Abstract
Graphene nanoparticles dispersions show immense potential as multifunctional agents for in vivo biomedical applications. Herein, we follow regulatory guidelines for pharmaceuticals that recommend safety pharmacology assessment at least 10 – 100 times higher than the projected therapeutic dose, and present comprehensive single dose response, expanded acute toxicology, toxicokinetics, and respiratory/cardiovascular safety pharmacology results for intravenously administered dextran-coated graphene oxide nanoplatelet (GNP-Dex) formulations to rats at doses between 1–500 mg/kg. Our results indicate that the maximum tolerable dose (MTD) of GNP-Dex is between 50 mg/kg ≤ MTD < 125 mg/kg, blood half-life < 30 minutes, and majority of nanoparticles excreted within 24 hours through feces. Histopathology changes were noted at ≥ 250 mg/kg in the heart, liver, lung, spleen, and kidney; we found no changes in the brain and no GNP-Dex related effects in the cardiovascular parameters or hematological factors (blood, lipid, and metabolic panels) at doses < 125 mg/kg. The results open avenues for pivotal preclinical single and repeat dose safety studies following good laboratory practices (GLP) as required by regulatory agencies for investigational new drug (IND) application.
Keywords: Graphene Nanoplatelets, Dextran, Toxicity, Dose Range, Biodistribution, Pharmacokinetics, Maximum Tolerated dose
1. Introduction
Carbon nanostructures such as fullerenes, metallofullerenes and carbon nanotubes have been widely investigated as multifunctional materials for applications in tissue engineering, molecular imaging, therapeutics, drug delivery, and biosensing [1–3]. Recently, graphene nanoparticles, (also known as graphene nanoplatelets (GNPs) or simply graphene oxide (GO), (herein referred as GNPs)) show promise in vitro and in vivo for drug/gene delivery and biological sensing/imaging applications due to their nanoscopic size, large specific surface area, and physicochemical properties [4–10]. For instance, GNPs could be loaded with aromatic drugs via Van der Waals (pi-pi stacking) interactions [5], or non-covalently complexed with cationic polymers such as polyethyleneimine via weak electrostatic interactions to facilitate plasmid DNA (pDNA) and small interfering RNA (siRNA) delivery [7]. GNPs could also be intercalated or covalently functionalized with important elements (e.g. manganese, iodine) in medicine to develop highly efficacious contrast agents for magnetic resonance imaging (MRI) [8, 9], computed tomography (CT) [10], and their intrinsic electromagnetic properties could be harnessed towards the development of probes for fluorescence [4], photoacoustic and thermoacoustic imaging [11]. There is now a wide body of research documenting the toxicology and pharmacology of fullerenes, metallofullerenes and carbon nanotubes (CNTs) [1–3, 12]. These studies on the various carbon nanostructures evaluate their safety for the above healthcare applications, or environmental/occupational health issues [12–14]. Reports to date show that the structure/shape (e.g. spherical, tubular), chemical composition (e.g. pristine, functionalized), synthesis method (e.g. chemical vapor deposition, oxidative exfoliation), and route of administration (e.g. intravenous, nasal) are key factors that influence toxicity and tissue response for carbon nanostructures [2, 3, 12, 15, 16]. For instance, multiwalled CNTs (MWCNTs) greater than 20 μm in length introduced directly into mesothelial lining of the body cavity of mice induced asbestos-like pathogenicity [13]. However, exposure of MWCNTs of lengths less than 20 μm did not induce a similar effect [13]. Multiple reports also recommend that pristine fullerenes or CNTs should be avoided for in vivo applications and have emphasized the importance of chemical functionalization of these nanoparticles to impart water dispersibility, reduce aggregation and improve stability in physiologic fluid as well as facilitate adequate excretion rates to prevent tissue accumulation [3, 12, 17, 18]. Compared to CNTs and fullerenes, fewer studies have assessed the in vitro [19–21], and in vivo [22, 23] biological effects of graphene nanoparticles.
Intravenous (IV) administration is a widely employed and preferred mode of systemically introducing pharmaceutical formulations for imaging, drug delivery or therapy. IV injections were employed in a subset of toxicological investigations of carbon nanostructures for such biomedical applications [1–3, 23–25]. In general, the maximum dosages of a test formulation in toxicity and biodistribution studies depend on concentration of the stock solution of the test formulation, and the maximum solution volume (typically 2 ml/kg for bolus and 4 ml/kg for slow IV injections) that can be injected without causing adverse side effects to the animals [26]. Hydrophobic carbon nanostructures have typically been covalently or non-covalently functionalized with moieties (functional groups, macromolecules) to improve water dispersibility and thus, allow higher doses. For small animal (rodents) toxicology studies that employed IV administration, the reported stock solution concentrations of water-dispersible carbon nanostructures are ≤ 10 mg/ml and maximum permissible doses (MPD) are in the units to low tens mg/kg range [2, 3, 23, 27, 28]. Additionally, for a given water-dispersed carbon nanostructure formulation, most investigations have focused on histopathology, and biodistribution, presenting little information on maximum tolerable doses (MTD), or assessment of other important issues such as respiratory and cardiovascular pharmacology safety. Consequently, the therapeutic indices of these formulations remain unknown. Furthermore, we found no published studies that explicitly followed the preclinical safety pharmacology guidelines of regulatory agencies such as the Food and Drug Administration (FDA), International Conference on Harmonization (ICH) and the European Medicines Agency (EMA) which recommend assessing toxicity at least 10 to 100 times higher than the projected therapeutic dose [29–32]. Such studies are necessary to receive regulatory approval for first-inhuman trials for any carbon nanostructure-based IV formulations for imaging, or therapeutic application.
Recently, we reported on synthesis, physiochemical characterization and in vitro studies (see Table S1&S2 in the supplementary information section) of GNPs non-covalently functionalized with the FDA-approved natural polymer dextran (hereafter called GNP-Dex) [9]. GNP-Dex nanoparticles are disc-shaped with an average diameter of ~100 nm (Figure S1), average thickness of ~3 nm and GNP: dextran weight ratio of 3:2 [9]. These nanoparticles are hydrophilic, and form stable colloidal dispersions in deionized water and biological fluids (buffers and blood) up to 100 mg/ml concentrations (Figure S2). To the best of our knowledge, this is the highest level of dispersion achieved for any water-dispersible graphene nanoparticle. To date, in vitro and in vivo studies of other formulations of water-dispersible GNPs, synthesized via covalent (including covalent functionalization with dextran), and non-covalent functionalization strategies have reported maximum concentrations of up to 1 mg/ml [5, 15, 23, 33, 34]. Lastly, GNP-Dex dispersions are iso-osmolar (upon addition of mannitol) and iso-viscous to blood [9].
According to FDA and ICH guidance documents, prerequisites for first-in-human trials of the GNP-Dex for IV biomedical applications (e.g. drug delivery or imaging contrast agent) include in vivo pre-clinical safety pharmacology studies in a rodent and non-rodent model. Thus, as a part of the preclinical in vivo safety pharmacology evaluation, here, we report dose response, expanded acute toxicology, toxicokinetics, and respiratory/cardiovascular safety pharmacology assessment of GNP-Dex in rats.
2. Materials and Methods
2.1. Animal care, dose ranges for expanded acute toxicity studies
All the experiments were performed according to the guidelines of Institutional Animal Care and Use Committee at Stony Brook University, NY. Acute toxicity (1-day, n=6) and expanded acute toxicity (30-days, n=8) studies were performed at the doses of 1, 25, 50, 125, 250, 500 mg/kg on Wistar male rats weighing 200–250 g. Mannitol (55 mg/ml), added to the GNP-Dex formulation to control the osmolality, was used as a control. The animals were anesthetized using isoflurane (1–2.5% mixture of O2/air, via inhalation). Single doses of GNP-Dex were administered intravenously via the tail vein and the animals were monitored for any adverse effects for 1-day or 30-days. After injection, animals were transferred into metabolic cages to collect urine and feces samples.
2.2. Transthoracic echocardiography and blood pressure measurement
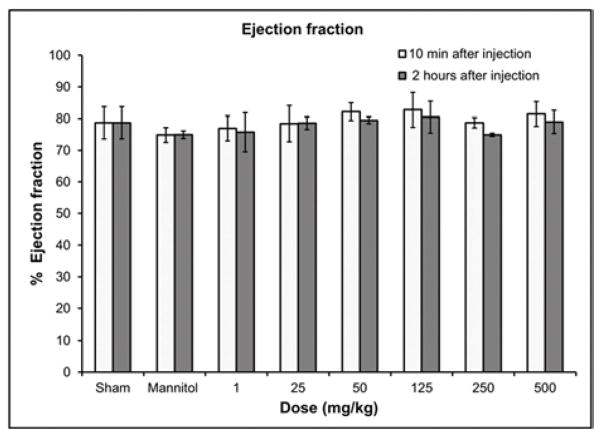
Transthoracic echocardiography with Doppler was performed with a high-resolution imaging System (Visual Sonics, Vevo 770, Toronto, Canada) using a 30-MHz linear array transducer (RMV 707B). GNP-Dex doses of 1, 25, 50, 125, 250 and 500 mg/kg and mannitol were injected into Wistar rats (n=4 at each dose for 30-day expanded acute toxicity group) under anesthesia (1–2.5% mixture of O2/air with isoflurane, via inhalation). Animals were placed on warming pads to maintain normothermia and their limbs were secured to electrocardiography sensors. Two-dimensional images were captured and recorded before injection as well as at 10 min and 2 hours post injection in parasternal long- and short-axis projections with 2D guided M-mode recordings at the mid-ventricular level in both views. Left ventricular (LV) dimensions (mass, volume, wall thickness) were measured in the M-mode view and % fractional shortening was calculated. Additionally heart rate, respiration rate, blood velocity, ejection fraction were recorded for both systolic and diastolic phase. An aortic arch or parasternal view (that is as parallel as possible with the sound beam) was used to obtain the pulsed-wave Doppler image to measure the blood flow velocity through the aorta. Visual Sonics software was used to calculate and analyze the data. Blood pressure was measured using a non-invasive occlusion type tail cuff system (Coda 6, Kent Scientific System, PA) 10 min and 2 hour after administration of GNP-Dex.
2.3. Biodistribution and elimination
One day and 30-day animals for the biodistribution studies received IV injections at 1, 25, 50, 125, 250, 500 mg/kg doses. Urine and feces samples were collected at 8 and 24 hours for the 1-day group, and daily up to a week for the 30-day group. Blood samples (~100 μl from tail vein) were collected at 0.5, 2, 4, 8 and 24 hours after injection for the 1-day group, and every 48 hours after injection up to one week post-injection for the 30-day group. At the end of the respective durations, animals were euthanized using 100% CO2 and brain, heart, liver, lungs, spleen and kidneys were harvested. Mn2+ ions generated during the synthesis of GNPs from the oxidizing agent KMnO4 get intercalated between graphene sheets. Hence, manganese served as a stable endogenous elemental tag to quantify GNP-Dex concentrations in blood, urine, feces and bio-distribution studies. The Mn2+ ions concentration in GNP-Dex, measured by inductively coupled plasma mass spectrometry (ICP-MS) was 0.064±0.010% [9]. The Mn2+ concentrations in the blood, urine, feces and various tissues were determined using ICP-MS as described below. The % injected dose values in the blood, urine, feces or various tissues were calculated using the formula
2.4. ICP-MS
Blood, urine, feces and organs from different animals at the same dose from the same group (day 1 or day 30) were pooled together, and chemically digested using trace metal grade 70% nitric acid (HNO3) (catalog # 02650, Sigma Aldrich, St Louis, MO), followed by trace metal grade 30% hydrogen peroxide (H2O2) (catalog # 95321, Sigma Aldrich, St Louis, MO) until only inorganic content was left. The digested samples were resuspended in trace metal grade 2% HNO3 and filtered through a 0.2 μ pore size filter. Samples were analyzed for Mn2+ concentration by setting the mass window in the range of 54.8–55.4 atomic mass units using ICP-MS (Thermo, Finnigan ELEMENT 2) with 100 μl sample volume.
2.5. Histopathology
Tissue samples were initially fixed in 4% paraformaldehyde for 48 hours and were dissected into 3 mm segments. All organs were dissected symmetrically in the same manner to help ensure consistency. The tissues were then dehydrated in graded ethanol and paraffin-embedded. 5 μm sections were cut using a microtome and were stained with Hematoxylin and Eosin (H&E) for histologic evaluation. Digital photomicroscopy was performed using a bright field microscope at 100X and 400X magnification. Histologic assessment of the tissue sections were performed by an experienced diagnostic and research anatomic pathologist (KRS).
2.6. Blood analysis
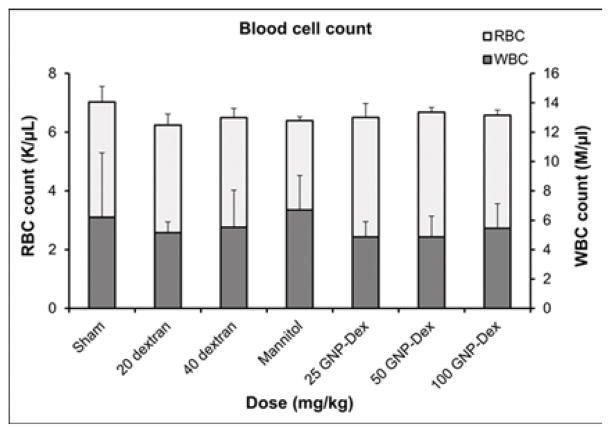
The animals were injected with GNP-Dex at doses of 25, 50 and 100 mg/kg (n=3 at each dose); dextran at doses 20 and 40 mg/kg (n=3 at each dose) and 55 mg/ml mannitol solution in water were used as controls. Animals were euthanized 1-day after injection and ~8 ml blood was collected from each animal by cardiac puncture for blood analysis. Serum chemistry test and complete blood panel analyses were performed in a Clinical Laboratory Improvement Amendments (CLIA) certified facility at Stony Brook University Hospital.
2.7. Statistical analysis
Data are expressed as mean± SE. All data were analyzed for statistical significance by one-way analysis of variance (ANOVA) followed by a Tukey post-hoc test using statistical analysis software GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). The criterion for statistical significance was p≤0.05.
3. Results
3.1. Dose responses
The acute toxicity study for GNP-Dex was performed in Wistar male rats in two studies. We injected GNP-Dex via the tail vein at doses between 1–500 mg/kg. One cohort (n=6/dose) was investigated for 1-day, and another one (n=8/dose) for 30-days. As shown in Table 1, for the 1-day group, mortality occurred at ≥ 125 mg/kg rapid bolus doses, within ~2 min after GNP-Dex administration. In the 30-day groups, mortality occurred at ≥ 250 mg/kg rapid bolus doses, again within ~2 min after GNP-Dex administration. Necropsies suggested that the cause of the death was pulmonary congestion (See necropsy report in supplementary information section). For both the day 1 and day 30 groups, the dosing rate of the GNP-Dex affected mortality observed at the higher doses (≥ 125 mg/kg). For instance, a rapid bolus (500 μl/15 sec) rate showed higher mortality than a slower (500 μl/5 min) injection rate. There were no changes in the body weight, breathing, hunching or unusual interactions with cage mates following injection of GNP-Dex at any dosages in either cohort.
Table 1.
Mortality observed in animals at injection rates 500 μl/15 sec and 500 μl/5 min at varying doses of GNP-Dex
| Mortality in animals (dead/total) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | 500 μl/15 sec (dead/total) | 500 μl/5 min (dead/total) | ||||||
| 1- day | 30- days | Total dead (day 1 and day 30 groups) | % of death | 1-day | 30- days | Total dead (day 1 and day 30 groups) | % of death | |
| Sham | - | 0/4 | 0/4 | 0 | 0/6 | - | 0/6 | 0 |
| 1 | - | 0/8 | 0/8 | 0 | 0/6 | - | 0/6 | 0 |
| 25 | - | 0/4 | 0/4 | 0 | 0/6 | 0/4 | 0/10 | 0 |
| 50 | - | - | - | - | 0/6 | 0/8 | 0/14 | 0 |
| 125 | 3/6 | 0/4 | 3/10 | 30 | - | 0/4 | 0/4 | 0 |
| 250 | 1/1 | 1/4 | 2/5 | 40 | 0/5 | 3/4 | 3/9 | 33 |
| 500 | 4/6 | 0/4 | 4/10 | 40 | - | 1/4 | 1/4 | 25 |
| Mannitol | 1/6 | - | 1/6 | 16.67 | - | 0/4 | 0/4 | 0 |
3.2. Histopathology
Tissue histopathology of major organs (brain, heart, liver, lung, kidney, spleen) was examined on all experimental and control animals. Histologic sections of experimental animals were compared with controls to identify GNP-Dex related effects. Figure 1 shows representative histologic section of a tissue from a rat injected with 250 mg/kg GNP-Dex and monitored for 1-day. The histology for several other doses is shown in the supplementary information section (Figure S3–S5). Table 2 summarizes the general observations in the histologic sections of major organs of 1-day and 30-day rats injected with ≥ 250 mg/kg GNP-Dex.
Figure 1.
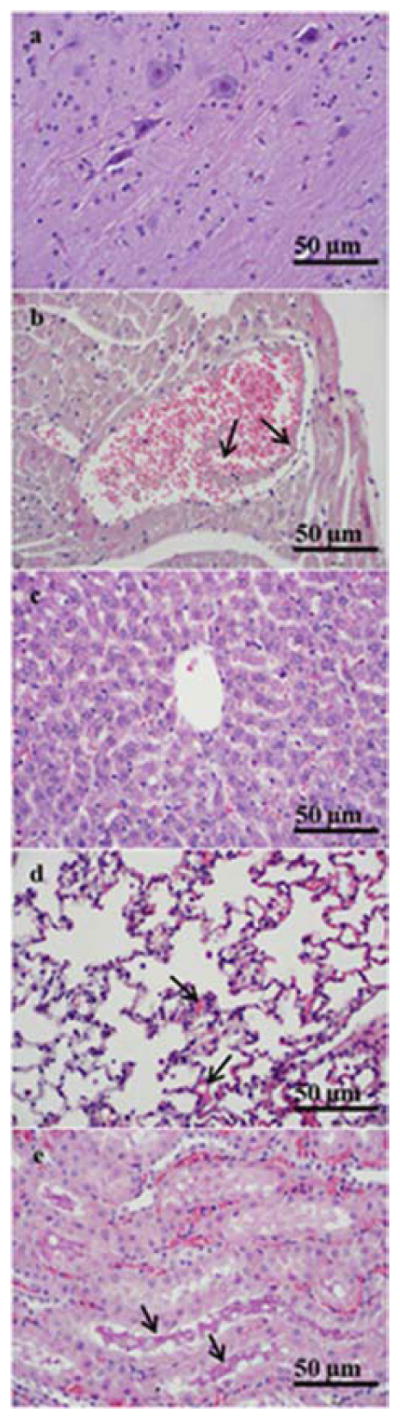
Representative high (400X) magnification photomicrographs illustrating histopathology of major organs from a day 1 animal at 250 mg/kg GNP-Dex dose. a) Cerebral cortex without diagnostic abnormalities. b) Myocardium with vascular congestion. A dilated vein containing red blood cells and amorphous debris (arrows) suggestive of the presence of GNP-Dex. c) Liver without diagnostic abnormalities. d) Pulmonary parenchyma with mild focal congestion in alveolar capillaries (arrows). e) Renal cortex with vascular congestion and proteinaceous casts in renal tubules (arrows).
Table 2.
Summary of tissue histopathology
| Organs | GNP-Dex related effects at ≥ 250 mg/kg |
| Brain | No GNP-Dex related effects |
| Heart | Focal congestion with patchy hyper-eosinophilia of cardiomyocytes |
| Liver | Centrilobular congestion |
| Lung | Mild focal congestion, focal granular brown pigment, consistent with aggregates of graphene nanoparticles, within capillaries and alveolar spaces |
| Kidney | Congestion, proteinaceous casts, rare foci of granular brown pigment within vascular spaces |
3.3. Cardiovascular safety
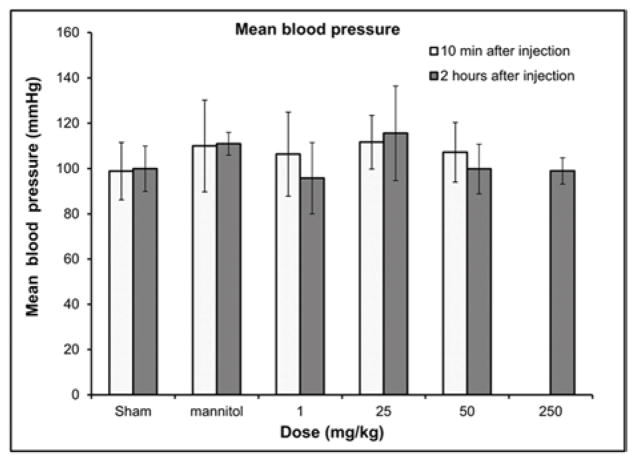
For respiratory and cardiovascular safety pharmacology studies, we monitored the blood pressure, respiration rate, heart rate and other important echocardiography parameters –blood velocity and % ejection fraction of the experimental and control rats (Figures 2 a–e). Other related parameters are included in the supplementary information (i.e., fractional shortening, systolic and diastolic left ventricular volume, intravascular septum thickness, left ventricular internal septum thickness and left ventricular posterior wall thickness, Figure S6 a–j). There were no statistically significant differences in blood pressure measurements (Figure 2a) (n=6, p<0.05) between the experimental (up to GNP-Dex doses of 250 mg/kg) and sham (no injection) group. For the rodents receiving 250 mg/kg and 500 mg/kg GNP-Dex injections, no blood pressure readings could be obtained up to 2 hours after the injection because the tails of all animals in these two high doses groups were taut, which impeded the tail cuff system’s ability to give reliable readings. At these high dosages, the animals also exhibited an impaired ability to walk (limping lower limbs) for the first few minutes after injection, a condition similar to intermittent claudication, where the legs are deprived of an oxygenated rich blood supply [35].
Figure 2.
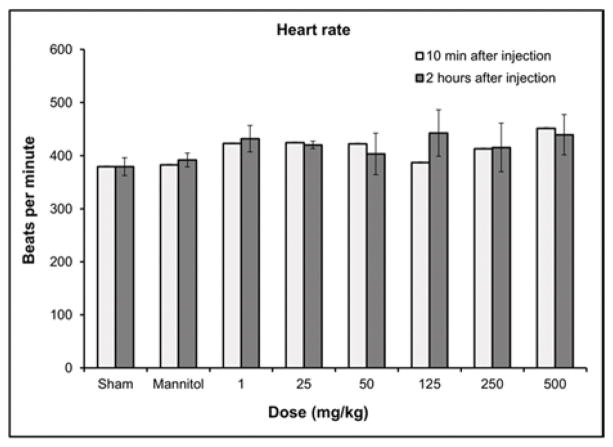
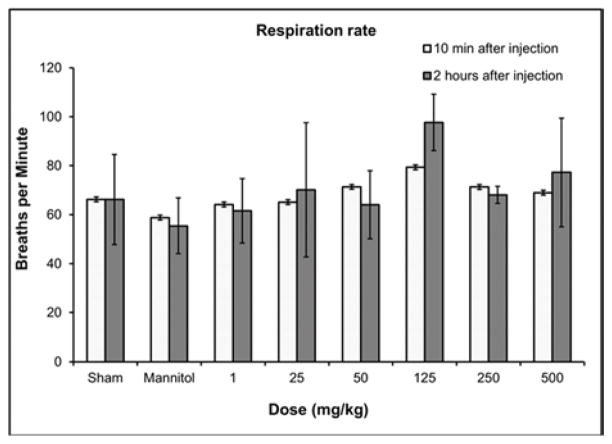
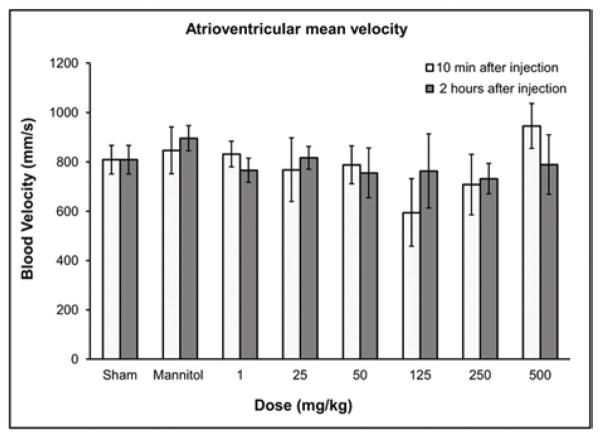
Hematological results from blood pressure and echocardiography measurements 10 min and 2 hours post injection of GNP-Dex (doses: 1–500 mg/kg). a) Blood pressure. b) Heart rate. c) Respiration rate. d) Atrioventricular mean blood velocity. e) % Ejection fraction.
In all surviving rats, there were no statistically significant differences in heart rate (Figure 2b), respiration rate (Figure 2c), atrioventricular mean blood velocity (Figure 2d) and % ejection fraction (Figure 2e) at IV doses up to 500 mg/kg following administration of GNP-Dex compared to controls. Additionally, as shown in Figure S6 there were no significant differences between experimental and control animals from 10 min post-dose through 2 hours post-dose at IV GNP-Dex doses up to 500 mg/kg for the other electrocardiographic parameters, fractional shortening, systolic or diastolic left ventricular volume, intravascular septum thickness, left ventricular internal septum thickness and left ventricular posterior wall thickness.
3.4. Blood analysis
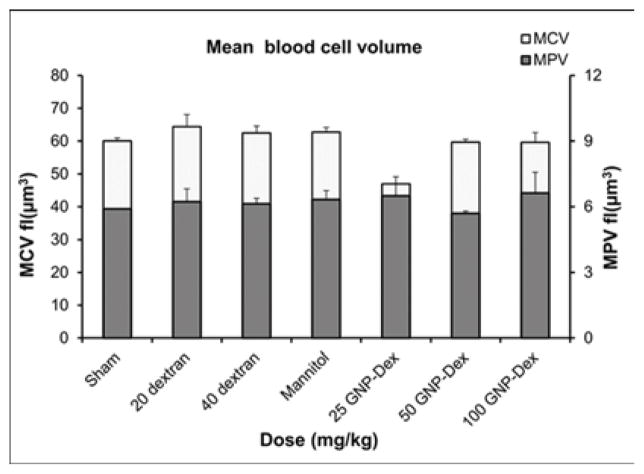
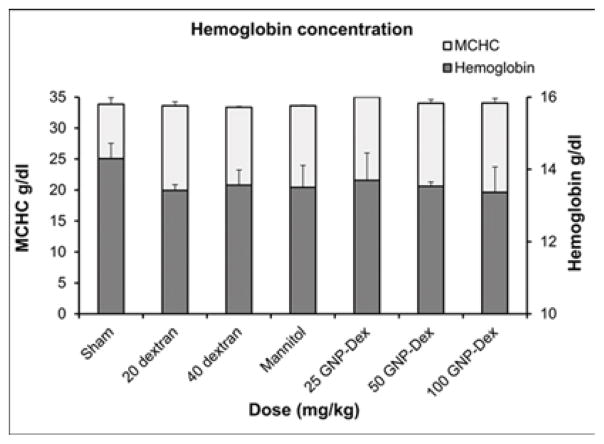
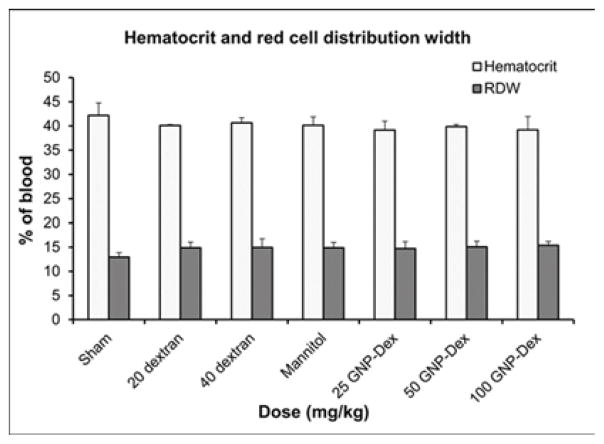
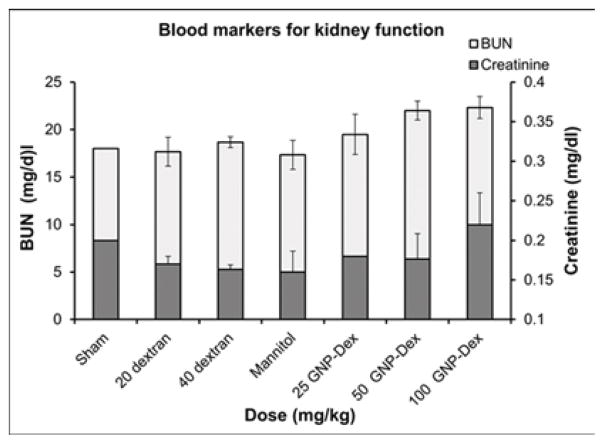
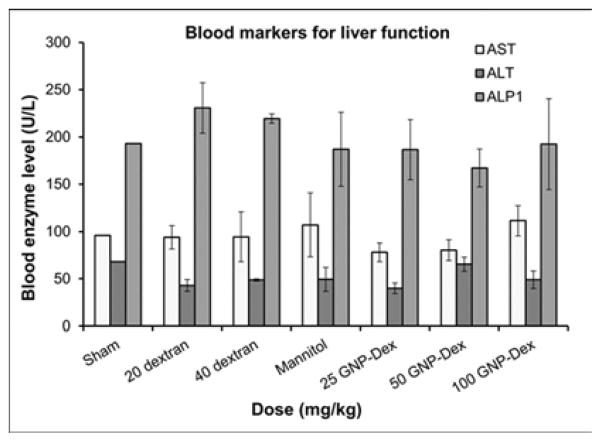
Hematological factors (blood cell count, lipid panel and metabolic panel) were analyzed at 25, 50 and 100 mg/kg doses. The maximum dose selected for this study was <125 mg/kg in order to assess if the GNP-Dex, at doses that show no test article related effects in terms of mortality, histopathological findings, or cardiovascular safety pharmacology studies would adversely affect the immune system or induce an inflammatory response. As the ratio of GNP:Dextran in GNP-Dex is 3:2 by weight, 20 mg/kg and 40 mg/kg doses of dextran that represent the equivalent amount of dextran in 50 and 100 mg/kg of GNP-Dex were selected as controls. Figure 3 a–i displays various hematological parameters for the experimental and control (rats injected with dextran or mannitol (the diuretic solution used to adjust the osmolality of GNP-Dex formulations)) animal groups, 1-day after administration of GNP-Dex. The results were also compared to the normal range of blood parameters of Wistar male rats (information provided by the vendor, Charles River Laboratories, Wilmington, MA, USA) [36]. Figure 3 a–d shows various parameters from the complete blood count analysis. Red blood cells (RBC), white blood cells (WBC) and blood cell volumes (i.e. mean corpuscular volume (MCV), mean platelet volume (MPV), mean corpuscular hemoglobin concentration (MCHC), hemoglobin, hematocrit and red cell distribution width (RDW) level) were within normal limits. Indicators of kidney function such as blood urea nitrogen (BUN) and creatinine (CRE) were also normal (Figure 3 e). Similarly, as shown in figure 3f and g, the established serum biochemistry assays for hepatic indicators (alkaline phosphatase (ALP), total protein (TP), albumin (ALB), alanine transaminase (ALT) and aspartate transaminase (AST)) measured 1-days after GNP-Dex injection showed no sign(s) of liver injury. Blood triglycerides (TG), cholesterol (CHO) and glucose (GLU) levels shown in supplementary figure S7a, blood ion concentrations of Na, K, Cl and CO2 as shown in supplementary figure S7b and blood protein (total protein (TP) and albumin) levels as shown in figure S7C were also in the healthy ranges.
Figure 3.
Blood Chemistry results following injection with GNP-Dex, dextran or mannitol 1 day after injection (n=3). a) Red blood cell (RBC) and White blood cell (WBC) count. b) Mean corpuscular volume (MCV) and mean platelet volume (MPV). c) Mean corpuscular hemoglobin concentration (MCHC) and total hemoglobin concentration. d) Hematocrit and red cells distribution width (RDW). e) Markers for kidney function - blood urea nitrogen (BUN) level and creatinine level. f) Markers for liver function - alkaline phosphatase (ALP), alanine transaminase (ALT) and aspartate transaminase (AST).
3.5. Biodistribution and elimination
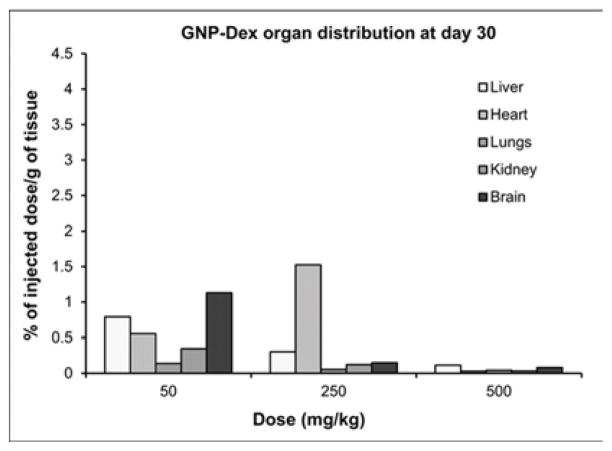
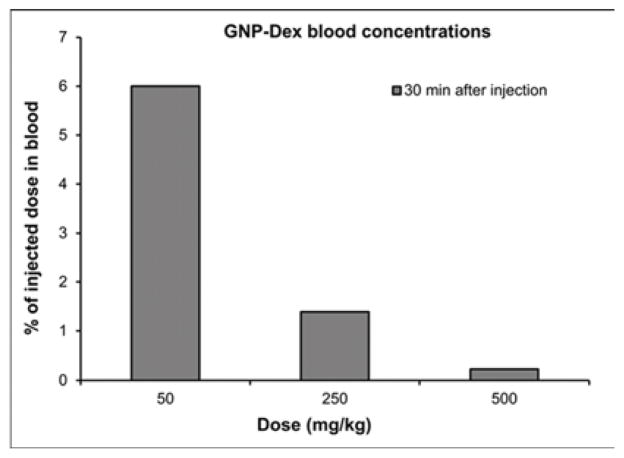
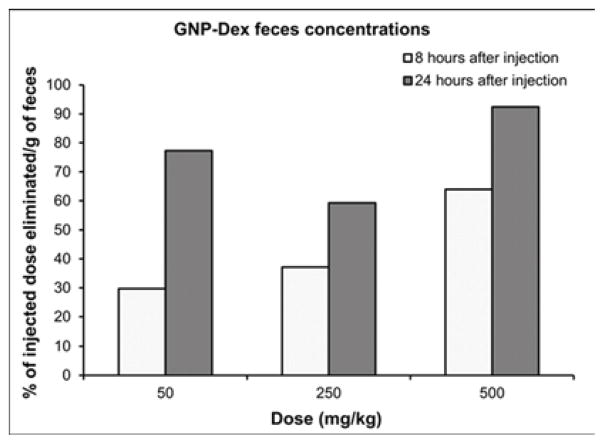
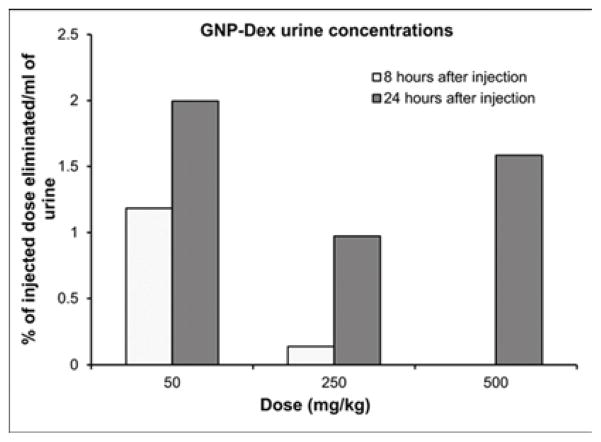
Tissue biodistribution, blood half-life, and elimination at representative low (25 or 50 mg/kg), medium (125 or 250 mg/kg) and high (500 mg/kg) doses of GNP-Dex are presented in Figure 4. Mn2+ concentrations in blood, and tissues, urine and feces samples of a number of animals were close to the limits of detection (LOD) of ICP-MS (1 PPB). Thus, data in Figure 4 are presented as pooled averages as proposed in previous studies [37] (see experimental section iv for details); variability and statistically significant differences between the groups could not be determined. Nevertheless, the results still allowed analysis of the trends. Figure 4 a and b show biodistribution of the nanoparticles in major vital organs at day 1 and day 30 respectively. The percent of GNP-Dex remaining in organs at day 1 was ~0.5–4%, and reduced to close to or the below limits of detection by day 30. In general, a higher percentage of GNP-Dex was detected in various organ at the low (25 mg/kg) dose, compared to the medium (125 mg/kg) and high (250 mg/kg) dose. Day 1 animals showed more GNP-Dex in heart and liver than in the kidney, lungs and brain. Day 30 animals showed more GNP-Dex in heart compared to other organs. The blood concentrations (Figure 4 c) of GNP-Dex at dosages of 50, 250 and 500 at 30 min were 6%, 1.5%, and less than 0.5% respectively. Figure 4 d and e show the elimination profile of GNP-Dex. No urine sample could be obtained at the 8 hour time point for day 1 group in the rats injected with 500 mg/kg. The average amount of GNP-Dex getting excreted through feces for the various doses was calculated to be ~60–90% over the period of 1-day post injection. A small fraction of GNP-Dex (0.5 to 2% of injected dose) was excreted by urine. No GNP-Dex could be detected in urine and feces samples analyzed at day 2 and beyond, in the 30-day group.
Figure 4.
Biodistribution and elimination of GNP-Dex in major organs. a) Biodistribution in organs at 1 day. b) Biodistribution in organs at 30 days. c) Blood retention of GNP-Dex. d) Elimination of GNP-Dex via feces. e) Elimination of GNP-Dex via urine
4. Discussion
Six escalating doses (low - 1, 25, 50 mg/kg, medium - 125, 250 mg/kg, and high - 500 mg/kg) were used. We assumed that, similar to other clinically approved pharmaceutical formulations for imaging or therapy (see supplementary information Table S3 for rationale); GNP-Dex could be intravenously administered at units to tens of mg/kg potential therapeutic dosages. Thus, the upper limit of GNP-Dex dose range in this study was 500 mg/kg, one to two orders of magnitude greater than the anticipated therapeutic dose. This dose is also the maximum permissible dose (MPD) that can be achieved using the highest GNP-Dex stock concentration of 100 mg/ml [9].
Necropsies of animals that died (Table 1) within ~2 min after GNP-Dex rapid bolus administration at ≥ 125 mg/kg (1 day group) or at ≥ 250 mg/kg (30 day group) suggested that the cause of the death was pulmonary congestion (See supplementary information section titled necropsy). The dosing rates also affected the mortality and the results suggest that slower dose rates are more preferable at these high dosages. For the rodents receiving 250 mg/kg and 500 mg/kg GNP-Dex injections, the absence of blood pressure readings up to 2 hours injections could be due to the injection of large numbers of GNP-Dex nanoparticles in a small dispersion volume (~500 μl) into the small diameter of the tail vein (~3.5 mm)[38]. The small size of the tail vein combined with the injection of large number of nanoparticles in a short time period could create temporary constriction at the site of injection, due to accumulation of the nanoparticles; thus, restricting the blood supply. Subsequently, as the GNP-Dex is absorbed into the circulation, the vasoconstriction is cleared, allowing blood pressure measurement. Based on these results and other literature reports [39–42], we hypothesize the following as the cause of observed mortality at the higher doses (> 125 mg/kg). As GNP-Dex gets into pulmonary micro- circulation during the first pass, a large number of nanoparticles in the very small diameter of the micro vessels (<100 μm) [43] could occlude blood vessel, hamper blood flow in the systemic circulation, and ultimately induce cardiac arrest. Alternatively, the local interaction of GNP-Dex with endothelium and smooth muscle cells could significantly increase the hydraulic conductivity of the blood vessels, lead to fluid accumulation in alveoli and pulmonary congestion, and eventually death of animals. Additional studies that thoroughly examine the biomarkers for lung inflammation and pulmonary edema following IV administration of GNP-Dex are necessary and currently underway to test these hypotheses.
Surviving animals showed no clinical signs of obvious toxicity (Figure 1 and Table 2). Essentially, histopathology changes were noted at ≥ 250 mg/kg in the heart, liver, lung, and kidney; congestion and the presence of brown granular debris/pigment were the most consistent findings across tissues. There were no apparent GNP-Dex-related effects on the brain. GNP-Dex dosages <125 mg/kg neither showed any test article related effects in terms of mortality, histopathological findings, respiratory, or cardiovascular safety parameters, nor adversely affect the immune system or induce an inflammatory response.
Multiple reports now demonstrate GNPs synthesized using the Hummer’s method or variation thereof leads to robust confinement (intercalation) of trace amounts (ppm levels) Mn2+ ions between the graphene sheets [8, 44, 45]. We had previously validated in vitro that the intercalated Mn2+ ions are thermally stable at physiological temperatures (37°C) in blood or biological buffers up to 30-days [9]. Thus, the intercalated Mn2+ ions do not separate from the GNP-Dex during the timespan of this study and served as stable endogenous elemental tags to perform ICP-MS elemental analysis and quantify GNP-Dex concentrations in blood, urine, feces and bio-distribution studies. ICP-MS elemental analysis is widely-used and an accepted technique for in vivo biodistribution studies as an alternative to relatively more expensive and hazardous radiolabelling methods [46, 47]. Additionally, even though radiolabeling is a widely used method for biodistribution and pharmacokinetics (PK) studies, the type and method of radiolabeling used can affect the PK profile [48]. As molecular weight of the test articles affects PK [49], introducing a radiolabeling agent in the test article can change its molecular weight and may alter PK profile. Due to these issues of the alternate method, we decided to exploit the presence of the endogenous Mn2+ tag in the test article for the biodistribution and PK studies, and employed the ICP-MS method. The % weight of manganese in the GNP-Dex samples was the average of 6 different samples of GNP-Dex was determined to be 0.064 %. The GNP-Dex showed a dose-dependent accumulation (~0.5–4%) in the major organs at day 1, with maximum organ accumulation at 50 mg/kg and the least accumulation with 500 mg/kg (Figure 4 a). Further, majority of the GNP-Dex accumulation were noted in liver, followed by heart, lungs, kidney and brain. However, they were present close to or below LOD levels by day 30, indicating removal from these organs (Figure 4 a and b). Based on these results and previous studies with GNPs, we hypothesize that over the time, the remaining small amount of GNP-Dex in the animal body is slowly engulfed by macrophages in the liver, uptaken by reticuloendothelial system (RES) and secreted into bile tract system. Additional experiments to validate this hypothesis are necessary. As the blood concentration (Figure 4 c) values of GNP- Dex at the various dosages were between 0.5 – 6 %; significantly less than 50%, we inferred the blood half-life of GNP-Dex to be within 30 min. However, we were unable to determine the precise blood half-life since the first time point of blood sampling was 30 minutes after injection. Further studies are currently underway to obtain the complete PK profile and determine the exact blood half-life value. The elimination results (Figure 4 d and e) indicate that GNP-Dex nanoparticles are eliminated through feces within 48 hours. The larger amounts of GNP-Dex in the feces than in urine suggest that these nanoparticles are excreted more through the bile than through the kidney.
Taken together, the results of this study indicate that the maximum tolerable dose (MTD) of GNP-Dex is between 50 mg/kg ≤ MTD < 125 mg/kg. The classic measure of toxicity, lethal dose (LD50- the dose at which mortality occurs in 50% of the study population) was not achieved even at the highest dose tested (500 mg/kg). However, it should be noted even though LD50 considers only mortality as an end point; valuable in forensic science, accidental exposure and environment safety investigations [50, 51]. For biomedical/healthcare applications, LD50 results are marginally informative and toxicologically inadequate as they do not take into account the factors affecting mortality, time frame of death from injection of test article, site and mode of action morbidity, and liver or kidney damage. Hence, reports related to toxicity of test article for biomedical applications have focused on assessment of factors affecting morbidity [50, 51]. Additionally, although there were no mortalities at doses ≤ 50 mg/kg even with bolus IV injections, slower injection is preferable. Our previous in vivo small animal (hamster cheek pouch model) vasoactivity studies indicate that at potentially high first pass concentrations (50 mg/ml) typically associated with bolus injections show transient arteriolar dilation without any endothelial dysfunction [52].
Other small animal (mouse) studies, have reported the toxicology of as-prepared GNP suspensions [27], and GNPs covalently functionalized with PEG [28] or dextran [23], intravenously injected at relatively low concentrations. As-prepared GNP suspensions at single IV doses between 0.1–0.25 mg/kg showed no adverse effects in mice; while repeated dosing in mice (up to 0.4 mg/animal (mouse) for 30-days) resulted in chronic toxicity with mortality in 4 out of 9 mice [27]. In another study, the blood half-life of as-prepared graphene oxide IV injected into mice at 10 mg/kg dosages was 5.3 hours. That formulation excreted through urine and showed pathological changes including pulmonary edema and granuloma formation over 14 days [24]. Mouse studies with GNPs water-dispersed via covalent functionalization with PEG or dextran, at one or two low doses (relative to the ones used in this study), neither showed any mortality nor significant adverse effects to the animals [23, 28]. The pharmacokinetics of PEG functionalized GNPs followed a two-compartment model, and had first and second phase half-lives 0.39 and 6.97 hours, respectively. Those nanoparticles excreted through both urine and feces over 7 days. Moreover, it did not show toxicity in blood chemistry, hematology and histologic studies at 20 mg/kg single dose in mice observed for 3 months [28]. Similarly, graphene covalently functionalized with dextran, also followed a two-compartment PK profile with first and second phase blood half-lives of 0.19 and 1.81 hours. The formulation excreted by both renal and fecal pathways, and did not elicit noticeable toxicity at 20 mg/kg single dose in mice monitored for 7 days [23].
The solubility and stability of the GNP-Dex formulation up to 100 mg/ml allowed us to perform acute toxicity studies at significantly higher dosages (25X greater) than with any other reported graphene IV toxicity studies (maximum 20 mg/kg) [28]. The results suggest that the safety profile of GNP-Dex is better (50 mg/kg ≤ MTD < 125 mg/kg), and the blood half-life is shorter (< 30 min) than the previous GNP formulations. The major route of elimination for GNP-Dex is fecal (90% of injected dose), while the other GNP formulations eliminate through both urine and feces. Similar to other GNP formulations [23, 24, 28], GNP-Dex shows more accumulation in liver, suggesting possible uptake by RES and eventual clearance by the bile system.
Our results open avenues for pivotal preclinical single and repeat dose safety studies following good laboratory practices (GLP) as required by regulatory agencies [32]; critical for the preclinical development of graphene nanoparticles-based formulations as multifunctional agents for imaging and therapy. Traditionally, pharmaceutical formulations possess single functionality (e.g. as imaging or therapeutic agent). For multifunctional IV pharmaceutical products, such as GNP-Dex, a significant portion of required safety studies are the same regardless of whether the ultimate focus is on development for systemic imaging or as a therapeutic agent. Thus, with burgeoning costs and developmental times for new healthcare technologies, the development of highly efficacious multifunctional graphene nanoparticle biomedical technologies could control costs and developmental times.
5. Conclusions
The maximum tolerable dose (MTD) of GNP-Dex is between 50 mg/kg ≤ MTD < 125 mg/kg. Slower injection is preferable than bolus IV injections. 1 day or 30 days post injection, GNP-Dex does not significantly affect the respiratory, cardiovascular or hematological factors (blood, lipid, and metabolic panels) at doses < 125 mg/kg. The formulations elicit minor changes in the heart, liver, lung, spleen, or kidney, and no changes in the brain. GNP-Dex shows a dose-dependent small accumulation (~0.5–4%) in the major organs at day 1 and negligible or no presence in these organs by day 30. However, they were present close to or below LOD levels by day 30, indicating removal from these organs by day 30. The nanoparticles have a blood half-life < 30 minutes, and are mainly excreted within 24 hours through feces. The results lay the foundation for pivotal preclinical single and repeat dose safety studies following good laboratory practices (GLP) as required by the regulatory agencies; critical for the investigational new drug (IND) applications for first-in-human trails.
Supplementary Material
Acknowledgments
This work was supported by Wallace H Coulter Foundation Translational Research Award, Fusion Award from the Stony Brook School of Medicine and the Office of the Vice President for Research, and the National Institute of Health grants to B.S. (1R41DK100205-01A1) and R.Z.L (DK62722). The authors thank Dr. Renee Pessin of RDP Consulting, Inc. for editorial help, Dr. David Hirschberg for performing the ICP-MS measurements, Ms. Shelagh Zegers for assistance with lyophilization and Dr. Maricedes Acosta-Martinez for providing metabolic cages for biodistribution studies.
Footnotes
Declaration of interests
Stony Brook University, along with its researchers, has filed patents related to the technology reported in this article. If licensing or commercialization occurs, the researchers are entitled to standard royalties. S. B. has financial interest in Theragnostic Technologies Inc., which, however, did not support this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakamura E, Isobe H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc Chem Res. 2003;36:807–15. doi: 10.1021/ar030027y. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Tabakman S, Welsher K, Dai HJ. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009;2:85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalwani G, Sitharaman B. Multifunctional fullerene- and metallofullerene-based nanobiomaterials. Nano Life. 2013;3:1342003. [Google Scholar]
- 4.Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1:203–12. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Robinson JT, Sun X, Dai H. Pegylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876–7. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Zhang X, Liu Z, Ma Y, Huang Y, Chen Y. High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J Phys Chem C. 2008;112:17554–8. [Google Scholar]
- 7.Feng L, Zhang S, Liu Z. Graphene based gene transfection. Nanoscale. 2011;3:1252–7. doi: 10.1039/c0nr00680g. [DOI] [PubMed] [Google Scholar]
- 8.Paratala BS, Jacobson BD, Kanakia S, Francis LD, Sitharaman B. Physicochemical characterization, and relaxometry studies of micro-graphite oxide, graphene nanoplatelets, and nanoribbons. PLoS One. 2012;7:e38185. doi: 10.1371/journal.pone.0038185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanakia STJ, Chowdhury SM, Lalwani G, Tembulkar T, Button T, Shroyer K, Moore W, Sitharaman B. Physicochemical characterization of a novel graphene based magnetic resonance imaging contrast agent. Int J Nanomedicine. 2013;8:2821–33. doi: 10.2147/IJN.S47062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalwani G, Sundararaj JL, Schaefer K, Button T, Sitharaman B. Synthesis, characterization, in vitro phantom imaging, and cytotoxicity of a novel graphene-based multimodal magnetic resonance imaging - x-ray computed tomography contrast agent. J Mat Chem C. 2014 doi: 10.1039/C4TB00326H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalwani G, Cai X, Nie L, Wang LV, Sitharaman B. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography. Photoacoustics. 2013;1:62–7. doi: 10.1016/j.pacs.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv Drug Deliv Rev. 2006;58:1460–70. doi: 10.1016/j.addr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–8. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 14.Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21:1166–70. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 15.Feng L, Liu Z. Graphene in biomedicine: Opportunities and challenges. Nanomedicine (Lond) 2011;6:317–24. doi: 10.2217/nnm.10.158. [DOI] [PubMed] [Google Scholar]
- 16.Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, Ananta JS, Morgant G, Szwarc H, et al. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to swiss mice. ACS Nano. 2010;4:1481–92. doi: 10.1021/nn901573w. [DOI] [PubMed] [Google Scholar]
- 17.Lacerda L, Herrero MA, Venner K, Bianco A, Prato M, Kostarelos K. Carbon-nanotube shape and individualization critical for renal excretion. Small. 2008;4:1130–2. doi: 10.1002/smll.200800323. [DOI] [PubMed] [Google Scholar]
- 18.Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4:627–33. doi: 10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]
- 19.Singh SK, Singh MK, Nayak MK, Kumari S, Shrivastava S, Gracio JJA, et al. Thrombus inducing property of atomically thin graphene oxide sheets. ACS Nano. 2011;5:4987–96. doi: 10.1021/nn201092p. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Yang S-T, Liu J-H, Dong E, Wang Y, Cao A, et al. In vitro toxicity evaluation of graphene oxide on a549 cells. Toxicol Lett. 2011;200:201–10. doi: 10.1016/j.toxlet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Sasidharan A, Panchakarla LS, Chandran P, Menon D, Nair S, Rao CNR, et al. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale. 2011;3:2461–4. doi: 10.1039/c1nr10172b. [DOI] [PubMed] [Google Scholar]
- 22.Yang K, Gong H, Shi X, Wan J, Zhang Y, Liu Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials. 2013;34:2787–95. doi: 10.1016/j.biomaterials.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Yang K, Feng L, Liu Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon. 2011;49:4040–9. [Google Scholar]
- 24.Zhang X, Yin J, Peng C, Hu W, Zhu Z, Li W, et al. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon. 2011;49:986–95. [Google Scholar]
- 25.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 26.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- 27.Wang K, Ruan J, Song H, Zhang J, Wo Y, Guo S, et al. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6:8. doi: 10.1007/s11671-010-9751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang K, Wan J, Zhang S, Zhang Y, Lee S-T, Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of pegylated graphene in mice. ACS Nano. 2011;5:516–22. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 29.ICH guidance on toxicokinetics: The assessment of systemic exposure in toxicity studies s3a. http://wwwichorg/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S3A/Step4/S3A_Guidelinepdf1994.
- 30.OECD guidelines for the testing of the chemicals-draft proposal for a revised tg 417: Toxicokinetics. http://wwwoecdorg/env/ehs/testing/41690691pdf2008.
- 31.M3(r2) nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. http://wwwfdagov/RegulatoryInformation/Guidances/ucm129520htm. http://www.fda.gov/RegulatoryInformation/Guidances/ucm129520.htm2010. [PubMed]
- 32.Guidance for industry, developing medical imaging drug and biological products, part 1: Conducting safety assessments. Center for Drug Evaluation and Research, Food and Drug Administration; 2004. http://wwwfdagov/cber/guidelineshtm. [Google Scholar]
- 33.Kim Y-K, Kim M-H, Min D-H. Biocompatible reduced graphene oxide prepared by using dextran as a multifunctional reducing agent. Chem Commun. 2011;47:3195–7. doi: 10.1039/c0cc05005a. [DOI] [PubMed] [Google Scholar]
- 34.Shan C, Yang H, Han D, Zhang Q, Ivaska A, Niu L. Water-soluble graphene covalently functionalized by biocompatible poly-l-lysine. Langmuir. 2009;25:12030–3. doi: 10.1021/la903265p. [DOI] [PubMed] [Google Scholar]
- 35.Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from the framingham heart study. Circulation. 1997;96:44–9. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 36.Baseline hematology and clinical chemistry values for charles river wistar rats. http://wwwcrivercom/files/pdfs/rms/wistar-rats/rm_rm_r_hematology_crl_wi_br_sex_age1998.
- 37.Krystek P. A review on approaches to bio distribution studies about gold and silver engineered nanoparticles by inductively coupled plasma mass spectrometry. Microchem J. 2012;105:39–43. [Google Scholar]
- 38.Staszyk C, Bohnet W, Gasse H, Hackbarth H. Blood vessels of the rat tail: A histological reexamination with respect to blood vessel puncture methods. Lab Anim. 2003;37:121–5. doi: 10.1258/00236770360563750. [DOI] [PubMed] [Google Scholar]
- 39.Inoue K. Promoting effects of nanoparticles/materials on sensitive lung inflammatory diseases. Environ Health Prev Med. 2011;16:139–43. doi: 10.1007/s12199-010-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34:559–67. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- 41.Phillips JI, Green FY, Davies JC, Murray J. Pulmonary and systemic toxicity following exposure to nickel nanoparticles. Am J Ind Med. 2010;53:763–7. doi: 10.1002/ajim.20855. [DOI] [PubMed] [Google Scholar]
- 42.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–99. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabuchi A, Mertens M, Kuppe H, Pries AR, Kuebler WM. Intravital microscopy of the murine pulmonary microcirculation. J Appl Physiol (1985) 2008;104:338–46. doi: 10.1152/japplphysiol.00348.2007. [DOI] [PubMed] [Google Scholar]
- 44.Panich AM, Shames AI, Sergeev NA. Paramagnetic impurities in graphene oxide. Appl Magn Reson. 2013;44:107–16. [Google Scholar]
- 45.Panich AM, Shames AI, Aleksenskii AE, Dideikin A. Magnetic resonance evidence of manganese–graphene complexes in reduced graphene oxide. Solid State Commun. 2012;152:466–8. [Google Scholar]
- 46.Lee L-W, So P-W, Price AN, Parkinson JRC, Larkman DJ, Halliday J, et al. Manganese enhancement in non-cns organs. NMR Biomed. 2010;23:931–8. doi: 10.1002/nbm.1513. [DOI] [PubMed] [Google Scholar]
- 47.Yicheng Ni CP, Bosmans H, Miao Y, Grant D, Baert AL, Marchal G. Comparison of manganese biodistribution and mr contrast enhancement in rats after intravenous injection of mndpdp and mncl2. Acta Radiol. 1997;38:700–7. doi: 10.1080/02841859709172402. [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa M, Staud F, Takemura S, Takakura Y, Hashida M. Pharmacokinetic evaluation of biodistribution data obtained with radiolabeled proteins in mice. Biol Pharm Bull. 1999;22:214–8. doi: 10.1248/bpb.22.214. [DOI] [PubMed] [Google Scholar]
- 49.Vexler VS, Clément O, Schmitt-Willich H, Brasch RC. Effect of varying the molecular weight of the mr contrast agent gd-dtpa-polylysine on blood pharmacokinetics and enhancement patterns. J Magn Reson Imaging. 1994;4:381–8. doi: 10.1002/jmri.1880040325. [DOI] [PubMed] [Google Scholar]
- 50.Sperling F, McLaughlin JL. Biological parameters and the acute ld50 test. J Assoc Off Anal Chem. 1976;59:734–6. [PubMed] [Google Scholar]
- 51.Zbinden G, Flury-Roversi M. Significance of the ld50-test for the toxicological evaluation of chemical substances. Arch Toxicol. 1981;47:77–99. doi: 10.1007/BF00332351. [DOI] [PubMed] [Google Scholar]
- 52.Sayan Mullick Chowdhury SK, Toussaint Jimmy, Frame Mary D, Dewar Anthony M, Shroyer Kenneth R, Moore William, Sitharaman Balaji. In vitro hematological and in vivo vasoactivity assessment of dextran functionalized graphene. Sci Rep. 2013;3 doi: 10.1038/srep02584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.