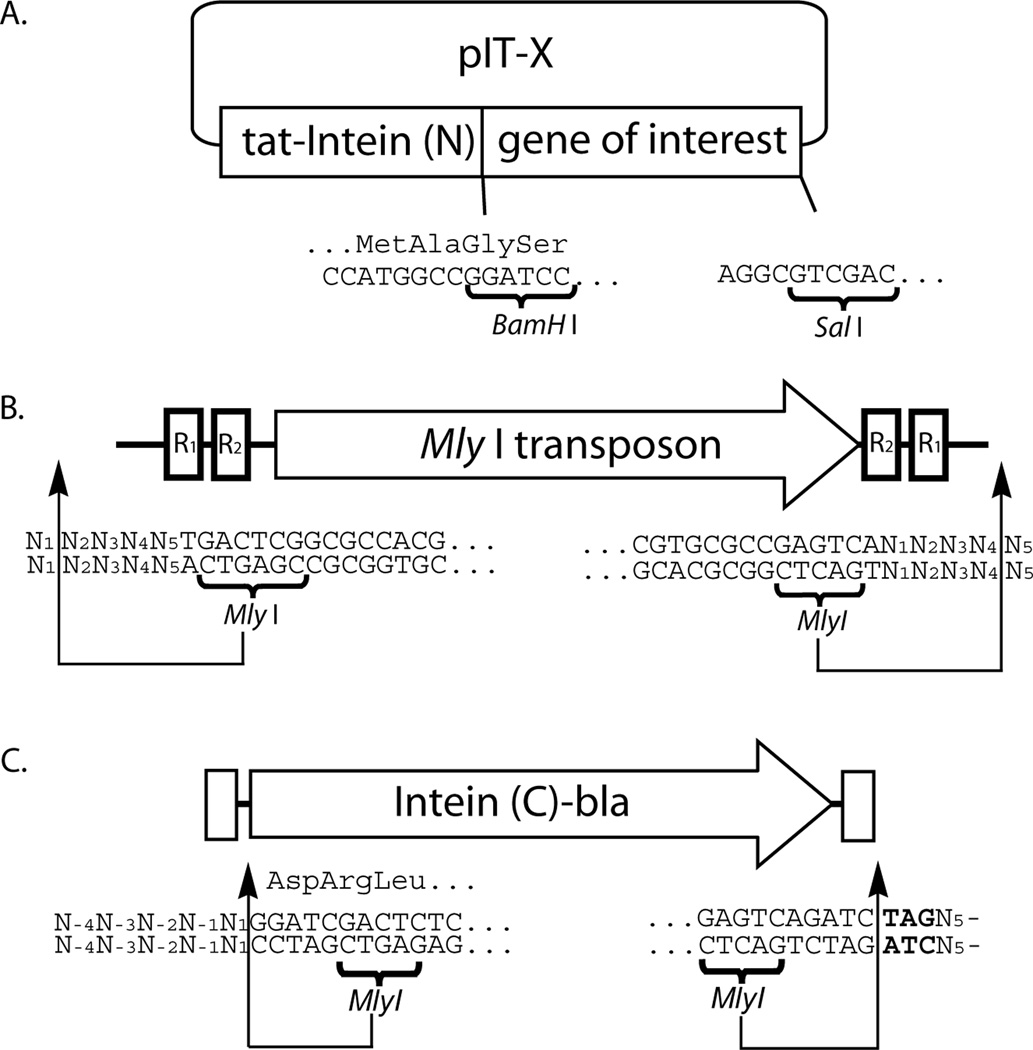
Figure 1.
Schematic diagram showing the DNA components described in this protocol with nucleotides numbered relative to original transposon insertion site. A) The cloning site of pIT-X indicating correct reading frame. Oligonucleotides used for protein of interest must match the correct reading frame. B) Mly I transposon showing positioning of restriction sites as it would insert into a segment of DNA. C) Reading-frame selectable linker showing restriction sites, frame, and amber stop codon scar in bold.
3. Methods
3.1. Generating a pIT-X vector containing gene of interest
Digest 2 µg of the pIT vector (6) using BamH I and Sal I. Gel-purify the 3.2 Kbp fragment using QIAEX II gel extraction kit (see Note 7).
Amplify the gene of interest using PCR to introduce a BamH I and Sal I at the 5' and 3'-ends, respectively.
Purify the PCR product using with the QIAquick PCR purification kit.
Digest the PCR products using BamH I and Sal I and gel-purify the digestion products using the QIAEX II gel extraction kit.
Ligate the purified digest of pIT vector and PCR product using T4 DNA ligase. Name the recombinant plasmid as pIT-X (see Note 8).
3.2. Transposition reaction
Prepare the Mly I transposon DNA by PCR as follows: 10 ng Entranceposon (M1-CamR) template, 0.2 mM dNTPs, 0.5 µM of primer MlyI transposon (which serves as the forward and reverse primer), 1× Phusion HF buffer (NEB) and 0.5 unit of Phusion DNA polymerase (NEB) in a 50 µL solution.
Incubate the reaction with the following cycle conditions: initial denaturation at 98 °C for 30 s, 30 cycles of 98 °C for 10 s, 60 °C for 30 s and 72 °C for 1 min and final extension at 72 °C for 10 min.
Purify the PCR product using with the QIAquick PCR purification kit.
Digest the Mly I transposon DNA as follows: 2 µg DNA, 10 U of Bgl II and 1×buffer O (Fermentas) in a 50 µL solution (see Note 9).
Load the digestion products on a 0.7% agarose gel and purify the 1.3 kbp fragment.
Load 1 or 2 µL of the purified transposon DNA on a 1% agarose gel and determine the concentration by comparison with a DNA standard.
Store the purified transposon DNA at −20 °C prior to use.
Perform a 20 µL transposition reaction with the following components: 400 ng of pIT-X plasmid DNA, 1.3 molar excess of Mly I transposon DNA (see Note 10), 1× reaction buffer (Finnzymes) and 1 U of MuA transpoase (Finnzymes).
Incubate the reaction at 30 °C for 4 hours.
Stop the reaction by adding SDS to a final concentration of 0.1% and heating at 75 °C for 10 min. Cool the reaction solution on ice.
Transform each 1 µL of products into 50 µL of electro-competent E. coli cells (see Note 11) and recover in 500 µL SOC at 37 °C for 1 hour.
Plate transformation cells on LB agar supplemented with 50 µg/mL kanamycin and 10 µg/mL chloramphenicol. Grow at 37 °C overnight.
For a gene of L bps, collect 9×(L+1,500) colonies from the transposition reaction. (see Note 12). Mix well the collected colonies in LB medium supplemented with 50 µg/mL kanamycin and 10 µg/mL chloramphenicol.
Save 5 tubes of 1 mL stock cells with an OD600 of >1.0 in 15% glycerol, stored at −80 °C (see Note 13). Extract the plasmid DNA from the remaining cells to build the pIT-X-transposon library.
3.3. Isolation of transposon insertions located in the gene of interest
Digest 2 µg of the transposon library DNA using Sal I and BamH I.
Load the digestion product on a 0.7% agarose gel and gel purify the two fragments using the QIAEX II gel extraction kit corresponding to the pIT vector backbone and the gene of interest with transposon insertions (see Note 14).
Ligate the two purified DNA fragments with T4 DNA ligase. Incubate at 16 °C for 16 hours. Inactivate the ligation product by heating at 70 °C for 10 min.
Transform the ligation products into electro-competent E. coli cells and plate cells on LB agar supplemented with 50 µg/mL kanamycin and 10 µg/mL chloramphenicol.
Collect 9×L colonies (see Note 15) and save cell stocks as above. Extract the plasmid DNA from the remaining cells to obtain the purified transposon library.
3.4. Creation of random triplet nucleotide deletions
Digest the purified transposon library DNA with Mly I as follows: 1 µg of DNA, 1 unit of FastDigest Mly I (Fermentas) (see Note 16) and 1×FastDigest buffer in 50 µL solution.
Incubate at 37 °C for 1 hour (see Note 17).
Load the digestion products on a 0.7% agarose gel and purify the larger fragment using the QIAEX II gel extraction kit. This corresponds to a linearized pIT-X library in which each DNA copy contains a random triplet nucleotide deletion in the gene of interest.
Load 1 or 2 µL of the purified deletion library DNA on a 0.7% agarose gel and determine the DNA concentration by comparison with DNA standard.
Store the purified DNA at −20 °C prior to use.
3.5. Linker ligation and reading-frame selection
Amplify the reading-frame selection linker by PCR as follows: 10 ng pInSALect template, 0.2 mM dNTPs, 0.5 µM of each primer LinkerFWD and LinkerFWD, 1× Phusion HF buffer (NEB) and 0.5 unit of Phusion DNA polymerase (NEB) in a total 50 µL solution.
Incubate the reaction with the following cycle conditions: initial denaturation at 98 °C for 30 s, 30 cycles of 98 °C for 10 s, 60 °C for 30 s and 72 °C for 1 min and final extension at 72 °C for 10 min.
Purify the PCR products with QIAquick PCR purification kit. Store at −20 °C prior to use (see Note 18).
Load 1 or 2 µL of the purified linker DNA on a 1% agarose gel and determine the concentration by comparison with a DNA standard.
Perform linker ligation in 100 µL total volume containing: 1 µg linker DNA, 500 ng of linearized pIT-X deletion library DNA, 1 µL of T4 DNA ligase and 1× T4 ligase buffer. This will produce a molar linker:library ratio of approximately 5:1. This can be adjusted appropriately given the size of pIT-X.
Incubate reaction at 16 °C for 16 hours. Heat-inactivate the ligation reaction at 70 °C for 10 min.
Pool all of the ligation product, ethanol-precipitate the DNA and resuspend in 10 µL of sterilized water.
Transform 1 µL of the concentrated ligation product into 50 µL electro-competent cells and recover in 500 µL SOC at 37 °C for 1 hour.
Plate cells on LB agar supplemented with 50 µg/mL kanamycin and 40 µg/mL ampicillin (see Note 19). Grow plates at 30 °C overnight (see Note 20).
Collect 9×L colonies and save stock cells as stated above. Extract plasmid DNA from remaining cells to obtain pIT-X-linker library. Store at −20 °C prior to use.
3.6. Generation of in-frame TAG mutations
Digest the linker-containing library DNA as follows: 1 µg of DNA, 1 unit of FastDigest Mly I (see Note 16) and 1×FastDigest buffer in 50 µL solution (see Note 21).
Incubate at 37 °C for 1 hour (see Note 17).
Load the digestion product on a 0.7% agarose gel and purify the larger fragment using QIAEX II gel extraction kit. This fragment corresponds to a linearized pIT-X library in which each DNA copy contains a random TAG codon mutation in the gene of interest.
Determine the concentration of purified TAG mutation library by comparison with a DNA standard on an agarose gel.
Perform a 10 µL intra-molecular ligation reaction as follows: ~20–30 ng of linearized TAG mutation library DNA, 1 µL T4 DNA ligase and 1× T4 ligase buffer.
Incubate reaction at 16 °C for 16 hours. Heat-inactivate ligase at 70 °C for 10 min.
Transform each 1 µL of the ligation products into electro-competent cells and recover at 37 °C for 1 hour.
Plate cells on LB agar supplemented with 50 µg/mL kanamycin. Grow at 37 °C overnight.
Collect 9×L colonies and save stock cells as stated above. Extract plasmid DNA from remaining cells to obtain pIT-X(TAG) mutation library. Store DNA at −20 °C.
Select individual colonies to verify mutations by DNA sequencing.
3.7. Cloning the TAG mutation library into an expression vector and production of mutant proteins
PCR amplify the TAG mutation library with primers appropriate for your expression vector and purify the PCR products with QIAquick PCR purification kit.
Digest the purified PCR products with desired restriction enzymes and gel-purify the digestion products using QIAEX II gel extraction kit.
Digest the desired expression vector using the same restriction enzymes and gel-purify the digestion products using QIAEX II gel extraction kit.
Ligate the purified PCR products and vector DNA using T4 DNA ligase as described above.
Incubate at 16 °C for 16 hours. Heat-inactivate the ligase at 70 °C for 10 min.
Transform the ligation products into electro-competent E. coli cells.
Plate cells on LB agar supplemented with the antibiotic corresponding to the selection marker in the expression vector.
Collect the 9×L colonies (see Note 15) and save stock cells as stated above. Extract the plasmid DNA from remaining cells (see Note 22).
Transform the collected library DNA into competent cells that are suitable for protein expression, such as those containing a pSUP plasmid for incorporation of unnatural amino acids (10) (see Notes 23 and 24).
Express the mutant proteins in liquid culture in the presence of desired unnatural amino acid at a final concentration of 2 mM. Depending on the ultimate use of the protein(s), screens or selections can be performed on individual clonal isolates or mixed populations.
Acknowledgements
The authors thank the National Institutes of Health (GM084396) for financial support. We are also grateful to Prof. Stefan Lutz (Emory University) for the plasmid pInSALect and Prof. Peter G. Schultz for the plasmid pSUP.
Footnotes
In this protocol, the Mly I transposon DNA is generated by PCR from Entranceposon (M1-CamR) DNA. Once constructed, this DNA can be inserted into a cloning vector such that it can be released from the vector by Bgl II digestion and contain a 5'-GATC overhang that is important for the transposon reaction (11). This will ensure correctly digested DNA is available for future use (Figure 1B).
The BamH I and Sal I sites in the genes of interest, if any, should be destroyed by site-directed mutagenesis for downstream manipulations.
The expression vector will be double transformed into an expression cell strain containing a plasmid for incorporation of unnatural amino acids (10). These plasmids contain a chloramphenicol selection marker and p15A origin of replication. Therefore any expression vector used for the protein of interest should have an alternative selection marker and origin of replication.
The forward primer should include a BamH I site before the start codon of the gene of interest. The number of oligonucleotides between the start codon and BamH I site should be adjusted such that the gene of interest will be in the same reading frame of the N-terminal fusion peptide from the pIT vector (Figure 1A).
The reverse primer should contain a Sal I site after the stop codon.
It is recommended to PAGE-purify this primer to eliminate truncated products. We have also found that including a phosphate at the 5'- end can increase the efficiency of linker ligation.
When gel slices are incubated with solubilization buffer (Qiagen), any temperature above 50 °C is not recommended. Higher temperatures denature double-stranded DNA and reduce the yield of gel-purification.
When inserted into pIT vector, the gene of interest is in-frame with an N-terminal fusion peptide (Figure 1A) which contains a Tat signal sequence and an N-terminal region of the VMA cis-splicing intein (VMA-N) (9).
It is not recommended to use excess DNA (e.g. > 2 µg) in Bgl II digestion. In our experience, excess DNA can result in incorrect or incomplete digestion products which will reduce the efficiency of transposon integrations.
Depending on the size of the target gene (and pIT-X), the amount of transposon DNA should be adjusted. It is important to maintain the molar excess below 2.0 to avoid formation of unstable complexes containing multiple transposon insertions.
When using competent cells with a transformation efficiency of 5×107 colonies/µg DNA, 20 µL of transposition products typically results in >80,000 colonies. Unused products can be stored at −20 °C for at least one month without any noticeable decrease of transformation yield.
To calculate the number of colonies required for full coverage, the number of allowed sites and site preference of transposon insertions must be considered. The number of insertion sites possible in the pIT vector backbone is 1,500 bps (2,900 of total length minus 600 bps of replication origin and 800 bps of kanamycin resistance gene). Therefore, there are (L+1,500) possible insertion sites for the pIT vector carrying a gene of L bps. Three-fold degeneracy 3×(L+1,500) of colonies are required to cover 95% of all the possible insertion events, assuming even distribution of insertions (12). To compensate for the site preference of transposon insertions, we assume that additional 3-fold degeneracy is sufficient to accommodate any intrinsic site preference of Mu transposase (13). Therefore, 3×3×(L+1,500) = 9×(L+1,500) colonies are sufficient to give a 95% coverage for a gene of L bps.
If needed, the whole tube (1 mL) of stock cells should be used for inoculation to maintain the library diversity.
As the transposon can insert both inside and outside of the gene of interest, a BamH I/Sal I digestion of the transposon library will result in four DNA fragments: i) vector backbone with transposon, ii) vector backbone, iii) gene of interest with transposon insertions and iv) gene of interest.
9L colonies is required for maintaining library diversity because transposon insertions outside of the gene of interest have been purged.
Under our experimental conditions, Fermentas FastDigest Mly I with FastDigest buffer cleaves DNA more precisely than NEB Mly I with NEBuffer 4 does, as judged by sequencing results of obtained mutants.
Longer incubation time is not recommended as overdigestion with Mly I can remove extra bases.
The PCR products of reading-frame selection linker contain the C-terminal region of VMA cis-splicing intein (VMA-C) and the β-lactamase gene (BLA), flanked by a Mly I site at each end and a TAG codon at the 3' end (Figure 1C).
When the linker DNA is inserted in-frame with the gene of interest, VMA-N from the pIT vector will be in the same reading-frame of VMA-C from the linker sequence. The VMA intein will self-splice and assemble the Tat signal peptide and BLA, which will subsequently confer cells ampicillin resistance.
Incubation at a reduced temperature of 30 °C is critical for correct intein-mediated splicing (9).
This Mly I digestion will release the linker sequence from the pIT-X library, leaving an in-frame TAG codon to fill the triplet nucleotide scar.
In the pIT-X plasmid, the N-terminal fusion peptide (Tat signal peptide and VMA-N) usually disrupts the native function of the protein of interest. Therefore, activity assay may not be directly performed under the context of pIT vector.
We have obtained the best results by creation of competent cells that already contain a plasmid for expressing a orthogonal aminoacyl-tRNA synthetase/tRNA pair. For example, one can transform BL21(DE3) with a pSUP plasmid and prepare electrocompetent cells using chloramphenicol selection. The TAG mutant library (in the expression vector) can then be transformed into these cells and selected for both plasmids. This approach results in higher protein expression yields and transformation efficiency.
We have found acceptable expression results using the pSUP series of plasmids(10) that express orthogonal aminoacyl-tRNA synthetase/tRNA pairs. Recently alternative plasmid systems(14) have been described that may result in improved yields depending on the protein being expressed. The choice of plasmid does not impact the methodology described here for TAG mutant library construction.
References
- 1.Cropp TA, Schultz PG. An expanding genetic code. Trends Genet. 2004;20:625–630. doi: 10.1016/j.tig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Wu N, et al. A genetically encoded photocaged amino acid. J Am Chem Soc. 2004;126:14306–14307. doi: 10.1021/ja040175z. [DOI] [PubMed] [Google Scholar]
- 3.Chin JW, et al. Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:11020–11024. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, et al. Addition of the keto functional group to the genetic code of Escherichia coli. Proc Natl Acad Sci U S A. 2003;100:56–61. doi: 10.1073/pnas.0234824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deiters A, et al. Site-specific PEGylation of proteins containing unnatural amino acids. Bioorg Med Chem Lett. 2004;14:5743–5745. doi: 10.1016/j.bmcl.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Daggett KA, Layer M, Cropp TA. A general method for scanning unnatural amino acid mutagenesis. ACS Chem Biol. 2009;4:109–113. doi: 10.1021/cb800271f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin AJ, et al. Expanded molecular diversity generation during directed evolution by trinucleotide exchange (TriNEx) Nucleic Acids Res. 2008;36:e77. doi: 10.1093/nar/gkn358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin AJ, et al. Expanded chemical diversity sampling through whole protein evolution. Mol Biosyst. 2009;5:764–766. doi: 10.1039/b904031e. [DOI] [PubMed] [Google Scholar]
- 9.Gerth ML, Patrick WM, Lutz S. A second-generation system for unbiased reading frame selection. Protein Eng Des Sel. 2004;17:595–602. doi: 10.1093/protein/gzh068. [DOI] [PubMed] [Google Scholar]
- 10.Ryu Y, Schultz PG. Efficient incorporation of unnatural amino acids into proteins in Escherichia coli. Nat Methods. 2006;3:263–265. doi: 10.1038/nmeth864. [DOI] [PubMed] [Google Scholar]
- 11.Haapa S, et al. An efficient and accurate integration of mini-Mu transposons in vitro: a general methodology for functional genetic analysis and molecular biology applications. Nucleic Acids Res. 1999;27:2777–2784. doi: 10.1093/nar/27.13.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick WM, Firth AE, Blackburn JM. User-friendly algorithms for estimating completeness and diversity in randomized protein-encoding libraries. Protein Eng. 2003;16:451–457. doi: 10.1093/protein/gzg057. [DOI] [PubMed] [Google Scholar]
- 13.Poussu E, et al. Probing the alpha-complementing domain of E. coli beta-galactosidase with use of an insertional pentapeptide mutagenesis strategy based on Mu in vitro DNA transposition. Proteins. 2004;54:681–692. doi: 10.1002/prot.10467. [DOI] [PubMed] [Google Scholar]
- 14.Young TS, et al. An enhanced system for unnatural amino acid mutagenesis in E. coli. J Mol Biol. 2010;395:361–374. doi: 10.1016/j.jmb.2009.10.030. [DOI] [PubMed] [Google Scholar]