Abstract
Objectives
An association between diabetes and pancreatic ductal adenocarcinoma (PDA) has long been recognized. Here we assess the impact of baseline hemoglobin-A1c (HbA1c) value in clinical outcomes of PDA patients.
Methods
HbA1c values were prospectively collected on 656 consecutive patients presenting to a pancreas multidisciplinary clinic from 2009–2012. Patients were diagnosed with benign pancreatic disease (BPD) or biopsy-confirmed resectable (R), borderline/locally advanced (BL), or metastatic (M) PDA. Excluded were those with prior treatment for PDA or a history of chronic DM (>1-year).
Results
Of 284 patients, 44 had benign disease, 62 R-PDA, 115 BL-PDA, and 63 M-PDA. Patients with malignant disease (R-, BL-, and M-PDA) collectively had a higher average HbA1c than patients with BPD (6.1% vs. 5.6%, p<0.001). Among patients with PDA (n=240), HbA1c≥6.5% was significantly associated with inferior overall survival (OS) compared to patients with HbA1c<6.5% (HR 1.74, OS 10.2 vs. 13.0 months, p=0.007), along with other known prognostic factors such as age≥65, ECOG≥1, CA19-9>90, tumor size >3cm, and disease stage. HbA1c≥6.5% remained in the final predictive model using backward elimination (HR 1.46, p=0.097) indicating that HbA1c ≥6.5% influences overall survival of patients with PDA even when accounting for other known prognostic factors.
Conclusions
HbA1c level at presentation is significantly higher in patients with PDA than patients with BPD and appears to affect survival.
Keywords: glycosylated hemoglobin, hemoglobin-A1c, diabetes mellitus, pancreatic cancer, survival
INTRODUCTION
An association between diabetes mellitus (DM) and pancreatic ductal adenocarcinoma (PDA) has been recognized for more than half a century,1 yet diagnostic and therapeutic applications of this relationship remain limited. Growing epidemiologic evidence suggests that patients with DM are at significantly greater risk for PDA. In a population-based study of 2,122 diabetic patients, the incidence of pancreatic cancer within 3 years of DM diagnosis was nearly 8 times that of the general, non-diabetic population.2
While long-standing DM may be a risk factor for developing PDA, new-onset DM may, conversely, be a manifestation of the cancer. PDA patients are significantly more likely to have new-onset DM(<2-year duration) than non-cancer controls4 and to have a significantly higher prevalence of DM than patients with other types of cancer, such as lung, breast, prostate, and colorectal cancers, as well as non-cancer patients.5
Moreover, dysglycemia itself may have negative impact on outcomes of hospitalized patients with cancer in terms of infection, mortality, length of stay, and toxicities. The risk of cancer death (due to stomach, liver, lung cancers) has been noted to be significantly higher among subjects with a high fasting plasma glucose (≥5.6 mmol/L), after adjustment for potentially confounding factors.3
Here we directly evaluate the relationship between glycosylated hemoglobin (HbA1c) – an objective and quantifiable measure of glucose intolerance—and pancreatic cancer in patients without a history of chronic DM presenting with suspected PDA. Specifically, we examine the potential utility of HbA1c level to discriminate between benign pancreatic disease (BPD) and stages of PDA and investigate the effect of impaired glucose tolerance on patient survival outcomes.
MATERIALS AND METHODS
Study Population and Data Collection
All patients included in this study were seen at a pancreatic multidisciplinary clinic (PMDC). The purpose of the clinic is to provide a comprehensive multi-specialist evaluation for patients with pancreatic diseases. With Institutional Review Board approval, HbA1c values were prospectively collected on patients seen at the PMDC between January 2009 and October 2012. Patients were required to provide written informed consent prior to study enrollment. A total of 656 patients presented to the PMDC during the period of the study. Based on multidisciplinary review, patients were diagnosed as having resectable (R), borderline resectable/locally advanced (BL), metastatic (M) pancreatic cancer, or benign disease. Diagnoses considered to be benign disease included autoimmune pancreatitis, intraductal papillary mucinous neoplasm (IPMN), or pancreatic cyst. Exclusion criteria included prior treatment with surgical resection or non-surgical antineoplastic therapies (n=306) and a self-reported history of type 1 DM (n=6) or type 2 DM (n=60) for longer than one year or of unknown duration. The final study cohort included 284 patients.
All patients underwent a pancreatic protocol three-dimensional (3D) computed tomography (CT) scan and had routine labs drawn, including complete blood count, complete metabolic profile, carbohydrate antigen 19-9 (CA19-9), and HbA1c, on the morning of the PMDC. All 3D CT studies were performed with a Definition Dual Source CT scanner (Siemens Medical Solutions, Malvern NJ) according to a standard protocol6. Demographics, medical history, and clinical information were obtained from the initial consult note recorded at the PMDC visit. Self-reported race, family history of pancreatic cancer (up to second-degree relative), performance status (as previously defined in Oken PMID: 7165009), and use of anti-hyperglycemic medication was also recorded to assess for interaction with HbA1c. Survival was determined and cross-checked by review of clinical follow-up information and the Social Security Death Index.
Statistical Analysis
All demographic and baseline data were summarized using descriptive statistics. Differences in HbA1c values among patients with benign, resectable, borderline resectable/locally advanced, and metastatic disease were assessed for significance using the Student t test. All p-values are reported as two-sided and the a priori level of significance was set at p≤0.05. Kaplan–Meier analysis was used to estimate time-to-event curves and survival rates. Univariate Cox regression analyses were performed to assess for an association between clinical factors/laboratory values and overall survival (OS). Characteristics that demonstrated a univariate association with survival at a significance level of p≤0.05 were entered as covariates into a multivariate proportional hazards regression model for OS and backward elimination was performed to generate the final model. The final proportional hazards regression model was used to estimate the hazard ratio (HR) for death attributable to each covariate. Analyses were performed using R software, version 2.15.2.
RESULTS
Study Cohort
The final study cohort included 284 patients seen at the PMDC who met the following criteria: (1) newly diagnosed with no prior history of anti-PDA treatment (including surgery); (2) either no prior history of DM or first diagnosed with DM <1 year prior to PMDC visit; (3) available HbA1c level from day of PMDC visit; (4) follow-up survival data available. Classified by disease stage, there were 44 patients (15%) with BPD, 62 with R-PDA (22%), 115 with BL-PDA (40%), and 63 with M-PDA (22%). The demographic and baseline characteristics of the study population are shown in Table 1.
Table 1.
Demographic and baseline characteristics of patients presenting to clinic with hemoglobin-A1c (HbA1c) values and no prior treatment for pancreatic diseases.
| Characteristic | Value | |||||
|---|---|---|---|---|---|---|
| Total (N=284) |
Benign (N=44) |
Resectable (N=62) |
Locally Advanced (N=115) |
Metastatic (N=63) |
||
| Median Age, y (IQR) | 66 (58–73) | 58 (46–68) | 66 (59–73) | 66 (60–73) | 67 (79–73) | |
| Age ≥ 65, No..(%) | 153 (53.9) | 15 (34.1) | 37 (59.7) | 65 (56.5) | 36 (57.1) | |
| Age < 65, No..(%) | 131 (46.1) | 29 (65.9) | 25 (40.3) | 50 (43.5) | 27 (42.9) | |
| Sex, No.(%) | ||||||
| Female | 136 (47.9) | 22 (50.0) | 27 (43.5) | 53 (46.1) | 34 (54.0) | |
| Male | 148 (52.1) | 22 (50.0) | 35 (56.5) | 62 (53.9) | 29 (46.0) | |
| Race/ethnicity, No. (%) | ||||||
| White | 245 | 34 (77.3) | 54 (87.1) | 102 (88.7) | 55 (87.3) | |
| Asian | 10 | 5 (11.4) | 1 (1.6) | 2 (1.7) | 2 (3.2) | |
| Black | 19 | 3 (6.8) | 5 (8.1) | 7 (6.1) | 4 (6.3) | |
| Hispanic | 4 | 0 (0) | 2 (3.2) | 1 (0.9) | 1 (1.6) | |
| Other | 6 | 2 (4.5) | 0 (0) | 3 (2.6) | 1 (1.6) | |
| ECOG, No. (%) | ||||||
| 0 | 128 (45.0) | 31 (70.4) | 30 (48.4) | 47 (40.9) | 20 (31.7) | |
| 1 | 95 (33.5) | 6 (13.6) | 20 (32.3) | 41 (35.7) | 28 (44.4) | |
| 2 | 12 (4.2) | 0 (0) | 1 (1.6) | 6 (5.2) | 5 (7.9) | |
| Not available | 49 (17.2) | 7 (15.9) | 11 (17.7) | 21 (18.3) | 10 (15.9) | |
| Family history, pancreatic cancer | 40 (14.1) | 6 (13.6) | 9 (14.1) | 17 (14.8) | 8 (12.7) | |
| Family history, diabetes | 29 (10.2) | 6 (13.6) | 8 (12.5) | 9 (7.8) | 6 (9.5) | |
| Ca 19-9 U/mL | ||||||
| ≥ 90 U/mL | 151 (56.7) | 0 (0.0) | 33 (53.2) | 86 (74.8) | 42 (66.7) | |
| < 90 U/mL | 133 (43.3) | 44 (100.0) | 29 (46.8) | 29 (25.2) | 21 (33.3) | |
| HbA1c category | ||||||
| < 6.5 % | 238 (83.8) | 42 (95.5) | 55 (88.7) | 96 (83.5) | 45 (71.4) | |
| ≥ 6.5% | 46 (16.1) | 2 (4.5) | 7 (11.3) | 19 (16.5) | 18 (28.6) | |
Disease Stage and HbA1c
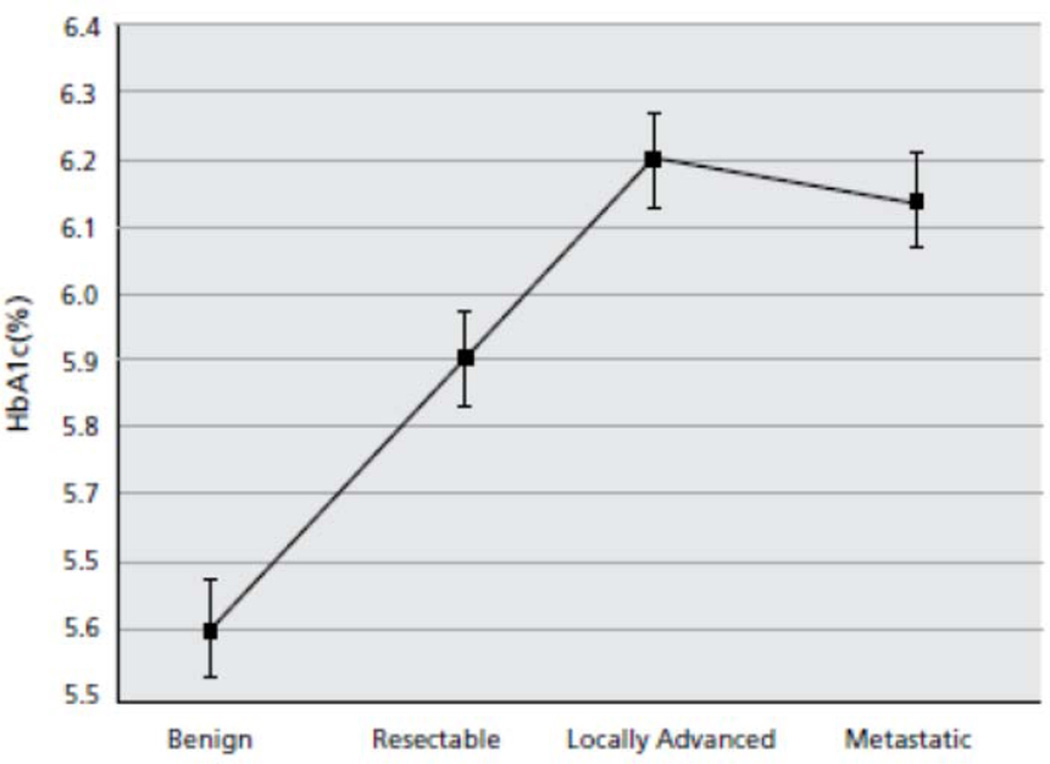
A plot of mean HbA1c values for each disease stage is shown in Figure 1. Mean HbA1c was 5.6% (SD, 0.5) for patients with BPD, 5.9% (SD, 1.0) for R-PDA, 6.2% (SD, 1.1) for BL-PDA, and 6.1% (SD, 0.8) for M-PDA. Patients with malignant disease (including R-, BL-, and M-PDA) collectively had higher HbA1c values on average at presentation than patients with BPD (6.1% vs. 5.6%, p<0.001). When each stage of PDA was compared separately to the BPD group, the difference in mean HbA1c values for patients with PDA versus patients with BPD became more significant as stage increased (R-PDA: 5.9% vs. 5.6%, p=0.048; BL-PDA: 6.2% vs. 5.6%, p=0.00043; M-PDA: 6.1% vs. 5.6%, p<0.0001). There was a trend towards higher HbA1c at presentation in patients with advanced PDA (BL and M) compared to patients with R-PDA (6.2% vs. 5.9%, p=0.100). The proportion of patients with HbA1c levels in the diabetic range (>6.4%) increased with more advanced stage of disease (4.5% of BPD, 11.3% of R-PDA, 16.5% of BL-PDA, and 28.6% of M-PDA).
Figure 1.
Plot of mean hemoglobin-A1c (HbA1c) based on disease status.
HbA1c Prognostic Value
Using univariate Cox regression analysis, inferior OS was found to be significantly associated with six factors (Table 2): (1) HbA1c ≥6.5% (HR=1.74, 95% CI 1.2–2.6, p=0.007); (2) age category ≥65 (HR=1.57, 95% CI 1.13, 2.20 p=0.008); (3) ECOG 2 compared with ECOG 0 (HR=2.28, 95% CI 1.09–5.25, p=0.030); (4) CA19-9 ≥ 90 U/mL (HR= 1.45, 95% CI 1.14–1.85, p=0.004); (5) tumor diameter ≥3 cm (HR=1.60, 95% CI 1.16–2.20, p=0.004); and (6) advanced disease stages BL-PDA compared to R-PDA (HR 1.71, 95% CI 1.12–2.64, p=0.014) and M-PDA compared to R-PDA (HR 2.70, 95% CI 1.70–4.29, p<0.001). These factors were used as covariates to construct the multivariable proportional hazards model for survival. Following multivariate analysis using backward elimination, the following factors were independently related to inferior OS (Table 3): HbA1c ≥6.5% (HR=1.46, 95% CI 0.93–2.27, p=0.097), age ≥65 (HR=1.64, 95% CI 1.11–2.41, p=0.012), CA19-9 >90 U/mL (HR 1.98, 95% CI 1.32–12.97, p=0.001), ECOG 1 compared to ECOG 0 (HR 1.35, 95% CI 0.92–1.99, p=0.131), ECOG 2 compared to ECOG 0, (HR 2.39, HR 1.04–5.51, p=0.041), and advanced disease stages BL-PDA compared to R-PDA (HR 1.36, 95% CI 0.84–2.21, p=0.217) and M-PDA compared to R-PDA (HR=1.93, 95% CI 1.14–3.27, p=0.014).
Table 2.
Associations between clinical factors/laboratory values and overall survival (OS) for 240 patients with pancreatic ductal adenocarcinoma in cohort. Median survival is shown in months with a 95% confidence interval (CI). All hazard ratios (HR) and p values are derived from univariate models.
| Characteristic | N | Median OS (months) | HR (95% CI) | P | |
|---|---|---|---|---|---|
| All cancer subjects | 240 | 12.4 [11.1, 13.6] | |||
| HbA1c category | |||||
| < 6.5 % | 196 | 13.02 [8.54, 17.50] | 1.00 | ||
| ≥ 6.5% | 44 | 10.22 [7.31, 13.13] | 1.74 (1.17, 2.60) | 0.007 | |
| Age category | |||||
| < 65 years | 102 | 18.1 [14.5, 21.8] | 1.00 | ||
| ≥ 65 years | 138 | 10.3 [7.5, 13.0] | 1.57 (1.13, 2.20) | 0.008 | |
| Sex | |||||
| Male | 126 | 12.4 [11.3, 13.5] | 1.00 | ||
| Female | 114 | 12.7 [7.6, 18.0] | 0.99 (0.72, 1.38) | 0.981 | |
| Race | |||||
| White | 211 | 12.4 [9.3, 15.4] | 1.00 | ||
| Non-White | 29 | 11.5 [9.7, 13.3] | 1.19 (0.74, 1.91) | 0.476 | |
| ECOG | |||||
| 0 | 97 | 16.2 [12.0, 20.3] | 1.00 | ||
| 1 | 89 | 10.9 [9.2, 12.5] | 1.40 (0.99, 2.00) | 0.059 | |
| 2 | 12 | 5.1 [1.5, 8.6] | 2.28 (1.09, 5.25) | 0.030 | |
| CA19-9 | |||||
| < 90 U/mL | 87 | 18.2 [11.6, 24.8] | 1.00 | ||
| ≥ 90 U/mL | 152 | 10.9 [11.1, 13.7] | 1.45 (1.14, 1.85) | 0.002 | |
| Current treatment for DM | |||||
| None | 228 | 12.4 [11.0, 13.7] | 1.00 | ||
| Oral medication | 7 | 10.4 [5.2, 15.6] | 2.38 (0.59, 9.66) | 0.224 | |
| Insulin | 5 | 23.2 [17.3, 29.1] | 2.54 (0.46, 13.97) | 0.284 | |
| Family History PDA | |||||
| No | 205 | 12.4 [11.3, 13.5] | 1.00 | ||
| Yes | 32 | 10.7 [3.6, 17.8] | 1.00 (0.67, 1.48) | 0.957 | |
| Family History DM | |||||
| No | 226 | 12.4 [11.1, 13.7] | 1.00 | ||
| Yes | 11 | 18.1 [3.1, 32.9] | 0.83 (0.59, 1.17) | 0.263 | |
| Tumor Diameter | |||||
| <3 cm | 77 | 18.2 [10.6, 25.8] | 1.00 | ||
| ≥ 3 cm | 143 | 11.2 [9.9, 12.53] | 1.60 (1.16, 2.20) | 0.004 | |
| Disease stage | |||||
| R-PDA | 62 | 20.5 [9.9, 31.1] | 1.00 | ||
| B-PDA | 115 | 13.0 [9.2, 16.8] | 1.72 (1.12, 2.64) | 0.014 | |
| M-PDA | 63 | 8.2 [7.1, 9.3] | 2.70 (1.70, 4.29) | <0.001 | |
Table 3.
Independent predictors for OS identified by the final multivariable proportional hazards model after backwards elimination. All factors showing a significant relationship to overall survival on univariate analysis were included in the initial model (HbA1c ≥6.5%, age ≥65 years, ECOG ≥1, CA19-9 >90 U/mL, tumor diameter ≥3cm, and disease stage; see Table 2).
| Characteristic | HR (95% CI) | P |
|---|---|---|
| HbA1c ≥ 6.5% | 1.46 (0.93, 2.27) | 0.097 |
| Age ≥ 65 years | 1.64 (1.11, 2.41) | 0.012 |
| CA19-9 > 90 U/mL | 1.98 (1.32, 12.97) | 0.001 |
| ECOG 0 vs. 1 | 1.35 (0.92, 1.99) | 0.131 |
| ECOG 0 vs. 2 | 2.39 (1.04, 5.51) | 0.041 |
| Disease stage R-PDA vs. BL-PDA | 1.36 (0.84, 2.21) | 0.217 |
| Disease stage R-PDA vs. M-PDA | 1.93 (1.14, 3.27) | 0.014 |
Median OS for all patients with PDA (n=240) was 12.4 months (95% CI, 11.1–13.6 months). Patients with HbA1c <6.5% had a significantly greater median OS at 13.0 months (95% CI 8.5–17.5 months) compared to those with HbA1c≥6.5% at 10.2 months (95% CI 7.3–13.1 months) (p<0.001).
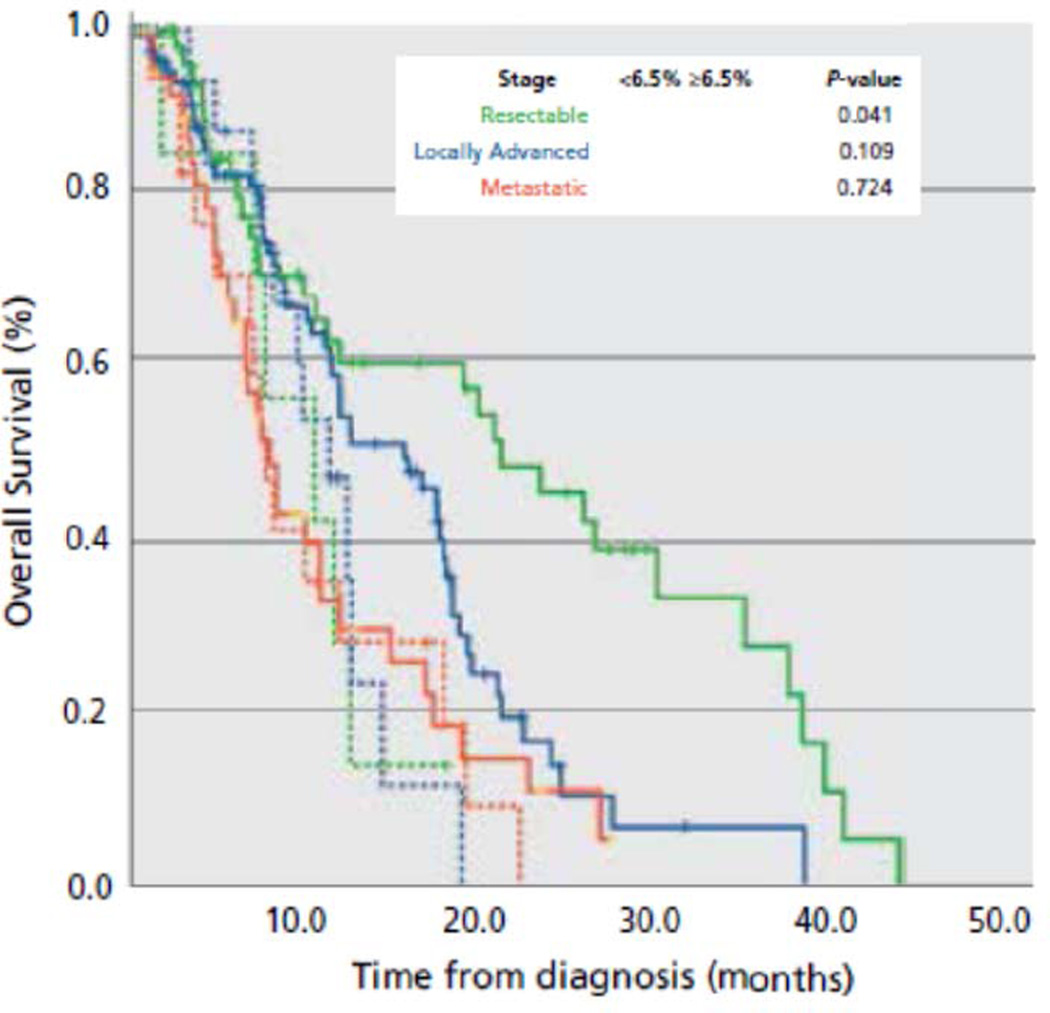
To evaluate the effect of HbA1c level on overall survival in each disease stage, we performed separate Kaplan-Meier analyses for R-PDA, BL-PDA, and M-PDA patients with HbA1c values ≥6.5% or <6.5% (Figure 2). Median OS was 20.5 months (95% CI, 9.9–31.1) in patients with R-PDA, 13.0 months (95% CI 9.3–16.8 months) in patients with BL-PDA, and 8.2 months (95% CI 7.1–9.3) in patients with M-PDA. OS was superior in R-PDA patients with HbA1c values <6.5 (21.9 months, 95% CI 14.0–29.8 months) compared to R-PDA patients with HbA1c values ≥6.5% (10.9 months, 95% CI 3.4–18.4 months) (p=0.041). BL-PDA patients with HbA1c values <6.5 (16.4 months, 95% CI 11.3–21.5 months) compared to BL-PDA patients with HbA1c values ≥6.5% (11.7 months, 95% CI 8.9–14.6 months) had improved OS; however, this did not approach significance (p=0.101). There was no difference in survival between M-PDA patients with baseline HbA1c <6.5% and those with baseline HbA1c ≥6.5% (P>>0.05).
Figure 2.
Kaplan-Meier curves for patients with resectable (green), locally advanced (blue), and metastatic (red) pancreatic ductal adenocarcinoma. Patients are stratified based on HbA1c level <6.5% (solid line) or ≥6.5% (dashed line) at the time of initial diagnosis.
DISCUSSION
Our study evaluates the impact of HbA1c level measured at presentation among patients with newly diagnosed pancreatic ductal adenocarcinoma on their clinical outcomes. Patients with pancreatic cancer often do not exhibit disease-specific symptoms until the cancer is at an advanced stage. Only 15–20% of patients are diagnosed early enough to qualify for surgical resection, while the remainder present with locally advanced or metastatic disease.7,8 DM, especially of new onset (<2-year duration), has previously been noted to be significantly more prevalent among PDA patients than age-matched controls.4 Lee and colleagues recently reported that PDA-associated DM could be discriminated from benign new-onset type 2 DM with 80.8% sensitivity and 67.6% specificity using the clinical factors of age, weight loss, BMI, and family history of DM.13 Our study highlights the significant relationship between HbA1c—an objective, quantifiable, and readily available marker of hyperglycemia—and PDA amongst a cohort of patients presenting with either BPD or PDA and no chronic history of DM. We show that while patients with benign pancreatic lesions, such as IPMNs or pancreatic cysts, or benign conditions, such as autoimmune pancreatitis, are more likely to present with a mean HbA1c value in the normal range (<5.7%), patients with PDA are more likely to present with a mean HbA1c in the pre-diabetic range (5.7–6.4%), and that the proportion of patients with diabetic-range HbA1c levels (>6.4%) increase with more advanced stage of disease. Our study also shows HbA1c as having an important impact on survival in patients with pancreatic cancer: HbA1c≥6.5 was significantly associated with inferior overall survival (OS) (HR 1.74, OS 10.2 vs. 13.0 months, p=0.007) in the univariate model and remained as an important factor following multivariate model analysis with backward elimination, by providing unique contributions to the fitting that could not be explained by the other variables. Hyperglycemia may indicate a different, more aggressive tumor biology that leads to worse survival outcomes in PDA patients. A diabetic-range serum HbA1c may serve as an initial marker of this difference.
PDA-associated DM presents a unique interface between endocrine regulation and tumorigenesis. Insulin and C-peptide measurements during glucose tolerance tests in pancreatic cancer patients suggest abnormal beta cell function and possible insulin resistance.14 When comparable physiologic insulin levels were achieved in patients with pancreatic cancer and age/weight-matched healthy controls via insulin infusion, Cersosimo and colleagues observed that total body glucose use was consistently lower in the pancreatic cancer patients, consistent with a state of insulin resistance.15 To evaluate beta-cell function in patients with pancreatic cancer, Basso and colleagues performed a glucagon stimulation test in patients with PDA, type 1 DM, type 2 DM, and healthy controls.16 Following glucagon stimulation, no significant increase was observed in C-peptide values of type 1 diabetics and pancreatic cancer patients, whereas significant increases occurred in controls and type 2 diabetics.16 These results suggest that insulin resistance and altered beta-cell function found in pancreatic cancer patients likely lead to hyperglycemia, supporting our finding of elevated HbA1c in patients with PDA compared to BPD.
Although the association between DM and PDA has been of interest to the medical community for some time, the pathophysiology of PDA-associated DM is not well understood. Emerging evidence suggests that insulin and insulin-like growth factor-1 (IGF-1) may be involved in tumorigenesis through their role in producing high energy intake, increased cell proliferation, and suppression of apoptosis.17 In vitro, insulin stimulates the growth of cancer cells, through interaction with IGF-1 receptors and its own receptors.18 In observational surveys on type 2 diabetes, insulin therapy is associated with an increased incidence of several forms of cancer, although it is difficult to discriminate the effect of confounders from that of insulin itself, and randomized trials do not confirm the increased risk associated with insulin therapy.19
On the other hand, it has been postulated that PDA-associated DM is a paraneoplastic phenomenon, caused by a tumor-secreted diabetogenic product.4 Conditioned medium from PDA cell lines not only inhibits insulin release from beta cell lines20,21 but also impairs glucose metabolism in peripheral tissues.22–24 Using matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) analysis, rat hepatocytes incubated with PDA cell line conditioned media were compared to rat hepatocytes incubated with unconditioned media and sera of patients with PDA, and a low molecular-weight, potential diabetogenic factor was identified.23,24 Aggarwal and colleagues recently proposed this factor to be adrenomedullin, a 52–amino acid peptide that is up-regulated in PDA cell lines, impairs insulin secretion from beta cells, and contributes to the insulin inhibitory effect of PDA cells in vitro and in vivo.25 In human, adrenomedullin is up-regulated at the gene level, at the protein level, and in the plasma of patients with PDA, especially those with DM.25
Independent studies have shown resolution of new-onset DM in PDA following tumor resection,4,26–29 consistent with the hypothesis that PDA-associated DM is caused by a diabetogenic tumor-secreted product. After pancreaticoduodenectomy, while DM resolved in 57% of patients with new-onset DM, its prevalence was unchanged in patients with longstanding DM4. These observations further support the notion that new-onset DM in pancreatic cancer patients may be driven by a tumor-secreted product.
Our study shows HbA1c to have an important impact on survival in patients with pancreatic cancer. Glycemic control has been said to reduce morbidity and mortality in specific groups of patients, such as critically ill patients. Van den Berghe and colleagues had shown that intensive insulin therapy to maintain blood glucose at or below 110 mg per deciliter reduced morbidity and mortality among patients in the surgical intensive care unit,30 and that the risk of subsequent death and disease was reduced in patients on tight glycemic control treated for three or more days in the medical intensive care unit.31 Dysglycemia may also have negative impact on outcomes of hospitalized patients with cancer in terms of infection, mortality, length of stay, and toxicities.32 A 31% reduction in overall relative risk of cancer (0.69; 95% confidence interval, 0.61–0.79) was found in subjects taking metformin compared with other antidiabetic drugs; this association was significant for pancreatic and hepatocellular cancer, and nonsignificant for colon, breast, and prostate cancer.33 Enhanced glycemic control may improve outcomes of pancreatic cancer patients, though this hypothesis must be tested in a prospective randomized fashion before any definitive conclusions can be drawn.
A notable limitation of our study is that the HbA1c level was obtained only at each patient’s initial visit to the PMDC, therefore only providing a snapshot of glycemic control at one point throughout the disease course. To further elucidate the role played by glycemic control in improving survival in PDA patients, it would be worthwhile to obtain HbA1c levels at regular intervals both during cancer treatment and at regular post-treatment follow-up visits.
CONCLUSIONS
Patients with PDA have higher HbA1c levels at presentation than patients with BPD, suggesting impaired glucose tolerance among patients with PDA. Severity of glucose intolerance appears to correlate with more advanced stage of disease at presentation and, moreover, to be independently related to survival.
Acknowledgments
Source of Funding: Funding received from National Cancer Institutes, P30 CA006973.
Footnotes
Conflicts of Interest: No conflict of interest declared.
REFERENCES
- 1.Green RC, Jr, Baggenstoss AH, Sprague RG. Diabetes mellitus in association with primary carcinoma of the pancreas. Diabetes. 1958;7:308–311. doi: 10.2337/diab.7.4.308. [DOI] [PubMed] [Google Scholar]
- 2.Chari ST, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirakawa Y, et al. Association between glucose tolerance level and cancer death in a general Japanese population: the hisayama study. Am. J. Epidemiol. 2012;176:856–864. doi: 10.1093/aje/kws178. [DOI] [PubMed] [Google Scholar]
- 4.Pannala R, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal G, Kamada P, Chari ST. Prevalence of Diabetes Mellitus in Pancreatic Cancer Compared to Common Cancers. Pancreas. 2012 doi: 10.1097/MPA.0b013e3182592c96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu H, et al. Comparison of conventional and 3-dimensional computed tomography against histopathologic examination in determining pancreatic adenocarcinoma tumor size: implications for radiation therapy planning. Radiother Oncol. 2012;104:167–172. doi: 10.1016/j.radonc.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J. Am. Coll. Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos EH, Cardin DB, Berlin JD. Treatment of early-stage pancreatic cancer. Oncology (Williston Park, N.Y.) 2011;25:182–189. [PubMed] [Google Scholar]
- 9.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann. Intern. Med. 1999;131:247–255. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 10.Canto MI, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin. Gastroenterol. Hepatol. 2004;2:606–621. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 11.Canto MI, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin. Gastroenterol. Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 12.Goggins M, Canto M, Hruban R. Can we screen high-risk individuals to detect early pancreatic carcinoma? J Surg Oncol. 2000;74:243–248. doi: 10.1002/1096-9098(200008)74:4<243::aid-jso1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, et al. New-onset diabetes patients need pancreatic cancer screening? J. Clin. Gastroenterol. 2012;46:58–61. doi: 10.1097/MCG.0b013e318238348c. [DOI] [PubMed] [Google Scholar]
- 14.Schwarts SS, Zeidler A, Moossa AR, Kuku SF, Rubenstein AH. A prospective study of glucose tolerance, insulin, C-peptide, and glucagon responses in patients with pancreatic carcinoma. Am J Dig Dis. 1978;23:1107–1114. doi: 10.1007/BF01072886. [DOI] [PubMed] [Google Scholar]
- 15.Cersosimo E, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–493. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Basso D, et al. Beta-cell function in pancreatic adenocarcinoma. Pancreas. 1994;9:332–335. doi: 10.1097/00006676-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Djiogue S, et al. Insulin resistance and cancer: the role of insulin and insulin-like growth factors. Endocr. Relat. Cancer. 2012 doi: 10.1530/ERC-12-0324. [DOI] [PubMed] [Google Scholar]
- 18.D’Esposito V, et al. Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia. 2012;55:2811–2822. doi: 10.1007/s00125-012-2629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannucci E. Insulin therapy and cancer in type 2 diabetes. ISRN Endocrinol. 2012:240–634. doi: 10.5402/2012/240634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Adrian TE, Westermark G, Gasslander T, Permert J. Dissociated insulin and islet amyloid polypeptide secretion from isolated rat pancreatic islets cocultured with human pancreatic adenocarcinoma cells. Pancreas. 1999;18:403–409. doi: 10.1097/00006676-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, et al. Dissociated secretion of islet amyloid polypeptide and insulin in serum-free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int. J. Pancreatol. 1997;21:157–164. doi: 10.1007/BF02822387. [DOI] [PubMed] [Google Scholar]
- 22.Basso D, et al. Altered glucose metabolism and proteolysis in pancreatic cancer cell conditioned myoblasts: searching for a gene expression pattern with a microarray analysis of 5000 skeletal muscle genes. Gut. 2004;53:1159–1166. doi: 10.1136/gut.2003.024471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basso D, et al. Putative pancreatic cancer-associated diabetogenic factor: 2030 MW peptide. Pancreas. 2002;24:8–14. doi: 10.1097/00006676-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Valerio A, et al. Glucose metabolic alterations in isolated and perfused rat hepatocytes induced by pancreatic cancer conditioned medium: a low molecular weight factor possibly involved. Biochem. Biophys. Res. Commun. 1999;257:622–628. doi: 10.1006/bbrc.1999.0521. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal G, et al. Adrenomedullin is Up-regulated in Patients With Pancreatic Cancer and Causes Insulin Resistance in β Cells and Mice. Gastroenterology. 2012;143:1510–1517. doi: 10.1053/j.gastro.2012.08.044. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Permert J, et al. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–107. [PubMed] [Google Scholar]
- 27.Permert J, et al. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–1050. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 28.Permert J, et al. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am. J. Surg. 1993;165:61–66. doi: 10.1016/s0002-9610(05)80405-2. discussion 66–67. [DOI] [PubMed] [Google Scholar]
- 29.Permert J, et al. Islet hormone secretion in pancreatic cancer patients with diabetes. Pancreas. 1997;15:60–68. doi: 10.1097/00006676-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Van den Berghe G, et al. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 31.Van den Berghe G, et al. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 32.Storey S, Von Ah D. Impact of malglycemia on clinical outcomes in hospitalized patients with cancer: a review of the literature. Oncol Nurs Forum. 2012;39:458–465. doi: 10.1188/12.ONF.458-465. [DOI] [PubMed] [Google Scholar]
- 33.Decensi A, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]