Abstract
Objective
In patients undergoing pelvic exenteration for recurrent gynecological malignancies, we assessed the performance of [18F]-FDG PET/CT for delineating disease extent and evaluated the association between quantitative FDG uptake metrics (SUVmax, total lesion glycolysis [TLG] and metabolic tumor volume [MTV]) and progression-free survival (PFS) and overall survival (OS).
Methods
Retrospective study of patients undergoing pelvic exenteration for gynecologic malignancies between January 2002 and November 2011 who had FDG PET/CT within 90 days before surgery. Two readers (R1, R2) independently determined the presence of bladder, rectum, vagina, cervix and pelvic side wall invasion and measured SUVmax, TLG and MTV in each patient. Areas under the curve (AUCs), for detecting organ invasion were calculated. Kaplan–Meier graphs were used to determine associations between FDG uptake and PFS/OS. Inter-reader agreement was assessed.
Results
33 patients (mean age 56 years, range: 28–81) were included; primary sites of disease were the cervix (n=18), uterus (n=8) and vagina/vulva (n=7). AUCs for organ invasion ranged from 0.74 to 0.96. There was a significant association between FDG uptake metrics incorporating tumor volume (TLG and MTV) and OS (p≤0.001) as well as between MTV and PFS (p=0.001). No significant association was identified between SUVmax and OS/PFS (p=0.604/0.652). Inter-reader agreement for organ invasion was fair to substantial (k=0.36–0.74) and almost perfect for FDG quantification (ICC=0.97–0.99).
Conclusion
In patients undergoing pelvic exenteration for recurrent gynecological malignancies, 18F-FDG PET/CT is useful for preoperative assessment of disease extent. Furthermore, quantitative metrics of FDG uptake incorporating MTV serve as predictive biomarkers of progression-free and overall survival in this population.
Keywords: Cervical cancer, Endometrial cancer, PET, Pelvic exenteration, Cancer recurrence, Total lesion glycolysis
Introduction
Historically, patients with recurrent gynecological disease have had very limited therapy options and a dismal prognosis [1]. Substantial improvement in outcomes has been achieved since the introduction of pelvic exenteration, first described by Brunschwig in 1948 [2]. Pelvic exenteration consists of the radical resection of all pelvic viscera involved by disease, such as the bladder, uterus, vagina, and/or rectum. Currently, 5-year overall survival for patients with recurrent gynecological disease of over 50% can be achieved with refinements in the surgical technique [3–5]. However, pelvic exenteration is a radical surgical procedure with potentially high rate of morbidity [6] and mortality [7], and therefore careful preoperative planning is essential to optimize the balance between the clinical benefit and potential morbidity. The extent of the surgical procedure can be tailored according to the organs involved, allowing for anterior (bladder and uterus), posterior (uterus and rectum), total perineal, or total pelvic exenterations to be performed as needed.
Preoperative evaluation of disease extent is usually performed through a combination of clinical assessment and imaging. Ultra-sound, computed tomography (CT) and magnetic resonance imaging (MRI) have all been used for anatomical delineation of local disease. Evaluation with all these techniques is often challenging in the setting of suspected gynecological cancer recurrence, as most patients in these group will have received treatment with surgery, chemotherapy, or irradiation at the time of their original diagnosis, posttreatment changes may be difficult to differentiate from recurrent disease [8,9]. More recently whole-body positron emission tomography (PET) using 18F-Fluorodeoxyglucose (18F-FDG) has been shown to be valuable for the detection of distant metastases in recurrent gynecological tumors. This is important because pelvic exenteration is typically contraindicated in the presence of extrapelvic sites of disease [10]. With increasing availability of PET/CT scanners and the advantages of hybrid anatomical and metabolic imaging with PET/CT, it is possible that the role of 18F-FDG PET/CT may be extended to the assessment of local extent of disease in addition to its established role in the assessment of metastatic disease. Furthermore, quantification of tumor 18F-FDG uptake on PET, which has been shown to correlate with patient outcomes in other clinical settings such as primary gynecological cancers [11–14], may also prove useful in the context of recurrent gynecological cancers undergoing pelvic exenteration. Therefore, the purpose of our study was to assess the performance of 18F-FDG PET/CT in determining disease extent and to evaluate the use of quantitative 18F-FDG uptake metrics (SUVmax, total lesion glycolysis (TLG) and metabolic tumor volume (MTV)) as predictive bio-markers for progression-free survival and overall survival in patients undergoing pelvic exenteration for recurrent gynecological malignancies.
Materials and methods
Patients
This retrospective study was approved by our Institutional Review Board, which waived the informed consent requirement. Inclusion criteria were: (i) biopsy-proven recurrent or persistent cancer of the cervical, uterine, vaginal, or vulvar malignancies; (ii) pelvic exenteration performed between January 2002 and November 2011, and (iii) 18F-FDG PET/CT performed less than 90 days prior to surgery (Fig. 1). No patients were excluded from analysis.
Image acquisition and data analysis
All patients were scanned on dedicated PET/CT systems (Discovery STE, LS or 690 [GE Medical Systems] (21 patients), Biograph 16 [Siemens Medical Systems] (10 patients) and Biograph CPS 1080 [Siemens Medical Systems] (2 patients)). A clinical imaging protocol was applied with an injection of approximately 400–455 MBq FDG after at least 6 h of fasting and documentation of blood glucose <200 mg/dL followed by a 73 +/− 13 minute uptake period. Subsequently, a low-dose, attenuation correction CT scan (120–140 kV, approximately 80 mA) was acquired, followed by PET emission images from the floor of the pelvis to the skull.
Two readers (IB, HAV), dually qualified in both diagnostic radiology and nuclear medicine, independently evaluated all studies, blinded to the clinical data and other imaging information. Readers recorded the presence of bladder, rectum, vagina, and cervix invasion using a 5-point scale (1: definitely no invasion, 2: probably no invasion, 3: indeterminate, 4: probably invasion and 5: definitely invasion). The criterion used for organ involvement was direct contact with an FDG-avid tumor as evidenced on the CT component of the examination, e.g. absence of a fat plane between the tumor and organ on CT. Similarly, pelvic sidewall invasion was diagnosed in the presence of direct contact between the tumor and obturator internus and/or piriformis muscle. Lymph nodes were considered malignant if there was uptake above background activity on PET and they were either (i) increased in size (e.g. >1.0 cm) or (ii) structurally abnormal (e.g. lack of fatty hilum, round shape, irregular margins) on CT. The presence of distant metastases was also recorded. PET images were analyzed with a lower threshold set at 0.0 g/mL and an upper threshold of 5.0 g/mL. Surgical histopathology served as the standard of reference for assessing local extent of disease.
For the assessment of quantitative 18F-FDG uptake, readers independently placed a volume-of-interest (VOI) over the dominant lesion in each patient. Care was taken to avoid inclusion of physiologic 18F-FDG uptake (e.g., excreted tracer in the bladder) within the tumor VOIs. The following quantitative metrics were recorded: (i) the highest standardized uptake value (SUV) within any voxel included in the tumor VOI (SUVmax); (ii) the metabolic tumor volume (MTV), defined as the sum of all voxels with an SUV above 42% of SUVmax and (iii) the total lesion glycolysis (TLG), defined as the metabolic tumor volume multiplied by the average SUV (SUVmean) of the same voxels. Voxels represent the 3 dimensional entity of imaging pixels, and their sizes determine the image resolution. Erdi et al. analyzed 1997 PET lesions in phantoms and determined the optimal cutoff form SUVmax for spherical lesions to delineate the true volume. This led to the widely accepted cutoff at 42% of SUVmax used in this study [15].
Statistical analysis
Receiver operating characteristic (ROC) curves were estimated separately for each organ using the 5-point evaluations and histopathologic reference standard. Areas under the curve (AUCs) were estimated using the trapezoidal rule. Sensitivity and specificity were estimated considering scores of 1–3 as negative for disease and suspicion scores of 4–5 as positive for disease. Patients were divided in groups above and below the median SUVmax, TLG, and MTV, and Kaplan–Meier curves were generated to determine associations between these quantitative metrics and progression-free survival and overall survival. Progression-free survival was defined as the interval from pelvic exenteration to the diagnosis of progression or death. Inter-reader agreement was assessed with weighted-kappa statistics and interclass correlation coefficients (ICC), for both tests values<0 were indicative for no agreement, values from 0 to 0.2 were interpreted as slight, 0.21 to 0.4 as fair, 0.41 to 0.6 as moderate, 0.61 to 0.8 as substantial and 0.81 to 1 as almost perfect agreement [16]. p-Values≤0.05 were considered significant. Statistical analysis was performed with the R software (version 2.13; R Foundation for Statistical Computing) and SAS software (version 9.3; SAS Institute).
Results
Tumor histology and patient characteristics
The study population consisted of 33 patients with a mean age of 56 years (range: 28–81 years) and diagnosed with carcinoma of the cervix (n=18), uterus (n=8), and vagina/vulva (n=7). All patients had received prior pelvic radiotherapy. In 21 patients (64%), previous hysterectomy had been performed. In one patient the rectum had previously been resected. The mean disease-free interval before pelvic exenteration of 37.2 months (range: 6.1–130 months). Median time between PET/CT and exenteration was 35 days (range 1–90 days). After a mean follow up of 26.6 months, 18 patients were alive without evidence of recurrent disease, 3 were alive with disease, and 12 had died.
Patient characteristics and tumor histology are summarized in Table 1.
18F-FDG PET/CT for assessment of local extent of disease
Histopathologic analysis revealed bladder invasion in 13 patients. This was correctly identified in 9 cases by reader 1 and in 10 cases by reader 2, yielding AUCs/sensitivities/specificities of 0.86/0.69/1.0 and 0.91/0.77/1.0 for readers 1 and 2, respectively. There was histo-pathological evidence of rectal invasion in 9 cases. Six of those 9 cases were considered either suspicious or consistent with rectal invasion by both readers, corresponding to AUCs/sensitivities/speci-ficities of 0.91/0.67/1.0 and 0.84/0.67/0.91 for readers 1 and 2, respectively (Fig. 2; Table 2). Cervical involvement by tumor was identified on histopathology in 7 cases. Both readers correctly diagnosed cervical invasion in 5 of the 7 cases. The AUC for cervical invasion was 0.84 for reader 1 and 0.79 for reader 2. The uterine corpus was only involved in one case, which was scored as definite invasion by both readers. There was histopathological evidence of invasion of the vaginal in 30 out of 33 patients. Reader 1 incorrectly classified one of the 3 patients without vaginal involvement on pathology as suspicious for vaginal involvement, while reader 2 had no false positive cases. False negative results for vaginal involvement were identified in 3 and 10 patients by reader 1 and 2 respectively. The vulva was invaded in 3 patients, 2 of which were identified by reader 1 and all 3 by reader 2. Pelvic side wall invasion was present in 5 patients, and this was correctly identified in 3/5 cases by reader 1 and in 4/5 cases by reader 2. Both readers had one false positive case for pelvic side wall involvement.
Table 2.
Accuracy for local extent of disease
| Reader 1: | AUC (95% CI) | Sensitivity (95%-CI; n) | Specificity (95%-CI; n) | PPV (95%-CI; n) | NPV (95%-CI; n) |
|---|---|---|---|---|---|
| Bladder | 0.86 [0.71-1.01] | 69.2% [38.6-90.9; 9/13] | 100.0% [83.2-100.0; 20/20] | 100.0% [66.4-100.0; 9/9] | 83.3% [62.6-95.3; 20/24] |
| Rectum | 0.91 [0.76-1.06] | 66.7% [29.9-92.5; 6/9] | 100% [85.2-100.0; 23/23] | 100% [54.1-100.0; 6/6] | 88.5% [69.8-97.6; 23/26] |
| Side Wall | 0.76 [0.50-1.03] | 60.0% [14.7-94.7; 3/5] | 96.0% [79.6-100.0; 24/25] | 75.0% [19.4-99.4; 3/4] | 92.3% [74.9-99.1; 24/26] |
| Cervix | 0.84 [0.52-1.16] | 71.4% [29.0-96.3; 5/7] | 75.0% [19.4-99.4; 3/4] | 83.3% [35.9-99.6; 5/6] | 60.0% [14.7-94.7; 3/5] |
| Vagina | 0.74 [0.35-1.14] | 90.0% [73.5-97.9; 27/30] | 66.7% [9.4-99.2; 2/3] | 96.4% [81.7-99.9; 27/28] | 40.0% [5.3-85.3; 2/5] |
| Reader 2: | AUC (95% CI) | Sensitivity (95%-CI; n) | Specificity (95%-CI; n) | PPV (95%-CI; n) | NPV (95%-CI; n) |
| Bladder | 0.91 [0.80-1.02] | 76.9% [46.2-94.7; 10/13] | 100.0% [83.2-100.0; 20/20] | 100% [69.2-100.0; 10/10] | 87.0% [66.3-97.2; 20/23] |
| Rectum | 0.84 [0.67-1.01] | 66.7% [29.9-92.5; 6/9] | 91.3% [72.0-98.9; 21/23] | 75.0% [34.9-96.8; 6/8] | 87.5% [67.6-97.3; 21/24] |
| Side Wall | 0.96 [0.88-1.03] | 80.0% [28.4-99.5; 4/5] | 96.0% [79.6-100.0; 24/25] | 80.0% [28.4-99.5; 4/5] | 96.0% [79.6-100.0; 24/25] |
| Cervix | 0.79 [0.47-1.10] | 85.7% [42.1-99.6; 6/7] | 75.0% [19.4-99.4; 3/4] | 85.7% [42.1-99.6; 6/7] | 75.0% [19.4-99.4; 3/4] |
| Vagina | 0.85 [0.77-0.93] | 66.7% [47.2-82.5; 20/30] | 100% [29.2-100.0; 3/3] | 100.0% [83.2-100.0; 20/20] | 23.1% [5.0-53.8; 3/13] |
| Inter-reader agreement (k) | |||||
| Bladder | 0.71 | ||||
| Rectum | 0.74 | ||||
| Side Wall | 0.55 | ||||
| Cervix uteri | 0.60 | ||||
| Vagina | 0.36 | ||||
18F-FDG PET/CT for assessment of lymph nodes and metastatic disease
There were 2 patients with lymph node metastases on histopathology. The location of the nodal involvement was perirectal in one case and the left common iliac region in the other. Both readers correctly classified the left iliac node as suspicious, but did not identify the perirectal node metastasis. The patient with perirectal nodal metastasis on pathology had a recurrent tumor in the vaginal cuff with gross recto-sigmoid invasion, correctly identified by both readers; however, there were extensive non-FDG avid inflammatory changes in the mesorectal and pericolonic fat which may have affected the detection of the metastatic node. False-positive lymph nodes were identified by both readers in 2 cases. In both cases the false positive nodes measured 1.0 cm and demonstrated mildly increased uptake compared to background activity. No distant metastases were identified.
Quantitative 18F-FDG PET metrics as predictive biomarkers
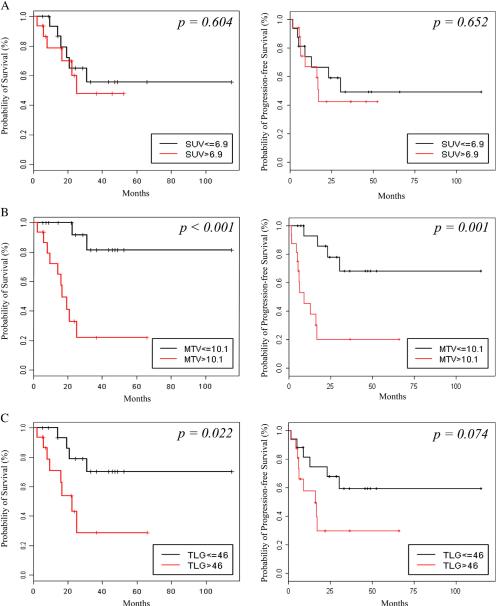
The median SUV max, MTV and TLG were 6.9, 10.1 mL and 46 g for reader 1 and 6.9, 10.1 mL and 42.8 g for reader 2 (Table 3). Using this median values as cutoffs, Kaplan–Meier analysis showed significant associations between MTV and overall survival (p<0.001) and TLG and overall survival (Reader 1: p=0.022, reader 2: p=0.021) but no significant association between SUVmax and overall survival (Reader 1: p=0.604, reader 2: p=0.652). Patients with an MTV above 10.1 mL also had a significantly shorter progression-free survival (Reader 1: p=0.001, reader 2: p=0.002) (Table 4, Fig. 3).
Table 4.
Kaplan-Meier survival for both readers:
| Overall survival | Median - cut off | Mean Months | SD | CI 95% | Significance (Log Rank (Mantel-Cox)) |
|---|---|---|---|---|---|
| R1 SUV max | ≤ 6.9 | 72.5 | 13.1 | 46.8-98.3 | |
| > 6.9 | 33.1 | 5.8 | 21.8-44.4 | 0.604 | |
| R1 MTV (mL) | ≤ 10.1 | 98.6 | 10.4 | 78.12-119.0 | |
| > 10.1 | 26.0 | 6.4 | 13.5-38.5 | < 0.001* | |
| R1 TLG (g) | ≤ 46 | 92.4 | 11.4 | 70.1-114.7 | |
| > 46 | 29.8 | 7.6 | 15.9-44.6 | 0.022* | |
| R2 SUV max | ≤ 6.9 | 79.6 | 12.7 | 54.7-104.5 | |
| > 6.9 | 29.9 | 5.6 | 18.9-40.9 | 0.198 | |
| R2 MTV (mL) | ≤ 10.1 | 98.8 | 10.3 | 78.6-119.1 | |
| > 10.1 | 26.3 | 6.4 | 13.7-38.7 | 0.001* | |
| R2 TLG (g) | ≤ 42.8 | 87.1 | 11.7 | 64.2-110.1 | |
| > 42.8 | 29.8 | 7.6 | 14.9-44.6 | 0.021* | |
| Progression-free survival | Median - cut off | Mean Months | SD | CI 95% | Significance (Log Rank (Mantel-Cox)) |
| R1 SUV max | ≤ 6.9 | 63.5 | 14.1 | 35.9-91.1 | |
| > 6.9 | 28.2 | 5.8 | 16.9-39.5 | 0.652 | |
| R1 MTV (mL) | ≤ 10.1 | 84.9 | 12.5 | 60.3-109.5 | |
| > 10.1 | 20 | 6.7 | 6.8-33.1 | 0.001* | |
| R1 TLG (g) | ≤ 46 | 74.2 | 13 | 48.7-99.7 | |
| > 46 | 26.7 | 7.6 | 11.8-41.5 | 0.74 | |
| R2 SUV max | ≤ 6.9 | 67.2 | 13 | 41.1-93.3 | |
| > 6.9 | 25.9 | 6.1 | 13.9-37.8 | 0.300 | |
| R2 MTV (mL) | ≤ 10.1 | 85.1 | 12.6 | 60.4-109.8 | |
| > 10.1 | 20 | 6.4 | 7.4-32.5 | 0.002* | |
| R2 TLG (g) | ≤ 42.8 | 74.2 | 13.0 | 48.7-99.7 | |
| > 42.8 | 36.7 | 7.6 | 11.8-41.5 | 0.74 | |
R1: reader 1, R2: reader 2, TLG: Total Lesion Glycolysis, MTV: Metabolic Tumor Volume, SD: Standard deviation, CI: Confidence interval
statistical significance
Fig. 3.
Kaplan–Meier curves for overall survival (left) and progression-free survival (right) using the median as a cutoff for each FDG uptake metric. A) SUVmax, B) Metabolic tumor volume (MTV) and C) Total lesion glycolysis (TLG).
The 18-month survival rate after pelvic exenteration was 93% in patients with a TLG≤46 g (the median) and 54% for patients with a TLG>46 g. For patients with an MTVb10.1 mL (the median) the 18-month survival was 100% versus 50% for patients with an MTV> 10 mL (Table 5). Two patient examples for lesions with low and high TLG and MTV are shown in Figs. 4 and 5.
Table 5.
Overall survival probability
| Median - cut off | 18-month Survival | CI 95% | 24-month Survival | CI 95% | |
|---|---|---|---|---|---|
| SUV max | ≤ 6.9 | 79.4% | 0.612-1.00 | 65% | 0.444-0.953 |
| > 6.9 | 69.9% | 0.488-1.00 | 59.9% | 0.375-0.958 | |
| MTV (mL) | ≤ 10.1 | 100% | 1.00-1.00 | 91.7% | 0.445-1.00 |
| > 10.1 | 49.5% | 0.288-0.849 | 33% | 0.151-0.721 | |
| TLG (g) | ≤ 46 | 93.3% | 0.815-1.00 | 79% | 0.604-1.00 |
| > 46 | 53.9% | 0.323-0.901 | 43.2 | 0.220-0.847 |
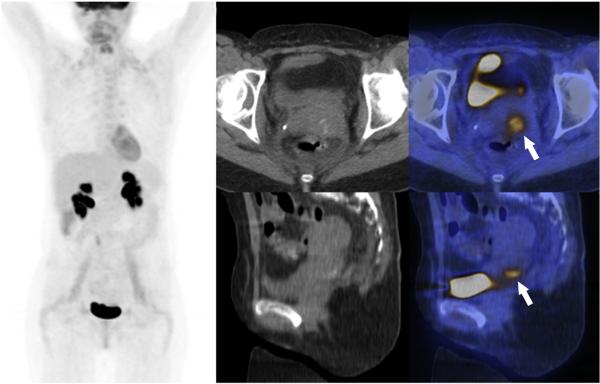
Fig. 4.
A) Coronal MIP of 18F-FDG PET images of a 44 year old woman diagnosed with recurrent cervical cancer initially treated with definitive chemoradiotherapy. The patient was without evidence of disease on her last follow-up, 4 years after total exenteration. B, C) Transverse CT and fused PET/CT images, with the recurrence (arrow), that was read as con-fined to the cervix, without any other organ involvement by both readers. D, E) Sagittal CT and fused PET/CT images illustrating the recurrence (arrow) confined to the cervix, what was confirmed on pathology. The tumor had a low metabolic activity SUVmax 4.1, MTV 6.6 mL and TLG 17 g.
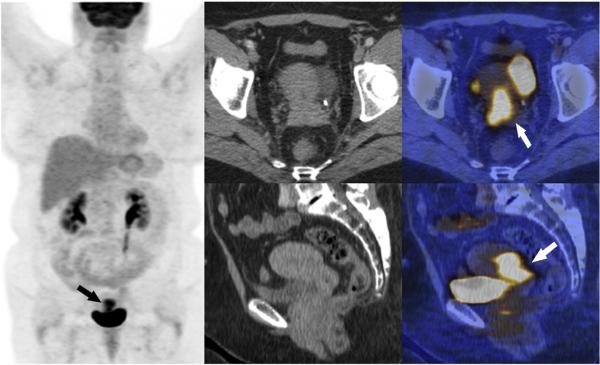
Fig. 5.
A) Coronal MIP of an FDG PET scan of a 56 year old woman diagnosed with recurrent cervical cancer 8 months after completion of definitive chemoradiotherapy. The patient recurred 9 months after total exenteration, with negative surgical margins and died 16 months later. B, C) Transverse CT and fused PET/CT images, with the recurrence (arrow) involving the entire cervix and extending to the lower uterine segment and vagina. D, E) Sagittal CT and fused PET/CT images illustrating the recurrence without direct contact of the hypermetabolic tumor to bladder or rectum (arrow). Pathology confirmed invasion of the cervix, uterus, and vagina. The tumor had a high metabolic activity SUVmax 10.8, MTV 23.3 mL and TLG 134 g.
Inter-reader agreement in 18F-FDG PET
Inter-reader agreement was substantial for invasion of the bladder, rectum, and cervix (k=0.60–0.74), moderate for invasion of pelvic side wall (k=0.49) and fair for invasion of the vagina (k=0.36) (Table 2). The FDG quantification with SUVmax, MTV, and TLG was observer independent with an almost perfect ICC of 0.99, 0.973, and 0.98, respectively.
Discussion
Our results suggest that 18F-FDG PET/CT is valuable for the assessment of local extent of disease in patients with recurrent gynecological cancers, with AUCs for the detection of pelvic organ and pelvic sidewall invasion ranging between 0.74 and 0.91 for 2 independent readers. The usefulness of PET for the detection of metastatic disease prior to pelvic exenteration for recurrent gynecological tumors was previously reported [10]; however, to the authors’ knowledge there are no studies in the literature evaluating the role of FDG PET/CT in evaluating the extent of disease within the pelvis, a critical aspect of treatment planning prior to pelvic exenteration. For instance, patients with documented disease in the bladder and/or uterus, but with sparing of the rectum, are eligible for anterior pelvic exenteration. Similarly, patients with rectal involvement but sparing of the bladder are eligible for posterior pelvic exenteration. In contrast, patients with documented involvement of the bladder and rectum require a total pelvic exenteration. These options have substantial implications for patients’ quality of life, as the need for a urostomy or a colostomy, and their associated comorbidities, may be obviated unless this is strictly necessary for tumor control.
We also identified a significant association between quantitative 18F-FDG uptake and overall and progression-free survival. The relationship between FDG uptake and clinical outcomes has been reported in multiple oncological settings, including primary gynecological cancers [11–14], but not specifically in patients with recurrent gynecological malignancies scheduled to undergo pelvic exenteration. A study of 96 patients with primary cervical carcinoma treated with radiotherapy showed a significant association between SUVmax of the primary tumor and overall and progression-free survival when an SUVmax cutoff of 10.2 was used [17]. Another study of patients with primary cervical cancer, in which a Cox proportional-hazards model for death from cervical cancer was used to evaluate tumor histology, lymph node metastasis, tumor volume, and SUVmax, showed that SUVmax of the primary tumor was the only significant independent factor (p=0.0027) [12]. The same group also showed that higher SUVmax in pelvic nodes of patients with primary cervical cancer was associated with an increased risk of developing pelvic disease recurrence (p=0.0035) and worse disease-specific (p=0.0230) and overall survival (p=0.0378) [13]. MTV and TLG have also been shown to predict survival in patients with primary cervical and endometrial carcinoma [14,18]. Interestingly, although we found an association between MTV, TLG and overall survival, there was no association between SUVmax and overall survival. We defined MTV as the sum of all voxels with an SUV above 42% of SUVmax and TLG as MTV multiplied by the average SUV of the same voxels [19]. This suggests that only metrics incorporating hypermetabolic tumor bulk offer significant prognostic information in patients with gyneco-logical cancer recurrence, whereas the usefulness of SUVmax, which cor responds to the highest SUV within any voxel included in the tumor VOI, may be limited in this patient population. SUVmax has been shown to have a high intrinsic variability, therefore the small sample size of our study might limit the prognostic value of SUVmax. The strongest associations between 18F-FDG uptake and outcomes were seen with MTV (p<0.001 for overall survival and p=0.001 for progression-free survival). Total lesion glycolysis was significantly associated with overall survival (p=0.022) but not with progression-free survival (p=0.074).
Another important finding from our study was the high inter-reader agreement achieved by 2 independent readers for the qualitative and quantitative analysis 18F-FDG PET. For detecting invasion of the most critical structures for surgical planning (bladder and rectum), inter-reader agreement was substantial (k=0.60–0.74) 14. Furthermore, there was almost perfect agreement in measurements of SUVmax, MTV and TLG with interclass correlation coefficients ranging between 0.97 and 0.99 14.
A potential limitation for 18F-FDG uptake as a predictive factor for outcome is the presence of inflammatory changes. Infection (e.g., radiation-induced superinfection) can lead to very high values for all quantitative 18F-FDG PET metrics. In this case, the high FDG uptake values did not correspond to an impaired outcome. There was also an 18F-FDG positive lymph node rated suspicious by both observers, showing only inflamma-tory changes on pathology. Excreted activity in the urinary bladder and urethra may interfere with the detection of bladder wall invasion. Similarly, urine contamination along vaginal wall could limit assessment. Placement of bladder catheters with saline irrigation of the bladder, which may eliminate these potential sources for false positive findings, was not used in these clinical routine cases. Nevertheless, both readers identified invasion of critical structures with high accuracy.
We acknowledge several limitations in our study. It is a retrospective analysis of patients who underwent pelvic exenteration at a single institution. Although this allowed detailed correlation between histopathology and PET/CT findings, we cannot extrapolate our results to patients with recurrent gynecological cancers who were not treated surgically, including those in whom distant metastases were identified preoperatively. Also, we cannot account for the theoretical possibility of disease progression in the time interval of 90 days between PET/CT and surgery, and how this would have affected our results. FDG quantification metrics depend on technical parameters and the chosen segmentation algorithm; a commonly used method to measure MTV and TLG is with a 42% threshold of SUVmax, based on a phantom study [16]. Our sample size was small, which limits the interpretation of our findings, particularly with regard to less frequently involved pelvic organs (e.g., vulva or vascular structures). Due to the small number, the median cutoff values determined in this study population cannot be generalized for other populations. However, no other studies have evaluated 18F-FDG PET/CT as a tool for preoperative determination of local disease extent and as a predictive biomarker for clinical outcomes after pelvic exenteration. Thus, our findings could form the basis of a larger prospective evaluation to further clarify the potential role of FDG PET in this patient population. Moreover, the encouraging results from our study, including the high inter-reader agreement, may be at least partially related to the fact that both readers had similar levels of experience with dedicated training in gynecological imaging and were dually trained in radiology and nuclear medicine. Such hybrid training is still unusual in most countries and healthcare settings.
Our findings highlight the importance of multimodality imaging in cancer diagnosis; it is hoped that future joint training programs will address the need for an increasing number of molecular imaging specialists with equal expertise in various modalities [20,21].
Conclusion
18F-FDG PET/CT is useful for preoperative assessment of disease extent in patients scheduled to undergo pelvic exenteration. This may help tailoring the surgical approach so that the risks and benefits of this technique are appropriately balanced. Furthermore, quantitative metrics of 18F-FDG uptake incorporating tumor volume serve as predictive biomarkers of survival in this patient population.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2013.01.017.
Supplementary Material
HIGHLIGHTS.
▶ FDG PET/CT had high accuracy for the evaluation of disease extent prior to pelvic exenteration for recurrent gynecological cancer.
▶ FDG uptake metrics incorporating tumor volume (total lesion glycolysis and metabolic tumor volume) are significantly associated with overall survival.
▶ No significant association was identified between SUVmaxand overall survival.
Footnotes
Conflict of interest statement
None.
References
- 1.Lopez MJ, Barrios L. Evolution of pelvic exenteration. Surg Oncol Clin N Am. 2005;14:587–606. doi: 10.1016/j.soc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer. 1948;1:177–83. doi: 10.1002/1097-0142(194807)1:2<177::aid-cncr2820010203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt AM, Imesch P, Fink D, Egger H. Indications and long-term clinical outcomes in 282 patients with pelvic exenteration for advanced or recurrent cervical cancer. Gynecol Oncol. 2012;125:604–9. doi: 10.1016/j.ygyno.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Park JY, Choi HJ, Jeong SY, Chung J, Park JK, Park SY. The role of pelvic exenteration and reconstruction for treatment of advanced or recurrent gynecologic malignancies: analysis of risk factors predicting recurrence and survival. J Surg Oncol. 2007;96:560–8. doi: 10.1002/jso.20847. [DOI] [PubMed] [Google Scholar]
- 5.Khoury-Collado F, Einstein MH, Bochner BH, Alektiar KM, Sonoda Y, Abu-Rustum NR, et al. Pelvic exenteration with curative intent for recurrent uterine malignancies. Gynecol Oncol. 2012;124:42–7. doi: 10.1016/j.ygyno.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Berek JS, Howe C, Lagasse LD, Hacker NF. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecol Oncol. 2005;99:153–9. doi: 10.1016/j.ygyno.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Andikyan V, Khoury-Collado F, Gerst SR, Talukdar S, Bochner BH, Sandhu JS, et al. Anterior pelvic exenteration with total vaginectomy for recurrent or persistent genitourinary malignancies: review of surgical technique, complications, and outcome. Gynecol Oncol. 2012;126:346–50. doi: 10.1016/j.ygyno.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Popovich MJ, Hricak H, Sugimura K, Stern JL. The role of MR imaging in determining surgical eligibility for pelvic exenteration. AJR Am J Roentgenol. 1993;160:525–31. doi: 10.2214/ajr.160.3.8430546. [DOI] [PubMed] [Google Scholar]
- 9.Dresen RC, Kusters M, Daniels-Gooszen AW, Cappendijk VC, Nieuwenhuijzen GA, Kessels AG, et al. Absence of tumor invasion into pelvic structures in locally recurrent rectal cancer: prediction with preoperative MR imaging. Radiology. 2010;256:143–50. doi: 10.1148/radiol.10090725. [DOI] [PubMed] [Google Scholar]
- 10.Husain A, Akhurst T, Larson S, Alektiar K, Barakat RR, Chi DS. A prospective study of the accuracy of 18Fluorodeoxyglucose positron emission tomography (18FDG PET) in identifying sites of metastasis prior to pelvic exenteration. Gynecol Oncol. 2007;106:177–80. doi: 10.1016/j.ygyno.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97:183–91. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738–44. doi: 10.1002/cncr.22974. [DOI] [PubMed] [Google Scholar]
- 13.Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer. 2010;116:1469–75. doi: 10.1002/cncr.24972. [DOI] [PubMed] [Google Scholar]
- 14.Liu FY, Chao A, Lai CH, Chou HH, Yen TC. Metabolic tumor volume by (18)F-FDG PET/CT is prognostic for stage IVB endometrial carcinoma. Gynecol Oncol. 2012;125:566–71. doi: 10.1016/j.ygyno.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Erdi YE. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer. 1997;80:2505–9. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2505::aid-cncr24>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. Measurement of observer agreement for categorical data. Bio-metrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 17.Xue F, Lin LL, Dehdashti F, Miller TR, Siegel BA, Grigsby PW. F-18 fluorodeoxyglucose uptake in primary cervical cancer as an indicator of prognosis after radiation therapy. Gynecol Oncol. 2006;101:147–51. doi: 10.1016/j.ygyno.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Chung HH, Kim JW, Han KH, Eo JS, Kang KW, Park NH, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol. 2011;120:270–4. doi: 10.1016/j.ygyno.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–71. doi: 10.1016/s1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 20.Hricak H, Choi BI, Scott AM, Sugimura K, Muellner A, von Schulthess GK, et al. Global trends in hybrid imaging. Radiology. 2010;257:498–506. doi: 10.1148/radiol.10100579. [DOI] [PubMed] [Google Scholar]
- 21.Delbeke D. SNM/ABNM joint position statement on optimizing training in nuclear medicine in the era of hybrid imaging. J Nucl Med. 2012 doi: 10.2967/jnumed.112.110346. 1978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.