Abstract
IL-12Rβ1 deficiency is an autosomal recessive disorder characterized by predisposition to recurrent and/or severe infections caused by otherwise poorly pathogenic mycobacteria and salmonella. IL-12Rβ1 is a receptor chain of both the IL-12 and the IL-23 receptor and deficiency of IL-12Rβ1 thus abolishes both IL-12 and IL-23 signaling. IL-12Rβ1 deficiency is caused by bi-allelic mutations in the IL12RB1 gene. Mutations resulting in premature stop codons, such as nonsense, frame shift, and splice site mutations, represent the majority of IL-12Rβ1 deficiency causing mutations (66%; 46/70). Also every other morbid mutation completely inactivates the IL-12Rβ1 protein. In addition to disease-causing mutations, rare and common variations with unknown functional effect have been reported in IL12RB1. All these variants have been deposited in the online IL12RB1 variation database (www.LOVD.nl/IL12RB1). In this article, we review the function of IL-12Rβ1 and molecular genetics of human IL12RB1.
Keywords: IL12RB1, IL-12Rβ1 deficiency, Mendelian Susceptibility to Mycobacterial Disease
Background
Interleukin-12 receptor β1 (IL-12Rβ1) deficiency is a rare autosomal recessive disorder characterized by predisposition to recurrent and/or severe disease caused by poorly pathogenic mycobacteria and salmonellae in otherwise healthy individuals [Fieschi et al., 2003; van de Vosse et al., 2004; de Beaucoudrey et al., 2010]. The infections with mycobacteria, such as poorly pathogenic non-tuberculous (environmental) mycobacteria or the live attenuated Mycobacterium bovis Bacille Calmette-Guérin strain (BCG) of the tuberculosis vaccine, usually occur only once in these patients [Fieschi et al., 2003]. Inversely, infections with various salmonella can occur repeatedly. Infections with other pathogens such as candida [Cardenes et al., 2010; de Beaucoudrey et al., 2010] and, occasionally, M. tuberculosis have also been observed [Caragol et al., 2003; Özbek et al., 2005; de Beaucoudrey et al., 2010; Tabarsi et al., 2011; Boisson-Dupuis et al., 2011].
IL-12Rβ1 is a common receptor chain of the IL-12 and the IL-23 receptors and deficiency of IL-12Rβ1 causes a profound defect in both IL-12 and IL-23 signaling. Upon infection with intracellular bacteria, phagocytes are activated and produce various cytokines including IL-12 and IL-23. The lack of IL-12 responses impairs the production of IFN-γ [de Jong et al., 1998; Altare et al., 1998; Feinberg et al., 2004]. Patients with defects in IFN-γ production or IFN-γ signaling are prone to Mendelian susceptibility to mycobacterial disease (MSMD) [van de Vosse et al., 2004]. In IL-12Rβ1 deficient patients also the development of IL-17 producing T cells (Th17 cells) was found to be impaired [de Beaucoudrey et al., 2008]. Although the exact involvement of IL-23 and Th17 cells is not clear, IL-23 and IL-17 are clearly implicated in various autoimmune and inflammatory disorders and are important for adaptive host defense against extracellular bacteria and Candida albicans [van de Vosse et al., 2009].
IL-12Rβ1 deficiency is caused by mutations in the IL12RB1 gene (MIM# 601604). Mutations in other genes, namely IL12B (MIM# 161561), IFNGR1 (MIM# 107470), IFNGR2 (MIM# 147569), STAT1 (MIM# 600555), IKBKG (MIM# 300248, better known as NEMO), IRF8 (MIM# 601565) and ISG15 (MIM# 147571) as well as certain CYBB (MIM# 300481) mutations also lead to severe disease mainly due to infections with poorly pathogenic mycobacteria [Filipe-Santos et al., 2006; van de Vosse et al., 2009; Hambleton et al., 2011; Bogunovic et al., 2012; Bustamante et al., 2011; Kong et al., 2013]. These disorders are often collectively referred to as Mendelian susceptibility to mycobacterial disease, or MSMD (MIM# 209950) although this term has over the years turned out not to fully cover the disorder because susceptibility to pathogens other than mycobacteria can also be observed [de Moraes Vasconcelos et al., 2005; Filipe-Santos et al., 2006; Sanal et al., 2007; Luangwedchakarn et al., 2009; Pedraza et al., 2010; Jirapongsananuruk et al., 2012]. IL12RB1 is the gene most often affected in MSMD patients. IL-12Rβ1 deficiency is an autosomal recessive disorder, which means that both alleles are mutated in patients. Family members carrying only one deleterious mutation do not have any overt clinical symptoms or a detectable immunological defect. Although complete IL-12Rβ1 deficiency leads in general to a milder form of MSMD than complete deficiency of for instance IFN-γR1 or IFN-γR2 [van de Vosse et al., 2004], the mortality rate is 30% with a mean age at death of 7.5 years [de Beaucoudrey et al., 2010]. The clinical phenotypes of IL-12Rβ1 deficiency and IL-12p40 deficiency [Prando et al., 2013] are so similar that they are considered clinical phenocopies.
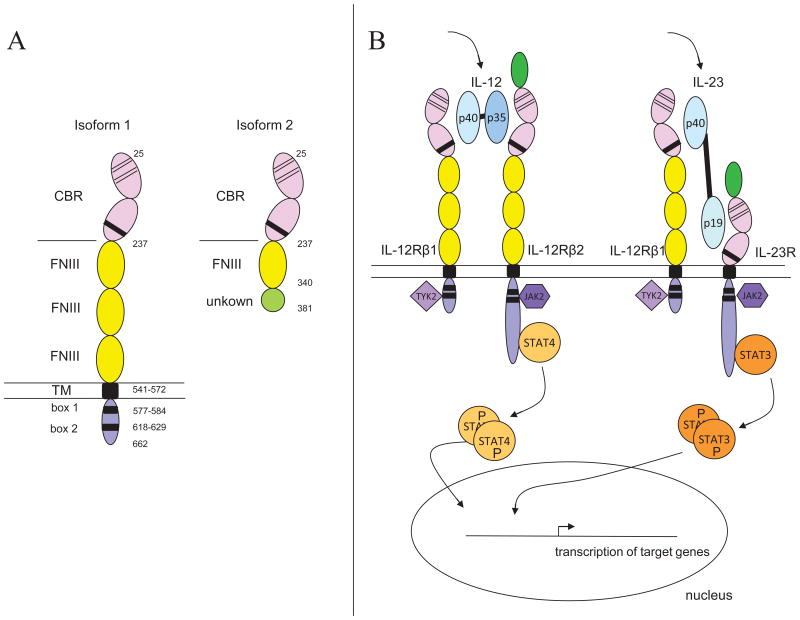
The IL12RB1 gene (also known as CD212) is located on chromosome 19p13.1 and spans an area of about 28 kb. Two mature ∼2.1 kb mRNAs are transcribed in about equal amounts from its 17 exons, with a small difference in length through alternative splicing of the last 13 bp of exon 16. These transcripts encode two protein isoforms of 662 and 660 amino acids. The 662 aa isoform is known as isoform 1. For the sake of clarity, we refer to the 660 aa isoform as isoform 1b. These two isoforms, both encoding full-length IL-12Rβ1 protein, only differ in the last amino acids which are KAKM and DR respectively [Chua et al., 1994; van de Vosse et al., 2003]. It is unknown whether this subtle difference in protein composition results in a functional difference. The 24 N-terminal amino acids encode a signal peptide that is cleaved off to generate mature proteins of 638 and 636 aa.
Mature, full-length IL-12Rβ1 has a large extracellular domain (aa 25-541) that consists of five Fibronectin type III repeats. The first two of these repeats (about 200 aa) form the cytokine binding region harboring two Cys-Cys pairs and the [STGL]xWSxWS motif, which are specific cytokine receptor family signatures. The other three Fibronectin type III repeats, each about 100 aa in length, make up the remainder of the extracellular domain. The extracellular domain is followed by a single 31 aa transmembrane domain and a short cytoplasmic portion (91 or 89 aa), containing box 1 (aa 577-584) and box 2 (aa 618-629) cytokine receptor motifs (Figure 1A). The molecular weight of the mature protein is 73 kD.
Figure 1.
(A) Schematic representation of the mature IL-12Rβ1 isoforms 1 and 2. The full-length IL-12Rβ1 receptor chain, isoform 1, contains a large extracellular segment comprised of five Fibronectin type III repeat regions (FNIII). The first two FNIII repeat regions together form the cytokine-binding region (CBR) that also contains the cytokine receptor signature (WSxWS motif and two Cys-Cys pairs). The other three FNIII repeat regions are required for proper dimerization. The extracellular domain is followed by a single transmembrane domain (TM). The intracellular part contains the box 1 and box 2 cytokine receptor motifs required for JAK-associated signal transduction. Isoform 2 contains the cytokine-binding region (CBR) and one more FNIII domain followed by a stretch of 41 aa with no similarity to known domains. (B) Schematic representation of signal transduction via the IL-12 and IL-23 receptors.
Another isoform (isoform 2) is transcribed from exon 1 to 9 plus a cryptic exon in intron 9. This isoform potentially encodes a 381 aa protein of which the last 41 aa are novel. This last stretch of amino acids has no similarity to any known functional domain. Isoform 2 lacks the transmembrane domain that is required for anchoring on the cell surface, as well as the intracellular domain required for signaling (Figure 1A). Also in mice, an isoform is known that lacks the transmembrane domain. This isoform (il12rb1Δtm) however is generated by the skipping of exon 14, an 97 bp internal exon, which results in a frameshift that replaces the last 178 aa by 108 other amino acids [Chua et al., 1995; Robinson et al., 2010].
The ratio between the expression of isoform 1 and isoform 2 was recently found to be lineage dependent: isoform expression ratio in PBMCs differs between donors, but also between the CD4+, CD8+ and CD56+ cell subsets of a single individual. These ratios could be shifted by activating the cells with PHA. Analysis of lung tissue from two sarcoidosis patients suggested that the ratio of isoform 1 over isoform 2 is increased in such patients compared to healthy controls [Ford et al., 2012]. Furthermore, ten additional minor splice variants of the IL12RB1 transcript were reported in human leukocytes. The expression of these transcripts could also be enhanced by in vitro stimulation of the cells with PHA [Ford et al., 2012].
The clinical phenotype of a large number of patients with IL-12Rβ1 deficiency has recently been reviewed extensively [de Beaucoudrey et al., 2010]. Here we discuss the molecular genetics of all known IL12RB1 mutations and variants and introduce the IL12RB1 variation database.
IL-12Rβ1 structure and function
IL-12Rβ1 is a receptor chain that in combination with the receptor chain IL-12Rβ2 can form the IL-12 receptor. Alternatively, in combination with IL-23R, it can form the IL-23 receptor. All three chains are transmembrane proteins that are expressed at the cell surface of certain cell types. While IL-12 and IL-23, as well as their receptors, are very similar in structure, signaling through both cytokines culminates into distinct effects: a difference in distribution of the receptor chains and a difference in the specific STAT (signal transducer and activator of transcription) units that are activated together result in distinct effects.
Upon binding of IL-12 to the extracellular part of the IL-12 receptor, the cytoplasmic proteins TYK2, directly interacting with IL-12Rβ1, and JAK2, interacting with IL-12Rβ2, are tyrosine phosphorylated. The phosphorylated TYK2 and JAK2 are necessary for the subsequent tyrosine phosphorylation and activation of STAT4 that is bound to IL-12Rβ2. STAT4, a transcription factor, subsequently homodimerizes, translocates to the nucleus, and binds to its target DNA to activate transcription of IFN-γ and other target genes (Figure 1B).
Signaling of IL-23 through the IL-23 receptor complex is very similar to IL-12 signaling; it also involves TYK2 and JAK2 tyrosine phosphorylation, but results mainly in STAT3 activation (Figure 1B). Originally STAT1, STAT3, STAT4 and STAT5 phosphorylation were reported in the Kit225 T cell line [Parham et al., 2002]. In primary CD4+ T cells and in T cell blasts containing an IL-23R expression construct only STAT1, STAT3 and STAT4 activation was observed [Che Mat et al., 2011; de Paus et al., 2008]. In primary NK-like T cells STAT3 and STAT4 were activated, while in primary NK cells none of these STATs were activated [van de Wetering et al., 2009]. Via activation of STATs, their nuclear translocation and subsequent binding to target DNA, IL-23 signaling results in transcription of IFN-γ and other target genes (Figure 1B).
Another important factor in the distinct responses to IL-12 and IL-23 is that IL-23 and IL-12 are produced in response to different stimuli and at different time-points. Both are produced by antigen-presenting cells, but IL-23 comes up directly in response to activation of pattern recognition receptors (PRRs) by pathogen-associated molecular patterns (PAMPs), while IL-12 is not produced unless an IFN-γ stimulus or CD40-costimulus is present as well [Verreck et al., 2004; van de Wetering et al., 2009].
Full length IL-12Rβ1 is constitutively expressed at low intensity on the surface of lymphocytes and can be highly upregulated by T cell activation or by stimulation with various interleukins, such as IL-2, IL-7 and IL-15 [Wu et al., 1997]. Full length IL-12Rβ1 is also expressed on dendritic cells [Robinson et al., 2010].
The expression of IL-12Rβ2 and IL-23R are the limiting factors in IL-12 and IL-23 signaling. IL12RB2 mRNA (encoding IL-12Rβ2) is not detectable in naïve T cells but can be induced by T cell activation and upregulated by IL-12 (Th1 cells) or downregulated by IL-4 (Th2 cells) [Rogge et al., 1999]. In PHA-stimulated PBMCs, IL-12Rβ2 protein could be upregulated by IL-12 and downregulated by IL-4, while after T cell activation not only IL-12 but also IFN-α and IFN-γ upregulated IL-12Rβ2 expression [Wu et al., 2008]. IL12RB2 transcripts as well as an IL-12 response were found in naïve B cells [Airoldi et al., 2002]. IL12RB2 mRNA was further detected in Th0, Th1, Th2, Th17 clones, with the Th17 clones indeed responding to IL-12 [Annunziato et al., 2007]. IL-17 itself was found to downregulate IL-12-induced IL12RB2 transcription in PBMCs [Toh et al., 2010].
The distribution of IL-23R is largely unknown due to the lack of an antibody that can directly detect its expression on the cell membrane. Based on IL-23 responsive cells it can however be concluded that IL-23R is present on activated T cells, memory T cells [Oppmann et al., 2000], NK-like T cells [van de Wetering et al., 2009] and Th17 cells [Cosmi et al., 2008].
The truncated IL-12Rβ1 isoform 2 protein will have a function very different from the full-length IL-12Rβ1 as it lacks the transmembrane and intracellular domains [van de Vosse et al., 2003]. In mice expression of the il12rb1Δtm transcript and the Il-12rβ1Δtm protein was induced in bone marrow-derived dendritic cells stimulated with mycobacteria or mycobacterial products but not with another pathogen or various cytokines [Robinson et al., 2010]. Il-12rβ1Δtm protein expression was also induced in mouse lungs upon M. tuberculosis infection and could in the first 2 weeks of infection be attributed to cd11c+ cells (mainly dendritic cells). This protein was found to enhance Il-12rβ1-dependent dendritic cell migration and promote M. tuberculosis specific T-cell activation. Analysis of human dendritic cells revealed that the IL-12Rβ1 isoform 2, similarly lacking the transmembrane domain, is also expressed in dendritic cells upon stimulation with mycobacteria and some other stimuli [Robinson et al., 2010]. The human isoform 2 was recently found to be localized in an intracellular compartment distinct from the ER [Ford et al., 2012]. Whether the human IL-12Rβ1 isoform 2 has a similar function as the mouse Il-12rβ1Δtm protein is still unknown.
Database
To provide an overview of all reported mutations and polymorphisms that affect the IL12RB1 transcript, an IL12RB1 variation database (www.LOVD.nl/IL12RB1) was created. This is a web-based database format for the collection, display and curation of DNA variations in specific genes. With the aim of creating the most complete and up-to-date information publicly available, clinicians and researchers may submit new sequence variants and introduce their patients carrying novel or known sequence variants into the database. New variation data is checked by the Mutalyzer program [Wildeman et al., 2008] to ensure a nomenclature following Human Genome Variation Society (HGVS) recommendations [den Dunnen and Antonarakis, 2000; den Dunnen and Antonarakis, 2001], and will be curated. All currently known IL12RB1 variant data from on-line available publications and abstracts have been entered in the mutation database, as well as many patients and mutations that have not yet been published.
The IL12RB1 gene has its own homepage with a summary of general information on the gene and the database, the chromosomal location of the gene, and the number of reported and unique variants in the database. Information on individual sequence variants (“view unique variants”) includes exon-intron location, DNA change, RNA change, protein change, predicted effect, and the first description of the variant. Patient information extrapolated from publications includes clinical diagnosis, pathogenicity of the sequence variant, mode of inheritance, ethnic origin, and the number of patients reported per family. The sequence variant tables can be displayed as a listing of unique variants or of nonpathogenic sequence variants only. Patient information can be displayed by looking at all sequence variants (“view all contents”) contained in the database. Hyperlinks to relevant gene and disease information as well as publications from the PubMed (http://www.ncbi.nlm.nih.gov/Pubmed) and OMIM (http://www.ncbi.nlm.nih.gov/Omim) databases are included.
IL12RB1 Mutations
Mutation distribution
Pathogenic IL12RB1 mutations were first reported in 1998 [de Jong et al., 1998; Altare et al., 1998] and have since been found in 198 individuals. These mutations are distributed across all exons except exon 16 and 17 wherein no mutations have been identified so far (Figure 2A). Seventy unique pathogenic mutations were identified (Table 1, Figure 2B).
Figure 2.
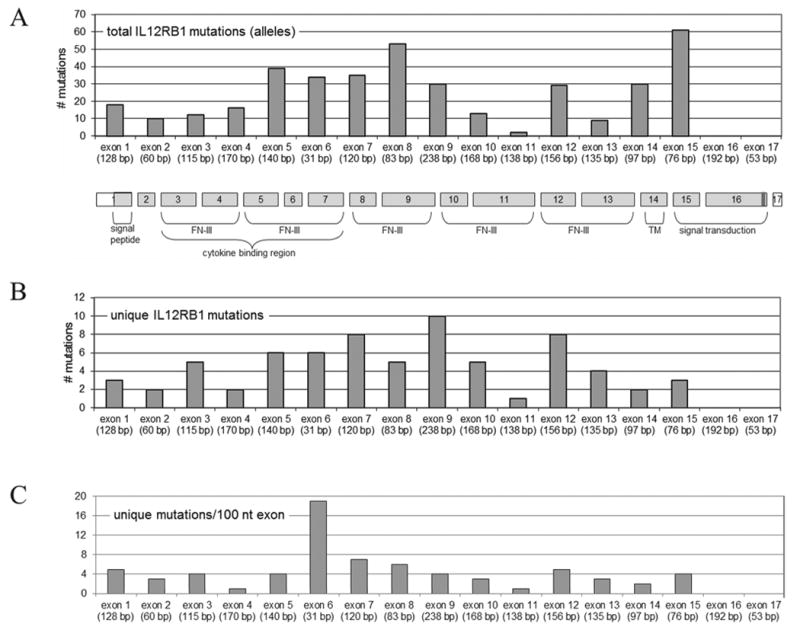
Genomic structure, encoded protein domains and mutation distribution in the IL12RB1 gene. Numbers and positions of total (A) and unique (B) variants are shown, as well as mutation distribution ratio as determined by dividing the number of unique mutations per exon by the exon length (C). FN III = Fibronectin type III repeat regions, TM = transmembrane domain. The coding region of the transcript is indicated in light grey. The last 13 bp of exon 16 (indicated in dark grey) are subject to alternative splicing (50:50 distribution). Note: mutations in splice sites and genomic deletions grouped under (first) affected exon.
Table 1. Deleterious mutations in the IL12RB1 gene.
| Region | DNA sequence change a | Protein change b | Effect/remarks | First description |
|---|---|---|---|---|
| Intron 1 | c.64+1G>T | premature stop codon? | affects splicinge | [Aytekin et al., 2011] |
| Intron 1 | c.64+2T>G | premature stop codon | affects splicing e | [de Beaucoudrey et al., 2010] |
| Intron 1 | c.64+5G>A | premature stop codons | 2 cryptic splice variants of intron 1 | [Elloumi-Zghal et al., 2002] |
| Exon 2 | c.69_72del (reported as c.65_68delCTGC) | p.Cys24GlufsX26 | [Haerynck et al., 2008] | |
| Exon 2 | c.94C>T | p.Gln32X | [de Jong et al., 1998] | |
| Exon 3 | c.169del | p.Ser57ValfsX73 | [de Beaucoudrey et al., 2010] | |
| Exon 3 | c.182A>G | p.Glu61Gly | L. Santos Argumedo - unpublished | |
| Exon 3 | c.184T>G | p.Cys62Gly | [de Beaucoudrey et al., 2010] | |
| Exon 3 | c.191G>A | p.Trp64X | J. Bustamante, S. Boisson-Dupuis - unpublished | |
| Exon 3 | c.230T>C | p.Leu77Pro | [Fieschi et al., 2003] | |
| Exon 4 | c.264C>G | p.Tyr88X | [de Beaucoudrey et al., 2010] | |
| Exon 4 | c.402C>A | p.Tyr134X | reported as: p.Y133X | [Luangwedchakarn et al., 2009] |
| Exon 5 | c.467_483delGTATGGAGTGGGAGACC | p.Val137GlyfsX8 | exon 5 absent in RNA | [Lichtenauer-Kaligis et al., 2003] |
| Exon 5 | c.512A>C | p.Gln171Pro | [Fieschi et al., 2003] | |
| Exon 5 | c.517C>T | p.Arg173Trp | [Boisson-Dupuis et al., 2011] | |
| Exon 5 | c.518G>C | p.Arg173Pro | [Aksu et al., 2001] | |
| Exon 5 | c.523C>T (reported as: 670C>T) | p.Arg175Trp | [Ozen et al., 2006] | |
| Intron 5 | c.549+2T>C | premature stop codon? | affects splicing e | [Fieschi et al., 2003] |
| Intron 5 | c.550-2A>G | p.Gly184SerfsX9 | exon 6 absent in RNA | [Elloumi-Zghal et al., 2002] |
| Exon 6 | c.556T>A | p.Cys186Ser | [Fieschi et al., 2003] | |
| Exon 6 | c.557G>A | p.Cys186Tyr | [Vinh et al., 2011] | |
| Exon 6 | c.558C>A | p.Cys186X | J. Bustamante, S. Boisson-Dupuis - unpublished | |
| Exon 6 | c.557_563delGCGGACCinsAGATATCA | p.Gly184SerfsX9 or p.Val137_Thr193del | exon 6 (55%) or 5 + 6 (45%) absent in RNA | [Lichtenauer-Kaligis et al., 2003] |
| Intron 6 | c.580+1G>A | premature stop codon? | affects splicing e | [de Beaucoudrey et al., 2010] |
| Exon 7 | c.592T>C | p.Cys198Arg | [Lichtenauer-Kaligis et al., 2003] | |
| Exon 7 | c.625C>T | p.Gln209X | [Jirapongsananuruk et al., 2012] | |
| Exon 7 | c.628_644dup | p.Gly216SerfsX32 | [Tanir et al., 2006] | |
| Exon 7 | c.631C>T | p.Arg211X | J. Bustamante, S. Boisson-Dupuis - unpublished | |
| Exon 7 | c.632G>C (reported as: 696G>C) | p.Arg211Pro | [Lee et al., 2009] | |
| Exon 7 | c.637C>T (reported as: 701C>T) | p.Arg213Trp | [Sakai et al., 2001] | |
| Exon 7 | c.658A>T | p.Ser220Cys | L. Santos Argumedo - unpublished | |
| Intron 7-13 | c.700+362_1619-944del | p.Asn235_Glu540del | genomic deletion of exon 8-13 | [Staretz-Haham et al., 2003] |
| Intron 7 | r.700_701ins21 | p.Pro233_Glu234insV GLVLIA | [Ramirez-Alejo et al., 2012] | |
| Exon 8 | c.710delC | p.Pro237HisfsX5 | [Jirapongsananuruk et al., 2012] | |
| Exon 8 | c.710dup (reported as: 711insC) | p.Gln238ThrfsX57 | [Tanir et al., 2006] | |
| Intron 8 | c.783+1G>A | p.Glu234AlafsX33 or p.Val137GlyfsX8 | exon 8 (75%) or 5+8 (25%) absent in RNA | [Lichtenauer-Kaligis et al., 2003] |
| Intron 8 | c.783+1G>C | p.Glu234AlafsX33 | exon 8 absent in RNA | [Altare et al., 1998] |
| Exon 9 | c.847C>T (reported as: 19602C>T) | p.Arg283X | [Schejbel et al., 2011] | |
| Exon 9 | c.853C>T | p.Gln285X | [Lee et al., 2008] | |
| Exon 9 | c.860A>T | p.His287Leu | L. Santos Argumedo - unpublished | |
| Exon 9 | c.872G>A | p.Cys291Tyr | [Franco et al., 2012] | |
| Exon 9 | c.913A>T | p.Lys305X | [Altare et al., 1998] | |
| Exon 9 | c.962C>A | p.Ser321X | [Fieschi et al., 2003] | |
| Exon 9 | c.983_999del | p.Leu328HisfsX37 | [de Beaucoudrey et al., 2010] | |
| Exon 9 | c.1007_1008delinsG | p.Ala336GlyfsX10 | [Fieschi et al., 2003] | |
| Exon 9 | c.1019_1020delCA | p.Thr340ArgfsX30 | M. Martinez-Gallo – unpublished | |
| Intron 9 | c.1021+1G>C | p.Pro262AsnfsX5 | exon 9 absent in RNA | [Özbek et al., 2005] |
| Exon 10 | c.1064_1066del (reported as: 1063_1065delACC) | p.Thr355del | [Tabarsi et al., 2011] | |
| Exon 10 | c.1100A>G | p.Tyr367Cys | [Fieschi et al., 2003] | |
| Exon 10 | c.1106T>C | p.Ile369Thr | [de Beaucoudrey et al., 2010] | |
| Exon 10 | c.1126C>T | p.Gln376X | [de Jong et al., 1998] | |
| intron 10 | c.1189+2T>A | premature stop codon? | affects splicing e | [de Beaucoudrey et al., 2010] |
| Intron 10 | c.1190-1G>A | premature stop codon? | affects splicing e | [Fieschi et al., 2003] |
| Exon 12 | unclear (reported as: 1336delC)c | premature stop codon | [Alangari et al., 2011] | |
| Exon 12 | c.1370dupA | p.His457GlnfsX2 | J. Bustamante, S. Boisson-Dupuis - unpublished | |
| Exon 12 | c.1386_1387del | p.Ser463CysfsX25 | [de Beaucoudrey et al., 2010] | |
| Exon 12 | c.1387_1392del | p.Ser463_Val464del | J. Bustamante, S. Boisson-Dupuis - unpublished | |
| Exon 12 | c.1425delC | p.Gly476AlafsX3 | [de Beaucoudrey et al., 2010] | |
| Exon 12 | c.1438G>T | p.Glu480X | [de Beaucoudrey et al., 2010] | |
| Exon 12 | c.1440_1447delins16 | premature stop codon e | [Fieschi et al., 2003] | |
| Exon 12 | c.1456C>T (reported as: 1520C>T) | p.Arg486X | [Asilsoy et al., 2009] | |
| Intron 12-13 | c.1483+182_1618+526del (reported as: c.1483+182_1619-1073del) | p.His496_Glu540del | [Fieschi et al., 2003] | |
| Exon 13 | c.1561C>G | p.Arg521Gly | [Potjewijd et al., 2012] | |
| Exon 13 | c.1561C>T | p.Arg521X | [de Beaucoudrey et al., 2010] | |
| Exon 13 | c.1593G>A d | p.Trp531X | [de Beaucoudrey et al., 2010] | |
| Exon 14 | c.1623_1624delinsTT | p.Gln542X | [Fieschi et al., 2003] | |
| Exon 14 | c.1706G>A | p.Gly569Asp | [de Beaucoudrey et al., 2010] | |
| Exon 15 | c.1749_1750dup (reported as: c.1745_1746insCA) | p.Pro584HisfsX37 | [Fieschi et al., 2003] | |
| Exon 15 | c.1765delG | p.Ala589ProfsX31 | [de Beaucoudrey et al., 2010] | |
| Intron 15 | c.1791+2T>G | p.Ala573LeufsX22 | exon 15 absent in RNA | [Cleary et al., 2003] |
Mutations are numbered in accordance with GenBank entry NM_005535.1, where +1 corresponds to the A of the ATG translation initiation codon. For intronic sequences, genomic sequence NG_007366.1 was used.
Amino acid changes are numbered in accordance with SwissProt entry P42701.
Position 1336 is a G not a C therefore exact location of mutation is unclear.
Mutation deduced from protein change, can also be c.1592G>A instead of c.1593G>A.
not specified.
Although at first glance the mutations appear to be distributed randomly across the exons (Figure 2B), when analyzing the number of unique mutations in relation to exon length, exon 6 contains a relatively high number of mutations (0.19 mutations/bp) compared to the average of 0.03 mutations/bp for all exons (Figure 2C).
Founder effects and global distribution
The seventy unique IL12RB1 mutations have a very uneven distribution: 41 mutations have thus far been reported only in one family while some other mutations occur very frequently. Of the mutations that are reported in at least four families, three were found in families from a single country only. This strongly suggests that these mutations have each arisen only once (Table 2). The two most common mutations, however, have both been found on three different continents.
Table 2. Frequently reported IL12RB1 mutations.
| Mutation | Effect on protein | Reported in | Location of patients or origin of their families |
|---|---|---|---|
| c.1791+2T>G | aberrant splicing | 27 patients from 17 families | 3 different continents |
| c.1623_1624delinsTT | p.Gln542X | 17 patients from 15 families | 3 different continents |
| c.783+1G>A | aberrant splicing | 17 patients from 12 families | 11 families from Turkey, one from Russia |
| c.64+2T>G | aberrant splicing | 7 patients from 6 families | 5 families from Turkey, one from Tunisia |
| c.264C>A | p.Tyr88X | 7 patients from 5 families | all from Saudi Arabia |
| c.518G>C | p.Arg173Pro | 6 patients from 6 families | all from Turkey |
| c.556T>A | p.Cys186Ser | 6 patients from 4 families | all from Qatar |
The most common IL12RB1 mutation, c.1791+2T>G, has been found in 27 patients from 17 families and was first described in an Iranian patient [Cleary et al., 2003] and has since been detected in patients from Spain, Sri Lanka, Iran, China, Turkey, Brazil, Ukraine, Saudi Arabia, Mexico and France [Caragol et al., 2003; Fieschi et al., 2003; Lee et al., 2008; de Beaucoudrey et al., 2010; Pedraza-Sanchez et al., 2010; Pedraza et al., 2010]. The wide distribution of this particular mutation at the 3′ splice site of exon 15 may suggest that it arose at multiple occasions in different individuals, perhaps due to a small mutation hotspot. This has however not been investigated yet.
The c.1623_1624delinsTT mutation has been reported in 17 patients from 15 families and was first identified in patients from Cyprus, France and Germany [Fieschi et al., 2003]. It has since been reported in patients from Argentina, Belgium, United States, Poland and the United Kingdom [Rosenzweig et al., 2006; Haerynck et al., 2008; Yancoski et al., 2009; Gruenberg et al., 2009; de Beaucoudrey et al., 2010]. A founder effect has been proposed for this mutation because its distribution is restricted to Europeans and to individuals from European ancestry living in former European colonies. Indeed, IL12RB1 haplotype analysis of five unrelated Argentinian patients and one Belgian patient carrying this mutation, revealed a common origin of the mutation about 19 generations or 475-years ago [Yancoski et al., 2009].
It has been noted before that global distribution of IL-12Rβ1 deficiency appears to be uneven, with the majority of patients originating from Europe, North Africa and the Middle East region while hardly any patients were identified in North America [van de Vosse and Ottenhoff, 2006]. This may be largely due to a difference in populations: the common practice of consanguineous marriages in North Africa and the Middle East region and the large numbers of immigrants from these regions in Europe. The majority of parents of IL-12Rβ1 deficient patients are known to be consanguineous (83 of 151 families), of the 83 known consanguineous families 65 originate from North Africa or the Middle East region.
Genotype-Phenotype Correlation
The various morbid IL12RB1 mutations have been shown to almost invariably (with exception of c.700+362_1619-944del) result in complete absence of expression of the full-length IL-12Rβ1 on the cell surface, while all morbid IL12RB1 mutations have the same cellular phenotype: complete absence of IL-12 and IL-23 responses. Because the cellular phenotype is the same for all mutations, no genotype-phenotype differences can be observed. Clinical phenotypes do however vary, due to differences in exposure to intracellular pathogens and the wide range of pathogenicity of these pathogens.
The majority of IL12RB1 mutations (66%; 46/70) has an obvious inactivating effect because they are mostly nonsense, frame shift, and splice site mutations resulting in premature stop codons in the extracellular (n=42) or transmembrane domain (n=1). Consequently, these mutations directly preclude expression of IL-12Rβ1 on the cell surface (Table 3). Three other mutations (c.1749_1750dup, c.1765delG and c.1791+2T>G) result in premature stop codons very early in the intracellular domain, leaving only 1, 11 and 16 aa respectively of the intracellular domain intact while replacing the remainder by a short stretch of random other amino acids.
Table 3. Overview of IL12RB1 mutation types.
| Mutation type | Number |
|---|---|
| small deletion, insertion or duplication resulting in a frameshift and a premature stop | 17 |
| nonsense mutation (single nucleotide substitution resulting in premature stop) | 17 |
| missense mutation (single nucleotide substitution resulting in amino acid change) | 19 |
| splice-site mutation (single nucleotide substitution) | 12 |
| large genomic, in frame deletion (one or more exons) | 2 |
| small, in frame deletion | 2 |
| in frame insertion | 1 |
| translocation | 0 |
| Total unique mutations | 70 |
Only four in-frame deletions are known. One is a single amino acid deletion, p.T355del, that results in a complete inability to respond to IL-12 [Tabarsi et al., 2011] because the corresponding protein is absent from the cell surface [de Beaucoudrey et al., 2010]. Another results in a two amino acid deletion, p.Ser463_Val464del, that was only very recently discovered, and that is also not expressed on the cell surface (S. Boisson-Dupuis and J. Bustamante, unpublished data). Of the two large in-frame deletions, c.1483+182_1618+526del results in the absence of exon 13, thereby removing a large part of the fifth extracellular Fibronectin repeat which results in the absence of the protein from the cell surface [de Beaucoudrey et al., 2010]. A large genomic deletion of exon 8 to 13 is one of the three exceptions to the rule that mutations in IL12RB1 preclude IL-12Rβ1 expression. This c.700+362_1619-944del mutation leaves the open reading frame intact [Staretz-Haham et al., 2003], resulting in a protein that lacks the three extracellular Fibronectin repeats between the cytokine binding region and the transmembrane domain. Although the shortened protein is detectable on the cell surface in significant amounts, it is not functional and leads to complete IL-12Rβ1 deficiency [Fieschi et al., 2004]. Another exception to the rule that mutations in IL12RB1 preclude IL-12Rβ1 expression, may be the mutation r.700_701ins21 which leads to the insertion of seven amino acids directly after the cytokine binding region. Preliminary data suggest it leads to diminishedIL-12Rβ1 expression as well as a diminished response to IL-12 (L. Santos Argumedo, unpublished data), the effect of this mutation has however not been proven yet.
All other mutations (27%; 19/70) are missense mutations, that is: nucleotide changes resulting in single amino acid substitutions (Table 3). We have previously shown, using a retroviral expression model system, that also these mutations invariably preclude, or very severely hamper, IL-12Rβ1 membrane expression and thus abrogate function [van de Vosse et al., 2005]. It is most likely that the quality control system in the endoplasmic reticulum, that precludes the transport of mutant, misfolded or incorrectly complexed proteins, also prevents the aberrant IL-12Rβ1 molecules from expression on the membrane.
Although no partial cellular phenotype has been found so far, two of the missense mutations, p.C198R and p.R521G, are detectable in minute amounts at the cell surface when overexpressed using a retroviral expression model [van de Vosse et al., 2005; Potjewijd et al., 2012]. Two patients with these mutations showed a relative mild clinical phenotype (p.C198R, 1 patient) [Lichtenauer-Kaligis et al., 2003] and late onset of disease (p.R521G, 1 patient) [Potjewijd et al., 2012]. Three other patients that were reported with the p.C198R mutation, also appear to have a relatively mild clinical phenotype [de Beaucoudrey et al., 2010]. Preliminary data on one recently identified missense mutation, p.S220C, suggests it may lead to diminished expression of the IL-12Rβ1 protein on patient' cells and a diminished but not absent IL-12 response (L. Santos Argumedo, unpublished data), the effect of this mutation has however not been proven yet.
Of the seventy mutations in Table 1, the first forty-seven affect not only the full-length IL-12Rβ1 protein (isoform 1) but also the shorter isoform 2 protein. We do not know the function and relevance of the human isoform 2 protein yet. If we suppose it has a function similar to the shorter isoform in mice, or that it is relevant in any other way, there may well be a clinical difference between patients in which only the full-length IL-12Rβ1 is affected versus patients in which both isoforms are affected. Therefore, we analyzed various parameters in a large group of patients (141) of which the clinical data were recently reviewed [de Beaucoudrey et al., 2010]. In 75 patients both isoforms are affected and in 65 patients only the full-length protein is affected. Compound heterozygous individuals with one mutation affecting both isoforms and the other affecting only isoform 1 were grouped with the individuals in which only isoform 1 is affected. Of one of the 141 patients the mutation is unknown. We compared mortality, current age, resistance to M. bovis BCG vaccination, number of individuals without mycobacterial infections or with only a local reaction to BCG vaccination, , the occurrence of candida infections, the occurrence of salmonella infections, and the number of asymptomatic individuals. The small differences observed between the two groups are neither significant, nor do they point in one direction, suggesting presence or absence of isoform 2 does not alter the clinical phenotype.
Penetrance of disease-causing mutations
Penetrance of IL-12Rβ1 deficiency is not always complete. Many symptomatic IL-12Rβ1 deficient patients (mean age at first infection: 2.4 years) have a sibling who has the same IL12RB1 genotype but who has not (yet) developed severe infections [de Beaucoudrey et al., 2010]. About 12% of the IL-12Rβ1 deficient individuals are asymptomatic, this may be merely due to a difference in exposure to the relevant pathogens - some IL-12Rβ1 deficient individuals do not develop disease until later in life [Tabarsi et al., 2011; Schejbel et al., 2011; Potjewijd et al., 2012] – resulting in a large range of the age at first infection (from 1 week to 31.7 years) [de Beaucoudrey et al., 2010]. It has however also been suggested that in order to develop mycobacterial infections, in IL-12Rβ1 deficient individuals also other minor immunodeficiencies need to be present [Schejbel et al., 2011].
Polymorphisms and unclassified sequence variants
In addition to the known morbid mutations, 115 variations have been reported in the IL12RB1 transcript. All have been included in the LOVD database. Note that of the 118 variants in the SNP database [NCBI SNP database, 2012] five are known deleterious mutations while two polymorphisms (leading to p.H438Y and p.G594E, see Table 4) have not yet been included. Most of the 115 variants in the LOVD database are unclassified: it is unknown whether they are deleterious mutations or harmless polymorphisms. Sixty-nine variations result in amino acid substitutions, nine of these (Table 4) were shown in a retroviral expression model system not to have a deleterious effect on IL-12Rβ1 expression or IL-12 responses ([van de Vosse et al., 2005] and unpublished data). Five of the other 46 variants, c.21G>A, c.152insC, c.288delG, c.633delA, and c.1136insG, are most likely pathogenic as they result in premature stop codons. Of the sixty non-synonymous single nucleotide variants (SNVs) of which the effect on IL-12Rβ1 protein function has not yet been determined experimentally we have determined the predicted effect with various computational tools (Supp. Table S1). Many are predicted to be deleterious and these are almost all rare variants, suggesting a selective constraint against these variants. In contrast, the high frequencies of the innocuous R156H, Q214R, M365T and G378R SNVs (Table 4) suggest an evolutionary advantage.
Table 4. IL12RB1 polymorphisms that result in amino acid changes*.
| SNPid | Nucleotide change | Amino acid change | Population frequency a (number of chromosomes tested) |
|---|---|---|---|
| rs11575925 | 222C>G | S74R | 0.4 % North America (4550) |
| 0.1 % European descent (1285) | |||
|
| |||
| rs147215816 | 271G>A | A91T | n.a.b |
|
| |||
| rs11575926 | 467G>A | R156H | 15 % European descent (1285) |
| 11.3 % North Americans (4550) | |||
| 0 % Asian, various (345) | |||
| 0 % Sub-Sahara African (238) | |||
|
| |||
| rs11575934 | 641A>G | Q214R | 37.5 % Asian, various (354) |
| 28.5 % European descent (1305) | |||
| 10.8 % Sub-Sahara African (120) | |||
|
| |||
| rs375947 | 1094T>C | M365T | 38.3 % Asian, various (180) |
| 28.7 % European descent (569) | |||
| 25.7 % Sub-Sahara African (226) | |||
| 14 % Mexican ancestry (100) | |||
|
| |||
| rs401502 | 1132G>C | G378R | 38.2 % Asian, various (296) |
| 35 % Europeans (286) | |||
| 18.6 % Sub-Sahara African (118) | |||
|
| |||
| ss539004547 | 1312C>T | H438Y | 0.9 % Japanese (112)c |
|
| |||
| rs11575935 | 1573G>A | A525T | 1.7 % Asian, various (120) |
| 0.4 % European descent (2040) | |||
|
| |||
| ss539004548 | 1781G>A | G594E | 0.9 % Japanese (112)c |
only polymorphisms included that have been analyzed in a retroviral expression model and were found not to have an effect on IL-12Rβ1 function [van de Vosse et al., 2005].
data from the SNP database [NCBI SNP database, 2012],
identified in a Chinese MSMD patient,
data from [Sakai et al., 2001].
Note: Two SNPs are still without rs-number, these have been requested (24-9-2012).
The two most common haplotypes of the IL12RB1 gene that can be found at roughly equal frequencies in many populations are: ‘QMG’ and ‘RTR’, where QMG refers to the tightly linked alleles c.641A, c.1094T, c.1132G, and RTR refers to c.641G, c.1094C, c.1132C. The designations QMG and RTR are derived from the amino acids substitutions p.Q214R, p.M365T and p.G378R encoded by these alleles. In a retroviral expression system of the full-length IL-12Rβ1 protein the QMG haplotype was a better responder to IL-12 than the RTR allele [van de Vosse et al., 2005], while no major differences were observed between the two haplotypes in response to IL-23 [de Paus et al., 2008]. In cells from healthy Japanese individuals who were homozygous for either the QMG or RTR haplotype a similar difference in IL-12-induced IFN-γ production was found: cells homozygous for the RTR haplotype responded less well to IL-12 [Akahoshi et al., 2003].
Associations of IL12RB1 polymorphisms with disease
Several IL12RB1 polymorphisms were reported to be associated with increased susceptibility to tuberculosis disease (TB). In a small cohort of TB patients (98 patients, 197 controls) from southern Japan the frequency of the QMG and RTR haplotypes was analyzed. In this study TB patients were more often homozygous for the RTR haplotype than controls (p<0.013) [Akahoshi et al., 2003]. Another small association study in the same population (86 patients, 265 controls) found similar associations of the RTR haplotype with pulmonary TB [Kusuhara et al., 2007]. In a family-based study in Morocco (101 families) nine polymorphisms were analyzed, including a representative polymorphism for the QMG/RTR haplotypes. Of these nine polymorphisms only two in the 5′ untranslated region of the gene showed a modest association with pulmonary TB: the variations c.-2C>T and c.-111A>T (p=0.013 and p=0.019) [Remus et al., 2004] (Supp. Table S2).
In a Korean cohort (115 patients, 151 controls) no associations were found of the p.Q214R, p.M365T, p.G378R or p.A525T polymorphisms with TB [Lee et al., 2005]. Also in a larger Indonesian cohort (382 patients, 437 controls) associations between TB and the p.Q214R (representing the common QMG/RTR haplotypes), c.A525T or c.-2C>T polymorphisms were not found [Sahiratmadja et al., 2007] (Supp. Table S2). Taken together, not one association between IL12RB1 polymorphisms and TB has thus far been reproduced in an ethnically different population. This may indicate that the reported associations are spurious findings due to small sample sizes, or that the populations analyzed are genetically too diverse. However, also in large genome-wide linkage studies and genome-wide association studies for TB IL12RB1 is not among the genes for which associations were found, suggesting that common IL12RB1 variations do not play a major role in adult TB susceptibility [Miller et al., 2004; Mahasirimongkol et al., 2012; Thye et al., 2010; Thye et al., 2012; Png et al., 2012].
Associations have also been reported between IL12RB1 polymorphisms and other infection- or immune-related diseases, such as the association of c.-111A>T and c.-2C>T with atopic dermatitis and the association of c.-111A>T with childhood asthma (382 atopic dermatitis patients, 304 childhood asthma patient, 658 controls) [Takahashi et al., 2005]. A large study (1946 patients, 1808 controls) even found an association of a tagging SNP, rs2305742, in IL12RB1 with non-Hodgkin lymphoma. The association was attributed to a subcohort from the United States but was not present in other subcohorts from the United States or from Australia [Lan et al., 2011] (Supp. Table S2).
Fourteen IL12RB1 polymorphisms, including one that can differentiate between the QMG/RTR haplotypes, were analyzed in a birth cohort of 913 Kenyan children for association with malarial anaemia. An association (p<0.003) was found between heterozygosity for an intronic IL12RB1 SNP, rs383483, and high density parasitaemia [Zhang et al., 2010]. A Chinese study analyzed four IL12RB1 polymorphisms, including three defining the two major haplotypes, in severe acute respiratory syndrome (SARS) (115 patients, 141 potentially exposed controls, 155 other controls). This study revealed that individuals with the c.1600C>T polymorphism (resulting in the amino acid substitution p.P534S) were at increased risk of developing SARS. This polymorphism was present on the RTR background, while the RTR haplotype itself (without the c.1600C>T variation) protected from developing SARS [Tang et al., 2008] (Supp. Table S2).
It is important to realize that most of these studies were performed in relatively small cohorts and were not yet, or could not be, replicated in larger cohorts and other ethnic populations. We should therefore be cautious when interpreting these data.
Diagnostic strategies
When a genetic defect is suspected in a patient with enhanced susceptibility to intracellular pathogens, the first analyses should be focused on determining whether the type 1 cytokine pathway is indeed affected. The strategy for these analyses has been outlined previously [van de Vosse et al., 2004; Feinberg et al., 2004]. Once a defect in the type 1 cytokine pathway is established, analysis of IL-12Rβ1 expression on the surface of T cells will, based on the currently known mutation spectrum, identify each individual with IL-12Rβ1 deficiency. However, to prevent missing a new type of mutation that does leave IL-12Rβ1 expression intact, measuring IL-12-induced IFN-γ production by activated T cells is advisable. When a defect is found, subsequent sequencing of the complete IL12RB1 transcript should identify the causative mutation. Because there is no known genotype-phenotype correlation in IL-12Rβ1 deficiency, the molecular diagnosis cannot be restricted to specific mutations or specific exons.
Future Prospects
We believe that the IL12RB1 variation database will help investigators and clinicians to quickly determine whether a variation is a known mutation, a known polymorphism or a variation with unknown effect requiring further analysis. The database will provide a complete and up-to-date overview of all reported variants in IL12RB1. Once many more patients with IL-12Rβ1 deficiency are identified, a genotype-phenotype correlation may still become apparent. Such a correlation could provide patients with a more accurate prognosis and could influence treatment choices. In addition, identification of the function and relevance of human isoform 2 may indicate whether or not absence of isoform 2 contributes to the clinical phenotype of IL-12Rβ1 deficiency.
Supplementary Material
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Meth. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airoldi I, Guglielmino R, Carra G, Corcione A, Gerosa F, Taborelli G, Trinchieri G, Pistoia V. The interleukin-12 and interleukin-12 receptor system in normal and transformed human B lymphocytes. Haematologica. 2002;87:434–442. [PubMed] [Google Scholar]
- Akahoshi M, Nakashima H, Miyake K, Inoue Y, Shimizu S, Tanaka Y, Okada K, Otsuka T, Harada M. Influence of interleukin-12 receptor β1 polymorphisms on tuberculosis. Hum Genet. 2003;112:237–243. doi: 10.1007/s00439-002-0873-5. [DOI] [PubMed] [Google Scholar]
- Aksu G, Tirpan C, Cavusoglu C, Soydan S, Altare F, Casanova JL, Kutukculer N. Mycobacterium fortuitum-chelonae complex infection in a child with complete interleukin-12 receptor beta 1 deficiency. Pediatr Infect Dis J. 2001;20:551–553. doi: 10.1097/00006454-200105000-00021. [DOI] [PubMed] [Google Scholar]
- Alangari AA, Al-Zamil F, Al-Mazrou A, Al-Muhsen S, Boisson-Dupuis S, Awadallah S, Kambal A, Casanova JL. Treatment of disseminated Mycobacterial infection with high-dose IFN-γ in a patient with IL-12Rβ1 deficiency. Clin Dev Immunol. 2011:691956. doi: 10.1155/2011/691956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppson O, Gollob JA, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asilsoy S, Bilgili G, Turul T, Dizdarer C, Kalkan S, Yasli H, Can D, Genel F, Sanal O. Interleukin-12/-23 receptor beta 1 deficiency in an infant with draining BCG lymphadenitis. Pediatr Int. 2009;51:310–312. doi: 10.1111/j.1442-200X.2009.02818.x. [DOI] [PubMed] [Google Scholar]
- Aytekin C, Dogu F, Tuygun N, Tanir G, Guloglu D, Boisson-Dupuis S, Bustamante J, Feinberg J, Casanova JL, Ikinciogullari A. Bacille Calmette-Guerin lymphadenitis and recurrent oral candidiasis in an infant with a new mutation leading to interleukin-12 receptor beta-1 deficiency. J Investig Allergol Clin Immunol. 2011;21:401–404. [PMC free article] [PubMed] [Google Scholar]
- Bogunovic D, Byun M, Durfee LA, Abhyankar A, Sanal O, Mansouri D, Salem S, Radovanovic I, Grant AV, Adimi P, Mansouri N, Okada S, et al. Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dupuis S, El Baghdadi J, Parvaneh N, Bousfiha A, Bustamante J, Feinberg J, Samarina A, Grant AV, Janniere L, El Hafidi N, Hassani A, Nolan D, et al. IL-12Rβ1 deficiency in two of fifty children with severe Tuberculosis from Iran, Morocco, and Turkey. PLoS ONE. 2011;6:e18524. doi: 10.1371/journal.pone.0018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, Grant AV, Marchal CC, Hubeau M, Chapgier A, de Beaucoudrey L, Puel A, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12:213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragol I, Raspall M, Fieschi C, Feinberg J, Larrosa MN, Hernández M, Figueras C, Bertrán JM, Casanova JL, Español T. Clinical tuberculosis in 2 of 3 siblings with interleukin-12 receptor β1 deficiency. Clin Infect Dis. 2003;37:302–306. doi: 10.1086/375587. [DOI] [PubMed] [Google Scholar]
- Cardenes M, Angel-Moreno A, Fieschi C, Sologuren I, Colino E, Molines A, Garcia-Laorden MI, Campos-Herrero MI, Andujar-Sanchez M, Casanova JL, Rodriguez-Gallego C. Oesophageal squamous cell carcinoma in a young adult with IL-12Rβ1 deficiency. J Med Genet. 2010;47:635–637. doi: 10.1136/jmg.2009.071910. [DOI] [PubMed] [Google Scholar]
- Che Mat NF, Zhang X, Guzzo C, Gee K. Interleukin-23-induced interleukin-23 receptor subunit expression is mediated by the Janus kinase/signal transducer and activation of transcription pathway in human CD4 T cells. J Interferon Cytokine Res. 2011;31:363–371. doi: 10.1089/jir.2010.0083. [DOI] [PubMed] [Google Scholar]
- Chua AO, Chizzonite R, Desai BB, Truitt TP, Nunes P, Minetti LJ, Warrier RR, Presky DH, Levine JF, Gately MK. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. J Immunol. 1994;153:128–136. [PubMed] [Google Scholar]
- Chua AO, Wilkinson VL, Presky DH, Gubler U. Cloning and characterization of a mouse IL-12 receptor-beta component. J Immunol. 1995;155:4286–4294. [PubMed] [Google Scholar]
- Cleary AM, Tu W, Enright A, Giffon T, de Waal Malefyt R, Gutierrez K, Lewis DB. Impaired accumulation and function of memory CD4 T cells in human IL-12 receptor β1 deficiency. J Immunol. 2003;170:597–603. doi: 10.4049/jimmunol.170.1.597. [DOI] [PubMed] [Google Scholar]
- Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, et al. Human interleukin 17–producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, Fieschi C, Stephan JL, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Janniere L, Rose Y, de SM, Kong XF, Filipe-Santos O, et al. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- de Moraes Vasconcelos D, Grumach AS, Yamaguti A, Andrade ME, Fieschi C, de Beaucoudrey L, Casanova JL, Duarte AJS. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the β1 subunit of the Interleukin (IL)-12/IL-23 receptor. Clin Infect Dis. 2005;41:e31–e37. doi: 10.1086/432119. [DOI] [PubMed] [Google Scholar]
- de Paus RA, van de Wetering D, van Dissel JT, van de Vosse E. IL-23 and IL-12 responses in activated human T cells retrovirally transduced with IL-23 receptor variants. Mol Immunol. 2008;45:3889–3895. doi: 10.1016/j.molimm.2008.06.029. [DOI] [PubMed] [Google Scholar]
- den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: A discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- den Dunnen JT, Antonarakis SE. Nomenclature for the description of human sequence variations. Hum Genet. 2001;109:121–124. doi: 10.1007/s004390100505. [DOI] [PubMed] [Google Scholar]
- Elloumi-Zghal H, Barbouche MR, Chemli J, Bejaoui M, Harbi A, Snoussi N, Abdelhak S, Dellagi K. Clinical and genetic heterogeneity of inherited autosomal recessive susceptibility to disseminated Mycobacterium bovis Bacille Calmette-Guérin infection. J Infect Dis. 2002;185:1468–1475. doi: 10.1086/340510. [DOI] [PubMed] [Google Scholar]
- Feinberg J, Fieschi C, Doffinger R, Feinberg M, Leclerc T, Boisson-Dupuis S, Picard C, Bustamante J, Chapgier A, Filipe-Santos O, Ku CL, de Beaucoudrey L, et al. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur J Immunol. 2004;34:3276–3284. doi: 10.1002/eji.200425221. [DOI] [PubMed] [Google Scholar]
- Fieschi C, Bosticardo M, de Beaucoudrey L, Boisson-Dupuis S, Feinberg J, Filipe Santos O, Bustamante J, Levy J, Candotti F, Casanova JL. A novel form of complete IL-12/IL-23 receptor β1-deficiency with cell surface-expressed non-functional receptors. Blood. 2004;104:2095–2101. doi: 10.1182/blood-2004-02-0584. [DOI] [PubMed] [Google Scholar]
- Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, Koch H, Richter D, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor β1 deficiency: medical and immunological implications. J Exp Med. 2003;197:527–535. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe-Santos O, Bustamante J, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, Casanova JL. Inborn errors of IL-12/23- and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Seminars in Immunology. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Ford NR, Miller HE, Reeme AE, Waukau J, Bengtson C, Routes JM, Robinson RT. Inflammatory signals direct expression of human IL12RB1 into multiple distinct isoforms. J Immunol. 2012;189:4684–4694. doi: 10.4049/jimmunol.1200606. [DOI] [PubMed] [Google Scholar]
- Franco J, Sierra JE, Perez CM, Wilches A, Restrepo A, Trujillo M, Garces C, Orrego JC, Rojas J, Arias AA, Casanova JL, Bustamante J. Protein-loosing enteropathy with hypogammaglobulinemia in a Colombian child with IL12RB1 deficiency presenting with disseminated BCG infection and chronic diarrhea. J Clin Immunol. 2012;32:S123. [Google Scholar]
- Gonzalez-Perez A, Lopez-Bigas N. Improving the Assessment of the Outcome of Nonsynonymous SNVs with a Consensus Deleteriousness Score, Condel. Am J Hum Genet. 2011;88:440–449. doi: 10.1016/j.ajhg.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg DA, Anover-Sombke S, Gern JE, Holland SM, Rosenzweig SD, Torgerson TR, Seroogy CM. Atypical presentation of IL-12 receptor β1 deficiency with pneumococcal sepsis and disseminated nontuberculous mycobacterial infection in a 19-month-old girl born to nonconsanguineous US residents. J Allergy Clin Immunol. 2009;125:264–265. doi: 10.1016/j.jaci.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerynck F, Holland SM, Rosenzweig SD, Casanova JL, Schelstraete P, De Baets F. Disseminated Mycobacterium avium infection in a patient with a novel mutation in the interleukin-12 receptor-β1 chain. J Pediatr. 2008;153:721–722. doi: 10.1016/j.jpeds.2008.05.050. [DOI] [PubMed] [Google Scholar]
- Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, et al. IRF8 Mutations and Human Dendritic-Cell Immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirapongsananuruk O, Luangwedchakarn V, Niemela JE, Pacharn P, Visitsunthorn N, Thepthai C, Vichyanond P, Piboonpocanun S, Fleisher TA. Cryptococcal osteomyelitis in a child with a novel compound mutation of the IL12RB1 gene. Asian Pac J Allergy Immunol. 2012;30:79–82. [PubMed] [Google Scholar]
- Kong XF, Vogt G, Itan Y, Macura-Biegun A, Szaflarska A, Kowalczyk D, Chapgier A, Abhyankar A, Furthner D, Djambas Khayat C, Okada S, Bryant VL, et al. Haploinsufficiency at the human IFNGR2 locus contributes to mycobacterial disease. Hum Mol Genet. 2013;22:769–781. doi: 10.1093/hmg/dds484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protocols. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- Kusuhara K, Yamamoto K, Okada K, Mizuno Y, Hara T. Association of IL12RB1 polymorphisms with susceptibility to and severity of tuberculosis in Japanese: a gene-based association analysis of 21 candidate genes. Int J immunogenet. 2007;34:35–44. doi: 10.1111/j.1744-313X.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- Lan Q, Wang SS, Menashe I, Armstrong B, Zhang Y, Hartge P, Purdue MP, Holford TR, Morton LM, Kricker A, Cerhan JR, Grulich A, et al. Genetic variation in Th1/Th2 pathway genes and risk of non-Hodgkin lymphoma: a pooled analysis of three population-based case-control studies. Br J Haematol. 2011;153:341–350. doi: 10.1111/j.1365-2141.2010.08424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Lee HS, Kim DK, Ko DS, Han SK, Shim YS, Yim JJ. Lack of an association between interleukin-12 receptor β1 polymorphisms and tuberculosis in Koreans. Respiration. 2005;72:365–368. doi: 10.1159/000086249. [DOI] [PubMed] [Google Scholar]
- Lee PP, Jiang LP, Wang XC, Chan KW, Tu WW, Lau YL. Severe mycobacterial infections in two pairs of Chinese siblings with interleukin-12 receptor β1 deficiency. Eur J Pediatr. 2008;167:213–232. doi: 10.1007/s00431-007-0430-2. [DOI] [PubMed] [Google Scholar]
- Lee WI, Huang JL, Lin TY, Hsueh C, Wong A, Hsieh MY, Chiu CH, Jaing TH. Chinese patients with defective IL-12/23-Interferon-γ circuit in Taiwan: partial dominant Interferon-γ receptor 1 mutation presenting as cutaneous granuloma and IL-12 receptor β1 mutation as Pneumatocele. J Clin Immunol. 2009;29:238–245. doi: 10.1007/s10875-008-9253-9. [DOI] [PubMed] [Google Scholar]
- Lichtenauer-Kaligis EG, de Boer T, Verreck FA, van Voorden S, Hoeve MA, van de Vosse E, Ersoy F, Tezcan I, van Dissel JT, Sanal O, Ottenhoff TH. Severe Mycobacterium bovis BCG infections in a large series of novel IL-12 receptor β1 deficient patients and evidence for the existence of partial IL-12 receptor β1 deficiency. Eur J Immunol. 2003;33:59–69. doi: 10.1002/immu.200390008. [DOI] [PubMed] [Google Scholar]
- Luangwedchakarn V, Jirapongsananuruk O, Niemela JE, Thepthai C, Chokephaibulkit K, Sukpanichnant S, Pacharn P, Visitsunthorn N, Vichyanond P, Piboonpocanun S, Fleisher TA. A novel mutation of the IL12RB1 gene in a child with nocardiosis, recurrent salmonellosis and neurofibromatosis type I: first case report from Thailand. Asian Pac J Allergy Immunol. 2009;27:161–165. [PubMed] [Google Scholar]
- Mahasirimongkol S, Yanai H, Mushiroda T, Promphittayarat W, Wattanapokayakit S, Phromjai J, Yuliwulandari R, Wichukchinda N, Yowang A, Yamada N, Kantipong P, Takahashi A, et al. Genome-wide association studies of tuberculosis in Asians identify distinct at-risk locus for young tuberculosis. J Hum Genet. 2012;57:363–367. doi: 10.1038/jhg.2012.35. [DOI] [PubMed] [Google Scholar]
- Miller EN, Jamieson SE, Joberty C, Fakiola M, Hudson D, Peacock CS, Cordell HJ, Shaw MA, Ramos F, Silveira F, Blackwell JM. Genome-wide scans for leprosy and tuberculosis susceptibility genes in Brazilians. Genes Immun. 2004;5:63–67. doi: 10.1038/sj.gene.6364031. [DOI] [PubMed] [Google Scholar]
- NCBI SNP database, dbSNP137. 2012 http://www.ncbi.nlm.nih.gov/snp/
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Özbek N, Fieschi C, Yilmaz BT, de Beaucoudrey L, Demirham B, Feinberg J, Bikmaz YE, Casanova JL. Interleukin-12 receptor β 1 chain deficiency in a child with disseminated tuberculosis. Clin Infect Dis. 2005;40:e55–e58. doi: 10.1086/427879. [DOI] [PubMed] [Google Scholar]
- Ozen M, Ceyhan M, Sanal O, Bayraktar M, Mesci L. Recurrent Salmonella bacteremia in interleukin-12 receptor β1 deficiency. J Trop Pediatr. 2006;52:296–298. doi: 10.1093/tropej/fml001. [DOI] [PubMed] [Google Scholar]
- Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL- 12Rβ1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- Pedraza S, Lezana JL, Samarina A, Aldana R, Herrera MT, Boisson-Dupuis S, Bustamante J, Pages P, Casanova JL, Picard C. Clinical disease caused by Klebsiella in 2 unrelated patients with interleukin 12 receptor β1 deficiency. Pediatrics. 2010;126:e971–e976. doi: 10.1542/peds.2009-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza-Sanchez S, Herrera-Barrios MT, Aldana-Vergara R, Neumann-Ordonez M, Gonzalez-Hernandez Y, Sada-Diaz E, de Beaucoudrey L, Casanova JL, Torres-Rojas M. Bacille Calmette–Guerin infection and disease with fatal outcome associated with a point mutation in the interleukin-12/interleukin-23 receptor beta-1 chain in two Mexican families. Int J Infect Dis. 2010;14(Supplement 3):e256–e260. doi: 10.1016/j.ijid.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Png E, Alisjahbana B, Sahiratmadja E, Marzuki S, Nelwan R, Balabanova Y, Nikolayevskyy V, Drobniewski F, Nejentsev S, Adnan I, van de Vosse E, Hibberd ML, et al. A genome wide association study of pulmonary tuberculosis susceptibility in Indonesians. BMC Med Genet. 2012;13:5. doi: 10.1186/1471-2350-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potjewijd J, de Paus RA, van Wengen A, Damoiseaux J, Verbon A, van de Vosse E. Disseminated Mycobacterium genavense infection in a patient with a novel partial interleukin-12/23 receptor β1 deficiency. Clin Immunol. 2012;144:83–86. doi: 10.1016/j.clim.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Prando C, Samarina A, Bustamante J, Boisson-Dupuis S, Cobat A, Picard C, AlSum Z, Al-Jumaah S, Al-Hajjar S, Frayha H, Al-Mousa H, Ben-Mustapha I, et al. Inherited IL-12p40 Deficiency: Genetic, Immunologic, and Clinical Features of 49 Patients From 30 Kindreds. Medicine (Baltimore) 2013;92:109–122. doi: 10.1097/MD.0b013e31828a01f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Alejo N, Santos-Argumedo L, Estrada-Garcia I, Blancas-Galicia L, Espinosa-Rosales F. An Insertion in IL-12/23 Receptor Beta 1 Chain Causes Mendelian Susceptibility to Mycobacterial Disease in a Mexican Patient. J Clin Immunol. 2012;32(2):398. [Google Scholar]
- Remus N, El Baghdadi J, Fieschi C, Feinberg J, Quintin T, Chentoufi M, Schurr E, Benslimane A, Casanova JL, Abel L. Association of IL12RB1 polymorphisms with pulmonary tuberculosis in adults in Morocco. J Infect Dis. 2004;190:580–587. doi: 10.1086/422534. [DOI] [PubMed] [Google Scholar]
- Robinson RT, Khader SA, Martino CA, Fountain JJ, Teixeira-Coelho M, Pearl JE, Smiley ST, Winslow GM, Woodland DL, Walter MJ, Conejo-Garcia JR, Gubler U, et al. Mycobacterium tuberculosis infection induces il12rb1 splicing to generate a novel IL-12Rβ1 isoform that enhances DC migration. J Exp Med. 2010;207:591–605. doi: 10.1084/jem.20091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1999;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig SD, Yancoski J, Bernasconi A, Krasovec S, Marciano BE, Casimir L, Berberian G, Simboli N, Rousseau M, Calle G. Thirteen years of culture-positive M. bovis-BCG infection in an IL-12Rβ1 deficient patient: Treatment and outcome. J Infect. 2006;52:e69–e72. doi: 10.1016/j.jinf.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Sahiratmadja E, Baak-Pablo R, de Visser AW, Alisjahbana B, Adnan I, van Crevel R, van Dissel JT, Ottenhoff TH, van de Vosse E. Association of polymorphisms in IL-12/IFN-γ pathway genes with susceptibility to pulmonary tuberculosis in Indonesia. Tuberculosis. 2007;87:303–311. doi: 10.1016/j.tube.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Sakai T, Matsuoka M, Aoki M, Nosaka K, Mitsuya H. Missense mutation of the interleukin-12 receptor β1 chain-encoding gene is associated with impaired immunity against Mycobacterium avium complex infection. Blood. 2001;97:2688–2694. doi: 10.1182/blood.v97.9.2688. [DOI] [PubMed] [Google Scholar]
- Sanal O, Turkkani G, Gumruk F, Yel L, Secmeer G, Tezcan I, Kara A, Ersoy F. A case of interleukin-12 receptor β-1 deficiency with recurrent Leishmaniasis. Pediatr Infect Dis J. 2007;26:366–368. doi: 10.1097/01.inf.0000258696.64507.0f. [DOI] [PubMed] [Google Scholar]
- Schejbel L, Rasmussen EM, Kemp HB, Lundsted AC, Nielsen KR, Obel N, Marquart H, Andersen AB. Combined IL-12 receptor and IgA deficiency in an adult man intestinally infested by an unknown, non-cultivable mycobacterium. Scand J Immunol. 2011;74:548–553. doi: 10.1111/j.1365-3083.2011.02603.x. [DOI] [PubMed] [Google Scholar]
- Staretz-Haham O, Melamed R, Lifshitz M, Porat N, Fieschi C, Casanova JL, Levy J. Interleukin-12 receptor β1 deficiency presenting as recurrent Salmonella infection. Clin Infect Dis. 2003;37:137–140. doi: 10.1086/375229. [DOI] [PubMed] [Google Scholar]
- Tabarsi P, Marjani M, Mansouri N, Farnia P, Boisson-Dupuis S, Bustamante J, Abel L, Adimi P, Casanova JL, Mansouri D. Lethal Tuberculosis in a previously healthy adult with IL-12 receptor deficiency. J Clin Immunol. 2011;31:537–539. doi: 10.1007/s10875-011-9523-9. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Akahoshi M, Matsuda A, Ebe K, Inomata N, Obara K, Hirota T, Nakashima K, Shimizu M, Tamari M, Doi S, Miyatake A, et al. Association of IL12RB1 promoter polymorphisms with increased risk of atopic dermatitis and other allergic phenotypes. Hum Mol Genet. 2005;14:3149ddi347–3159. doi: 10.1093/hmg/ddi347. [DOI] [PubMed] [Google Scholar]
- Tang F, Liu W, Zhang F, Xin ZT, Wei MT, Zhang PH, Yang H, Ly H, Cao WC. IL-12RB1 genetic variants contribute to human susceptibility to Severe Acute Respiratory Syndrome infection among Chinese. PLoS ONE. 2008;3:e2183. doi: 10.1371/journal.pone.0002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanir G, Dogu F, Tuygun N, Ikinciogullari A, Aytekin C, Aydemir C, Yuksek M, Boduroglu E, de Beaucoudrey L, Fieschi C, Feinberg J, Casanova JL, et al. Complete deficiency of the IL-12 receptor β1-chain: three unrelated Turkish children with unusual clinical features. Eur J Pediatr. 2006;165:415–417. doi: 10.1007/s00431-005-0078-8. [DOI] [PubMed] [Google Scholar]
- Thye T, Owusu-Dabo E, Vannberg FO, van Crevel R, Curtis J, Sahiratmadja E, Balabanova Y, Ehmen C, Muntau B, Ruge G, Sievertsen J, Gyapong J, et al. Common variants at 11p13 are associated with susceptibility to tuberculosis. Nat Genet. 2012;44:257–259. doi: 10.1038/ng.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thye T, Vannberg FO, Wong SH, Owusu-Dabo E, Osei I, Gyapong J, Sirugo G, Sisay-Joof F, Enimil A, Chinbuah MA, Floyd S, Warndorff DK, et al. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat Genet. 2010;42:739–741. doi: 10.1038/ng.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh ML, Kawashima M, Hot A, Miossec P, Miossec P. Role of IL-17 in the Th1 systemic defects in rheumatoid arthritis through selective IL-12Rβ2 inhibition. Ann Rheum Dis. 2010;69:1562–1567. doi: 10.1136/ard.2009.111757. [DOI] [PubMed] [Google Scholar]
- van de Vosse E, de Paus RA, van Dissel JT, Ottenhoff TH. Molecular complementation of IL-12Rβ1 deficiency reveals functional differences between IL-12Rβ1 alleles including partial IL-12Rβ1 deficiency. Hum Mol Genet. 2005;14:3847–3855. doi: 10.1093/hmg/ddi409. [DOI] [PubMed] [Google Scholar]
- van de Vosse E, Hoeve MA, Ottenhoff TH. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect Dis. 2004;4:739–749. doi: 10.1016/S1473-3099(04)01203-4. [DOI] [PubMed] [Google Scholar]
- van de Vosse E, Lichtenauer-Kaligis EG, van Dissel JT, Ottenhoff TH. Genetic variations in the interleukin-12/interleukin-23 receptor (β1) chain, and implications for IL-12 and IL-23 receptor structure and function. Immunogenetics. 2003;54:817–829. doi: 10.1007/s00251-002-0534-9. [DOI] [PubMed] [Google Scholar]
- van de Vosse E, Ottenhoff TH. Human host genetic factors in mycobacterial and Salmonella infection: lessons from single gene disorders in IL-12 / IL-23 dependent signaling that affect innate and adaptive immunity. Microbes Infect. 2006;8:1167–1173. doi: 10.1016/j.micinf.2005.10.032. [DOI] [PubMed] [Google Scholar]
- van de Vosse E, van Dissel JT, Ottenhoff TH. Genetic deficiencies of innate immune signaling in human infectious disease. Lancet Infect Dis. 2009;9:688–698. doi: 10.1016/S1473-3099(09)70255-5. [DOI] [PubMed] [Google Scholar]
- van de Wetering D, de Paus RA, van Dissel JT, van de Vosse E. IL-23 modulates CD56+CD3- Natural Killer Cell and CD56+/CD3+ Natural Killer T Cell function differentially from IL-12. Int Immunol. 2009;21:145–153. doi: 10.1093/intimm/dxn132. [DOI] [PubMed] [Google Scholar]
- Verreck FA, de Boer T, Langenberg DML, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal Malefyt R, Ottenhoff THM. Human IL-23 producing type-1 macrophages promote but IL-10 producing type-2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DC, Schwartz B, Hsu AP, Miranda DJ, Valdez PA, Fink D, Lau KP, Long-Priel D, Kuhns DB, Uzel G, Pittaluga S, Hoover S, et al. Interleukin-12 receptor β1 deficiency predisposing to disseminated Coccidioidomycosis. Clin Infect Dis. 2011;52:e99–e102. doi: 10.1093/cid/ciq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman M, van Ophuizen E, den Dunnen JT, Taschner PE. Improving sequence variant descriptions in mutation databases and literature using the Mutalyzer sequence variation nomenclature checker. Hum Mutat. 2008;29:6–13. doi: 10.1002/humu.20654. [DOI] [PubMed] [Google Scholar]
- Wu CY, Gadina M, Wang K, O'Shea J, Seder RA. Cytokine regulation of IL-12 receptor β2 expression: differential effects on human T and NK cells. Eur J Immunol. 2008;30:1364–1374. doi: 10.1002/(SICI)1521-4141(200005)30:5<1364::AID-IMMU1364>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Wu CY, Warrier RR, Wang X, Presky DH, Gately MK. Regulation of interleukin-12 receptor β1 chain expression and interleukin-12 binding by human peripheral blood mononuclear cells. Eur J Immunol. 1997;27:147–154. doi: 10.1002/eji.1830270122. [DOI] [PubMed] [Google Scholar]
- Yancoski J, Rocco C, Bernasconi A, Oleastro M, Bezrodnik L, Vrátnica C, Haerynck F, Rosenzweig SD. A 475 years-old founder effect involving IL12RB1: A highly prevalent mutation conferring Mendelian Susceptibility to Mycobacterial Diseases in European descendants. Infect Genet Evol. 2009;9:574–580. doi: 10.1016/j.meegid.2009.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Prather D, vanden Eng J, Crawford S, Kariuki S, ter Kuile F, Terlouw D, Nahlen B, Lal AA, Slutsker L, Udhayakumar V, Shi YP. Polymorphisms in genes of interleukin 12 and its receptors and their association with protection against severe malarial anaemia in children in western Kenya. Malar J. 2010;9:87. doi: 10.1186/1475-2875-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.