Introduction
In sexually mature females, ovulation is the process whereby an egg ready to be fertilized is released from the ovary. Fully grown antral follicles are composed of mural granulosa and cumulus cells and contain a germ cell, the oocyte, which has developed the competence to mature into a fertilizable egg (Fig. 1). Ovulation is triggered by a surge of the gonadotropin luteinizing hormone (LH) secreted by the pituitary. During the periovulatory period, the oocyte completes maturation and major phenotypic changes take place in the mural granulosa and cumulus cells, culminating with rupture of the follicle and release of the matured egg. Malfunction of any step of this complex process causes impairment of fertility. Thus, a better understanding of the cellular and molecular events associated with ovulation is of paramount importance to improve assisted reproduction, as well as to develop new strategies for fertility control.
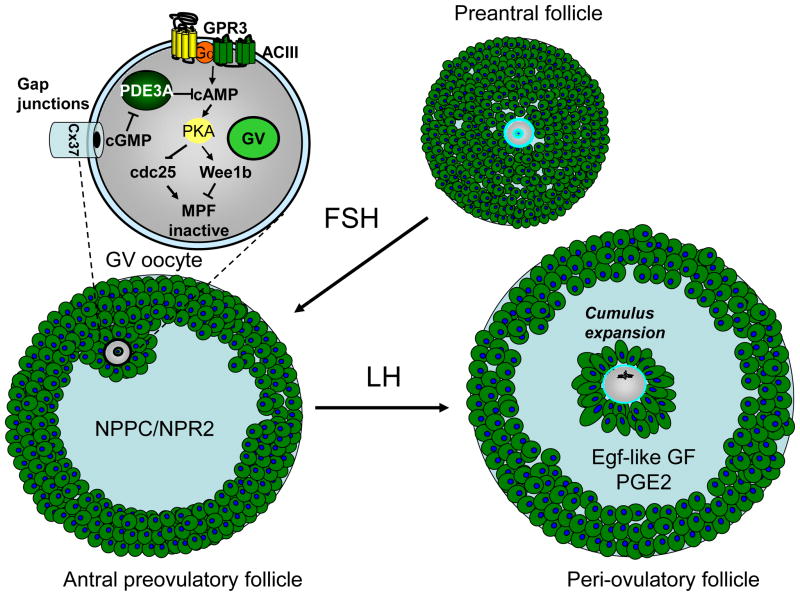
Fig. 1. Schematic representation of follicles at different stages of maturation and primary regulatory signals.
During preantral follicle growth, oocytes are not competent to reenter meiosis. With the formation of the antrum, the NPPC/NPR2 paracrine regulation becomes active and maintains oocytes arrested in meiotic prophase via regulation of intrafollicular and oocyte cGMP. With the LH surge, a switch in paracrine regulation takes place with inactivation of the NPPC/NPR2 module and activation of EGF-like growth factors and the PGE2 paracrine regulations. The enlargement of a GV oocyte included in the figure summarized the components of the cAMP signaling pathway involved in meiotic arrest and functional in an antral preovulatory follicle.
With the present review, we will summarize the current knowledge of the molecular and signaling events associated with ovulation in mammals, focusing primarily on the signaling in the follicle that controls oocyte maturation. Oocytes enter a specialized cell cycle (meiosis) during fetal life and are arrested in prophase I until reproductive maturity, after which oocytes from selected gonadotropin-responsive follicles reenter the cell cycle and complete meiosis upon fertilization. Thus, an oocyte may remain arrested in meiotic prophase for periods up to 40 years or more in women. This blockade in the meiotic cell cycle is under the control of the somatic cells of the follicle, because oocytes or cumulus-oocyte complexes that are removed from the mature follicle resume meiosis spontaneously (Pincus and Enzmann 1935; Edwards 1965). Moreover, only somatic cells of the follicle express the molecular machinery necessary to respond to LH. Therefore, somatic-germ cell communications are critical for maintaining the meiotic arrest and to induce meiotic maturation. The primary focus of this review will be the mechanisms and signals mediating these local regulations in the follicle.
Oocyte meiotic arrest and its control
A well established concept is that meiotic arrest of the oocyte is dependent on high concentrations of the second messenger cyclic AMP (cAMP) [(Cho et al. 1974; Dekel and Beers 1978), reviewed in(Conti et al. 2002)] Given the dependence of oocyte meiotic arrest on the interaction of the oocyte with granulosa cells, a widely held view was that cAMP was provided to the oocyte by somatic cells through gap junctions (Dekel 1988). Indeed, inhibitors of gap junction permeability induced oocyte maturation (Sela-Abramovich et al. 2006), suggesting that this connection is necessary to transfer an inhibitory factor to the oocyte, cAMP being the most plausible candidate. Several studies have provided evidence that cAMP generated in somatic cells is transferred to the oocyte (Vivarelli et al. 1983; Bornslaeger and Schultz 1985; Salustri et al. 1985). A caveat of these studies is that PDE inhibitors had to be used to demonstrate this transfer. More recently and using a cAMP sensor expressed exclusively in the oocyte, Webb et al have shown that treatment of cumulus oocyte complexes with FSH which stimulates cAMP accumulation in cumulus cell causes an increase in cAMP also in oocytes; more importantly, under conditions where gap junctions are closed the oocyte transfer is abolished (Webb et al. 2002). Surprisingly, cAMP levels remain constant when gap junctions are closed, suggesting cAMP production by the oocyte. Indeed, recent data indicate that the entire molecular machinery required to produce cAMP is expressed in mammalian oocytes and that the activity of these endogenous components is sufficient to maintain cAMP at levels that prevent maturation (see below).
G-protein coupled receptors and the transduction machinery are functional in oocytes
Analysis of the oocyte transcriptome indicates that several GPCRs are expressed by rodent oocytes (Mehlmann et al. 2004; Hinckley et al. 2005). However, the function of most of these receptors remains unknown except for a cluster of receptors that includes GPR3 and GPR12. These GPCRs were initially identified as receptors with considerable constitutive activity, but that could be further activated by S1P (Kostenis 2004). As the true physiological ligand for these receptors may not be correctly identified, they should still be regarded as orphan receptors (Yin et al. 2009). Studies with genetic or oligonucleotide-mediated ablation of GPR3 in the mouse, and GPR12 in the rat, have provided evidence that these receptors are required to maintain high cAMP levels in the oocyte and meiotic arrest (Mehlmann et al. 2004; Hinckley et al. 2005; Ledent et al. 2005). GPR3 is also expressed on Xenopus and human oocytes, suggesting a conserved function across species (Deng et al. 2008; DiLuigi et al. 2008). Oocytes lacking these receptors display leaky meiotic arrest and premature reentry into the cell cycle as soon as the oocyte acquires the ability to mature (Mehlmann et al. 2004; Ledent et al. 2005). A phenotype reminiscent of premature ovarian failure was also reported for the GPR3 null mice by Ledent et al. (Ledent et al. 2005).
Downstream of these receptors, the role of a Gs protein has been demonstrated by neutralizing antibody injection (Mehlmann et al. 2002). In addition, associated adenylyl cyclases have been characterized biochemically and their function assessed genetically (Horner et al. 2003). These findings have provided a molecular explanation to earlier observations demonstrating cAMP synthesis by the oocyte (Olsiewski and Beers 1983; Urner et al. 1983). Thus, all the membrane machinery required for cAMP generation is expressed in oocytes and its function is required to maintain meiotic arrest.
It has long been known that inhibitors of phosphodiesterases (PDEs) were able to maintain meiotic arrest in oocytes removed from the follicle (Dekel and Beers 1978; Dekel and Beers 1980; Vivarelli et al. 1983; Bornslaeger et al. 1984). A major PDE expressed in mouse, rat, monkey and human oocytes is the product of the PDE3A gene (Richard et al. 2001; Shitsukawa et al. 2001; Jensen et al. 2002; Nogueira et al. 2006). Both pharmacological and genetic evidence indicate that the activity of this enzyme is critical for meiotic resumption (Tsafriri et al. 1996; Masciarelli et al. 2004). In the mouse and rat ovarian follicle, this enzyme is primarily expressed in the oocyte and not in granulosa cells. This property has allowed for the use of selective inhibitors to manipulate cAMP levels in the oocyte without affecting the somatic compartment (Tsafriri et al. 1996). Whether this compartmentalized expression is retained in other species is not clear, as PDE3 activity has been detected in cumulus cells of pig follicles (Sasseville et al. 2007).
Control of the meiotic block downstream of cAMP
High levels of cAMP in the oocyte suppress the activation of MPF via the action of cAMP-dependent protein kinase A (PKA) (Maller and Krebs 1977; Maller and Krebs 1980; Bornslaeger et al. 1986) (see Fig 1). The activity of MPF, a complex of Cdc2 and cyclin B, is negatively regulated by the phosphorylation of two highly conserved residues of Cdc2, Thr14 and Tyr15. These inhibitory phosphorylations are catalyzed by the Wee1 kinases, whereas dephosphorylation of these residues is dependent on the Cdc25 phosphatases (Lew and Kornbluth 1996). Recent studies show that PKA directly regulates the activity of both Cdc25 phosphatases and Wee1 kinases (Han et al. 2005; Zhang et al. 2008; Pirino et al. 2009; Oh et al. 2010). In GV-arrested oocytes, PKA-mediated phosphorylation of Cdc25B negatively regulates its function by sequestering it in the cytoplasm in a complex with 14-3-3 protein (Zhang et al. 2008; Pirino et al. 2009). Phosphorylation of Wee1B by PKA increases Wee1B activity and thereby inhibits the activity of MPF in the nucleus. Although MPF is mainly localized in the cytoplasm of GV-arrested oocytes, the cytoplasmic localization may be the result of rapid nuclear export and slow nuclear import (Marangos and Carroll 2004; Reis et al. 2006). Thus, Wee1B prevents the activation of MPF that is shuttling into the nucleus during meiotic arrest. On the other hand, Myt1 inhibits the activity of MPF localized in the cytoplasm, as demonstrated by morpholino oligonucleotide knockdown of Myt1 (Oh et al. 2010). When cAMP levels decrease and thereby inactivate PKA in the oocyte, Cdc25B is translocated to the nucleus (Oh et al. 2010). The accumulation of the phosphatase in the nucleus promotes the activation of MPF that is shuttling into the nucleus. In the nucleus, the activated MPF promotes the export of Wee1B to the cytoplasm. Therefore, meiotic arrest is maintained by cAMP-mediated activation of PKA via the direct regulation of the kinase and phosphatase that regulate MPF activity. These two branches of the regulatory loop likely work in a synergistic fashion to maintain an inactive MPF during meiotic arrest. A third component controlling meiotic arrest may be the equilibrium between synthesis and degradation of Cyclin B. Blockade of the APC activity responsible for cyclin B degradation causes meiotic resumption in a subset of oocytes at the GV stage (Homer et al. 2009; Holt et al. 2010; Holt et al. 2011).
Maintenance of cAMP levels that prevent maturation in the oocyte: role of cGMP
The finding that the machinery for cAMP accumulation is expressed in its entirety in the mammalian oocyte has consolidated the idea that this second messenger is produced autonomously by the oocyte at levels sufficient to maintain meiotic arrest. However, this view needs to be reconciled with the finding that a mammalian oocyte, once removed from the follicle, is unable to maintain the meiotic arrest. To resolve these discrepancies, two hypotheses have been tested experimentally. One is that receptors involved in cAMP generation in the oocyte are regulated by signals originating in the somatic compartment. As mentioned above, GPR3 and GPR12 are the two main GPCRs regulating cAMP generation in mouse and rat oocytes, respectively. Although these receptors are thought to bind several lipid moieties, subsequent data have cast doubt on the specificity of these effects and these receptors remain orphan receptors (Yin et al. 2009). Thus, it has not been possible to directly test whether ligands are produced by granulosa cells nor to demonstrate these are necessary to maintain meiotic arrest. However, these receptors have considerable constitutive activity when expressed in a heterologous system, and some data support the idea that the cAMP signals produced by these receptors in the oocyte do not depend on ligands from somatic cells. Freudzon et al (Freudzon et al. 2005) have used the subcellular localization of Gsα as a readout of the activity of GPR3 in the oocytes (Gsα translocates to the cytoplasm upon receptor activation). By comparing the localization in follicle enclosed and follicle cell-free oocytes, they concluded that GPR3 activity does not require surrounding follicular cells. These findings are consistent with the absence of a follicular stimulus activating GPR3, with the caveat that the assay may not be sufficiently sensitive and quantitative to detect subtle changes in activity.
An additional possibility is that cAMP levels non-permissive to maturation are maintained in the oocytes via regulation of the endogenous PDE3A. Changes in PDE3 activity have been detected in oocytes during maturation (Richard et al. 2001), although the cause of this activation was unclear. More recently, a property of PDE3A has recently become the focus of further investigation (Shitsukawa et al. 2001). This enzyme hydrolyzes both cAMP and cGMP. However, the velocity of cAMP hydrolysis is one order of magnitude higher than that for cGMP hydrolysis. Given these kinetic properties, this enzyme behaves as a cGMP-inhibited cAMP-hydrolyzing PDE (Manganiello et al. 1990); work done initially in platelets has conclusively shown that cAMP levels are affected by cGMP inhibition of this PDE (Maurice and Haslam 1990). Thus, the possibility exists that cGMP, if present in the oocyte, may inhibit PDE3A and therefore contribute to maintaining cAMP levels above a certain threshold to maintain meiotic arrest.
As we will describe further below, recent data strongly support this view. However, it should be noted that clues that cGMP plays an important role in meiotic arrest can be traced back to reports published over the last three decades. In light of the most recent findings, all these pieces of information fit nicely in a model that may explain most of the facets of meiotic arrest in rodents. For instance, work done more than three decades ago in porcine oocytes indicated the presence of a factor in the follicular fluid that inhibited meiotic maturation (Tsafriri et al. 1976). This factor was partially characterized as a protein with molecular weight of about 2000. A subsequent report showed that porcine follicular fluid increased cGMP in the follicle (Kolena et al. 1993) even though the data were interpreted more in context with luteinization of granulosa cells. In hamster ovaries, cGMP levels have an opposite pattern of accumulation than cAMP during the estrous cycle, being highest in diestrus and lowest in proestrus/estrus when oocytes mature (Hubbard 1980). Also, oocyte cGMP levels were measured and shown to decrease roughly at the same time as oocyte cAMP levels (Tornell et al. 1990a). These authors further showed that atrial natriuretic peptide, which increases cGMP in the cumulus-oocyte complex, prevented rat oocyte maturation (Tornell et al. 1990b). Additional data suggest that pharmacological manipulations elevating cGMP levels in the oocyte maintain meiotic arrest (Tornell et al. 1984; Zhang et al. 2005a; Zhang et al. 2005b). In the converse experiment, inhibition of soluble guanylyl cyclase with ODQ caused oocyte maturation in follicle cultures and this effect was reversed by 8-Br-cGMP (Sela-Abramovich et al. 2008). Thus, a substantial amount of data suggested a role for cGMP for maintenance of meiotic arrest. Three recent papers have reignited interest in ovarian cGMP and have provided key observations on how meiotic arrest may be maintained in the antral follicle (Norris et al. 2009; Vaccari et al. 2009; Zhang et al. 2010).
A pool of cGMP required for maintenance of meiotic arrest is present in GV arrested oocytes. Injection of PDEs that hydrolyze only cGMP in isolated mouse oocytes or in follicle-enclosed oocytes causes oocytes to re-enter the cell cycle (Norris et al. 2009; Vaccari et al. 2009). More importantly, maturation does not occur when PDE3A knockout oocytes are injected or when PDE3 activity is blocked with an inhibitor, strongly suggesting that cGMP functions upstream of PDE3A. In addition, cGMP concentrations of about 1 μM have been measured by RIA or by FRET measurements (Norris et al. 2009; Vaccari et al. 2009). Genetic evidence for a paracrine loop that controls cGMP levels in the follicle has been recently reported by Eppig and collaborators (Zhang et al. 2010). Inactivation of either the NPPC ligand or the cognate receptor NPR2 in two genetic mouse models results in premature oocyte maturation, phenocopying the GPR3 ablation. Moreover, cGMP is decreased in these knockouts and the peptide derived from NPPC prevents spontaneous maturation (Zhang et al. 2010).
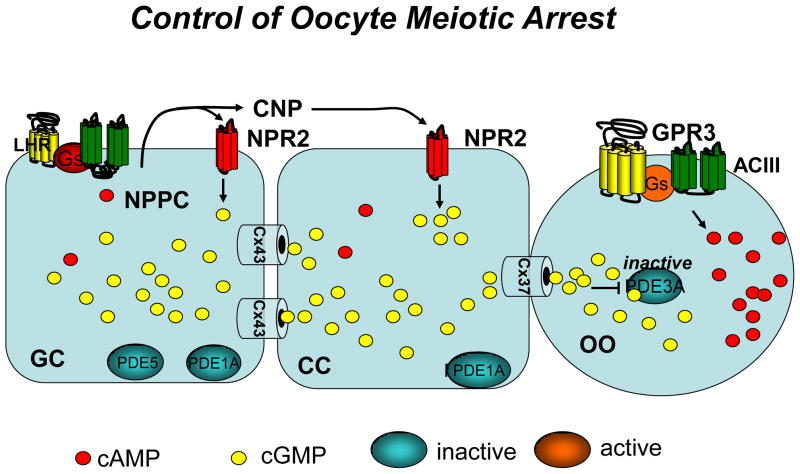
Taken together, these findings support the following model for maintenance of meiotic arrest in murine oocytes (see Fig. 2). Granulosa cells express NPPC, which is the precursor for CNP. Although direct evidence of secretion by granulosa cells is lacking, it is likely that CNP accumulates in the follicular extracellular space and activates NPR2. Activated NPR2 causes accumulation of cGMP in the granulosa cell compartment. This diffuses to the oocyte and maintains PDE3A in an inactive state. Since little cAMP accumulates in the follicle prior to LH stimulation, it is likely that cGMP, rather than cAMP, may be the diffusing molecule critical for maintaining the meiotic arrest. It remains to be determined why in earlier reports maturation could be blocked by ANP which does not bind NPR2. It is also unclear why soluble guanylyl cyclase inhibitors, which should not affect receptor guanylyl cyclases, are able to induce maturation in the rat. This may indicate species differences in the NPR expressed, or that under certain conditions ANP can bind and activate NPR2. In a recent report, ANP showed no effect on mouse oocyte maturation (Zhang et al. 2010) and our unpublished observations suggest that ANP does not increase cGMP in granulosa cells (Zamah et al submitted).
Fig. 2. Model summarizing the cyclic nucleotide signaling pathways involved in maintenance of meiotic arrest in the mouse follicle.
The presence of cGMP PDEs in granulosa cells is inferred from biochemical and inhibitor data. Although NPR2 expression in cumulus cells may be higher than in mural cells, mRNA for this receptor is detectable also in mural granulosa cells. GC, granulosa cells; CC, cumulus cells; OO, oocyte. The major connexin subunit expressed in the oocyte is connexin 37 whereas connexin 43 is expressed in the somatic compartment. Heterodimers of connexin 37/43 mediate some communication between the oocyte and cumulus cells.
LH surge and associated signals
The LH surge terminates the program of FSH-dependent steroidogenesis and granulosa cell growth, while promoting differentiation of somatic cells into luteal cells. At the same time, LH induces the expression of genes required for follicle rupture and ovulation, and activates multiple signaling cascades leading to oocyte maturation.
The LHR belongs to the group of GPCRs that are dependent on interaction with Gsα and activation of adenylyl cyclase to produce cAMP (Rajagopalan-Gupta et al. 1998; Richards 2001). Since most of the LH effects are reproduced by pharmacological activation of adenylyl cyclase, for instance with forskolin, it is accepted that an increase in cAMP is the primary signal that mediates the biological effects of the LH surge. The initial cAMP signal activates a number of signaling pathways and related kinases, all having important function during ovulation. However, numerous other signals are also activated by LH in the follicle. A scheme summarizing these pathways is reported in Fig. 3. Some of these are mediated by LHR interaction with other G proteins. In rodents, the LH receptor (LHR) is expressed predominantly by theca and mural granulosa cells (Amsterdam et al. 1975; Peng et al. 1991). Therefore, paracrine signaling and intercellular communications must be essential for cumulus-oocyte complex response to the LH surge. For instance, LH-dependent local production of prostaglandins plays an essential role for ovulation (reviewed in (Robker et al. 2000).
Fig. 3. Scheme of LH Receptor-activated signaling pathways regulating oocyte meiotic maturation.
The scheme summarizes our current knowledge on how the signal emanating from the LHR receptor branches to regulation of different signaling pathways. Question marks indicate that the intracellular mechanism linking a signal to downstream responses has not been established. The figure is an updated version of a figure published by Hsieh et al (Hsieh et al. 2011).
LH activation of PLC and inositol phosphate signaling
Early studies in rat and bovine ovaries showed that LH stimulates phosphoinositide (PI) turnover in intact granulosa cells in culture (Davis et al. 1983; Davis et al. 1986; Herrlich et al. 1996). In addition, several reports have documented that LHR activation is associated with an increase in intracellular Ca2+ in granulosa cells (Veldhuis 1987). Using a model of recombinant receptors expression in Xenopus oocytes, it has been shown that LH causes an increase in Ca2+-activated Cl− current, as measured by the two-microelectrode voltage-clamp method (Gudermann et al. 1992). This indicates that Ca2+ mobilization from intracellular stores occurs in this reconstitution system after LHR occupancy, probably as a consequence of LHR coupling to Gq and phospholipase (PLC) endogenous to the frog oocyte. In most cases, the high concentration of LH required to elicit these signals indicated that this coupling may be important at the time of the LH surge. The physiologic role of LH-dependent Ca2+ signals in ovulation has not been explored in detail, but a report indicates that at least in the mouse, this pathway may not be necessary for oocyte maturation (Mehlmann et al. 2006). Conversely, LH-dependent Ca2+ signals may play a role in luteinization, since PKC seems to play an important role in this transition (Morris and Richards 1995).
LH regulation of PI3-kinases and downstream pathways
Work from several laboratories has shown that gonadotropins may activate powerful signaling pathways involved in cell replication independently of PKA (Richards 2001). Granulosa cell stimulation by LH is accompanied by an increase in phosphorylation and therefore activation of the protein kinase AKT, a kinase downstream of the PI3K pathway. Class I PI3Ks (Phosphatidylinositol 3-kinases) are a family of signal transduction enzymes which catalyze the 3′ phosphorylation of the inositol ring of phosphatidyinositols to produce PIP3. PIP3 recruits AKT to the membrane, promoting its phosphorylation. An elegant study by Andric and Ascoli using immature rat granulosa cells transfected with mutant LH receptors showed that LH-mediated AKT phosphorylation is independent of cAMP and inositol phopshate production (Andric and Ascoli 2008). PI3K may also be linked to activation of other effectors such as RAS (Ramjaun and Downward 2007). LH can activate the small GTP-binding protein RAS in an MA10 mouse Leydig cell line (Shiraishi and Ascoli 2006). Similar observations on activation of RAS downstream kinases have been reported for FSH (Wayne et al. 2007). The amount of GTP bound to RAS, which is a measure of its activation, increases markedly but transiently after stimulation with an ovulatory dose of hCG (Fan et al. 2008). Moreover, granulosa cell specific knockout of RAS causes a major disruption of the ovulatory process, in addition to having a major effect on follicle growth (Fan et al. 2009a; Fan et al. 2009b). The mechanisms of LH activation of RAS are not well defined but they may be both direct and indirect through activation of tyrosine kinase receptors (see below).
LH regulation of cGMP levels in the follicle
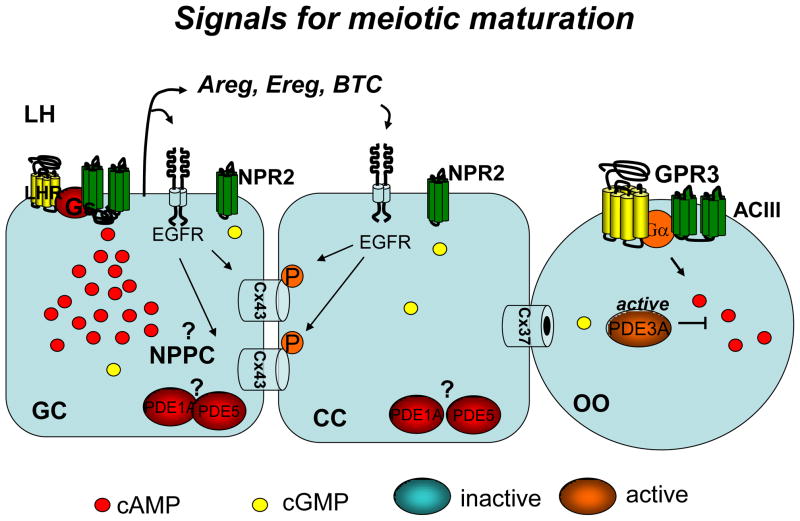
Although some earlier observations suggested that LH regulates follicular cGMP (Davis et al. 1986), recent data using complementary approaches clearly show that LH causes a profound decrease in cGMP in both granulosa cells and the oocyte (Norris et al. 2009; Vaccari et al. 2009), thus providing a clue to how signals for maturation may develop. The decrease in oocyte cGMP levels is in a range sufficient to cause several fold activation of PDE3A. The mechanism by which activation of LHR causes a marked decrease in cGMP is at present unknown. Preliminary data from our laboratory indicate that Nppc mRNA is under the control of the gonadotropins FSH and LH (Zamah et al in preparation), but other mechanisms including desensitization of NPR2 are most likely involved. Some of the effects on cGMP may be indirect and dependent on activation of the epidermal growth factor (EGF) network (see below) since EGF-like growth factors produce a decrease in cGMP similar to LH (Norris et al. 2008; Vaccari et al. 2009) (Fig. 4).
Fig. 4. Model summarizing the different signaling pathways in the ovarian follicle mediating the LH-induced oocyte maturation.
Question marks indicate putative changes not yet substantiated by experimental data. P in a red circle indicates phosphorylation. The inactivation of NPR2 may be consequent to decrease in ligand production by granulosa cells or desensitization of the receptor through unknown mechanisms. Activation of a cGMP-specific phosphodiesterase may also contribute to the decrease in cGMP in the follicle.
LH transactivation of the EGF network
In recent years, the discovery that the LH surge is associated with activation the EGF signaling network has provided new insight and raised further questions on how the LH signal is propagated from the mural granulosa cells to the cumulus-oocyte complex for the induction of oocyte reentry into the cell cycle and ovulation. A growing body of evidence supports a critical role for LHR transactivation of the EGFR in the regulation of these processes, with recent studies aimed at defining the downstream steps involved.
EGF has long been known to induce oocyte maturation in vitro (Dekel and Sherizly 1985), and to improve oocyte competence (De La Fuente et al. 1999), yet presence in the preovulatory follicle and follicular fluid remained inconclusive (Westergaard and Andersen 1989; Reeka et al. 1998; Hsieh et al. 2009). It is now known that the EGF-like growth factors amphiregulin (AREG), epiregulin (EREG) and betacellulin (BTC), rather than EGF, are rapidly and transiently induced in the somatic cells of the preovulatory follicle by LH (Park et al. 2004; Sekiguchi et al. 2004; Ashkenazi et al. 2005). LH/hCG induction of these EGF-like growth factors has been reported in multiple species (Fru et al. 2007; Wang et al. 2007; Chen et al. 2008; Lindbloom et al. 2008; Inoue et al. 2009; Zamah et al. 2010). The EGF-like growth factors are released from the cell surface as mature, soluble peptides by proteolytic cleavage of the ectodomain, and when added to cumulus-oocyte complexes or follicles in culture, activate the EGFR on the surfaces of the somatic cells to promote oocyte meiotic resumption and cumulus expansion. Inhibition of growth factor shedding by the matrix metalloprotease inhibitors GM6001 or TAPI-1 blocks the LH effects on maturation and cumulus expansion (Ashkenazi et al. 2005; Panigone et al. 2008).
Pharmacological inhibition of EGFR activity prevents these LH-induced events (Park et al. 2004; Ashkenazi et al. 2005), demonstrating that the EGFR is clearly involved. In addition, transcripts for the other members of the Egfr/Erbb1 family, namely Erbb2, Erbb3 and Erbb4, have recently been detected in granulosa cells and cumulus cells from human preovulatory follicles 36 hours after hCG stimulation, although at much lower levels than for Egfr mRNA (Zamah et al. 2010). ERBB2, which has no known ligand, becomes phosphorylated in mouse ovaries after hCG stimulation (Noma et al. 2010; Kim et al. 2011). Therefore, it is possible that upon ligand binding, EGFR forms not only homodimeric complexes, but perhaps also heterodimers with one or more of the other ERBB receptors in the follicle. That different ligand-receptor complexes may form likely determines the specificity of the signals produced. However, the physiological role for ERBB2, ERBB3 and/or ERBB4 in the ovary remain to be determined experimentally in vivo.
Studies using different mouse models of disruption of the EGF signaling network underscore a physiological role for this pathway in propagating the LH signal in the preovulatory follicle (Hsieh et al. 2007). Mice null for one of the EGF-like growth factors have a mild ovarian phenotype, likely due to compensation by the other EGF-like growth factors (Hsieh et al. 2007; Kim et al. 2011). A more profound effect on ovarian function was observed in mice null for amphiregulin and homozygous for the hypomorphic Egfrwa2 allele (Areg−/− Egfrwa2/wa2) (Hsieh et al. 2007). Oocyte maturation, cumulus expansion and ovulation are all impaired in these double mutant mice. In the human, AREG was found to be the most abundant EGF-like growth factor to accumulate in the follicular fluid of ovulatory follicles 36 hours after hCG stimulation (Inoue et al. 2009; Zamah et al. 2010). Human follicular fluid from hCG-stimulated follicles was capable of inducing cumulus expansion and oocyte maturation in cultured mouse cumulus-oocyte complexes (Zamah et al. 2010). However, immunodepletion of AREG, but not of other EGF-like growth factors, blocked the ability of the follicular fluid to promote both these events. In addition, AREG levels were significantly lower in follicular fluid from follicles yielding an immature oocyte or an oocyte that developed into an aberrant embryo, than in fluid from follicles producing healthy oocytes (Zamah et al. 2010). However, others have observed an inverse correlation between AREG and oocyte quality, raising the possibility of more complex relationship with AREG production (Inoue et al. 2009) The exact pathway(s) downstream of the LHR that control EGF-like growth factor expression and release are an area of active research, but have yet to be fully determined. Taken together, the findings in the mouse and human strongly support a critical role for the EGF-like growth factors in normal oocyte maturation and ovulation.
In a study from our laboratory, the mouse preovulatory follicle culture model was used to investigate the timing of LH transactivation of the EGFR in relation to the onset of oocyte meiotic resumption (Panigone et al. 2008). With this study, LH was found to induce the expression of Areg and Ereg mRNAs, as well as the phosphorylation of EGFR, as early as 30 minutes after stimulation, a time that precedes the onset of oocyte meiotic resumption by at least 1 hour. Areg and Ereg mRNA levels and EGFR phosphorylation increased and were maximal in follicles after 2 hours of LH stimulation. The protein synthesis inhibitor puromycin blocked LH-induced but not AREG-induced EGFR phosphorylation and oocyte GVBD, indicating that de novo protein synthesis was required. Thus, the initial activation of the EGF network by LH is rapidly amplified and maintained over time and likely serves as a mechanism to propagate and promote the LH signal throughout the follicle. Indeed, the importance for amplification, stabilization, and propagation of EGFR signals can be seen, for example, in the regulation of ERK1/2 activity in the follicle (see below).
LH regulation of MAPK
LH-dependent phosphorylation and activation of ERK1/2 has been demonstrated in granulosa cells of different species (Cameron et al. 1996; Carvalho et al. 2003; Su et al. 2003; Tajima et al. 2003; Panigone et al. 2008). Further studies showed that LH-dependent ERK 1/2 activation occurred downstream of cAMP and was dependent on PKA activation (Seger et al. 2001). We now know that multiple signaling cascades downstream of the LHR, including the EGFR and possibly PKC pathways (Woods and Johnson 2007) may also mediate ERK1/2 activation.
In the mouse, LH induces the phosphorylation of ERK1/2 in preovulatory follicles within 30 minutes, and phosphorylation levels are increased after 2 hours of stimulation (Panigone et al. 2008). This activation of ERK1/2 occurs first in the mural granulosa cells, and then, over time, also in the cumulus cells. LH-induced ERK1/2 phosphorylation was inhibited ~50–60% when preovulatory follicles were preincubated with the EGFR tyrosine kinase inhibitor AG1278, or with GM6001 or TAPI-1, matrix metalloprotease inhibitors that prevent growth factor shedding (Panigone et al. 2008; Hsieh et al. 2011). Reduced levels of phosphorylated ERK1/2 were also observed in Areg−/− Egfrwa2/wa2 follicles that were stimulated with LH for 2 hours, compared to wild type. Immunostaining for phosphorylated ERK1/2 in hCG-stimulated Areg−/− Egfrwa2/wa2 was decreased in the mural granulosa cells of preovulatory follicles compared to wild-type, and ERK1/2 activation in the cumulus cells was delayed and also reduced(Hsieh et al. 2011). Using a pharmacological approach, Reizel et al. (Reizel et al. 2010) found that blocking EGFR activity the last 15 minutes of incubation with LH for different time intervals resulted in reduced phosphorylation levels of ERK1/2, suggesting that sustained EGFR activity was required to maintain chronic ERK1/2 phosphorylation. Together, these observations show that LH transactivation of the EGFR is important for regulating ERK1/2 activation in the follicle. Because LH-induced ERK1/2 phosphorylation is not completely prevented in the Areg−/− Egfrwa2/wa2 follicles, this suggests that additional pathways are involved in ERK1/2 activation.
Different experimental models have been used to evaluate the role of ERK1/2 in the ovarian follicle. In cultured mouse follicles, the MEK inhibitor U0126 caused little inhibition of LH-induced oocyte germinal vesicle breakdown (GVBD) when used at 10 μM, a concentration sufficient to prevent LH-induced phosphorylation of ERK1/2, but blocked both LH-induced GVBD and cumulus expansion when used at 100 μM, a concentration that could produce nonspecific effects (Su et al. 2003). However, when ERK1/2 was disrupted specifically in mouse granulosa cells, oocyte maturation, cumulus expansion and ovulation failed to occur in response to hCG, indicating a necessary role for these kinases in vivo (Fan et al. 2009b). Notably, activation of ERK1/2 in cumulus cells with GDF9 alone was not sufficient to stimulate oocyte maturation in cultured cumulus-oocyte complexes (Su et al. 2003). Reduced but measureable levels of phosphorylated ERK1/2 are induced by LH in Areg−/− Egfrwa2/wa2 follicles, yet oocyte meiotic resumption is impaired (Hsieh et al. 2007). Together, these studies suggest that ERK1/2 is necessary but not sufficient to induce oocyte maturation. Another possible interpretation, however, is that a specific pool of ERK1/2 activated by EGFR signaling is required to promote oocyte reentry into the cell cycle, but this remains to be proven.
An additional member of the MAPK superfamily that may play an important function in LH signaling is p38MAPK. Inhibition of p38MAPK activity resulted in impaired meiotic resumption and abnormal cumulus expansion of in vitro maturation of porcine COCs (Yamashita et al. 2009). Three of the four p38MAPK isoforms, p38MAPKβ, p38MAPKγ, and p38MAPKδ, have been knocked out in mice, which display normal female fertility. More recently, Liu et al have generated mice with granulosa cell-specific knockout of the p38MAPKα isoform (Liu et al. 2010). In this model, female mice retained fertility, with the most notable alteration being aberrant EGF-like growth factor (specifically Areg and Ereg) gene expression patterns within the mural granulosa and cumulus cells. Interestingly, in vivo COC expansion and ovulation in these mice are normal, whereas in vitro COCs fail to expand normally. This could be overcome by addition of EGF-like growth factors to the in vitro culture media, suggesting that p38MAPK does have a role in LH-induced EGF network transactivation, but it is not essential for this function in vivo.
LH regulation of gap junction permeability
Gap junctions play an important role in signaling between somatic cells of the follicle, as well as between the cumulus cells and the developing oocyte. The gap junctions between different cells are distinguishable based on the dominant connexin protein present in the junction. Connexin-43 (Cx43) is the predominant connexin present in gap junctions connecting granulosa cells to granulosa cells, whereas Connexin-37 (Cx37) is the major connexin present in junctions between the cumulus cells and oocyte (Beyer et al. 1989; Simon et al. 1997). In the preovulatory follicle, LH induces decreased permeability of Cx43 gap junctions but not of Cx37 gap junctions (Norris et al. 2008). LH induces the phosphorylation of Cx43 on specific serine residues, and this phosphorylation is MAPK-dependent (Norris et al. 2008). Phosphorylation of Cx43 is associated with gap junction closure, which under experimental conditions appears sufficient to induce oocyte meiotic resumption, presumably by blocking passage of an inhibitory signal to the COC. In mutant mouse models with inactivating mutations of EGFR, LH-induced Cx43 phosphorylation is impaired (Andric et al. 2010)(Hsieh et al. 2011). Subsequent work further demonstrated that LH-induced EGFR transactivation is required for gap junction closure (Norris et al. 2010). However, other as yet undefined signals appear able to promote maturation in the absence of gap junction closure, since a MEK inhibitor (UO126) which prevents gap junction closure does not prevent LH-mediated meiotic resumption (Norris et al. 2008). It is possible that this additional signal may be the decrease in cGMP.
Conclusions
The available information summarized above clearly shows that a complex array of signaling cascades is activated by LH at the time of oocyte maturation and ovulation. These pathways act in parallel or sequentially and undoubtedly produce profound and often irreversible changes in the cells of the follicle. Some of the pathways we have described are intracellular, whereas others require extracellular events including the release of factors that act in an autocrine or paracrine fashion. All these events are necessary to propagate the LH signal from the periphery of the follicle to the center where the oocyte resides. The spatial dimension of signal propagation within the follicle has received little attention, but there is no doubt that exploring how different cellular domains function in time and space will provide a better understanding of the ovulatory process.
Highlights.
cAMP signaling controls MPF and prevents cell cycle reentry in the oocyte
cGMP signaling intersects with cAMP signaling to control meiotic arrest
the ovulatory LH signal activates multiple signaling pathways in the follicle
LH signals are propagated through the ovulatory follicle via release of paracrine factors
Acknowledgments
Our studies on the signaling of ovulation and oocyte maturation are supported by NIH grants R01 RO1-GM080527, RO1HD052909, and U54-HD055764. AMZ is supported by a WRHR scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsterdam A, Koch Y, Lieberman ME, Lindner HR. Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol. 1975;67(3):894–900. doi: 10.1083/jcb.67.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andric N, Ascoli M. Mutations of the lutropin/choriogonadotropin receptor that do not activate the phosphoinositide cascade allow hCG to induce aromatase expression in immature rat granulosa cells. Mol Cell Endocrinol. 2008;285(1–2):62–72. doi: 10.1016/j.mce.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andric N, Thomas M, Ascoli M. Transactivation of the epidermal growth factor receptor is involved in the lutropin receptor-mediated down-regulation of ovarian aromatase expression in vivo. Mol Endocrinol. 2010;24(3):552–560. doi: 10.1210/me.2009-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146(1):77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108(2):595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger EA, Mattei PM, Schultz RM. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Development Biology. 1986;114:453–462. doi: 10.1016/0012-1606(86)90209-5. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Schultz RM. Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. BiolReprod. 1985;33:698–704. doi: 10.1095/biolreprod33.3.698. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Wilde MW, Schultz RM. Regulation of mouse oocyte maturation: Involvement of cyclic AMP phosphodiesterase and calmodulin. Dev Biol. 1984;105:488–499. doi: 10.1016/0012-1606(84)90306-3. [DOI] [PubMed] [Google Scholar]
- Cameron MR, Foster JS, Bukovsky A, Wimalasena J. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5′-monophosphates in porcine granulosa cells. Biol Reprod. 1996;55(1):111–119. doi: 10.1095/biolreprod55.1.111. [DOI] [PubMed] [Google Scholar]
- Carvalho CR, Carvalheira JB, Lima MH, Zimmerman SF, Caperuto LC, Amanso A, Gasparetti AL, Meneghetti V, Zimmerman LF, Velloso LA, Saad MJ. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology. 2003;144(2):638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhou B, Yan J, Xu B, Tai P, Li J, Peng S, Zhang M, Xia G. Epidermal growth factor receptor activation by protein kinase C is necessary for FSH-induced meiotic resumption in porcine cumulus-oocyte complexes. J Endocrinol. 2008;197(2):409–419. doi: 10.1677/JOE-07-0592. [DOI] [PubMed] [Google Scholar]
- Cho WK, Stern S, Biggers JD. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool. 1974;187(3):383–386. doi: 10.1002/jez.1401870307. [DOI] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187(1–2):153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Davis JS, Farese RV, Clark MR. Stimulation of phospholipid synthesis by luteinizing hormone in isolated rat granulosa cells. Endocrinology. 1983;112(6):2212–2214. doi: 10.1210/endo-112-6-2212. [DOI] [PubMed] [Google Scholar]
- Davis JS, Weakland LL, West LA, Farese RV. Luteinizing hormone stimulates the formation of inositol trisphosphate and cyclic AMP in rat granulosa cells. Evidence for phospholipase C generated second messengers in the action of luteinizing hormone. Biochem J. 1986;238(2):597–604. doi: 10.1042/bj2380597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente R, O’Brien MJ, Eppig JJ. Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum Reprod. 1999;14(12):3060–3068. doi: 10.1093/humrep/14.12.3060. [DOI] [PubMed] [Google Scholar]
- Dekel N. In Vitro fertilization and other assisted reproduction. Acad Sci; New York: 1988. Regulation of oocyte maturation : the role of cAMP; pp. 211–216. [DOI] [PubMed] [Google Scholar]
- Dekel N, Beers WH. Rat oocyte maturation in vitro: relief of cyclic AMP inhibition by gonadotropins. Proc Natl Acad Sci U S A. 1978;75(9):4369–4373. doi: 10.1073/pnas.75.9.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel N, Beers WH. Development of the rat oocyte in vitro: inhibition and induction of maturation in the presence or absence of the cumulus oophorus. Dev Biol. 1980;75(2):247–254. doi: 10.1016/0012-1606(80)90160-8. [DOI] [PubMed] [Google Scholar]
- Dekel N, Sherizly I. Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology. 1985;116(1):406–409. doi: 10.1210/endo-116-1-406. [DOI] [PubMed] [Google Scholar]
- Deng J, Lang S, Wylie C, Hammes SR. The Xenopus laevis isoform of G protein-coupled receptor 3 (GPR3) is a constitutively active cell surface receptor that participates in maintaining meiotic arrest in X. laevis oocytes. Mol Endocrinol. 2008;22(8):1853–1865. doi: 10.1210/me.2008-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLuigi A, Weitzman VN, Pace MC, Siano LJ, Maier D, Mehlmann LM. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol Reprod. 2008;78(4):667–672. doi: 10.1095/biolreprod.107.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208(5008):349–351. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Paquet M, Wang J, Lydon JP, DeMayo FJ, Richards JS. Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009a;69(16):6463–6472. doi: 10.1158/0008-5472.CAN-08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009b;324(5929):938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135(12):2127–2137. doi: 10.1242/dev.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol. 2005;171(2):255–265. doi: 10.1083/jcb.200506194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fru KN, Cherian-Shaw M, Puttabyatappa M, VandeVoort CA, Chaffin CL. Regulation of granulosa cell proliferation and EGF-like ligands during the periovulatory interval in monkeys. Hum Reprod. 2007;22(5):1247–1252. doi: 10.1093/humrep/del519. [DOI] [PubMed] [Google Scholar]
- Gudermann T, Nichols C, Levy FO, Birnbaumer M, Birnbaumer L. Ca2+ mobilization by the LH receptor expressed in Xenopus oocytes independent of 3′,5′-cyclic adenosine monophosphate formation: evidence for parallel activation of two signaling pathways. Mol Endocrinol. 1992;6(2):272–278. doi: 10.1210/mend.6.2.1314958. [DOI] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15(18):1670–1676. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Herrlich A, Kuhn B, Grosse R, Schmid A, Schultz G, Gudermann T. Involvement of Gs and Gi proteins in dual coupling of the luteinizing hormone receptor to adenylyl cyclase and phospholipase C. J Biol Chem. 1996;271(28):16764–16772. doi: 10.1074/jbc.271.28.16764. [DOI] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287(2):249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Holt JE, Tran SM, Stewart JL, Minahan K, Garcia-Higuera I, Moreno S, Jones KT. The APC/C activator FZR1 coordinates the timing of meiotic resumption during prophase I arrest in mammalian oocytes. Development. 2011;138(5):905–913. doi: 10.1242/dev.059022. [DOI] [PubMed] [Google Scholar]
- Holt JE, Weaver J, Jones KT. Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development. 2010;137(8):1297–1304. doi: 10.1242/dev.047555. [DOI] [PubMed] [Google Scholar]
- Homer H, Gui L, Carroll J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science. 2009;326(5955):991–994. doi: 10.1126/science.1175326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258(2):385–396. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27(5):1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Thao K, Conti M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS One. 2011;6(6):e21574. doi: 10.1371/journal.pone.0021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med. 2009;27(1):52–61. doi: 10.1055/s-0028-1108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CJ. Ovarian cAMP and cGMP fluctuations in the hamster during the oestrous cycle. J Reprod Fertil. 1980;59(2):351–355. doi: 10.1530/jrf.0.0590351. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Miyamoto S, Fukami T, Shirota K, Yotsumoto F, Kawarabayashi T. Amphiregulin is much more abundantly expressed than transforming growth factor-alpha and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2009;91(4):1035–1041. doi: 10.1016/j.fertnstert.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Jensen JT, Schwinof KM, Zelinski-Wooten MB, Conti M, DePaolo LV, Stouffer RL. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum Reprod. 2002;17 (8):2079–2084. doi: 10.1093/humrep/17.8.2079. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee H, Threadgill DW, Lee D. Epiregulin-dependent amphiregulin expression and ERBB2 signaling are involved in luteinizing hormone-induced paracrine signaling pathways in mouse ovary. Biochem Biophys Res Commun. 2011;405(2):319–324. doi: 10.1016/j.bbrc.2011.01.039. [DOI] [PubMed] [Google Scholar]
- Kolena J, Danisova A, Matulova L, Scsukova S. Stimulatory action of porcine follicular fluid on granulosa cell secretion of cyclic GMP. Exp Clin Endocrinol. 1993;101(4):262–264. doi: 10.1055/s-0029-1211242. [DOI] [PubMed] [Google Scholar]
- Kostenis E. Novel clusters of receptors for sphingosine-1-phosphate, sphingosylphosphorylcholine, and (lyso)-phosphatidic acid: new receptors for “old” ligands. J Cell Biochem. 2004;92(5):923–936. doi: 10.1002/jcb.20092. [DOI] [PubMed] [Google Scholar]
- Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, Vassart G. Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci U S A. 2005;102(25):8922–8926. doi: 10.1073/pnas.0503840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol. 1996;8(6):795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- Lindbloom SM, Farmerie TA, Clay CM, Seidel GE, Jr, Carnevale EM. Potential involvement of EGF-like growth factors and phosphodiesterases in initiation of equine oocyte maturation. Anim Reprod Sci. 2008;103(1–2):187–192. doi: 10.1016/j.anireprosci.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan HY, Wang Y, Richards JS. Targeted disruption of Mapk14 (p38MAPKalpha) in granulosa cells and cumulus cells causes cell-specific changes in gene expression profiles that rescue COC expansion and maintain fertility. Mol Endocrinol. 2010;24(9):1794–1804. doi: 10.1210/me.2010-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JL, Krebs EG. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1977;252(5):1712–1718. [PubMed] [Google Scholar]
- Maller JL. Regulation of oocyte maturation. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- Manganiello VC, Smith CJ, Degerman E, Belfrage P. Cyclic GMP-inhibited Cyclic Nucleotide Phosphodiesterase. JJ. 1990:87–116. [Google Scholar]
- Marangos P, Carroll J. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction. 2004;128(2):153–162. doi: 10.1530/rep.1.00192. [DOI] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114(2):196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DH, Haslam RJ. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. MolPharmacol. 1990;37:671–681. [PubMed] [Google Scholar]
- Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297(5585):1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kalinowski RR, Ross LF, Parlow AF, Hewlett EL, Jaffe LA. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol. 2006;299(2):345–355. doi: 10.1016/j.ydbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306(5703):1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- Morris JK, Richards JS. Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology. 1995;136(4):1549–1558. doi: 10.1210/endo.136.4.7895665. [DOI] [PubMed] [Google Scholar]
- Nogueira D, Ron-El R, Friedler S, Schachter M, Raziel A, Cortvrindt R, Smitz J. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod. 2006;74(1):177–184. doi: 10.1095/biolreprod.105.040485. [DOI] [PubMed] [Google Scholar]
- Noma N, Kawashima I, Fan HY, Fujita Y, Kawai T, Tomoda Y, Mihara T, Richards JS, Shimada M. LH-induced neuregulin 1 (NRG1) Type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol. 2010;25(1):104–116. doi: 10.1210/me.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135(19):3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140(5):655–662. doi: 10.1530/REP-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136(11):1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Han SJ, Conti M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J Cell Biol. 2010;188(2):199–207. doi: 10.1083/jcb.200907161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsiewski PJ, Beers WH. cAMP synthesis in the rat oocyte. Dev Biol. 1983;100(2):287–293. doi: 10.1016/0012-1606(83)90223-3. [DOI] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22(4):924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129(6):3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- Pincus G, Enzmann EV. The Comparative Behavior of Mammalian Eggs in Vivo and in Vitro : I. The Activation of Ovarian Eggs. J Exp Med. 1935;62(5):665–675. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirino G, Wescott MP, Donovan PJ. Protein kinase A regulates resumption of meiosis by phosphorylation of Cdc25B in mammalian oocytes. Cell Cycle. 2009;8(4):665–670. doi: 10.4161/cc.8.4.7846. [DOI] [PubMed] [Google Scholar]
- Rajagopalan-Gupta RM, Lamm ML, Mukherjee S, Rasenick MM, Hunzicker-Dunn M. Luteinizing hormone/choriogonadotropin receptor-mediated activation of heterotrimeric guanine nucleotide binding proteins in ovarian follicular membranes. Endocrinology. 1998;139(11):4547–4555. doi: 10.1210/endo.139.11.6302. [DOI] [PubMed] [Google Scholar]
- Ramjaun AR, Downward J. Ras and phosphoinositide 3-kinase: partners in development and tumorigenesis. Cell Cycle. 2007;6(23):2902–2905. doi: 10.4161/cc.6.23.4996. [DOI] [PubMed] [Google Scholar]
- Reeka N, Berg FD, Brucker C. Presence of transforming growth factor alpha and epidermal growth factor in human ovarian tissue and follicular fluid. Hum Reprod. 1998;13(8):2199–2205. doi: 10.1093/humrep/13.8.2199. [DOI] [PubMed] [Google Scholar]
- Reis A, Chang HY, Levasseur M, Jones KT. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol. 2006;8(5):539–540. doi: 10.1038/ncb1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizel Y, Elbaz J, Dekel N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol. 2010;24(2):402–411. doi: 10.1210/me.2009-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard FJ, Tsafriri A, Conti M. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod. 2001;65(5):1444–1451. doi: 10.1095/biolreprod65.5.1444. [DOI] [PubMed] [Google Scholar]
- Richards JS. Perspective: the ovarian follicle--a perspective in 2001. Endocrinology. 2001;142(6):2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O’Malley BW, Espey LL, Richards JS. Ovulation: a multi-gene, multi-step process. Steroids. 2000;65(10–11):559–570. doi: 10.1016/s0039-128x(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Salustri A, Petrungaro S, De Felici M, Conti M, Siracusa G. Effect of follicle-stimulating hormone on cyclic adenosine monophosphate level and on meiotic maturation in mouse cumulus cell-enclosed oocytes cultured in vitro. Biol Reprod. 1985;33(4):797–802. doi: 10.1095/biolreprod33.4.797. [DOI] [PubMed] [Google Scholar]
- Sasseville M, Cote N, Vigneault C, Guillemette C, Richard FJ. 3′5′-cyclic adenosine monophosphate-dependent up-regulation of phosphodiesterase type 3A in porcine cumulus cells. Endocrinology. 2007;148(4):1858–1867. doi: 10.1210/en.2006-1257. [DOI] [PubMed] [Google Scholar]
- Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, 3rd, Amsterdam A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J Biol Chem. 2001;276(17):13957–13964. doi: 10.1074/jbc.M006852200. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T, Mizutani T, Yamada K, Kajitani T, Yazawa T, Yoshino M, Miyamoto K. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33(1):281–291. doi: 10.1677/jme.0.0330281. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147(5):2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Galiani D, Nevo N, Dekel N. Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action. Biol Reprod. 2008;78 (6):1111–1118. doi: 10.1095/biolreprod.107.065490. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Ascoli M. Activation of the lutropin/choriogonadotropin receptor in MA-10 cells stimulates tyrosine kinase cascades that activate ras and the extracellular signal regulated kinases (ERK1/2) Endocrinology. 2006;147(7):3419–3427. doi: 10.1210/en.2005-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitsukawa K, Andersen CB, Richard FJ, Horner AK, Wiersma A, van Duin M, Conti M. Cloning and characterization of the cyclic guanosine monophosphate-inhibited phosphodiesterase PDE3A expressed in mouse oocyte. Biol Reprod. 2001;65(1):188–196. doi: 10.1095/biolreprod65.1.188. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385(6616):525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263(1):126–138. doi: 10.1016/s0012-1606(03)00437-8. [DOI] [PubMed] [Google Scholar]
- Tajima K, Dantes A, Yao Z, Sorokina K, Kotsuji F, Seger R, Amsterdam A. Down-regulation of steroidogenic response to gonadotropins in human and rat preovulatory granulosa cells involves mitogen-activated protein kinase activation and modulation of DAX-1 and steroidogenic factor-1. J Clin Endocrinol Metab. 2003;88(5):2288–2299. doi: 10.1210/jc.2002-020913. [DOI] [PubMed] [Google Scholar]
- Tornell J, Billig H, Hillensjo T. Resumption of rat oocyte meiosis is paralleled by a decrease in guanosine 3′,5′-cyclic monophosphate (cGMP) and is inhibited by microinjection of cGMP. Acta Physiol Scand. 1990a;139(3):511–517. doi: 10.1111/j.1748-1716.1990.tb08953.x. [DOI] [PubMed] [Google Scholar]
- Tornell J, Brannstrom M, Hillensjo T. Different effects of cyclic nucleotide derivatives upon the rat oocyte-cumulus complex in vitro. Acta Physiol Scand. 1984;122(4):507–513. doi: 10.1111/j.1748-1716.1984.tb07539.x. [DOI] [PubMed] [Google Scholar]
- Tornell J, Carlsson B, Billig H. Atrial natriuretic peptide inhibits spontaneous rat oocyte maturation. Endocrinology. 1990b;126(3):1504–1508. doi: 10.1210/endo-126-3-1504. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol. 1996;178(2):393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Pomerantz SH, Channing CP. Inhibition of oocyte maturation by porcine follicular fluid: partial characterization of the inhibitor. Biol Reprod. 1976;14 (5):511–516. doi: 10.1095/biolreprod14.5.511. [DOI] [PubMed] [Google Scholar]
- Urner F, Herrmann WL, Baulieu EE, Schorderet-Slatkine S. Inhibition of denuded mouse oocyte meiotic maturation by forskolin, an activator of adenylate cyclase. Endocrinology. 1983;113(3):1170–1172. doi: 10.1210/endo-113-3-1170. [DOI] [PubMed] [Google Scholar]
- Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81(3):595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD. Mechanisms subserving hormone action in the ovary: role of calcium ions as assessed by steady state calcium exchange in cultured swine granulosa cells. Endocrinology. 1987;120(2):445–449. doi: 10.1210/endo-120-2-445. [DOI] [PubMed] [Google Scholar]
- Vivarelli E, Conti M, De Felici M, Siracusa G. Meiotic resumption and intracellular cAMP levels in mouse oocytes treated with compounds which act on cAMP metabolism. Cell Differ. 1983;12(5):271–276. doi: 10.1016/0045-6039(83)90023-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Ying Wang C, Yan Kwok AH, Leung FC. Epidermal growth factor (EGF) receptor ligands in the chicken ovary: I. Evidence for heparin-binding EGF-like growth factor (HB-EGF) as a potential oocyte-derived signal to control granulosa cell proliferation and HB-EGF and kit ligand expression. Endocrinology. 2007;148(7):3426–3440. doi: 10.1210/en.2006-1383. [DOI] [PubMed] [Google Scholar]
- Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21(8):1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- Webb RJ, Marshall F, Swann K, Carroll J. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase a in mammalian oocytes. Dev Biol. 2002;246(2):441–454. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- Westergaard LG, Andersen CY. Epidermal growth factor (EGF) in human preovulatory follicles. Hum Reprod. 1989;4(3):257–260. doi: 10.1093/oxfordjournals.humrep.a136883. [DOI] [PubMed] [Google Scholar]
- Woods DC, Johnson AL. Protein kinase C activity mediates LH-induced ErbB/Erk signaling in differentiated hen granulosa cells. Reproduction. 2007;133(4):733–741. doi: 10.1530/REP-06-0261. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Hishinuma M, Shimada M. Activation of PKA, p38 MAPK and ERK1/2 by gonadotropins in cumulus cells is critical for induction of EGF-like factor and TACE/ADAM17 gene expression during in vitro maturation of porcine COCs. J Ovarian Res. 2009;2:20. doi: 10.1186/1757-2215-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009;284(18):12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamah AM, Hsieh M, Chen J, Vigne JL, Rosen MP, Cedars MI, Conti M. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010;25 (10):2569–2578. doi: 10.1093/humrep/deq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330(6002):366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Tao Y, Xia G, Xie H, Hong H, Wang F, Lei L. Atrial natriuretic peptide negatively regulates follicle-stimulating hormone-induced porcine oocyte maturation and cumulus expansion via cGMP-dependent protein kinase pathway. Theriogenology. 2005a;64(4):902–916. doi: 10.1016/j.theriogenology.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tao Y, Zhou B, Xie H, Wang F, Lei L, Huo L, Sun Q, Xia G. Atrial natriuretic peptide inhibits the actions of FSH and forskolin in meiotic maturation of pig oocytes via different signalling pathways. J Mol Endocrinol. 2005b;34(2):459–472. doi: 10.1677/jme.1.01673. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Xu XY, Li XS, Yu M, Yu AM, Zong ZH, Yu BZ. Protein kinase A modulates Cdc25B activity during meiotic resumption of mouse oocytes. Dev Dyn. 2008;237(12):3777–3786. doi: 10.1002/dvdy.21799. [DOI] [PubMed] [Google Scholar]