Abstract
In this study, we explore reward-based decision making and electrodermal responding (EDR) among children with autism spectrum disorder (ASD) during a children’s gambling task. In addition, we examine whether individual behavioral and EDR responses predict social communication, repetitive symptoms, parent reports of executive function, and behavioral challenges. The ability to form advantageous strategies for long-term gain is of interest for children with ASDs, who exhibit both difficulty with executive function and atypical responses to reward. Twenty-one children ages 6–7 years with ASD and no intellectual disability and 21 age- and IQ-matched typically developing children participated. Both groups exhibited a similar pattern of gambling selections, but children with ASD showed less knowledge of the reward contingencies of the decks after playing. In addition, although EDR was similar between groups in anticipation of selections, children with ASD exhibited greater EDR during feedback about rewards as the task progressed. Children with ASD who exhibited the greatest increases in EDR were more likely to exhibit repetitive symptoms, particularly rituals and the need for sameness, as well as internalizing behaviors and reduced executive function in other settings.
Keywords: autism, reward, executive function, decision-making, repetitive behavior, internalizing, electrodermal response
Autism spectrum disorder (ASD) is characterized by difficulties with social and communicative function and the presence of repetitive behaviors. In addition to these symptoms, executive functioning (EF) is impaired in autism from preschool through adulthood (see Hill, 2004; Kenworthy et al., 2009; Pennington & Ozonoff, 1996 for reviews). Executive function encompasses a range of interrelated cognitive processes, such as planning for the future and complex decision-making, which underlie problem solving and goal-directed, controlled behavior and rely heavily on development of the prefrontal cortex (e.g., Casey et al., 2000; Zelazo & Müller, 2002). Within the prefrontal cortex, there is evidence for at least two distinct and interconnected networks—one related to regulation of cognition and the other related to regulation of motivation and emotion (Gläscher et al., 2012; Stuss, 2011). The latter is mediated by the ventral medial prefrontal cortex (vmPFC), which has extensive connections with limbic structures involved in emotion processing, such as the amygdala. The vmPFC cortex is also involved with processing magnitudes of reward, adjusting action when reward value changes, integrating reward and loss information into behavior, and the experience of pleasure (e.g., Bechara et al., 1999; Diekhof et al., 2012; Grabenhorst & Rolls, 2011; Holland & Gallagher, 2004). Impairments in emotion processing, motivation and reward have been implicated in ASD (Dawson, 1996; Dawson et al., 2005; Kohls et al., 2012a) and the vmPFC is proposed to have a role in the etiology of ASD (Bachevalier & Loveland, 2006; Dawson et al., 1998).
A variety of tasks have been developed to examine EF during the experience of strong emotions. These so-called “hot” executive tasks involve stimuli with explicit affective content or explicit rewards to highlight appetitive and aversive contingencies for performance (Kerr & Zelazo, 2004; Metcalfe & Mischel, 1999). One such measure, the gambling task, assesses aspects of executive function including decision-making and flexibility in the context of explicit rewards. It was developed to measure the real-world difficulties in decision-making, inflexibility, and inability to incorporate emotional cues into cognitive processes observed among patients with damage to the vmPFC (Bechara et al., 1994). Unlike many traditional measures of executive function, the gambling task requires weighing personally relevant rewards and penalties in the face of uncertainty. Participants select freely from decks of cards, all of which indicate money lost and gained, with varying magnitudes and frequencies of reward and punishment. Decks that have a net gain over time are considered advantageous or “safe,” whereas those with a net loss are disadvantageous or “risky.” The task provides for dissociation between immediate and future consequences and measures the ability to make adaptive goaldirected decisions. In the advantageous decks, immediate rewards are generally smaller but large losses are less frequent, resulting in a long-term gain. Healthy adult participants respond by picking more frequently from these decks as the task progresses, and the ability to develop an advantageous selection bias may begin by integrating autonomic cues to develop a “hunch” (Damasio et al., 1991). Consistent with this account, healthy adults begin to exhibit anticipatory electrodermal responding (EDR) after they gain experience with the decks but before they are able to articulate which decks are risky or develop a conscious strategy (Bechara et al., 1996; 1997). Such anticipatory EDR is greatest in anticipation of risky decks, and EDR amplitude is positively correlated with better behavioral performance (Carter & Pasqualini, 2004; Crone et al., 2004). In addition to EDR before making a selection, work with nonhuman primates suggests that EDR immediately after a selection reflects expectancy of reward contingencies (Amiez et al., 2003), such that anticipatory EDR likely reflects a combination of cognitive and appetitive responding. Healthy adults also exhibit EDR in response to feedback following card selection, which is interpreted as monitoring the balance of wins and losses and updating the representation of contingencies (Crone et al., 2004). EDR during the feedback phase is larger on trials with net losses, EDR magnitude corresponds with magnitude rather than frequency of losses, and EDR during feedback is not predictive of performance (Crone et al., 2004). Patients with vmPFC damage do not generate anticipatory EDR despite regular responses to feedback (Bechara et al., 1996), whereas patients with damage to the amygdala fail to produce EDR in anticipation of risky selections and in response to feedback (Bechara et al., 1999). Thus, the gambling task separately evaluates response to feedback and anticipation of future contingencies, though it is thought that the ability to anticipate contingencies is reliant on the ability to first monitor feedback outcomes. In summary, the gambling task provides a means of investigating anticipation and reactivity to reward contingencies in the context of EF.
To date, there are only three reported investigations of the gambling performance of individuals with ASD—all with adolescents and adults (Johnson et al., 2006; South et al., 2008; Yechiam et al., 2010). In the first study, groups did not differ in advantageous selections or in their EDR during the anticipation phase of gambling trials, but post-selection EDR responses to feedback differed for the disadvantageous deck with more frequent losses (Johnson et al., 2006). In the second study, South et al. (2008) compared the behavioral responses of adults with ASD and typical development and found no differences in the rate of advantageous choices as the task progressed. In the third study, Yechiam et al. (2010) found that adolescents with ASD made fewer advantageous selections than adolescents without ASD as the task progressed, and switched between the four decks more frequently. Together, these studies demonstrate that adults with ASD have comparable behavioral performance but reduced EDR to feedback, whereas adolescents with ASD make fewer advantageous selections and fewer consecutive selections from the same deck. To our knowledge, however, the behavior and EDR of children with ASD has not been examined during gambling.
Although typical gambling task performance is protracted and continues to improve into young adulthood (Crone et al., 2005; Hooper et al., 2004; Huizenga et al., 2007), gambling tasks have been developed that are sensitive to individual differences in performance as early as preschool (Crone & van der Molen, 2004; Kerr & Zelazo, 2004). These tasks account for developmental differences in working memory and sensitivity to rewards by reducing the number of decks and increasing the frequency of feedback about rewards and losses (Crone et al., 2005). The Children’s Gambling Task, developed by Kerr and Zelazo (2004), consists of two-decks, provides relatively frequent feedback contingencies, and provides information about wins and losses sequentially. This task is sensitive to developmental differences between 3- to 4-year-olds and between 5-year-olds and younger children, with older children choosing more advantageously in later blocks (Bunch et al., 2007; Hongwanishkul et al., 2005; Kerr & Zelazo, 2004). In contrast to these findings, one group failed to find developmental differences in advantageous selections among 3–6-year-olds using a four-deck task rather than the CGT (Garon & Moore, 2004). However, they subsequently found a significant effect of age using a simplified two-deck, 40 trial version of their original task (Garon & Moore, 2007). Questioning children about the decks’ contingencies revealed age-related differences among preschoolers in their ability to articulate explicit knowledge of the task (Garon & Moore, 2004; 2007). Finally, measurement of EDR from 8–18-year-olds during a more complex 4-deck version revealed increased anticipatory EDR to decks with frequent losses by older adolescents whereas EDR was linked to feedback about loss magnitude across the age range (Crone & van der Molen, 2007).
Our first goal in conducting this study was to determine whether young children with ASD differ from age- and IQ-matched typically developing children in their ability to make advantageous reward-based decisions, one aspect of “hot” EF, or in their conceptual knowledge of a gambling task. We explored possible differences using the Children’s Gambling Task (Kerr & Zelazo, 2004) and the Conceptual Knowledge questions developed by Garon and Moore (2004). Prior work indicates that adolescents with ASD made fewer advantageous selections despite similar performance between adults with ASD and comparison groups (Johnson et al., 2006; South et al., 2008; Yechiam et al., 2010). Testing children is important in order to understand how reward guides behavior earlier in development. Furthermore, investigating effects of reward on future behavior of young children with ASD may be clinically informative to those planning behavioral interventions that use reward to shape behavior of preschoolers and young school-aged children (Kohls et al., 2012a). Given the finding of less advantageous decision making by adolescents with ASD (Yechiam et al., 2010), delayed ability to learn abstract rules from rewards in 3–6 year olds with ASD but not later in development (Dawson et al., 1998; Jones et al., 2013), and theorized disruption of the vmPFC-limbic pathway early in the etiology of ASD (Bachevalier & Loveland, 2006), we hypothesized that young children with ASD would make fewer advantageous selections as the gambling task progressed and, as a result, have worse explicit knowledge of the task compared to typically developing children.
Our second goal was to compare EDR responses of children with ASD and typically developing children during wins, losses, and anticipation. The ability to process reward outcomes and to anticipate future outcomes has been implicated in ASD (Kohls et al., 2012a), and the gambling task provides information about both. We examined EDR to feedback and anticipation. Among typically developing children, EDR during feedback is generally similar in children and adolescents (Crone & van der Molen, 2004). Given the earlier maturation of EDR responses to gambling feedback in typical development, reduced EDR to gambling feedback among adults with ASD (Johnson et al., 2006), and recent work suggesting aberrant responses to nonsocial rewards by children with ASD (Cascio et al., 2012; Scott Van-Zeeland et al., 2010), we hypothesized that the feedback phase would be sensitive to differences between children with ASD and typical development. However, findings are inconsistent with respect to under- versus over-responsiveness, so we did not have a strong prediction about enhanced versus reduced response. In terms of anticipation, EDR follows a more protracted course in typical development (Crone & van der Molen, 2004). Yet, in the present study, this phase began at the time of selection and lasted until receipt of feedback, which corresponds to the time course of EDRs related to expecting rewards recorded from nonhuman primates (Amiez et al., 2003) and may have a different developmental course. No differences in anticipatory EDR were detected among adults with ASD during the gambling task (Johnson et al., 2006), though imaging and eventrelated potentials indicate reduced neural activity in anticipation of non-social rewards among children and adults with ASD (Dichter et al., 2012; Kohls et al., 2011; Kohls et al., 2012b). Thus, we predicted reduced anticipatory EDR among children with ASD.
Our final goal was to explore potential correlates of individual behavioral and EDR responses including social communication, repetitive symptoms, parent reports of EF, and internalizing behavioral challenges such as anxiety. We were particularly interested in examining the pattern of behavioral and EDR responses for each child (i.e., slope) in response to ongoing feedback about reward contingencies. First, because the gambling task was designed to assess real-world social and repetitive difficulties exhibited by adults with brain damage, we examined correlations with symptoms of ASD in both the social and repetitive domains. Altered reward circuitry may contribute to autism symptoms due to a failure to associate reward value with social interactions and a tendency to be overly rewarded by repetitive behaviors and interests (Dawson et al., 2005; Dichter et al., 2012; Klin et al., 2007; Lam et al., 2008; Neuhaus et al., 2010). Second, prior work suggests reward responding in ASD may be affected by anxiety, with a different pattern of reactivity and behavior among highly risk averse participants (Johnson et al., 2006; South et al., 2011). Thus, given the higher prevalence of anxiety among individuals with ASD (van Steensel et al., 2011), we were also interested in exploring whether response style or EDR during gambling related to internalizing behaviors, which includes anxiety. Finally, the gambling task is thought to be sensitive to real-world executive dysfunction in adults. Kenworthy et al. (2008) have emphasized the importance of measuring real-world EF impairments among individuals with ASD via experimental measures and parent reports of abilities in daily life such as the BRIEF questionnaire (Gioia et al., 2000). In order to understand whether gambling is related to an ecologically meaningful measure of EF in children with ASD, we compared performance on the gambling task to BRIEF scores.
Method
Participants
Twenty-one children with idiopathic ASD and 21 typically developing children participated. Both groups contained 15 boys and 6 girls, reflecting the sex distribution of autism. Valid EDR recordings were obtained from 18 (13 males) children with ASD and 19 (15 male) children with typical development as detailed below. All children were between the ages of 6;0–7;11 years at enrollment and groups were matched on age, t(40) = 0.75, p = .46, Cohen’s d=0.27. There were no group differences in household income, number of people in the household, or parent education (see Table 1 for descriptive statistics; there were no significant diagnostic group differences in age, sex, or IQ for children with valid EDR recordings, all ps > .13).
Table 1.
Descriptive Statistics by Group
| ASD (n=21) | TD (n=21) | t | Cohen’s d | |
|---|---|---|---|---|
| Age in months | 82.0 (7.1); 72–94 | 80.3 (7.6); 72–95 | 0.8 ns | 0.23 |
| Differential Ability Scales-2 standard scores | ||||
| GCA | 104.0 (11.6); 87–133 | 109.1 (7.2); 100–131 | −1.7 ns | −0.53 |
| Verbal | 107.1 (10.8); 89–125 | 109.0 (9.5); 94–123 | −0.6 ns | −0.19 |
| Nonverbal | 99.1 (13.0); 76–125 | 103.4 (8.0); 89–124 | −1.3 ns | −0.40 |
| Spatial | 104.5 (13.4); 87–148 | 111.1 (8.0); 93–127 | −1.9 ns | −0.60 |
| Vineland Adaptive Behavior Scales-II standard score | ||||
| Adaptive Composite | 85.7 (7.6); 71–96 | 99.9 (3.7); 92–106 | −7.7*** | −2.38 |
| Behavior Assessment System for Children-2 (T score with clinical cut-off = 65) | ||||
| Beh. Symptom Inde | 65.8 (10.0); 50–86 | 48.7 (6.8); 35–64 | 6.5*** | 2.00 |
| Autism Diagnostic Interview-Revised (ADI-R) | ||||
| Social | 19.6 (4.7); 8–26 | |||
| Communication | 17.4 (4.7); 7–24 | |||
| Repetitive Behavior | 7.3 (3.0); 1–12 | |||
| Autism Diagnostic Observation Schedule (ADOS) | ||||
| Total | 13.3 (3.9); 8–25 | |||
Notes. Means are reported with SD in parentheses, followed by ranges. ADI-R criteria are published for the WPS version and based on Le Couteur, Lord, and Rutter (2003); current research criteria include children who are −2 points on any one of the domains. ADOS criteria are determined based Gotham et al. (2007) by combining scores of Social Affect + Restricted and Repetitive Behavior scales.
p < .05,
p≤ .01,
p ≤ .001.
Children were recruited as part of a larger study via participant registries, flyers, mailings, local service providers and parent groups. To obtain the final matched sample, 18 additional children (11 ASD, 7 comparison) were recruited. Exclusionary criteria were assessed with a screening interview during enrollment and included cognitive impairment, medical disorders or injuries with implications for the central nervous system (e.g., serious head injury or major illness affecting the brain), seizures, major physical abnormalities, and significant sensory or motor impairment. For control children, additional criteria included birth or developmental abnormalities, learning or language disabilities, current or past history of psychiatric or neurological disorders, and family history of ASD. Screening for significant developmental delays and psychopathology was conducted via the Vineland-2 interview (Sparrow et al., 1984) and the Behavior Assessment System for Children (BASC-2; Reynolds & Kamphaus, 2002). As expected, groups differed on these measures and all typically developing children fell within the average, non-clinical range (see Table 1). The study was conducted with approval from the University Human Subjects Division.
Diagnostic and Cognitive Assessments
Cognitive ability was assessed using the school age core of the Differential Ability Scales-Second Edition (DAS-2; Elliott, 2007). All children had DAS-2 General Conceptual Ability standard scores (comparable to Full Scale IQ) of 85 or above. The groups did not differ on General Conceptual Ability or Verbal Ability composite scores (see Table 1). Diagnoses of autism or a related spectrum disorder were based on the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al., 2003), the Autism Diagnostic Observation Schedule (ADOS; Gotham et al., 2007; Lord et al., 2002), and clinical judgment of autism symptoms per DSM-IV-TR diagnostic criteria (American Psychiatric Association, 2000). The first author, who established research reliability on the ADOS and ADI-R, conducted all evaluations.
Experimental Assessment of Behavior
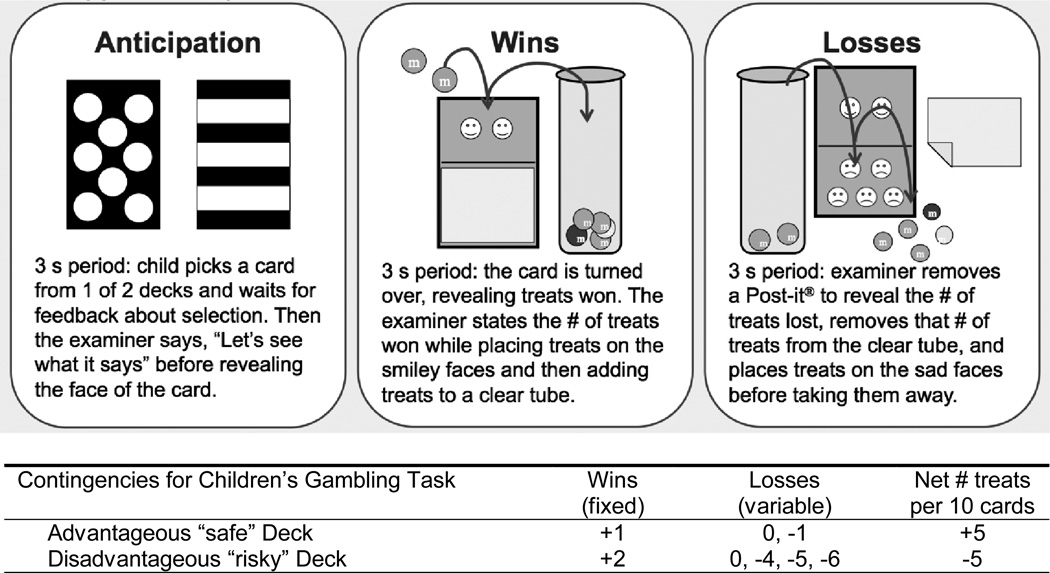
Prior to the Children’s Gambling Task (CGT; Kerr & Zelazo, 2004), parents were asked to rate their children’s most preferred small foods from a list of 20 snack items. Children were then offered a variety non-social rewards including items rated highest by their parent, other small foods, Yu-Gi-Oh cards, stickers and pennies and allowed to select their favorite. One child in each group selected Yu-Gi-Oh cards, one typically developing child selected pennies, and all others selected foods such as Skittles, Jelly Bellies, Tings, and M & Ms. During the CGT, participants were seated across from the examiner who administered the task. Children were directed to win as many treats as possible while choosing cards freely from two visually distinct decks (see Figure 1). On its face, each card had happy and sad faces to represent treats won or lost, respectively. Reward values were constant within each deck, whereas losses occurred unpredictably and varied in magnitude following the order used by Kerr and Zelazo (2004). The risky deck offered more rewards per card along with larger losses for a net disadvantage, whereas the safe deck offered fewer rewards and smaller losses for a net advantage. To illustrate the contingencies, the examiner played three consecutive cards from each deck (order of the first selection was counterbalanced). For each of 40 trials, happy faces were revealed first and treats won were placed on top of the happy faces and then in a clear cylinder. Then, a Post-it® note was removed to reveal sad faces and the appropriate number of treats lost (if any) was removed from the cylinder and placed on top of the sad faces. The placement of treats into a narrow, clear cylinder allowed children to concretely track their net gains or losses throughout the CGT–a feature that illustrated rewards over time. Following Kerr and Zelazo (2004, p. 151), the examiner narrated immediate wins and losses without commenting on the child’s performance. The number of advantageous choices in each 10 trial block was the dependent variable.
Figure 1.
The Children’s Gambling Task
Children’s conceptual knowledge of the task was assessed following the protocol used by Garon and Moore (2004). At the conclusion of the gambling task, children were asked four questions, “Now that we are done with the game, which deck was the best to pick from,” “Why do you think this was the best to pick from,” “Which deck was the worst to pick from,” and “Why was this deck the worst to pick from?” Responses to these items yielded a conceptual knowledge total score ranging from zero to four.
Assessment of Electrodermal Responding
Continuous recordings of the EDR signal were collected with a sampling rate of 200 Hz using a Biopac MP150 system (Goleta, CA) with appropriate signal conditioning and amplification. To measure EDR, two standard 0.8 cm2 Ag-AgCl electrodes were adhered to the thenar eminence of the participant’s nondominant hand with masking collars and NaCl electrolyte solution. In order to stabilize participants’ hands and reduce movement artifacts, children were then invited to wear a glove and stick it to the table using Velcro. Placing electrodes took less than 5 min. Baseline responses were recorded for 120 s while children sat quietly across the table from the experimenter and looked at a picture. Following the baseline, the Children’s Gambling Task was administered. Using flags in Biopac AcqKnowledge software, a research assistant manually marked the onset of three phases within each CGT trial in real time in order to time-lock them to the EDR recording: (1) anticipation beginning with the child’s selection of the card followed by the examiner saying, “Let’s see what it says” and flipping the card; (2) wins beginning with the examiner’s flip of the card followed by labeling the winnings, and (3) losses beginning with the removal of the Post-it® note to reveal losses followed by labeling the losses. Each phase was intended to last a minimum of 3 s, and responses were scored for the first 3 s of each phase. Occasionally, the wins or losses phases began before the full 3 s of the prior phase elapsed. When this occurred, any events that occurred were scored as part of the newly initiated phase rather than the prior one.
The electrodermal signal was recorded from all 42 children. However, one file (TD) was lost due to experimenter error, one file was lost due to an equipment failure (ASD) and one file was excluded due to significant movement artifacts throughout the recording (ASD). Two additional subjects (1 TD, 1 ASD) provided data during the CGT but did not have baseline data.
The largest positive fluctuation in signal amplitude was hand-scored as the EDR. Criteria for a response included: (1) a fluctuation beginning when slope becomes positive until it peaks and becomes negative; (2) falling within the 3 s response period; and (3) exceeding .05 µS. Criteria for mechanical and movement artifacts were jagged or sharp fluctuations. During the 120 s baseline, the largest response for each 3 s period (40 total), if present, was then averaged across four 30 s blocks. All children had at least one response during baseline. Mean fluctuation did not differ by group across the 120 s baseline, t(35) = 1.48, p = .15, d = 0.48, or during the final 30 s block (i.e., from 90–120 s), t(35) = 1.74, p = .09, d = 0.581. During each trial of the CGT, the largest response during each condition (Anticipation, Wins, and Losses), if present, was scored. Trial scores were averaged for each of the four blocks (10 trials/block). Although group differences were not detected during baseline, the level of non-task related responding was controlled for each child by computing individual difference scores for EDR fluctuations during the CGT. Difference scores were computed by subtracting the mean EDR during the 120 s baseline from the mean EDR for Anticipation, Wins, and Losses in each block.
Measures of Symptom Expression, Behavioral Challenges and Executive Function
Current symptoms of ASD were measured using the ADOS, which reflects clinical observations of symptoms in social-affect and in repetitive behaviors and interests. Repetitive symptoms are often more difficult to observe during clinical evaluation, so additional parent reports of current symptoms were obtained via the Repetitive Behavior Scale-Revised (RBS-R; Bodfish et al., 1999). The RBS-R yields a total score from Stereotypy, Self-Injury, Compulsions, Rituals, Sameness, and Restricted Behavior scales. Internalizing and externalizing behaviors were assessed via the Behavior Assessment System for Children (BASC-2; Reynolds and Kamphaus, 2004). Finally, executive dysfunction in daily life was measured with the Behavior Rating Inventory of Executive Function (BRIEF; Gioia et al., 2000), which yields the Metacognition Index, Behavioral Regulation Index, and a Global Executive Composite. Two parents of children with ASD declined to complete the BRIEF; data were available for all children with ASD for all other measures. Groups differed on parent reports of repetitive behavior (RBS-R), internalizing behaviors (BASC-2), and EF (BRIEF) (see Table 2).
Table 2.
Observed and parent reported symptoms and behavior with Means, SDs, and Ranges
| ASD | TD | t | |
|---|---|---|---|
| ADOS Social Affect | 9.9 (3.3); 5–17 | ||
| ADOS Restricted/Repetitive | 3.4 (1.8); 0–8 | ||
| Repetitive Behavior Scale-Revised | 23.3 (18.3); 1–65 | 2.7 (3.0); 0–11 | 5.1*** |
| BASC-2 Externalizing | 56.5 (7.8); 44–75 | 51.9 (8.0); 42–69 | 1.9 ns |
| BASC-2 Internalizing | 58.0 (16.3); 39–110 | 49.7 (8.9); 35–67 | 2.1* |
| BRIEF Global Executive Composite | 66.1 (6.1); 57–76 | 53.2 (6.6); 40–64 | 6.4*** |
| BRIEF Behavioral Regulation | 61.4 (8.5); 41–72 | 50.1 (8.4); 36–63 | 4.2*** |
| BRIEF Metacognition | 66.5 (6.4); 53–77 | 54.6 (6.7); 42–64 | 5.8*** |
Notes. Group differences were examined using T-tests. Raw scores are reported for the ADOS and RBS-R. BASC-2 T scores are reported and have a clinical cut off ≥ 70 and At Risk range from 60–69. T scores are reported for the BRIEF and have a clinical cut off of ≥ 65.
p < .05,
p ≤ .01,
p ≤ .001.
Data Analyses
To explore behavioral and EDR response patterns across Blocks 1–4 of the CGT, multilevel models were created using Hierarchical Linear Modeling (HLM 6.08; Raudenbush et al., 2004). HLM may be used to analyze growth trajectories for individual participants, with the advantage of flexibility in the number of observations because nesting occurs within individual participants. This is particularly useful for the EDR data because children were not expected to have a response for every block of each condition when card selection was taken into consideration. For instance, comparison of responses to the advantageous versus disadvantageous decks would result in differing numbers of opportunities for EDR depending on the number of selections each child made from the two decks as the task progressed. Multilevel modeling estimates both within- and between-subjects effects on trajectories of repeated measurement over time and uses full maximum likelihood to accommodate missing data. The model for behavioral responses during the CGT is presented below:
-
Level 1:
CGTij = π0j + π1j(Blockij) + rij
-
Level 2:
π0j = β00 + β01(ASD vs. TD) + u0j
π1j = β10 + β11(ASD vs. TD) + u1j
At Level 1, repeated observations for each participant were modeled as random effects. For example, in the equation above, the Children’s Gambling Task represents repeated observations of CGT selections across the four 10-trial blocks (i.e., the dependent variable) for each child. Level 2 equations tested the significance of the intercept (β0j) and slope (β1j) at Level 1. Diagnostic group was entered as a dummy coded fixed effect and mean-centered.
Group differences in passing rates in conceptual knowledge during the CGT were assessed via non-parametric Chi-square analyses. To explore the relation between individual slopes during the CCT and symptoms, behavioral challenges and executive dysfunction, correlations were computed. Individual ordinary least square estimates of the slope parameter (Level 2) were generated by HLM for these correlation analyses.
Results
Behavior of Children with ASD versus Typical Development
We first examined whether young children with ASD differed from age- and IQ-matched typically developing children in their ability to make advantageous choices on the CGT. The groups did not differ in their overall selection from the safe deck (i.e., mean-centered intercept), β01 = 0.33, t(40) = 1.16, p = .26, or in their advantageous selections as the task progressed (i.e., slope), β11 = −0.18, t(40) = −1.32, p = .20. Instead, both groups made increasingly advantageous selections from the safe deck in later blocks, β10 = 0.33, t(40) = 2.38, p = .02.
We also compared explicit knowledge of children when questioned at the end of the CGT. Children who correctly answered at least three of the four Conceptual Knowledge questions were scored as passing. Five children with ASD versus 13 typically developing children passed. The proportion who passed differed by group, χ2(1, N = 42) = 6.22, p = .01, Φ = 0.39. We explored whether the explicit impression formed (i.e., Conceptual Knowledge) corresponded with decision making during gambling. For children with typical development, more advantageous choices later in the task (i.e., larger slopes) corresponded with better explicit knowledge, r(21) = .55, p < .01. In contrast, children with ASD who made more advantageous choices over time did not have better explicit knowledge, r(21) = .11, p = .63. However, the difference between these correlation coefficients was not significant, Fischer r-to-z = 1.52, p = .13.
Electrodermal Responding of Children with ASD versus Typical Development
We first tested group differences in EDR to feedback about wins relative to baseline. In general, EDR increased across blocks during the task, β10 = 0.03, t(35) = 2.74, p = .01, although slopes differed between groups, β11 = 0.02, t(35) = 2.03, p = .05. Electrodermal responding to wins increased throughout the task for children with ASD, whereas EDR did not increase for typically developing children. Furthermore, the difference in overall EDR (i.e., mean-centered intercepts) between groups approached significance, β01 = −.06, t(35) = −1.83, p =.08.
During the loss condition, the groups did not differ on overall EDR (i.e., intercepts), β01 = −0.06, t(35) = −1.24, p = .22. The effect of slope approached significance between groups, β11 = 0.03, t(35) = 1.78, p = .08. Furthermore, because both decks included cards with zero treats lost, the model was recomputed to include only the trials in which one or more treats were lost (i.e., actual losses). Again, the groups did not differ in mean-centered intercepts, β01 = 0.02, t(35) = 0.33, p = .74, or slopes, β11 = 0.01, t(35) = 0.85, p = .40.
During anticipation, there were no group differences in mean-centered intercepts, β01 = −0.02, t(35) = −0.55, p = .59, or slopes, β11 = 0.02, t(35) = 1.00, p = .32. Both groups exhibited increasing EDR during the CGT, β10 = 0.04, t(35) = 2.00, p = .05. Electrodermal responding in anticipation of cards from the risky deck versus the safe deck was also examined. Slopes increased in the HLM model that included anticipatory EDR only when the risky deck was selected, β10 = 0.07, t(34) = 2.16, p = .04, but not when the safe deck was selected, β10 = 0.03, t(35) = 1.60, p = .12. Groups did not differ in anticipatory EDR to the risky deck on either intercepts, β01 = −0.02, t(34) = −0.26, p = .80, or slopes, β11 = 0.02, t(34) = 0.71, p = .48.
Associations between CGT Responses, Symptoms and Behavior among Children with ASD
Within the ASD group, advantageous selections during the CGT and explicit knowledge were not correlated significantly with severity of symptoms, challenging behaviors or EF (see Table 3). However, several significant associations were found between these measures and EDR. Increasing EDR slopes during anticipation were associated with lower ADOS social symptoms. Conversely, larger increases in EDR to wins as the CGT progressed were associated with more repetitive behaviors on the RBS-R. To follow-up, we explored correlations between EDR slopes to wins and the repetitive symptom subdomains of sameness, ritualistic, compulsive and stereotyped behaviors. Increased EDR to wins corresponded with higher levels of ritualistic behaviors, r(18) = .60, p < .01, and need for sameness, r(18) = .49, p = .04, but not compulsive or stereotyped behavior. In terms of behavioral challenges (BASC-2), higher levels of internalizing behaviors corresponded with increasing EDR to wins, losses and anticipation. Externalizing behavior did not relate to EDR. Finally, increasing EDR to wins corresponded with higher levels of global executive dysfunction and greater difficulties with metacognition.
Table 3.
Pearson correlations among children with ASD for gambling behavior and EDR, symptoms, and behavioral function
| Conceptual | CGT Choices | Win EDR | Loss EDR | Anticipation | |
|---|---|---|---|---|---|
| Knowledge | Slope | Slope | Slope | EDR Slope | |
| ADOS Social Affect | –.01 | .38 | –.22 | –.14 | –.49* |
| ADOS Repetitive | .07 | .26 | .08 | –.37 | .06 |
| RBS-R Repetitive Total | –.09 | –.07 | .51* | .01 | .40 |
| BASC-2 Internalizing | –.05 | –.12 | .73*** | .51* | .72*** |
| BASC-2 Externalizing | –.07 | .22 | .47 | .38 | .40 |
| BRIEF Behav. Regulation | –.26 | .08 | .36 | .00 | .13 |
| BRIEF Metacognition | –.03 | .16 | .64** | –.06 | .38 |
| BRIEF Global Executive | –.20 | .19 | .54* | –.06 | .22 |
p < .05,
p ≤ .01,
p ≤ .001.
Discussion
This study built on prior investigations of gambling in ASD by comparing behavioral responses, conceptual knowledge, and EDR of children with ASD and age- and IQ-matched comparison children during the Children’s Gambling Task. In addition, associations between these measures and symptoms, behavioral challenges and real-world executive functioning ability were examined within the ASD group. Contrary to our predictions, the goal-directed decision making of young children with ASD was comparable to the pattern of card selections of an age and IQ-matched comparison group. Both groups made increasingly advantageous choices, which is consistent with the pattern of performance exhibited by typically developing preschoolers as early as age four (Kerr & Zelazo, 2004) and suggests components of EF such as goal-directed decision making and flexibility in the context of explicit rewards did not differ between groups. Similar results with adults with ASD (Johnson et al., 2006; South et al., 2008) suggest this aspect of EF is relatively preserved throughout development among individuals with ASD without cognitive impairment. Despite this, fewer children with ASD were able to communicate explicit knowledge of the decks’ contingencies at the conclusion of the task. Consistent with prior work (Garon & Moore, 2004), typically developing children who had better explicit knowledge made more advantageous choices as the CGT progressed. Conceptual knowledge did not correspond with advantageous selections among children with ASD, which suggests that children with ASD may have difficulty articulating their explicit strategies resulting from the implicit experience of rewards.
Second, groups were similar in EDR at baseline, in response to losses, and during anticipation. As with healthy adults (Bechara et al., 1996; 1997) and adults with ASD (Johnson et al., 2006), children with ASD and typical development had a similar pattern of increasing EDR throughout the CGT in anticipation of the risky deck, but not the safe deck. Indeed, children with ASD who had increased EDR during anticipation (i.e., the pattern exhibited by typically developing individuals) exhibited fewer social-affective symptoms–a result consistent with the positive relation between associative learning of stimulus-reward contingencies and social ability among younger, lower-IQ children with ASD (e.g., Dawson et al., 1998). The timing of the anticipation phase in our study paralleled an investigation linking EDR between the behavioral response and receipt of reward to anticipation of expected, behaviorally contingent rewards (Amiez et al., 2003). Together with behavioral results, these findings suggest that children with ASD were, on average, equally able to adjust decision-making strategies over time in order to obtain more advantageous reward outcomes and form anticipatory EDR related to expected feedback contingencies. Nonetheless, individual differences within the ASD group were meaningfully related to social ability such that more typical anticipatory EDR responses corresponded with less social impairment.
Interestingly, the groups differed in their EDR during wins as the CGT progressed. Children with ASD had increasing EDR during feedback about winnings, whereas typically developing children did not. Thus, in the CGT, which separately presented feedback about wins and losses, children with ASD appear to have an enhanced reactivity to non-social rewards over time, but equal EDR to losses. This differs from adults with ASD who tended to have reduced EDR to losses (Johnson et al., 2006). The finding of increased EDR to positive, non-social rewards over time among children with ASD is consistent with fMRI evidence of enhanced activation of the bilateral insula and anterior cingulate among hungry children with ASD relative to comparison children while viewing images of food (Cascio et al., 2012). There is also support for stronger vmPFC activation in individuals with ASD relative to comparison groups for other nonsocial rewards including money (Schmitz et al., 2008; Scott Van-Zeeland et al., 2010) and images of intense interest (Dichter et al., 2012). Yet, enhanced neural responding to non-social rewards has not been consistently detected with fMRI (Dichter et al., 2012; Kohls et al., 2012b) and has not been detected via event-related potentials (Larson et al., 2011; McPartland et al., 2012) via the feedback-related negativity (FRN) component, which is thought to index monitoring of rewards. These mixed results highlight the need for more systematic investigation of response to nonsocial rewards and losses in children with ASD, given methodological differences in reward type and significance in the context of the ongoing task as well as measurement modality. Critically, the current investigation, which revealed a different pattern of biological response to reward over time rather than an overall effect of group, offers an additional clue about these mixed results. It may be useful to examine dynamic reward responding and monitoring over time in order to detect effects such as satiation and the ability to exert cognitive control over hedonic input.
Finally, among children with ASD, individual differences in EDR during the CGT, but not behavioral selections or conceptual knowledge, correlated with severity of symptoms, behavior challenges, and level of real-world EF. Internalizing behavior was associated with greater increases in EDR for all three phases of the CGT. Of particular interest, increasing EDR while receiving positive rewards distinguished diagnostic groups and corresponded with increased repetitive symptoms, especially ritualistic behaviors and the need for sameness. Specifically, children with ASD who had larger increases in EDR during wins over time had more difficulty with novelty, interruptions, and new routines, and were more ritualistic in daily activities, travel, play and communication topics. Increasing EDR during wins was also linked to worse overall executive function, particularly metacognition, which involves initiating, planning, integrating and coordinating in order to accomplish complex, novel tasks. We speculate that those children with ASD who had the largest increases in EDR over time may have been ‘caught up’ in the experience of ‘liking’ one salient contingency (in this case, winning treats and collecting them in a container), at the expense of attending to other aspects of the environment (see Neuhaus et al., 2010), which would represent a failure of the EF system to flexibly monitor, integrate, and shift between stimuli in the face of rewards. This could contribute to repetitive behaviors if certain stimuli or behavioral patterns are overly rewarding to children with ASD and anxiety if novelty is experienced as more disruptive and unexpected.
However, it is important to note that an alternative explanation is possible. The separation of feedback for wins and losses in the CGT represents an important difference from adult versions of the gambling task, which simultaneously present feedback about rewards and losses. Consequently, the Children’s Gambling task, which separates wins from losses, may result in EDR responses during the wins phase that actually reflect prolonged or delayed anticipation of uncertain losses in the upcoming loss phase, rather than increased excitement over accumulating treats (i.e., monitoring wins). Thus, increased EDR for children with ASD during the wins phase may represent anxiety about impending negative feedback. We speculate that individuals with ASD who are more concerned with poor performance and sensitive to negative feedback may exhibit strongest skin responses just before losses are revealed rather than immediately following their decision. The observed correlations between EDR during the wins phase and internalizing behaviors and insistence on sameness would be consistent with a subgroup of adults with ASD who appeared more motivated to avoid negative outcomes than to seek rewards (Johnson et al., 2006). Further, South et al., (2011) demonstrated children with ASD who had higher anxiety and IQ exhibited more risk-taking behavior (i.e., focus on immediate rewards), which they interpreted as an attempt to avoid failure. Thus, it may be that increased EDR during the wins phase is a combination of sensitivity to immediate rewards and concern for anticipated losses. We suggest that anxiety about potential failure in new environments and with new people may also contribute to a desire for sameness and development of ritualistic behaviors in ASD. Finally, a recent fMRI study of adults with generalized anxiety disorder (GAD) without ASD may help explain the pattern of increasing EDR across task conditions among children with ASD who had the highest levels of internalizing behaviors. In a modified high-risk gambling game, Yassa and colleagues (2012) found that EDR increased across task blocks among adults with GAD relative to controls and that the increase was associated with reduced amygdala activation and increased activity in the bed nucleus of the stria terminalis. These authors interpreted their findings as a shift away from the acute stress response of the amygdala to a more sustained anxious response.
The present findings may have important implications for treatment and understanding how children with ASD learn. Importantly, among high-IQ children with ASD who have similar behavioral responses during a learning task, two key differences emerged. First, children with ASD were less able to articulate their experience accurately. This suggests that tasks in which learning is assumed to occur implicitly may not translate to explicit knowledge for children with ASD, even when verbal reasoning abilities are similar to their peers. Second, children with ASD were more reactive to feedback. Thus, the use of nonsocial reinforcement during learning and intervention may have different effects for children with ASD. Kohls and colleagues (2012a) highlight the discrepancy between reliance on reward-based behavioral interventions for young children with ASD and the limited understanding of reward systems in these children. Behavioral and physiological tasks offer a means for investigating the development of these neural systems in young children who may have difficulty complying with the demands of neuroimaging.
The current study provides novel information about autonomic reactivity in response to reward, anticipation of behavioral consequences, and the development of explicit awareness of implicit learning experiences in young children with ASD. Yet, future work is needed to address remaining questions. This study is limited by a relatively small sample size of 6- and 7-year olds high in cognitive ability. Although our sample size is comparable to those in previously published papers, replication with a larger sample will be important to understand whether the pattern of responding suggests a meaningful cluster of individual differences. Reduced performance during gambling (Yechiam et al., 2010) by adolescents with ASD raises the possibility that a more challenging task would have been more sensitive to group differences in young children with ASD. Mixed results from neuroimaging suggest possible variability in the reward system related to context, reward type and age, so extension of this line of research to a wider range of developmental levels is critical. Finally, dynamic tasks that allow for better isolation of negative anticipation of losses and response to rewards will be useful in clarifying the significance of individual differences in arousal in the context of reward with respect to anxiety, repetitive behavior and metacognition in children with ASD.
Acknowledgements
American Psychological Association and International Society of Autism Research Dissertation Awards to the first author, a Robert C. Bolles Graduate Fellowship in Psychology to the first author, and a Cure Autism Now Young Investigator Award to the second author supported this project. The study does not necessarily reflect the views of the funding agencies and is the sole responsibility of the authors. We thank Jasleen Tiwana, Amandeep Kaur Virk and Dana Kamara for research assistance, Sheila Crowell for consultation regarding EDR recording and statistical analysis, and Jessica Greenson and Milani Smith for clinical supervision. We especially thank the participants and their families.
Footnotes
The number of responses was also calculated during baseline, and did not differ by group across the entire 120 s, or during the final 30 s.
The authors have no conflict of interest.
These data represent a portion of the first author’s doctoral dissertation and preliminary results were presented at the International Meeting for Autism Research, Chicago, IL in May 2009.
Literature Cited
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition, Text Revision. Washington, DC: Author; 2000. [Google Scholar]
- Amiez C, Procyk E, Honoré J, Sequeira H, Joseph J-P. Reward anticipation, cognition, and electrodermal activity in the conditioned monkey. Experimental Brain Research. 2003;149:267–275. doi: 10.1007/s00221-002-1353-9. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience & Biobehavioral Reviews. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–12. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FW, Lewis MH. The Repetitive Behavior Scale-Revised. Western Carolina Center Research Reports. 1999 [Google Scholar]
- Bunch KM, Andrews G, Halford GS. Complexity effects on the children’s gambling task. Cognitive Development. 2007;22:376–383. [Google Scholar]
- Carter S, Pasqualini MCS. Stronger autonomic response accompanies better learning: A test of Damasio’s somatic marker hypothesis. Cognition and Emotion. 2004;18:901–911. [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock JL, Newsom CR, Cowan RL, Benningfield MM, Rogers BP, Cao A. Response of neural reward regions to food cues in autism spectrum disorders. Journal of Neurodevelopmental Disorders. 2012;4:2–11. doi: 10.1186/1866-1955-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Crone EA, Bunge SA, Latenstein H, van der Molen MW. Characterization of children’s decision making: Sensitivity to punishment frequency, not task complexity. Child Neuropsychology. 2005;11:245–263. doi: 10.1080/092970490911261. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Developmental changes in real life decision making: Performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Developmental Neuropsychology. 2004;25:251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- Crone EA, Somsen RJ, van Beek B, van der Molen MW. Heart rate and skin conductance analysis of antecedents and consequences of decision making. Psychophysiology. 2004;41:531–540. doi: 10.1111/j.1469-8986.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Development of decision making in school-aged children and adolescents: Evidence from heart rate and skin conductance analysis. Child Development. 2007;78:1288–1301. doi: 10.1111/j.1467-8624.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Somatic markers and the guidance of behavior. In: Eisenberg LH, Benton A, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. pp. 217–228. [Google Scholar]
- Dawson G. Neuropsychology of autism: A report on the state of the science. Journal of Autism and Developmental Disorders. 1996;26:179–184. doi: 10.1007/BF02172008. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J. Neuropsychological correlates of early symptoms of autism. Child Development. 1998;69:1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude-An activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales. 2nd Ed. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- Garon N, Moore C. Complex decision-making in early childhood. Brain and Cognition. 2004;55:158–170. doi: 10.1016/S0278-2626(03)00272-0. [DOI] [PubMed] [Google Scholar]
- Garon N, Moore C. Awareness and symbol use improves future-oriented decision making in preschoolers. Developmental Neuropsychology. 2007;31:39–59. doi: 10.1207/s15326942dn3101_3. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Child Neuropsychology. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, et al. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The autism diagnostic observation schedule: revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure, and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Sciences. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Current Opinions in Neurobiology. 2004;14:148. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hongwanishkul D, Happaney KR, Lee WSC, Zelazo PD. Assessment of hot and cool executive function in young children: Age-related changes and individual differences. Developmental Neuropsychology. 2005;28:617–644. doi: 10.1207/s15326942dn2802_4. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: Implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Huizenga HM, Crone EA, Jansen BJ. Decision-making in healthy children, adolescents and adults explained by the use of increasingly complex proportional reasoning rules. Developmental Science. 2007;10:814–825. doi: 10.1111/j.1467-7687.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Yechiam E, Murphy RR, Queller S, Stout JC. Motivational processes and autonomic responsivity in Asperger’s disorder: Evidence from the Iowa Gambling Task. Journal of the International Neuropsychological Society. 2006;12:668–676. doi: 10.1017/S1355617706060802. [DOI] [PubMed] [Google Scholar]
- Jones EJH, Webb SJ, Estes A, Dawson G. Rule learning in autism: The role of reward type and social context. Developmental Neuropsychology. 2013;38:58–77. doi: 10.1080/87565641.2012.727049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychological Review. 2008;18:320–338. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A, Zelazo PD. Development of “hot” executive function: The Children’s Gambling Task. Brain and Cognition. 2004;55:148–157. doi: 10.1016/S0278-2626(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Klin A, Danovitch JH, Merz AB, Volkmar F. Circumscribed interests in higher functioning individuals with austim spectrum disorders: An exploratory study. Research and Practice for Persons with Severe Disabilities. 2007;32:89–100. [Google Scholar]
- Kohls G, Chevallier C, Troiani V, Schultz RT. Social ‘wanting’ dysfunction in autism: Neurobiological underpinnings and treatment implications. Journal of Neurodevelopmental Disorders. 2012a;4:10. doi: 10.1186/1866-1955-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Schulte-Rüther,Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Konrad K. Atypical brain responses to reward cues in autism as revealed by event-related potentials. Journal of Autism and Developmental Disorders. 2011;41:1523–1533. doi: 10.1007/s10803-011-1177-1. [DOI] [PubMed] [Google Scholar]
- Kohls G, Schulte-Rüther M, Nehrkorn B, Müller K, Fink GR, Kamp-Becker I, et al. Reward system dysfunction in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012b doi: 10.1093/scan/nss033. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49:1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, South M, Krauskopf E, Clawson A, Crowley MJ. Feedback and reward processing in high-functioning autism. Psychiatry Research. 2011;187:198–203. doi: 10.1016/j.psychres.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- McPartland JC, Crowley MJ, Perszyk DR, Mukerji CE, Naples AJ, Wu J, Mayes LC. Preserved reward outcome processing in ASD as revealed by event-related potentials. Journal of Neurodevelopmental Disorders. 2012;4:1–9. doi: 10.1186/1866-1955-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus E, Beauchaine TP, Bernier R. Neurobiological correlates of social functioning in autism. Clinical Psychology Review. 2010;30:733–748. doi: 10.1016/j.cpr.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows. Skokie, IL: Scientific Software International, Inc.; 2004. [Google Scholar]
- Reynolds CR, Kamphaus RW. BASC-2 Behavior Assessment System for Children, second edition manual. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- Rutter M, Le Couteur A, Lord C. ADI-R: The Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DG. Neural correlates of reward in autism. British Journal of Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Dana J, White SE, Crowley MJ. Failure is not an option: Risk-taking is moderated by anxiety and also by cognitive ability in children and adolescents diagnosed with an autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41:55–65. doi: 10.1007/s10803-010-1021-z. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, Suchy Y, Kesner RP, McMahon WM, Lainhart JE. Is amygdale impairment in autism specific for social information? Journal of the International Neuropsychological Society. 2008;14:42–54. doi: 10.1017/S1355617708080107. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti D. Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Stuss DT. Functions of the frontal lobes: Relation to executive functions. Journal of the International Neuropsychological Society. 2011;17:759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- van Steensel FJA, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clinical Child and Family Psychology Review. 2011;14:302–317. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CEL, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of Psychiatric Research. 2012;46:1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Arshavsky O, Shamay-Tsoory SG, Yaniv S, Aharon J. Adapted to explore: Reinforcement learning in autistic spectrum conditions. Brain and Cognition. 2010;72:317–324. doi: 10.1016/j.bandc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Müller U. Goswami U. Handbook of childhood cognitive development. Oxford: Blackwell; 2002. Executive function in typical and atypical development; pp. 445–469. [Google Scholar]