Abstract
Peer antisocial behavior robustly predicts adolescents’ own behavior but not all adolescents are equally vulnerable to their peers’ influence and genetic factors may confer vulnerability. This study used data of n = 3081 adolescents from the Avon Longitudinal Study of Parents and Children (ALSPAC) to examine whether BDNF, a polymorphism that affects psychological functioning, moderates the association between affiliation with aggressive peers at age 10 and own aggression at age 15. A significant gene-environment interaction was found, where those who affiliated with aggressive peers in childhood showed increased risk for being aggressive in adolescence if they carried the BDNF met-met variant compared to val-val carriers. Our findings underline the importance of both biological and social factors for adolescent development.
Keywords: aggression, peers, BDNF, adolescence, ALSPAC
During adolescence youths spend increasingly less time being supervised by adults (Paikoff & Brooks-Gunn, 1991) and more time interacting with peers (Larson & Richards, 1991). This is also the period when individuals are most vulnerable to the influence of their peers (Brechwald & Prinstein, 2011; Steinberg & Monahan, 2007). Studies provide ample evidence that affiliation with deviant friends increases own antisocial behavior, even after accounting for peer-related confounders such as peer rejection (e.g., Snyder et al., 2005; 2010) and family-related factors (Vitaro, Brendgen, & Tremblay, 2000). In fact, one of the strongest predictors of antisocial behavior in adolescence is peer behavior (van Lier, Wanner, & Vitaro, 2007b). Despite the crucial role of peers in adolescence, the influence of deviant peers on adolescent antisocial behavior is not uniformly strong. Individual differences in sensitivity to peer influence have been ascribed to relationship and individual factors such as impulsivity (Snyder et al., 2010).
Studies have also begun to examine the degree to which individual variation in genetic factors may elevate or attenuate the influence of deviant peers. Quantitative genetic studies have shown that sensitivity to friends’ influence is partly heritable (e.g., van Lier et al., 2007a) and, more recently, studies have begun to identify the specific genes involved. For example, Lee (2011) used data from the National Longitudinal Study of Adolescent Health (Add Health) to examine the interplay between the monoamine-oxidase-A (MAOA) gene and deviant peer affiliation and reported that genotype moderated the association between affiliation with deviant peers and own aggressive behavior. Latendresse and colleagues (2011) used data from the Child Development Project (CDP) to examine interaction effects between peer antisocial behavior measured at age 12 and CHRM2, a gene that had previously been linked to personality traits (e.g., Hendershot, Bryan, Feldstein, Claus, & Hutchinson, 2011) and alcohol related disorders (e.g., Jung et al., 2011). Latendresse et al. showed that youth carrying the minor CHRM2 allele were more likely to report externalizing problems if they had associated with antisocial peers. Although research into biological moderation of peer effects is still scarce (Brendgen, 2012), these two studies clearly point at the necessity to take specific genetic effects into account when trying to understand how certain youth might be more likely to show antisocial behavior following affiliation with deviant peers, and support and extend a vast literature of gene-environment interactions.
The present study adds to the above through examining the brain-derived neurotrophic factor (BDNF) gene, a polymorphism located on chromosome 11p13-14, which regulates the secretion of brain-derived neurotrophic factor in the brain. The functional BDNF polymorphism consists of a valine to methionine substitution at position 66 (val66met) with BDNF secretion being reduced in met- compared to val-alleles (Hong, Liou, & Tsai, 2011). BDNF is a key mediator of neuronal plasticity in the prefrontal cortex and hippocampus in humans (Calabrese, Molteni, Racagni, & Riva, 2009). The polymorphism is implied in regulation of responses to stress through its effect on the hypothalamic-pituitary-adrenal (HPA) axis (Colzato, van den Wildenberg, van den Does, & Hommel, 2011; Gatt et al., 2009; Shalev et al., 2009). In detail, met-allele carriers showed greater HPA axis activity and higher levels of anxiety, nervousness, and insecurity, and increased substance abuse following stress induction (Colzato et al., 2011). BDNF also affects susceptibility to environmental stressors in the prediction of depressive symptoms (Wichers et al., 2008) and impulsive aggression (Wagner, Baskaya, Dahman, Lieb, & Tadic, 2010). In both studies, met-allele carriers were more vulnerable to environmental risk than val-val carriers. Moreover, carriers of the met-allele scored higher on introversion (Terraciano et al., 2010) and show an increased risk for psychopathological disorders related to and including aggression (e.g., Spalletta et al., 2010) and impulsivity (Oades et al., 2008).
Associations between genetic factors and complex behaviors are increasingly studied from a neurological perspective. Raine’s (2008) observation that BDNF is important for expressing conduct problems under conditions of (social) stress is supported by studies in this realm. For instance, stressful social environments have been linked to neural processes, including functioning of the neural stress response system as well as brain regions involved in emotion regulation (Meyer-Lindenberg & Tost, 2012). Peer groups are of particular importance in adolescence and high levels of aggression within them may constitute a source of pressure and social stress. Supporting this assumption, Paus et al. (2008) observed differences in brain morphology in children with high versus low resistance to peer influence. The expression of brain-derived neurotrophic factor was discussed as a potential force driving these differences, which underlines the hypothesis that the BDNF polymorphism may modulate adolescents’ peer experiences and the effects of deviant peer affiliation.
Building on previous studies of genetic factors in vulnerability to peer influence and of associations between the BDNF polymorphism and psychopathology, the current study tested whether adolescents carrying one, and particularly two met-alleles would be more vulnerable to the influence of aggressive peers, and hence at increased risk for aggression themselves, controlling for their own childhood aggression.
Effects of gender
Gender was included as a potential moderator for several reasons. First, boys use physical aggression more than girls (Card, Stucky, Sawalani, & Little, 2008; Hyde, 1984; Lahey et al., 2000). While it is not clear whether gender moderates peer influence on aggressive behavior (e.g., Mears, Ploeger, & Warr, 1998), our study focusses on peer aggression in pre-adolescence, a life stage in which peer relations are largely gender-segregated (Rose & Rudolph, 2006). Girls may be assumed to spend time mainly with other girls and may therefore be less exposed to (and affected by) aggressive behavior in their peer environment. In addition, gender moderates to some extent the social function of aggression in the peer context, that is, the use of aggressive behavior as a mean to achieve popularity and status (Prinstein & Cillessen, 2003; Salmivalli, Kaukiainen, & Lagerspetz, 2000; Vaillancourt & Hymel, 2006). Finally, differences between males and females have been reported with respect to the effects of BDNF on psychopathology (e.g., Verhagen et al., 2010), including the role this polymorphism plays as a moderator of stressful life experiences. For instance, Van Oostrom et al. (2012) showed that BDNF interacted with stressful life events in childhood in the prediction of affective memory bias, a measure related to depression; however, this association was only found in males and not in females. Taking into account gender-specific patterns in use of aggressive behavior and effects of BDNF and responding to calls for attending to gender specificity in gene-environment interaction studies in the prediction of psychopathology (Caspi & Moffitt, 2006; Dunn, Uddin, Subramanian, Smoller, Galea, & Koenen, 2011), we examined potential effects of gender.
Our analyses were guided by the following hypotheses: First, we assumed that own mid-adolescent aggression is positively predicted by affiliation with aggressive peers in childhood. Second, we hypothesized this association to be moderated by BDNF genotype. Consistent with previous conceptualization of BDNF, we assumed an additive model, that is, increasing risk with every met-allele (Lipsky & Marini, 2007). The results of this study would support a diathesis-stress model if BDNF met-allele carriers were found to be more vulnerable to the effects of affiliating with aggressive friends than carriers of the val-val homozygous variant. This should be especially the case for carriers of the met-met variant. Third, we tested gender differences in an exploratory manner.
Method
Participants
Participants for the current study represent a subsample of the Avon Longitudinal Study of Parents and Children (ALSPAC), an ongoing population-based study designed to investigate the effects of a wide range of influences on the health and development of children. Pregnant women residing in the southwest of England who had an estimated date of delivery between April 1, 1991, and December 31, 1992, were invited to participate. The initial study cohort consisted of 14,541 pregnancies and 13,971 singletons or twins still alive at 12 months of age. A total of n = 9244 individuals were genotyped for BDNF, of which n = 3081 also had data on measures of socioeconomic status, single-parent status, peer, and own antisocial behavior at ages 10 and 15. When compared with the 1991 UK National Census Data, the ALSPAC sample was found to be similar to the UK population as a whole, except that the sample showed a slightly higher proportion of married or cohabiting mothers and families who were house owner-occupiers, and (consistent with the area where the study is based) a smaller proportion of mothers from ethnic minorities (4.1% versus 7.6%). Note that only participants of British ancestry were included in the present analyses. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the local Research Ethics Committee. Detailed information about ALSPAC is available from Boyd et al. (2012) and online (http://www.bris.ac.uk/alspac). Parents gave written consent for children in this study.
It should be noted that due to the design of ALSPAC, not all adolescents participated in each assessment (Boyd et al., 2012), resulting in varying numbers of cases for the different measures. Moreover, as is common in longitudinal studies, cases lost to attrition were disadvantaged by contrast with those who continued to participate in the study, particularly with regard to a variety of socio-demographic indicators. Compared to adolescents who did not participate in the age 15 assessment, adolescents with data present at age 15 were less likely to come from single-parent families (χ2 = 43.39, p < .001) or families with a higher SES as assessed through occupational classification (χ2 = 59.94, p < .001). We further compared cases with information on age 15 aggression to those without on all other study measures and found that data presence was more likely for girls (χ2 = 42.16, p < .001), for those who were not involved in aggressive behavior at age 10 (χ2 = 29.06, p < .001) and did not affiliate with aggressive peers (χ2 = 28.16, p < .001). No differences in data presence at age 15 was found for BDNF. Extensive information on attrition within the ALSPAC study is provided by Boyd et al. (2012).
Assessment
Aggressive behavior
Adolescents reported on their engagement in aggressive activities at ages 10 and 15. At age 15, a 5-item scale (‘threatened to hurt someone they know’, ‘hit, spat, or thrown stones at someone they know’, ‘hit, kicked, or punched someone’, ‘stolen money or property that someone was carrying’, ‘hurt or injured animals on purpose’) was used. This measure reflects the different forms of physically aggressive behaviors in a diagnosis of Conduct Disorder as suggested by the American Psychiatric Association in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (American Psychiatric Association, 1994). For each item, adolescents indicated how often they had engaged in it in the past 12 months, with response categories from 0 = never, 1 = just once, 2 = 2–4 times, and 3 = 5 or more times. The internal reliability of the scale was acceptable, Cronbach’s α = .72. The majority of adolescents reported no engagement at all (59% of adolescents) but 10% of adolescents reported engaging in several forms of aggressive behavior. A sum score (M = 1.37, SD = 2.29, range = 0–15) was used as dependent variable in subsequent analyses.
At age 10, two items were used to assess aggression (‘getting into fights’ and ‘being cruel to animals’), rated in yes/no format and assessing lifetime occurrence (e.g. ‘have you ever…’). Because of the small number of items we dichotomized sums into 0 (no engagement) versus 1 (engagement in one or more behaviors). Ten percent of adolescents reported aggressive behavior at age 10 (10.12% got into fights, 0.6% had been cruel to animals).
Aggressive behavior in peers
Also at age 10, participants rated their peer’s aggression using the same 2-item measure. Participants were asked about their peers’ behavior in a collective manner (e.g., ‘have your friends been in a fight’). Twenty-nine percent of children reported their peers having been in fights and 6.25% reported peer cruelty to animals. As for adolescents’ age 10 self-reports, we dichotomized sum scores into 0 (no peer aggression) and 1 (any peer aggression). This resulted in 31% of peers being assigned a score of 1.
In addition, we controlled for single-parent status and socioeconomic status in all analyses. Both demographic indicators were assessed from mothers in the beginning of the study and have been used in other studies using the same sample (e.g., Barker, in press). Both covariates were dichotomized and indicated whether or not a mother was a single parent (4.9%) and whether or not a child grew up in a family of low socioeconomic status according to the Registrar General’s social class scale. Families in classes IV and V were considered to have a low SES (12.1%).
Genotyping for BDNF
DNA extraction and genotyping in this sample are described in detail in Jones et al. (2000). The frequencies for the val-val, val-met, and met-met genotypes were 66.2% (n = 1135), 29.1% (n = 499) and 4.7% (n = 81) for boys and 67.0% (n = 1294), 29.5% (n = 569), and 3.6% (n = 69) for girls. The BDNF polymorphism was in Hardy-Weinberg equilibrium for both boys (χ2 = 0.582, p = 0.45) and girls (χ2 = 0.147, p = 0.70). Supporting an additive risk model, val-homozygotes were assigned a score of 0, val-met heterozygotes were assigned a score of 1, and met-met homozygotes were assigned a score of 2. Thus, each met-allele was assumed to add to individual genetic risk as has been found with regards to other phenotypes, including aggression (Spalletta et al., 2010) and in interplay with maternal care on personality traits (Suzuki et al., 2011).
Analytic strategy
Analyses were carried out using negative binomial regression models to account for skew in aggressive behavior at age 15 and results were confirmed using the bootstrappping procedure in Stata 12. The regression models proceeded in two steps. In the first step, we examined the main effects of BDNF genotype and peer aggressive behavior on adolescent own aggressive behavior, controlling for gender, own aggression at age 10, low SES, and single-parent status. We estimated main effects both in a combined as well as in separate models to clarify the independent contributions of peer aggression and genotype. In the second step, the interaction between genotype and friends’ behaviors was added to the model to test whether the effect of peer aggressive behavior differed by BDNF genotype. Main effect of BDNF and its interaction with peer aggression were estimated as contrast between val-val (baseline) and val-met and val-val and met-met variants. Significant interactions were followed up using simple slope models (Aiken & West, 1991). That is, we re-estimated the association between peer aggression at age 10 and own mid-adolescent aggression on different levels of BDNF, while controlling for all covariates that were part of the original model.
Finally, we examined whether gender further moderated the interaction between BDNF genotype and peer behavior by testing whether a three-way interaction term would reach statistical significance while also estimating all main effects (covariates and predictors) and all potential two-way interaction effects.
Regression coefficients are presented in unstandardized form and as Incidence Rate Ratios (IRR). IRR refer to the risk of higher (or chance of lower) levels of aggression at age 15 given an increase or decrease in the predictor. In other words, IRR indicate a one-unit increase or decrease in outcome (e.g., engaging in two instead of one aggressive acts) given a one-unit increase in the predictor variable. Because our main predictors are categorically coded, a one-unit increase in peer aggression means that this behavior is present versus absent in peers. For BDNF, each unit increase refers to an additional met-allele. The interpretation of IRR is similar to Odds Ratio, that is, a coefficient of 1.20 indicates a 20% elevated risk to increase by one unit in the outcome whereas an IRR of 0.85 indicates a 15% lower score on the outcome variable given a one-unit increase on the predictor variable.
Results
Associations between study measures
We first examined frequency differences between BDNF genotype variants for peer aggression and covariates. These analyses were conducted to identify potential gene-environment correlations, which indicate that exposure to an environmental risk is associated with genotype (e.g., risk for affiliation with aggressive peers at age 10 would differ as a function of BDNF). Frequency differences such as these need to be tested prior to examining gene-environment interaction effects. No differences were found, thus ruling out the possibility that adolescents selected themselves into aggressive peer context as a function of their genotype. Table 1 shows bivariate correlations among all study measures. As expected, participants’ aggression was fairly stable and at both measurement waves associated with peer aggression. Own and peer aggression at age 10 overlapped considerably. Boys were more aggressive than girls and reported higher peer aggression.
Table 1.
Bivariate Spearman rho Correlations Between Study Measures
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1) Own aggression age 15 | ||||||
| 2) Own aggression age 10 | .19*** | |||||
| 3) Peer aggression age 10 | .21*** | .43*** | ||||
| 4) BDNF (5) | −.02 | .01 | .01 | |||
| 5) Gender (6) | −.26*** | −.24*** | −.28*** | −.01 | ||
| 6) Low SES | .02 | .03* | .06** | −.02 | .03 | |
| 7) Single parent | .06*** | .04*** | .02 | −.02 | .02 | .05*** |
Note:
BNDF is coded as follows: 0=val-val, 1=val-met, 2=met-met; gender is coded as follows: 1=male, 2=female.
p<.001. Low SES is coded according to Standard Occupational Classification Table, participants coded as class IV and V were coded 1= low SES group. Single parent was coded 1 when mothers indicated to not have a partner. Coefficients in the table represent Spearman ρ coefficients for correlations of nonparametric data.
Effects of peer aggression and genotype on mid-adolescence own aggression
As presented in Step 1 in Table 2, peer aggression in late childhood predicted aggressive behavior in mid-adolescence, above and beyond the effects of own aggression, gender, single-parent status, and SES. The IRR suggests that presence of aggressive peers increased the risk for a one-unit increase in own mid-adolescent aggression by 1.43 times or 43%. Contrasts between the different genotype variants revealed no differential risk. We also conducted separate main effect models in which either BDNF or peer aggression constituted the focal predictor while controlling for all covariates. These separate models confirmed the effects found in the combined model. In detail, after controlling for all covariates used in the combined model, BDNF did not significantly predict aggression at age 15 (with val-val variant as comparison group: IRR val-met = 0.92, p = .23; IRR met-met = 0.82, p = .24). The main effect model for peer aggression, in contrast, yielded a significant effect (IRR = 1.41, p < .001) similar in size to the combined model.
Table 2.
Regression Model Predicting Aggressive Behavior at age 15.
| Aggression at 15 | ||||||
|---|---|---|---|---|---|---|
| Step 1 | Step 2 | |||||
| B | IRR | p | B (SE) | IRR | p | |
| Gender | −.78 | 0.46 | <.001 | −.79 | 0.46 | <.001 |
| Own aggression age 10 | .41 | 1.51 | <.001 | .42 | 1.53 | <.001 |
| Low SES | .16 | 1.17 | .181 | .16 | 1.17 | .175 |
| Single parent | .28 | 1.32 | .173 | .28 | 1.32 | .173 |
| Peer aggression age 10 | .36 | 1.43 | <.001 | .27 | 1.32 | .003 |
| BDNF (val-val baseline) | ||||||
| Val-met | −.10 | 0.91 | .178 | −.16 | 0.86 | .085 |
| Met-met | −.25 | 0.78 | .127 | −.59 | 0.55 | .010 |
| Peer aggression age 10 x BDNF (val-val baseline) | ||||||
| Val-met | .16 | 1.18 | .276 | |||
| Met-met | .73 | 2.07 | .035 | |||
Note:
All models are based on negative binomial regressions. Coefficients are presented in unstandardized (B) and standardized (IRR) form. Gender is coded as follows: 1=male, 2=female. Low SES is coded according to Standard Occupational Classification Table, participants coded as class IV and V were coded 1= low SES group. Single parent was coded 1 when mothers indicated to not have a partner.
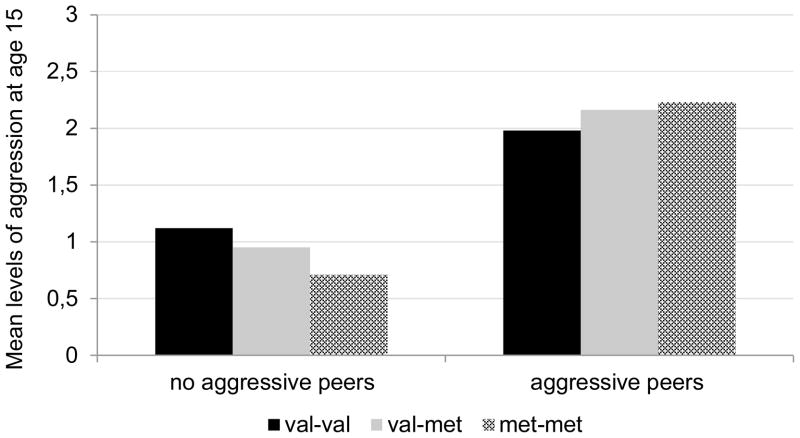
We next estimated a model in which BDNF, peer aggression, and the interaction term of BDNF and peer aggression predicted adolescents’ aggression in mid-adolescence, again controlling for adolescents’ own prior aggression, single-parent status, SES, and gender (Step 2 in Table 2). As noted above, the effect of BDNF was assessed by contrasting the val-val variant with val-met and met-met variants, both for main genotype effect and as part of the interaction with aggressive peers. As depicted in Table 2, genotype interacted with peer aggression such that a significant contrast was yielded between val-val and met-met carriers. To probe this interaction, we computed simple slope models (Aiken & West, 1991). These analyses showed a more pronounced effect of peers on adolescent’s aggression in met-met carriers (IRR = 2.11, p < .00) than val-val carriers (IRR = 1.30, p = .004). The risk for carriers of val-met carriers lay in between both homozygous variants (IRR = 1.66, p < .001) but did not differ significantly from the risk for val-val variant carriers as shown in Step 2 in our regression model. To illustrate this effect, the different levels of own aggression in presence versus absence of aggressive peers are depicted separately by genotype in Figure 1.
Figure 1.
Mean levels of Aggression at age 15 by BDNF Genotype and Presence or Absence of Peer Aggression at age 10. The scale refers to actual scores and represents over 90% of all values in the sample.
Finally, we examined whether the interaction between genotype and peer aggression worked differently for boys and girls. For this model, all main effects (i.e., BDNF, peer aggression, gender) as well as all two-way interaction effects (BDNF x peer aggression, gender x peer aggression, gender x BDNF) were entered into the model simultaneously with the three-way interaction term (BDNF x peer aggression x gender). The three-way interaction effect failed to reach significance, all other estimates were similar to prior analyses.
Given that a dominant effect of the met-allele has been reported in some studies (e.g., Wagner et al., 2010), we additionally computed regression models in which we combined both met-allele carrier groups. No significant main or interaction effect was found (results not tabled but available from first author).
Discussion
This study is one of only a handful of studies into genetic moderation of the link between peer aggressive behavior and later own aggression. In this context, we examined BDNF, a candidate gene with effects on a wide range of personality and psychopathological measures. In line with our hypothesis, we found that affiliation with aggressive peers in late childhood bore greater risk for adolescent aggressive behavior when individuals carried the met-met variant of the BDNF genotype compared to val-val homozygote carriers. We hypothesized a diathesis-stress model, assuming an additive effect for BDNF in which carriers of the val-met, and particularly met-met variants were assumed to be at linearly increasing risk for mid-adolescent aggression if they had affiliated with aggressive peers in late childhood. Our analyses partly supported this hypothesized significant interaction between genotype and exposure to aggressive peers.
In detail, we found that the regression of own aggression on earlier peer aggression was steeper for carriers of two met-alleles compared to carriers of two val-alleles. Although our measure of own aggression was not the same for assessments at age 10 and 15 and we thus only partly capture the increase in aggression, this pattern suggests that particularly carriers of two met-alleles are vulnerable to the effects of affiliation with aggressive peers. Noting that we computed an additive model while some previous studies combined val-met and met-met carriers for sample size reasons, our results nonetheless show similarity to previous findings: Wichers et al. (2008) showed that childhood adversity predicted later depression more strongly in met-carriers than in individuals who did not carry this low-functioning allele. Similarly, Aguilera et al. (2009) found met-allele carriers to be at higher risk for depressive symptoms following sexual abuse in childhood than was the case for carriers of the val-val genotype. These results suggest that BDNF, although in our study not linked to aggression directly, may be a marker for sensitivity to environmental effects.
A potential mechanism through which BDNF affects sensitivity to the environment is by influencing the experience of stressful conditions. BDNF has been discussed as moderator of the HPA axis, the pathway that regulates responses to stress (Colzato et al., 2011; Gatt et al., 2009; Shalev et al., 2009). Peer aggression may be perceived as stressful for different reasons: 1) it may be threatening and induce fear (i.e., of being the target of aggressive behavior), 2) it can induce pressure to show behavioral conformity and put individuals at increased risk for ostracism by normative peers and punishment by adults, and 3) it can induce competition and negative hierarchy among peer groups (Prinstein & Cillessen, 2003; Salmivalli et al., 2000; Vaillancourt & Hymel, 2006). With regard to specific biological pathways, BDNF may act in two ways: genotypic variation may affect individual sensitivity to stress caused by aggression in peers and it may also affect cortisol levels which then translate into individual differences in own use of aggression. BDNF plays an important role in cortisol stress response (e.g., Colzato et al., 2011); hence, on experience of aggressive peers, some youths may experience greater arousal as a function of BDNF variant and more inclination to comply with (aggressive) peer norms. Indeed, prior studies have discussed associations between HPA axis functioning, cortisol levels, and aggression (for a review see Pavlov, Christiakov, & Chekhonin, 2012). Thus, future studies that track instances of peer aggression, collect real-time cortisol level measures, and assess participants’ perceived susceptibility to peer influence in conjunction with differential genotype are complex but needed to understand the exact biological and psychological pathways that we tentatively discuss here.
Limitations and future directions
Despite the insight into the interplay of peer environment and BDNF in the prediction of aggression in adolescence, our results need to be interpreted with several limitations in mind. Our study is based on self-reports thus refers to adolescents’ own accounts of their peers’ aggressive behaviors, which biases the validity of this measure. Notably, the moderate overlap between adolescents’ own and their peers behavior at age 10 is in line with other studies that assessed deviant peer group affiliation in a general sense (e.g., Weerman, 2011). Main effects of peer on adolescents’ own later aggressive behavior were modest but it should be noted that these measures were assessed with a time difference of approximately five years and spanning life stages with potentially very different peer contexts (i.e., primary school in late childhood versus secondary school and considerably more spare time contact to peers outside of school). The measures of aggressive behavior at age 10 (both own and peer) were limited in their variance, a consequence of small number of items. Moreover, although we were able to control for own prior behavior, the measures of aggression at ages 10 and 15 differed and thus prevented us from examining actual change. A more similar control would have been highly desirable for a more rigorous research design.
In addition to using more refined measures of the environment, future studies that go beyond single candidate genotypes are necessary. We are not alone in finding negligible main effects of genotype on a phenotype of sizable heritability (Maher, 2008) and it is possible that inclusion of additional markers will increase the size of this effect. In addition, the interplay between genotypes and environmental factors may function via endophenotypes such as personality factors. Future studies are encouraged to include measures that are more proximally associated with specific genetic factors and also with distal outcomes to illuminate the pathways through which candidate genes moderate environmental factors.
Other unmeasured factors may have accounted for the association between childhood peer and mid-adolescent own aggression. A range of demographic features (school environment, neighborhood) or individual factors that promote affiliation with aggressive peers and also own aggressive behavior are feasible confounders of the associations described here. Furthermore, this study was based on a cohort sample and aggression both in peers as well as adolescents themselves was not very common. It is possible that the additive effect of BDNF would have been more pronounced and statistically significant in a clinical sample.
Similar to many other longitudinal studies, ALSPAC has faced attrition over time. Because predictors of attrition (see Boyd et al., 2012) are also predictors of deviant peer affiliation and aggression in adolescence, our sample almost certainly underrepresents adolescents who show severe problem behavior. This, however also means that risk associations found in the current study are likely to have been attenuated. We also note that simulation studies suggest that while attrition inevitably affects estimates of prevalence, it is less likely to impact on associations among variables (Wolke et al, 2009).
Notwithstanding these limitations, this study contributed to the continuous effort to understand how social and biological factors work together to impact adolescents’ behavioral development. We showed that young people who affiliate with aggressive peers in late childhood are at particular risk for engaging in such behavior themselves, but that this association was even more pronounced for met-met variant carriers of the BDNF gene compared to val-val carriers. Thus, genetic markers play a non-negligible role in the search for factors that make some adolescents more vulnerable to peer effects on aggressive behavior.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council (Grant ref: 74882) the Wellcome Trust (Grant ref: 076467) and the University of Bristol provide core support for ALSPAC. Access to ALSPAC was supported by a grant from the National Institute of Child and Human Development (1R01 HD068437-01A1) to E.D. Barker.
Contributor Information
Tina Kretschmer, Email: t.kretschmer@rug.nl, University of Groningen, The Netherlands (1), King’s College, London, Institute of Psychiatry, London, UK (2), Faculty of Behavioural and Social Sciences, Grote Rozenstraat 31, 9712 TG Groningen, The Netherlands.
Frank Vitaro, Email: frank.vitaro@umontreal.ca, University of Montréal, Canada, 3050 boul. Édouard-Montpetit, local B-234, Montréal, QC H3T 1J7, Canada.
Edward D. Barker, Email: t.barker@bbk.ac.uk, Birkbeck, University of London, UK, Department of Psychological Sciences, Birkbeck, University of London, London, WC1E 7HX, United Kingdom
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, Van Os J, Fañanás L. Early adversity and 5-HTT/BDNF genes: new evidence of gene–environment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Testing and interpreting interactions in multiple regression. Newbury Park, California: Sage; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Barker ED. The duration and timing of maternal depression as a moderator of the relationship between dependent interpersonal stress, contextual risk and early child dysregulation. Psychological Medicine. doi: 10.1017/S0033291712002450. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A, Golding J, MacLeod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Smith GD. Cohort Profile: The ‘Children of the 90s’ – the index offspring of the Avon Longitudinal Study of Parents and Children. International Journal of Epidemiology. 2012 doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechwald WA, Prinstein MJ. Beyond homophily: A decade of advances in understanding peer influence processes. Journal of Research on Adolescence. 2011;21:166–179. doi: 10.1111/j.1532-7795.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendgen M. Genetics and peer relations: A review. Journal of Research on Adolescence. 2012;22:419–437. [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: A link between stress and mood disorders. Psychoneuroendocrinology. 2009;34:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Card NA, Stucky BD, Sawalani GM, Little TD. Direct and indirect aggression during childhood and adolescence: A meta-analytic review of gender differences, intercorrelations, and relations to maladjustment. Child Development. 2008;79:1185–1229. doi: 10.1111/j.1467-8624.2008.01184.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nature Reviews Neuroscience. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Van den Wildenberg W, Van der Does WAJ, Hommel B. BDNF val66met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology. 2011;36:1562–1569. doi: 10.1016/j.psyneuen.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Dunn EC, Uddin M, Subramanian SV, Smoller JW, Galea S, Koenen KC. Research review: Gene–environment interaction research in youth depression – a systematic review with recommendations for future research. Journal of Child Psychology and Psychiatry. 2011;52:1223–1238. doi: 10.1111/j.1469-7610.2011.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Bryan AD, Feldstein SW, Claus ED, Hutchinson KE. Preliminary evidence for associations of CHRM2 with substance use and disinhibition in adolescence. Journal of Abnormal Child Psychology. 2011;39:671–681. doi: 10.1007/s10802-011-9511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CJ, Liou YJ, Tsai SJ. Effects of BDNF polymorphisms on brain functioning and behavior in health and disease. Brain Research Bulletin. 2011;86:287–297. doi: 10.1016/j.brainresbull.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Hyde JS. How large are gender differences in aggression? A developmental meta-analysis. Developmental Psychology. 1984;20:722–736. [Google Scholar]
- Jones RW, Ring S, Tyfield L, Hamvas R, Simmons H, Pembrey M, Golding J the ALSPAC Study Team. A new human genetic resource: a DNA bank established as part of the Avon longitudinal study of pregnancy and childhood (ALSPAC) European Journal of Human Genetics. 2000;8:653–660. doi: 10.1038/sj.ejhg.5200502. [DOI] [PubMed] [Google Scholar]
- Jung MH, Park BL, Lee BC, Ro Y, Park R, Shin HD, Bae JS, Choi IG. Association of CHRM2 polymorphisms with severity of alcohol dependence. Genes, Brain, and Behavior. 2011;10:253–256. doi: 10.1111/j.1601-183X.2010.00663.x. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Schwab-Stone M, Goodman SH, Waldman ID, Canino G, Rathouz PJ, Miller PJ, Jensen PS. Age and gender differences in oppositional behavior and conduct problems: A cross-sectional household study of middle childhood and adolescence. Journal of Abnormal Psychology. 2000;109:488–503. [PubMed] [Google Scholar]
- Larson R, Richards MH. Daily companionship in late childhood and early adolescence: changing developmental contexts. Child Development. 1991;62:284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Latendresse SJ, Bates JE, Goodnight JA, Lansford JE, Budde JP, Goate A, Dick DM. Differential susceptibility to adolescent externalizing trajectories: examining the interplay between CHRM2 and peer group antisocial behavior. Child Development. 2011;82:1797–1814. doi: 10.1111/j.1467-8624.2011.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE. Deviant peer affiliation and antisocial behavior: Interaction with Monoamine Oxidase A (MAOA) genotype. Journal of Abnormal Child Psychology. 2011;39:321–332. doi: 10.1007/s10802-010-9474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Annals of the New York Academy of Sciences. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nature Neuroscience. 2012;15:663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- Mears DP, Ploeger M, Warr M. Explaining the gender gap in delinquency: Peer influence and moral evaluations of behavior. Journal of Research in Crime and Delinquency. 1998;35:251–266. [Google Scholar]
- Oades RD, Lasky-Su J, Christiansen H, Faraone SV, Sonuga-Barke EJ, Banaschewski T, Chen W, Asherson P. The influence of serotonin-and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): findings from a family-based association test (FBAT) analysis. Behavioral and Brain Functions. 2008;4:48–62. doi: 10.1186/1744-9081-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Population Censuses and Surveys. Standard Occupational Classification. Vol. 3: Social Classifications and Coding Methodology. London: HMSO; 1991. [Google Scholar]
- Paikoff RL, Brooks-Gunn J. Do parent-child relationships change during puberty? Psychological Bulletin. 1991;110:47–66. doi: 10.1037/0033-2909.110.1.47. [DOI] [PubMed] [Google Scholar]
- Pavlov KA, Chistiakov DA, Chekhonin VP. Genetic determinants of aggression and impulsivity in humans. Journal of Applied Genetics. 2012;53:61–82. doi: 10.1007/s13353-011-0069-6. [DOI] [PubMed] [Google Scholar]
- Paus T, Toro R, Leonard G, Lerner JV, Lerner R, Perron M, Pike GB, Steinberg L. Morphological properties of the action-observation cortical network in adolescents with low and high resistance to peer influence. Social Neuroscience. 2008;3:303–316. doi: 10.1080/17470910701563558. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Cillessen AH. Forms and functions of adolescent peer aggression associated with high levels of peer status. Merrill-Palmer Quarterly. 2003;49:310–342. [Google Scholar]
- Raine A. From genes to brain to antisocial behavior. Current Directions in Psychological Science. 2008;17:323–328. [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin. 2006;132:98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmivalli C, Kaukiainen A, Lagerspetz K. Aggression and sociometric status among peers: Do gender and type of aggression matter? Scandinavian Journal of Psychology. 2000;41:17–24. doi: 10.1111/1467-9450.00166. [DOI] [PubMed] [Google Scholar]
- Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Ebstein RP, Kaitz M. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34:382–388. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Snyder J, Schrepferman L, Oeser J, Patterson G, Stoolmiller M, Johnson K, Snyder A. Deviancy training and association with deviant peers in young children: Occurrence and contribution to early-onset conduct problems. Development and Psychopathology. 2005;17:397–413. doi: 10.1017/s0954579405050194. [DOI] [PubMed] [Google Scholar]
- Snyder J, McEachern A, Schrepferman L, Just C, Jenkins M, Roberts S, Lofgreen A. Contribution of peer deviancy training to the early development of conduct problems: Mediators and moderators. Behavior Therapy. 2010;27:317–328. doi: 10.1016/j.beth.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Morris DW, Angelucci F, Rubino IA, Spoletini I, Bria P, Martinotti G, Corving AP. BDNF Val66Met polymorphism is associated with aggressive behavior in schizophrenia. European Psychiatry. 2010;25:311–313. doi: 10.1016/j.eurpsy.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Developmental Psychology. 2007;43:1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Matsumoto Y, Shibuya N, Sadahiro R, Kamata M, Goto K, Otani K. The brain-derived neurotrophic factor Val66Met polymorphism modulates the effects of parental rearing on personality traits in healthy subjects. Genes, Brain, and Behavior. 2011;10:385–391. doi: 10.1111/j.1601-183X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Tanaka T, Sutin AR, Deiana B, Balaci L, Sanna S, Olla N, Costa PT. BDNF Val66Met is associated with introversion and interacts with 5-HTTLPR to influence neuroticism. Neuropsychopharmacology. 2010;35:1083–1089. doi: 10.1038/npp.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt T, Hymel S. Aggression and social status: The moderating roles of sex and peer-valued characteristics. Aggressive Behavior. 2006;32:396–408. [Google Scholar]
- Van Lier P, Boivin M, Dionne G, Vitaro F, Brendgen M, Koot H, Tremblay RE, Pérusse D. Kindergarten children’s genetic vulnerabilities interact with friends’ aggression to promote children’s own aggression. American Academy of Child and Adolescent Psychiatry. 2007a;46:1080–1087. doi: 10.1097/CHI.0b013e318067733e. [DOI] [PubMed] [Google Scholar]
- Van Lier P, Wanner B, Vitaro F. Onset of antisocial behavior, affiliation with deviant friends, and childhood maladjustment: A test of the childhood- and adolescent-onset models. Development and Psychopathology. 2007b;19:167–185. doi: 10.1017/S0954579407070095. [DOI] [PubMed] [Google Scholar]
- Van Oostrom I, Franke B, Rijpkema M, Gerritsen L, Arias-Vásquez A, Fernández G, Tendolkar I. Interaction between BDNF Val66Met and childhood stressful life events is associated to affective memory bias in men but not women. Biological Psychology. 2012;89:214–219. doi: 10.1016/j.biopsycho.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vásquez A, Buitelaar JK, Franke B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Molecular Psychiatry. 2010;15:260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Brendgen M, Tremblay RE. Influence of deviant friends on delinquency: Searching for moderator variables. Journal of Abnormal Child Psychology. 2000;28:313–325. doi: 10.1023/a:1005188108461. [DOI] [PubMed] [Google Scholar]
- Wagner S, Baskaya O, Dahmen N, Lieb K, Tadic A. Modulatory role of the brain-derived neurotrophic factor Val66Met polymorphism on the effects of serious life events on impulsive aggression in borderline personality disorder. Genes, Brain, and Behavior. 2009;9:97–102. doi: 10.1111/j.1601-183X.2009.00539.x. [DOI] [PubMed] [Google Scholar]
- Weerman FM. Delinquent peers in context: A longitudinal network analysis of selection and influence effects. Criminology. 2011;49:253–286. [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietinck R, van Os J. The BDNF Val(66)Met x 5-HTTLPR x child adversity interaction and depressive symptoms: an attempt at replication. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:120–123. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]
- Wolke D, Waylen A, Samara M, Steer C, Goodman R, Ford T, Lamberts K. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. British Journal of Psychiatry. 2009;195:249–256. doi: 10.1192/bjp.bp.108.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]