Abstract
The purpose of this study was to test the hypothesis that chronic stress in a negative social and psychological state plays a critical role in pancreatic cancer development and progression. In this study, we created a new stress model system to determine the effects of chronic stress on pancreatic cancer progression. Here, we show that chronic stress not only causes depression in mice, most likely attributed to an elevated level of epinephrine, but also induces pancreatic cancer progression. We provide evidence that the pancreatic cancer progression induced by chronic stress could be blocked to a significant degree by β2-AR inhibitor ICI118 551 or HIF-1α inhibitor 2-methoxyestradiol. Moreover, establishment of pancreatic cancer in mice exposed to chronic stress was accompanied by up-regulation of the expression of MMP-2, MMP-9, and VEGF, mediated by a HIF-1α-dependent β-AR signaling pathway. Our data suggest that the β2-AR-HIF-1α axis regulates stress-induced pancreatic tumor growth and angiogenesis. This study may have a therapeutic or preventive potential for the patients with pancreatic cancer who are especially prone to psychosocial stress challenges.
Keywords: Angiogenesis, β2-AR, HIF-1α, pancreatic cancer, regulatory axis, stress
INTRODUCTION
Pancreatic cancer is a deadly disease with an escalating incidence rate in recent years. Over an estimated 43,920 new cases of pancreatic cancer were diagnosed, and 37,390 patients died of this disease in the USA in 2012 [1]. Epidemiological data show that chronic stress associated with a negative social and psychological state, e.g., depression, might be a risk factor for cancer development and progression [2–4]. However, the underlying biological mechanisms for the stress:pancreatic cancer link are not well understood. Conceivably, it might be suggested that the risk of stress on effecting pancreatic cancer may be additionally modulated by cultural and ethnicity considerations. In China, cancer diagnosis is rarely directly communicated to patients by oncologists out of concern that stress and social stigma might inadvertently compromise the efficacy of management and treatment of the disease.
Currently there are few studies have been reported about the impact of stress on tumor development using animal models. Using a mouse model implanted with melanoma fibrosarcoma, Hasegawa and Saiki showed that psychosocial stress augmented tumor development through β-adrenergic activation [5]. Yang et al. also suggested that the activation of the hypothalamic-pituitary-adrenal (HPA) axis induced by stress regulates matrix metalloproteinase (MMPs) levels, which play an important role in tumor development and progression, as well as metastasis [6]. Later, Thaker et al. demonstrated that chronic stress promotes tumor growth and angiogenesis in a xenografted mouse model of ovarian carcinoma using a physical restraint system [7]. They further showed that β2-adrenergic receptor (β2-AR) is a critical mediator for the stress effects in their tested mouse model of ovarian carcinoma using gene specific siRNA [7]. In addition, they revealed that VEGF plays a key role in mediating stress-induced acceleration in tumor growth [7]. However, the downstream of the β2-AR signals, which is responsible for the stress-induced tumor growth has not been studied. Whether stress serves as a mechanism to promote pancreatic cancer progression is not clear. In this study, we used the physical constraint model of Thaker et al. [7]. In addition, we created a new terrifying noise stress model to mimic the noise environment to determine the effects of the chronic stress on pancreatic cancer progression in vivo.
Our data show that 1) chronic stress induces murine depression and promotes pancreatic cancer progression, and 2) HIF-1α is a downstream target of β2-AR signal pathway. These results suggest that β2-AR-HIF-1α is a major regulatory axis for stress-induced pancreatic tumor growth and angiogenesis.
MATERIALS AND METHODS
Cell Culture and Major Reagents
The MIA PaCa-2 and BxPC-3 human pancreatic cancer cell lines (the American Tissue Type Collection, USA) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, USA) supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), 0.1 mM nonessential amino acids, 0.2 mM glutamine, 1 mM pyruvate, and 10% heat-inactivated fetal bovine serum (FBS). All antibodies including anti-Ki-67, anti-CD31, anti-VEGF, anti-MMP-2, anti-MMP-9, anti-HIF-1α, and anti-β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). ICI118 551 (a β2-AR antagonist) and, 2-methoxyestradiol (a HIF-1α inhibitor) were obtained from Sigma Chemical Co. Ltd (USA).
Animals and Chronic Stress Models
Male, athymic nude mice (6 weeks old) weighing 14–20 g were obtained from the Experimental Animal Center of Xi'an Jiaotong University (Xi'an, China). The mice were reared in a professional chamber, a room (specific pathogen-free) with controlled temperature, light at 24°C, and 12 h/12 h light / dark, respectively. A UV lamp installed in the professional chamber was used to keep sterile conditions as nude mice parenting requires a sterile environment. There is a buffer room between the professional chamber and the mouse raising room. The food, water, and bedding used by mice were sterilized. Mice were grouped as 8/group. Before initiating the experiment, all mice acclimatized to a pulverized diet for 7 days. The experimental protocol was in accord with National Institutes of Health guidelines. We used both constraint stress model based on previous studies (2 h/once/day) [8] and new terrifying noise stress model that we designed for this study. The terrifying noise stress model was created as following: one, two, and three female or male SD rats (7 weeks, 210–260 g, obtained from the Experimental Animal Center of Xi'an Jiaotong University) were put, respectively, in a cage (first, we put one rat into the cage, then took it out and put two rats into the cage, finally took two out of the cage and put three rats into the cage) with a charged board for jumping. Surrounded by glass, the board is covered with copper grid, l mA AC passing, 10 w fluorescent lamps on the ceiling of the cage. The animals were given a 5 min adaptation period and, then, given an electric shock and flash, while gradually increasing the voltage to 40–60 V. The screaming sounds in response to the electric shock were recorded. The lengths of recording time were 49 s (for one rat), 66 s (for two rats), 102 s (for three rats). The recorded terrifying noise on CD-ROM, namely the screaming sounds from rats by electric shock, was used to stress the mice (6 h /once / day). The periodic immobilization stress continued for 7 weeks. After tumor sizes reached approximately 50 mm3, mice were treated with or without drugs (i.e., inhibitors). Drugs were injected s.c. around the solid tumors or given as intragastric administration.
Xenografted Pancreatic Tumor Mouse Model with Stress
The stress models were prepared by inoculating pancreatic cancer MIA PaCa-2 or BxPC-3 cells (1 × 106) in 200 µl PBS into the right flank region of the mice using a 27-gauge needle via s.c. injection 2 weeks after the initiation of stress. The control group was inoculated with pancreatic cancer cells without being subjected to the aforementioned two types of stress. The tumor volume was measured every 3 days during the experimental period and expressed as volume, which was calculated using the following formula: V=1/2×ab2 (a=large diameter, b=small diameter). Finally animals were sacrificed at the indicated time and primary tumors were excised. Half of the tumor tissue was formalin fixed and paraffin embedded for immunohistochemistry. The other half was frozen in liquid nitrogen and stored at −80°C.
The Open Field Tests (OFT) and Morris Water Maze Test
The open field tests (OFT) were conducted based on previous study [9]. The open field consisted of a cardboard box (80 cm2 × 40 cm) with black walls and a white floor, which was divided into 5 cm × 5 cm squares. The mice were placed at the center of the floor. The numbers of locomotor activities (at least three paws in a quadrant) were recorded for 3 min. OFT was operated and scored by trained and experienced observers who were blind to the treatments of the mice. Each mouse was tested individually, only once.
The procedures of Morris water maze were the same as used in an earlier study [10]. The maze was a circular pool made of black glass fiber with a diameter of 180 cm and a wall height of 60 cm, which was filled with water at a temperature of 25 °C to a depth of 32 cm. The circular escape platform made of transparent Plexiglas (diameter =10 cm) was placed 40 cm from the pool’s edge, and submerged 1.5 cm below the surface of the water. During all trials, the location of the hidden platform was kept in the same position. Animals were given three swim trials (maximally 120 s each) daily starting from four different positions. Animals were trained for 3 days. The parameters such as escape latency time, path length, and swim speed were recorded by a video system.
Enzyme-Linked Immunosorbent Assay (ELISA)
Epinephrine levels in serum were examined using ELISA according to the manufacturer’s instructions (Rapidbio Company, Shanghai, China.). Briefly, ELISA plates were coated with 100 µl serum albumin for 20 min at 25°C. Then, the dilution samples (0.1 ml) were placed on plates for 1 h at 37°C. After washing with PBS-Tween 20 (pH 7.4), plates were blocked with antirat epinephrine for 20 min at 25°C, and then, were incubated with TMB (3, 3’, 5, 5 -tetramethylbenzidine) solution as substrate. After 15 min incubation at room temperature, a stop solution was added, and OD was measured in an ELISA reader at 450 nm. Each sample was analyzed in triplicate.
Immunohistochemical Analysis of Ki-67, CD31, VEGF, MMP-2, and MMP-9 in Tumor Samples
The expression levels of Ki-67, CD31, VEGF, MMP-2, and MMP-9 in the tumor tissues were determined by immunohistochemistry as previously described [7]. Tissue sections (4 µm) were incubated overnight with primary antibody at a proper concentration. The next day, the slides were incubated for 30 min with biotinylated goat anti-rabbit IgG, followed by incubation with peroxidase-conjugated streptavidin for 20 min at room temperature. Color was developed using 0.02% of 3, 3’-diaminobenzidine (DAB) in 50 mM Tris–HCl buffer (pH 7.6) for 5–7 min. Finally, the sections were counterstained with hematoxylin, rinsed with water, dehydrated, cleared, and coverslipped. In the case of negative controls, the primary antibody was replaced with normal goat or rabbit serum. The known carcinomas in the breast were used as the positive controls. Angiogenesis was determined by CD31 staining. Microvessel density in the tumor tissues was assessed in blind analysis according to the established methods described previously [7]. The microvessels were counted in 1 mm×1 mm fields of the tissue, and the numbers obtained were averaged. Microvessel density was expressed as vessel number/mm2.
RT-PCR and Real-Time PCR
Total RNA was extracted from tumors using TRIzol reagent (GIBCO BRL, NY, USA). First-strand cDNA was synthesized from 2 µg of total RNA using the RevertAid Kit (Fermentas MBI, USA). The PCR primers sets were designed as following.
VEGF (140 bp), forward 5’-CTGGGCTGTTCTC GCTTCG-3’ and reverse 5’-CTCTCCTCTTCCTT CTCTTCTTCC-3’;
MMP-9 (111 bp), forward 5’-TGGTCCTGGTGCT CCTGGTG-3’ and reverse 5’-GCTGCCTGTCGG TGAGATTGG-3’;
MMP-2 (241 bp), forward 5’-ACCCTCAGAGCCA CCCCTAA-3’ and reverse 5’-AGCCAGCAGTGA AAAGCCAG-3’;
β-actin (179 bp), forward 5’-ATCGTGCGTGACA TTAAGGAGAAG-3’ and reverse 5’-AGGAAGG AAGGCTGGAAGAGTG-3’.
The PCR conditions included an initial cDNA synthesis reaction at 42°C for 1 h, followed by a denaturation step for 5 min at 94°C and 22 cycles: 30 s at 94°C, 30 s at 55°C, and 30 s at 72°C. After the last cycle, a final extension was performed at 72°C for 10 min. The housekeeping gene β-actin was used as an internal control.
Real-time quantitative PCR was carried out with Platinum SYBR Green qPCR SuperMix UDG (Invitrogen, USA) using the Rotor-Gene RG-3000 (Corbett Research, Doncaster Victoria, Australia). Prior to amplification, the samples were incubated at 95°C for 10 min, and each amplification cycle consisted of denaturation for 45 s at 95°C, annealing for 30 s at 55°C, and extension for 30 s at 72°C. The quantity of MMP-2, MMP-9, VEGF, and β-actin in each sample was determined using a standard curve generated by serial dilution. The PCR signals were quantitated by densitometric analysis using Quantity One analysis software. Final histogram results were reported as target gene absolute value / β-actin absolute value.
Western Blotting
Pancreatic tumor tissues (75–100 mg/mouse) derived from the control and experimental mice were minced and incubated on ice for 30 min in 0.5 ml of ice-cold whole-cell lysate buffer (10% NP40, 5 M NaCl, 1 M HEPES, 0.1 M EGTA, 0.5 M EDTA, 0.1 M PMSF, 0.2 M sodium orthovanadate, 1 M NaF, 2 µg/ml aprotinin, and 2 µg/ml leupeptin). The minced tissue was homogenized using a Dounce homogenizer and centrifuged at 16,000 × g at 4°C for 10 min. The protein was separated by 10% SDS-PAGE and electrotransferred onto nitrocellulose membranes. After being blocked with 5% non-fat milk in TBST (20 mM Tris, 150 mM NaCl, and 0.2% Tween-20, pH 7.6), the membranes were incubated with primary antibodies at 4°C overnight, followed by 1:2000 horseradish peroxidase (HRP)-conjugated secondary antibody for 2 h. Immunoreactive bands were visualized using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The Western blot signals were quantitated by densitometric analysis using Total Lab Nonlinear Dynamic Image analysis software (Nonlinear, USA). Final histogram results were indicated as target gene absolute value / β-actin absolute value.
Statistical Analysis
Each experiment was performed at least three times. Data were presented as their mean values ± standard deviation, and differences were evaluated using Student’s t-test or ANOVA. P < 0.05 was considered to be statistically significant.
RESULTS
The Effect of Chronic Stress on Mice’s Behavior
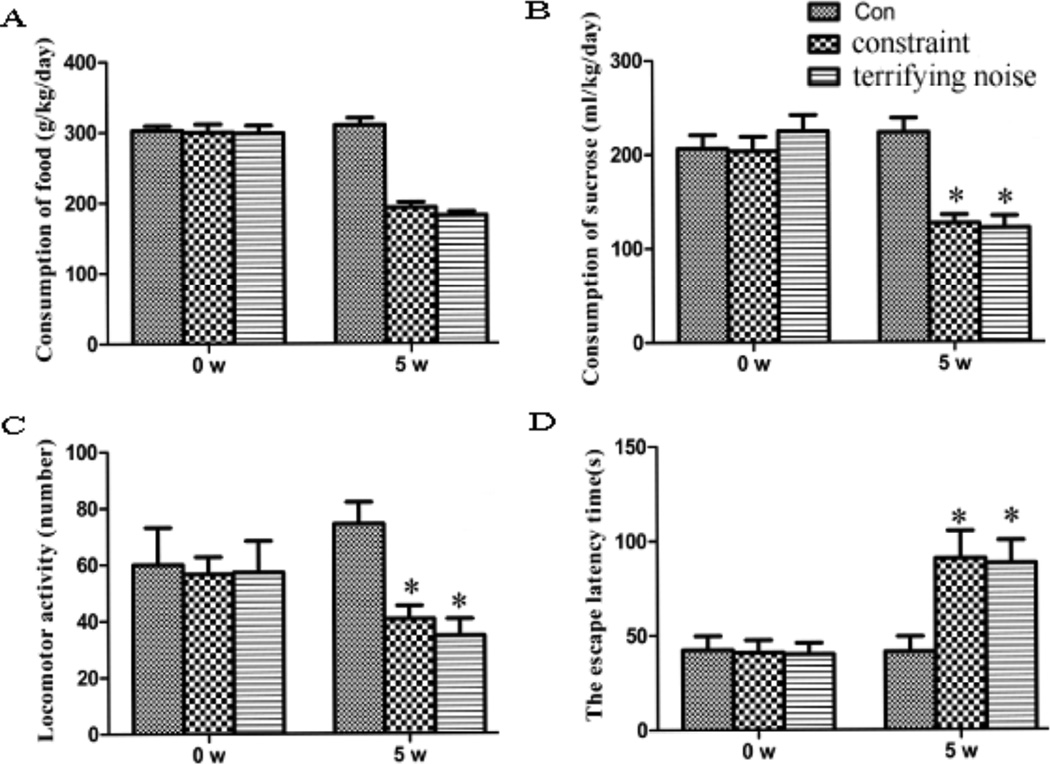
To assess the effects of terrifying sound stress and constraint stress models on pancreatic cancer progression, we measured changes of mice’s behavior and found that the mice under stress conditions consumed less food and sucrose water, exhibited decreased activities and body weight, and showed lower interest and anhedonia, which are core symptoms of depression (Fig. 1). The data indicate that both terrifying sound stress and constraint stress models evoke social stress reactions with prominent psychosocial components mimicking emotional alterations.
Fig. (1).
Effect of chronic stress on mouse behavior. The behavior of mice in each group was assessed by their consumption of food (A), their consumption of 1% sucrose (B), their locomotor activities (C) and their memory ability (D). Results are expressed as mean±SEM (n=8/group). *P < 0.05 compared with the control group.
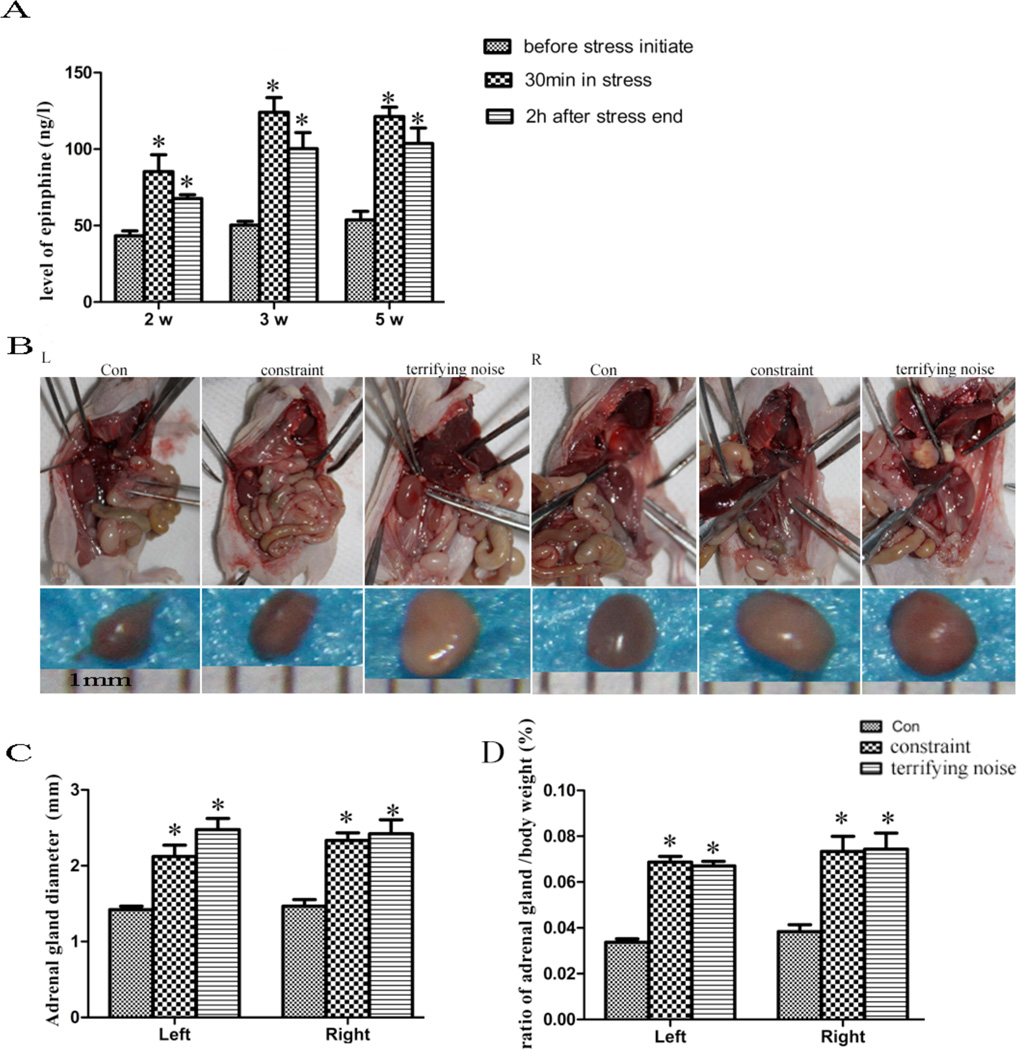
Chronic Stress Up-regulates Blood Concentration of Epinephrine and Increases Adrenal Gland Size and Weight
Previous studies have shown that stressors such as social isolation can activate the HPA axis, which leads to the release of catecholamines, glucocorticoids, and other stress hormones from the adrenal gland, brain, and sympathetic nerve terminals [11, 12]. To assess the effects of chronic stress on sympatho-adrenal-medullary activity, we measured epinephrine concentration in the plasma, and the size and weight of the adrenal glands in the control and stressed mice. As shown in Fig. (2A), the concentrations of epinephrine in the mice in constraint and terrifying sound stress groups are higher than those in the control group (P < 0.05). The mean diameters of adrenal glands are smaller in the mice in the control group compared to the mice in the constrained and terrifying sound stress groups (P < 0.05) (Fig. 2B, C). Furthermore, the ratio of adrenal gland/body weight in the stress groups is higher than the one in the control group (P < 0.05) (Fig. 2D). Our results reveal that chronic stress causes the release of stress hormones.
Fig. (2).
Effect of chronic stress on the levels of epinephrine, and size and weight of adrenal glands. (A) the concentration of epinephrine in serum from mice was measured by ELISA at before stress initiation, after 30 min stress, and 2 h after stress ending in 2 w (14 d), 3 w (21 d), and 5 w (35 d) during the 7-week stress period. Results are expressed as mean±SEM. *P < 0.05 compared with the control group. (B) the diameter of adrenal gland from each mouse in the control and stress groups after 5 w. (C) quantification of the adrenal gland diameter. (D) the ratio of adrenal gland/body weight.
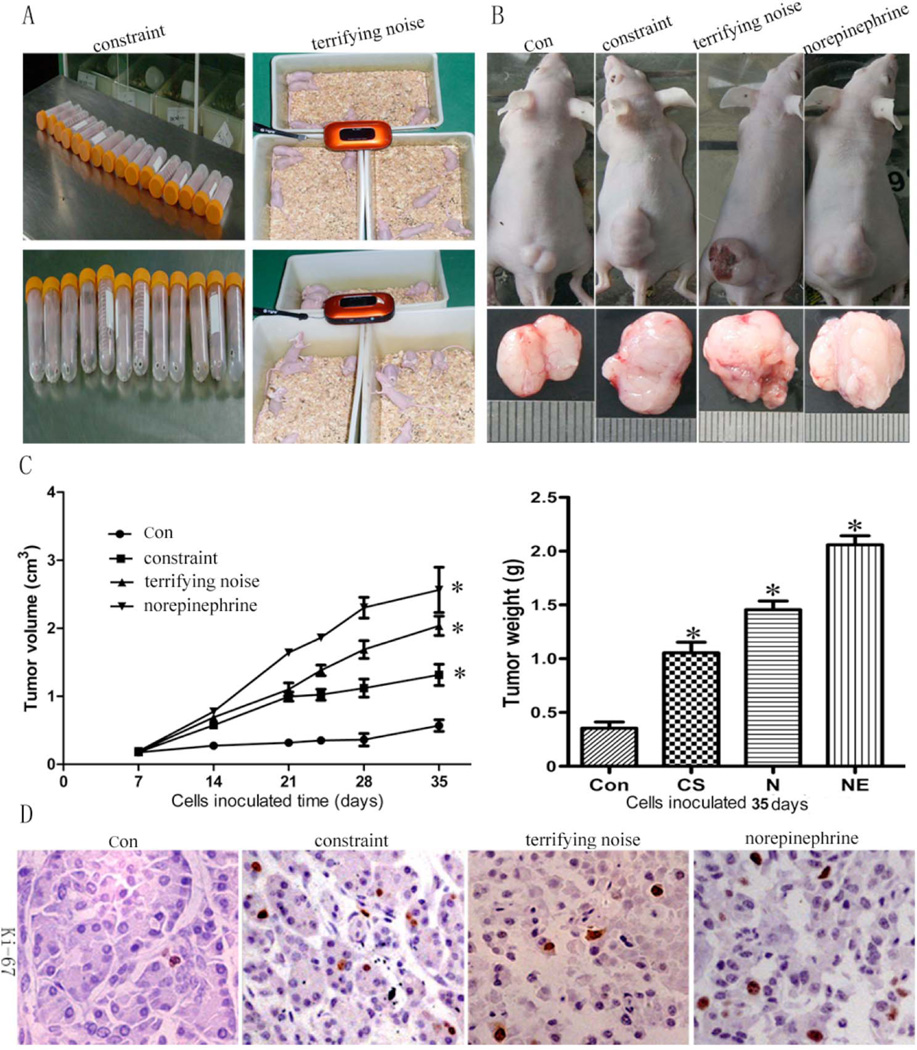
Chronic Stress Stimulates Tumor Growth
To determine whether chronic stress has stimulative effects on tumor growth, a xenografted human pancreatic tumor mouse model using MIA PaCa-2 and BxPC-3 cells was created by inoculating tumor cells after the mice experienced 2 w of stress (the selection of these cells is based on our prior study showing MIA PaCa-2 and BxPC-3 cells highly expressed the beta-receptor). To determine the effect of stress hormone on tumor growth, we also included a group of mice that, after the tumor cells were implanted, received norepinephrine as a positive control (5 mg/kg, once/day). As indicated in Fig. (3A–C), the data from mice implanted with BxPC-3 cells after 35 days show that the mean tumor volumes in the constraint stress group (1.29 ± 0.11 mm3), the terrifying sound stress group (2.02 ± 0.18 mm3), and the norepinephrine treatment group (2.37 ± 0.24 mm3) are significantly higher than in the control group (0.57 ± 0.09 mm3) (P < 0.05 for all). The terrifying sound stress is more effective in stimulating tumor growth than constraint stress, and norepinephrine has the strongest effect on stimulating tumor growth compared to both stress models. Additional experiments from mice implanted with MIA PaCa-2 cells showed similar effects on tumor growth (Supplemental Fig. 1). These data suggest that chronic stress promotes pancreatic tumor growth in vivo. To further confirm this effect, tumor tissues were stained for Ki-67, a marker for cell proliferation. The results show that stress significantly up-regulated the expression of Ki-67 in the tumors from the mice under stress (P < 0.05) (Fig. 3D). Our data indicate that stress has a promotive effect on tumor growth.
Fig. (3).
Chronic stress stimulates pancreatic tumor growth in xenografted mice. MIA PaCa-2 and BxPC-3 cells were inoculated 2 w after the initiation of stress. (A) stress models. (B) tumor diameter in control and stress groups after 5 w. (C) the tumor volume and weight. (D) the expression of Ki-67 in the tumor tissues (× 400). Results are expressed as mean±SEM. *P < 0.05 compared with the control group.
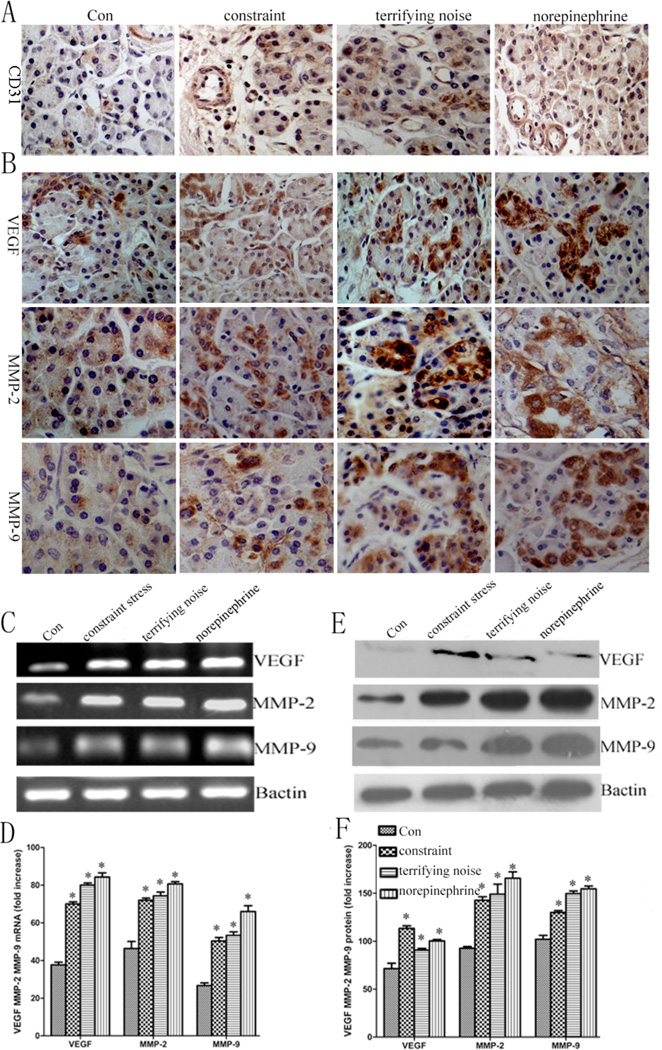
Chronic Stress Increases Tumor Angiogenesis
To further examine whether the chronic stress stimulates tumor angiogenesis, we evaluated the distribution of blood vessels in solid tumors using immunohistochemistry. Mice were grouped (n=8) as: (a) control group, (b) constraint, (c) terrifying noise, and (d) norepinephrine. We observed that the densities of blood vessels (CD31) in the tumor tissues from the mice within the two stress groups and the norepinephrine treated group are higher than that from the mice in the control group (P < 0.05) (Fig. 4A) (Supplemental Fig. 2), suggesting that chronic stress promotes tumor growth through angiogenesis. Our study also reveals that the expression of VEGF, MMP-2, and MMP-9 in the tumor tissues from the mice in both stress groups and the norepinephrine treated group are higher than that of the control (Fig. 4B). Furthermore, stress induced angiogenesis is associated with significant elevation of VEGF MMP-2, MMP-9 at mRNA, and protein levels by RT-PCR/real-time PCR, and Western blotting, respectively (Fig. 4C–F).
Fig. (4).
Tumor angiogenesis increases in chronically stressed animals. (A) microvessel density was higher in the stress groups than in the control, the vessel density was calculated statistically using Image Pro Plus 6.0 software (Media Cybernetics Inc., Bethesda, MD, USA). (B) the expression levels of VEGF, MMP-2, and MMP-9 in the tumor samples from control and stressed animals were determined by immunohistochemistry, all photographs were taken at 400×. (C) the expression of VEGF, MMP-2, and MMP-9 at mRNA level in the tumor samples. (D) quantification of mRNA by RT-PCR. (E) the protein expression of VEGF, MMP-2, and MMP-9 was determined using western blot analysis. (F) quantification of western blot data. Data from at least three independent experiments with duplicate determinations are expressed as means ± SEM. *P < 0.05 vs control.
Effects of β2-AR on Pancreatic Tumor Growth
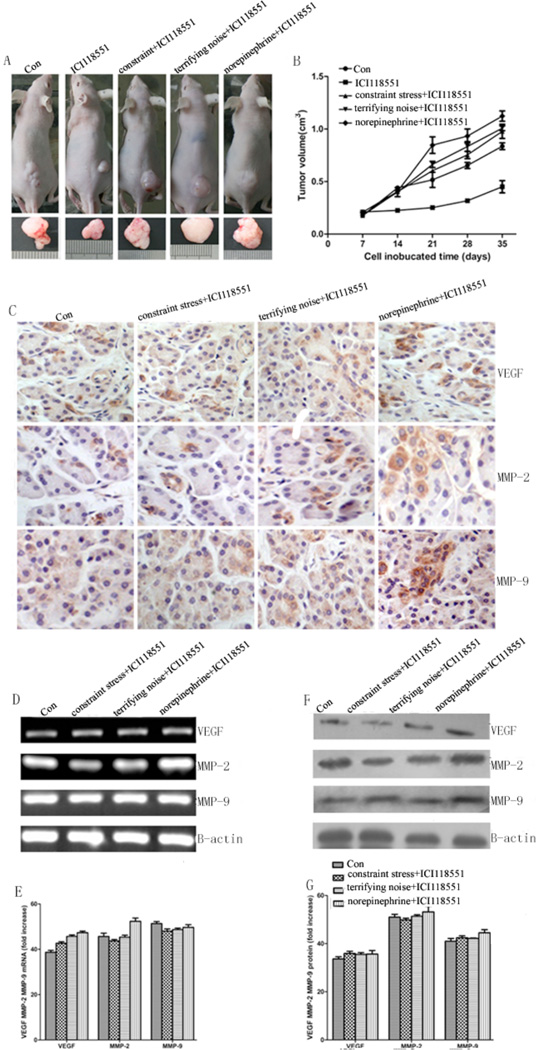
To evaluate the role of activation by epinephrine, one of the major stress hormones in mediating pancreatic tumor growth under stress condition in vivo, we determined the effect of ICI118 551, a β2-AR antagonist, on tumor growth using both the constrained stress model and the terrifying sound stress model. After inoculating the tumor cells for 7 d, mice were treated with either (a) PBS, (b) 10 mg/kg ICI118 551, (c) constraint stress + 10 mg/kg ICI118 551, (d) terrifying noise + 10 mg/kg ICI118 551, or (e) 5 mg/kg norepinephrine + 10 mg/kg ICI118 551. The tumor volumes were measured every 3–4 days. As shown in Fig. (5A, B), the β2-AR antagonist ICI118 551 blocks the stimulating effects induced by stress on tumor growth and the expression of VEGF, MMP-2, and MMP-9 in the tumor tissues of mice using both the constrained stress model and the terrifying sound stress model (Fig. 5C–G). The mean tumor volumes in the group with constraint stress + ICI118 551 group (0.81 ± 0.05 mm3), in the group with terrifying sound stress + ICI118 551 group (0.82 ± 0.04 mm3) are not significantly higher than in the control group (0.72 ± 0.02 mm3) (P > 0.05), while the mean tumor volumes in the ICI118 551 group (0.44 ± 0.05 mm3) is smaller than the control group, showing statistical significance (P = 0.034). The results implicate a critical role for β2-AR activity in chronic stress effects on tumor growth.
Fig. (5).
Effect of β2-AR inhibition on pancreatic cancer growth and the expression of VEGF, MMP-2, and MMP-9 at mRNA and protein levels in the control and chronically stressed animal. Beginning 7 d after tumor cell injection, we simultaneously treated mice with PBS, ICI118 551 (p.o.). (A) representative tumors in control and intervention groups after 5 w. (B) quantification of tumor volume. (C) the expression levels of VEGF, MMP-2, and MMP-9 in the tumor tissues. All photographs were taken at 400×. (D) the mRNA expression of VEGF, MMP-2, MMP-9. (E) quantification of mRNA by RT-PCR. (F) the protein expression of VEGF, MMP-2, and MMP-9 was determined using Western blot analysis. (G) quantification of western blot data. Data from at least three independent experiments with duplicate determinations are expressed as means ± SEM.
The HIF-1α is a Key Player in Stress-induced Tumor Growth
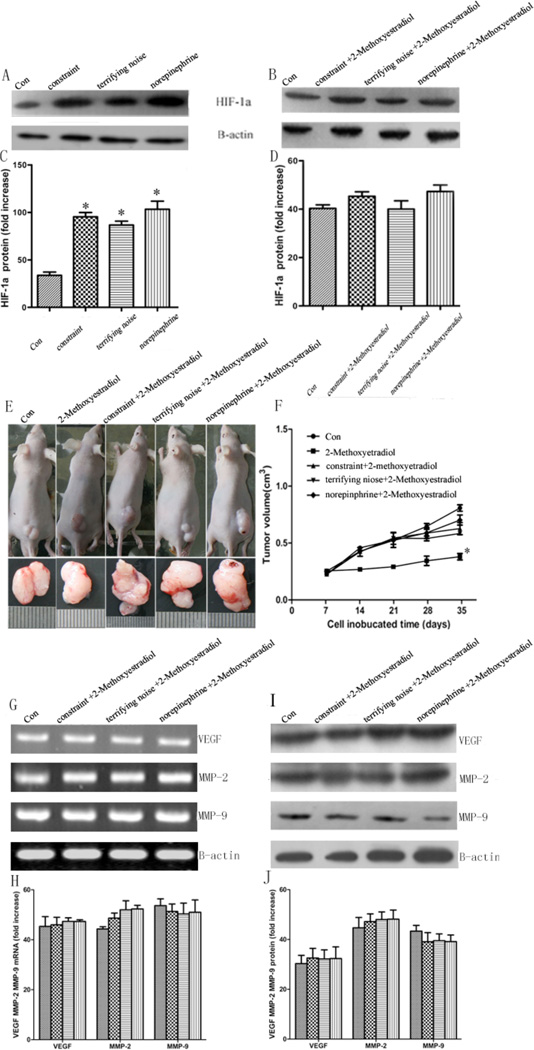
In our previous in vitro study, the activation of β2-AR induced by catecholamine could modulate the expression of VEGF and MMPs via HIF-1α [13]. To explore the molecular mechanisms of the stress-induced pancreatic tumor growth, we then evaluated the effect of chronic stress on the expression of HIF-1α in pancreatic tumor tissues by Western blot analysis. Our results show that chronic stress promotes the expression of HIF1-α, and this effect is blocked by β2-AR antagonist ICI118 551 (Fig. 6A–D). To further determine if HIF-1α is involved in stress-induced tumor growth, we explored the effect of HIF-1α inhibitor 2-methoxyestradiol on the BxPC-3 xenografted mice. After implanting the tumor cells for 7 days, mice were treated with: (a) PBS, (b) 10 mg/kg 2-methoxyestradiol, (c) constraint stress +10 mg/kg 2-methoxyestradiol, (d) terrifying noise +10 mg/kg 2-methoxyestradiol, (e) 5 mg/kg norepinephrine +10 mg/kg 2-methoxyestradiol, and then the tumor volume was measured every 3–4 days. As shown in Fig. (6E, F), 2-methoxyestradiol blocks the effects of stress on tumor growth and the expression of VEGF, MMP-2, and MMP-9 in the tumor tissues from the mice in the chronic stress groups (Fig. 6G–J) and by real-time PCR (Supplemental Fig. 3).. The mean tumor volumes in the constraint stress + 2-methoxyestradiol group (0.57 ± 0.06 mm3) and the terrifying sound stress + 2-methoxyestradiol group (0.68 ± 0.09 mm3) are not higher than in the control group (0.52 ± 0.05 mm3) (P > 0.05), while the mean tumor volumes in the 2-methoxyestradiol group (0.34 ± 0.02 mm3) is smaller than the control group (P = 0.027). These results suggest that HIF-1α plays a critical role in chronic stress mediating tumor growth.
Fig. (6).
Effect of HIF-1α on pancreatic cancer growth in vivo and the expression of VEGF, MMP-2, and MMP-9 at mRNA and protein levels. (A, C) HIF-1α protein in the chronic stress groups is higher than control group. (B, D) The stressed mice were treated with ICI118 551 abolish this effect. (E) tumor diameter in the control and intervention groups after 5 w. (F) quantification of tumor volume. (G) the mRNA expression of VEGF, MMP-2, and MMP-9 was determined using RT-PCR. (H) quantification of mRNA by RT-PCR. (I) the protein expression of VEGF, MMP-2, and MMP-9 was determined using Western blot analysis. (J) quantification of western blot data. Data from at least three independent experiments with duplicate determinations are expressed as means ± SEM. *P < 0.05 vs control.
DISCUSSION
Long exposure to a psychosocial stress is thought to play a role in the etiology of many diseases [14, 15], especially, in the initiation, development, and progression of cancer [16]. Although stressful life events related to the development of cancer is still controversial, a number of epidemiological and clinical studies over past years have linked stressful life events with increased cancer risk and decreased survival probability [17, 18]; while various coping strategies for reducing stress can decrease the cancer recurrence after therapy and increase survival time [19, 20]. Due to stress factor’s special characteristics, studying the effect on cancer pathogenesis under stress environment is limited. In this study, we first demonstrated that a terrifying sound stress model can be used to mimic social stress and is a suitable tool to study the effects of chronic stress on hormone related diseases. By using our new model systems, we reveal that chronic stress results in the depressive behavior of mice and an elevated level of epinephrine as well as induces pancreatic tumor growth in the xenografted mice. We also provide evidence that HIF-1α, as a critical downstream effector of the β2-AR regulatory system, may also play an important role in the chronic stress induced tumor growth.
Increasing evidence shows that there is an association between depressive symptoms and increased morbidity and/or mortality for several chronic diseases, including cancer. In our study, we observed that chronic social stress initially induces emotional alterations, especially depressive symptoms, then produces psychological depressive symptoms resulting in endocrine functional change, which leads to cancer-related mortality. We found that the mice under stress conditions took less food, consumed less sucrose water, decreased activities and body weight, and showed lower interest and anhedonia, which are core symptoms of depression.
The mechanism studies regarding chronic stress on tumor progression have been focused on the immune function, a key component of the stress response involving activation of the HPA axis, and the sympathetic nervous system (SNS), leading to catecholamines, glucocorticoids, and other stress hormones release that can inhibit many aspects of cellular immune function such as lymphocyte trafficking, T cells, natural killer cell activity [21–24]. However, recent studies using chronic stress and orthotopic mouse model of ovarian cancer suggested that stress could affect tumor growth and progression via β-AR-cAMP-PKA signaling pathways, which is independent of the effects on the immune system [7]. The results indicated that neuroendocrine stress response hormone could directly regulate the growth of ovarian carcinomas in vivo. In addition, our previous in vitro studies indicated that activation of the β2-AR signal pathway could enhance pancreatic cancer progression [25] and HIF1-α plays a critical role in regulating angiogenesis and metastasis related genes such as MMP-2, MMP-9, and VEGF [26–28]. In this study, we used xenografted human pancreatic cancer mice under constraint or terrifying noise stress environment to determine the role of catecholamines in the chronic stress induced tumor growth and demonstrated that β2-AR-HIF-1α is a novel regulatory axis for stress-induced pancreatic tumor growth and angiogenesis by using β2-AR inhibitor ICI118 551 and HIF-1α inhibitor 2-methoxyestradiol. The possible molecular pathways involved in the stress mouse model of pancreatic cancer are illustrated in Fig. (7). It is worth mentioning that both our current study and previous studies used immunodeficiency mice to examine the effects of chronic stress on tumor progression, which do not allow us to evaluate the influence of immune system to tumor growth induced by stress. Therefore, employing genetically engineered immunocompetent murine model such as Kras/p53/pdxCre or KPC mice in pancreatic cancer warrants further investigation, which is under way in our laboratory, on effects and mechanism of the immune system on tumor growth affected by stress.
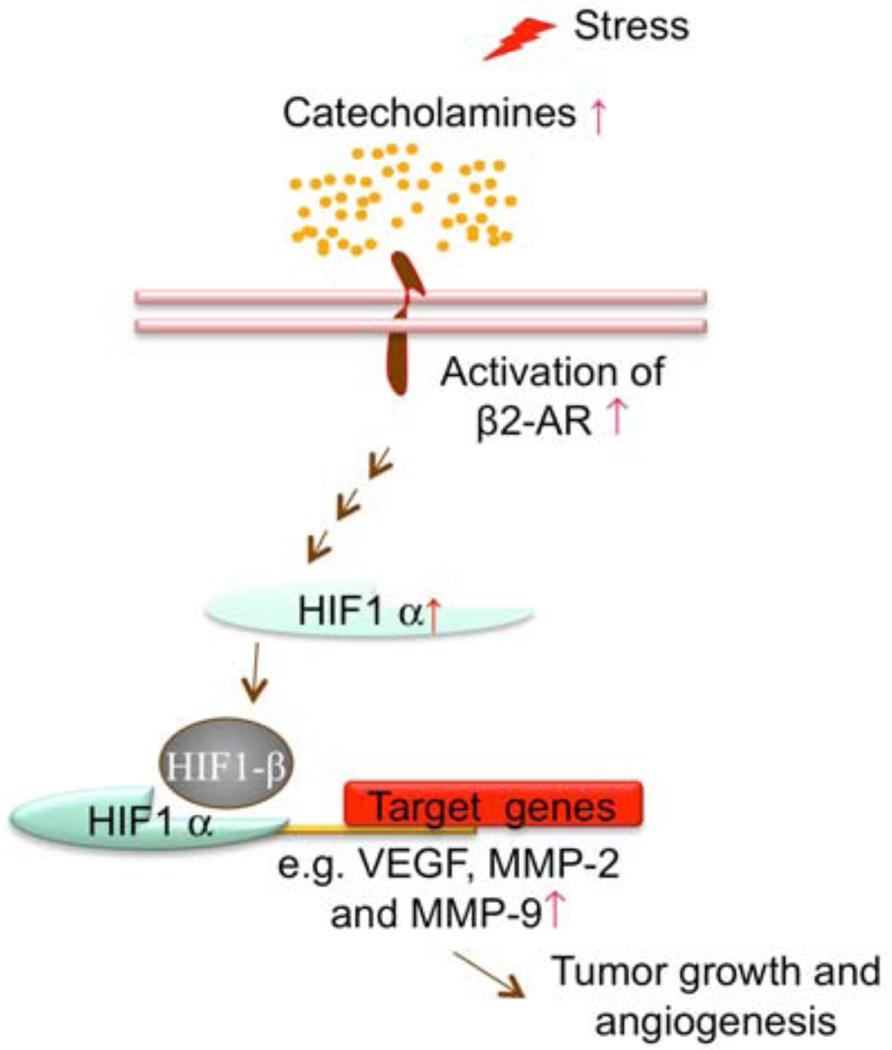
Fig. (7).
β-AR signaling pathways model in pancreatic cancer. Agonist such as catecholamines binding to the β-AR initiates a signaling cascade. The signaling ultimately regulates the downstream gene expression through the HIF-1α regulatory system.
In summary, we established a new noise model for studying the relationship between stress and human disease progression and have successfully applied this model to determine the correlation of stress and pancreatic cancer progression. We revealed that behavioral stressors can enhance the pathogenesis of pancreatic cancer in vivo via elevated levels of stress hormones. Our data also demonstrated that the activation of the β2-AR pathway by stress hormones results in up-regulated expression of HIF-1α. Subsequently, high levels of HIF-1α increase the expression of its downstream target genes related to angiogenesis such as MMP-2, MMP-9, and VEGF, which promote pancreatic cancer growth and angiogenesis. Taken together, we have found that β2-AR-HIF-1α is a novel regulatory axis for stress-induced pancreatic cancer growth and angiogenesis. Thus, the potential utility of β2-AR or HIF-1α inhibitors as anticancer therapies may be an effective strategy for patients with pancreatic cancer, especially for patients who are subject to psychosocial stress due to their cancer diagnosis and treatment.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by Major Scientific Grand of Shaanxi 13115 (2010ZDKG-49), National Nature Science Foundation of China (NSFC) (81172360, 81201824) (Q. Ma), pilot project grants (E. Wu) from Program Project Grants from the National Center for Research Resources (NCRR; P20 RR020151 and the National Institute of General Medical Sciences (NIGMS; P20 GM103505 and P30 GM103332-01) from the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NSFC, NIH, NCRR, or NIGMS. The authors would like to extend their thanks to Dr. R. Craig Schnell (North Dakota State University) for his thoughtful reading.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Yan C, Siegel D, Newsome J, et al. Antitumor indolequinones induced apoptosis in human pancreatic cancer cells via inhibition of thioredoxin reductase and activation of redox signaling. Mol Pharmacol. 2012;81(3):401–410. doi: 10.1124/mol.111.076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang JA, Sun LM, Muo CH, et al. The analysis of depression and subsequent cancer risk in Taiwan. Cancer Epidemiol Biomarkers Prev. 2011;20(3):473–475. doi: 10.1158/1055-9965.EPI-10-1280. [DOI] [PubMed] [Google Scholar]
- 3.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11):1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control. 2010;21(2):191–199. doi: 10.1007/s10552-009-9449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa H, Saiki I. Psychosocial stress augments tumor development through β-Adrenergic activation in mice. Jpn J Cancer Res. 2002;93:729–735. doi: 10.1111/j.1349-7006.2002.tb01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang EV, Bane CM, MacCallum RC, et al. Stress-related modulation of matrix metalloproteinase expression. J Neuroimmunol. 2002;133:144–150. doi: 10.1016/s0165-5728(02)00270-9. [DOI] [PubMed] [Google Scholar]
- 7.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 8.Van Kampen M, Kramer M, Hiemke C, et al. The chronic psychosocial stress paradigm in male tree shrews: evaluation of a novel animal model for depressive disorders. Stress. 2002;5(1):37–46. doi: 10.1080/102538902900012396. [DOI] [PubMed] [Google Scholar]
- 9.Xiu LJ, Lin HM, Wei PK, et al. The effect of chronic mild stress on tumor bearing rats’ behavior and its mechanism. Neurosci Lett. 2010;473(1):1–4. doi: 10.1016/j.neulet.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Turkmen S, Lundgren P, Birzniece V, et al. 3 βeta-20 beta-dihydroxy-5 alpha-pregnane (UC1011) antagonism of the GABA potentiation and the learning impairment induced in rats by allopregnanolone. Eur J Neurosci. 2004;20(6):1604–1612. doi: 10.1111/j.1460-9568.2004.03610.x. [DOI] [PubMed] [Google Scholar]
- 11.Hermes GL, Delgado B, Tretiakova M, et al. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci USA. 2009;106(52):22393–22398. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antoni MH, Lutgendorf SK, Cole SW, et al. Opinion - The influence of bio-behavioural factors on tumor biology: pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu HT, Ma QY, Zhang D, et al. HIF-1 alpha links beta-adrenoceptor agonists and pancreatic cancer cells under normoxic condition. Acta Pharmacologica Sinica. 2010;31(1):102–110. doi: 10.1038/aps.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moles A, Bartolomucci A, Garbugino L, et al. Psychosocial stress affects energy balance in mice: Modulation by social status. Psychoneuroendocrinology. 2006;31(5):623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Danelia M, Trapaidze D. Psychosocial work environment and coronary heart disease. Georgian Med News. 2005;121:56–58. [PubMed] [Google Scholar]
- 16.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 17.Lutgendorf SK, DeGeest K, Dahmoush L, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25(2):250–255. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayr M, Schmid RM. Pancreatic cancer and depression: myth and truth. BMC Cancer. 2010;10:56. doi: 10.1186/1471-2407-10-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galway K, Black A, Cantwell M, et al. Psychosocial interventions to improve quality of life and emotional wellbeing for recently diagnosed cancer patients. Cochrane Database Syst Rev. 2012;14(11):CD007064. doi: 10.1002/14651858.CD007064.pub2. CD007064. pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen PB, Jim HS. Psychosocial interventions for anxiety and depression in adult cancer patients: Achievements and challenges. CA Cancer J Clin. 2008;58(4):214–230. doi: 10.3322/CA.2008.0003. [DOI] [PubMed] [Google Scholar]
- 21.Lekander M. The immune system is affected by psychological factors High stress levels can change susceptibility to infection and allergy. Lakartidningen. 1999;96(44):4807–4811. [PubMed] [Google Scholar]
- 22.Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97(23):1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: Immunological basis and implications for psychoneuroimmunology. Brain Behav Immun. 2003;17(1):S27–S36. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- 24.Parker J, Klein SL, McClintock MK, et al. Chronic stress accelerates ultraviolet-induced cutaneous carcinogenesis. J Am Acad Dermatol. 2004;51(6):919–922. doi: 10.1016/j.jaad.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Ma Q, Hu H, et al. b2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NF-kB and AP-1. Cancer Biol Ther. 2010;10(1):19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- 26.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12(2):369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9(12):4514–4521. [PubMed] [Google Scholar]
- 28.Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95(4):808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.