Abstract
Hallucinogens have been part of spiritual practice for millennia, but controversy surrounding their mind-manifesting effects led to their proscription by the mid-20th century, largely without evidence of harm or toxicity and despite nascent data suggesting therapeutic utility in treating depressive illnesses. This review explores their pharmacodynamic actions and the current limited data on their clinic effectiveness. These drugs appear to exert their psychedelic effects through their agonist or partial agonist activity at the serotonergic 5-HT2A receptor, though they also have affinity for other metabotropic serotonin receptors. Hallucinogen binding affects a wide range of intracellular signalling pathways, the precise nature of which remains incompletely understood. They alter the serotonergic tone of brainstem raphe nuclei that project through the brain; they interact with receptors in the prefrontal cortex altering connectivity patterns and intracellular functioning; and they disrupt inhibitory control of sensory input via the thalamus to the cortex. The serotonergic system has long been implicated in anxiety and depressive disorders, and is a major target of most existing antidepressants. Classical hallucinogens alter the functioning of this system, but not in the same way current medications do: whilst there are identified receptors and neurotransmitter pathways through which hallucinogens could therein produce therapeutic effects, the neurobiology of this remains speculative at this time. There is currently an extremely limited but growing literature on hallucinogen safety and clinical application. The drugs appear well tolerated by healthy controls and clinical populations, and the rapid tolerance to repeated administration might reduce the possibility of dependency. Clinical trials reported over the past decade have generally shown positive therapeutic potential, but they are notably few in number. Legislative policy has had a freezing effect on evaluation of these compounds, a better understanding of which might improve our knowledge of the processes involved in consciousness, the neuropathology of depression, and potentially open up new pharmacological therapies.
Keywords: 5-HT2A, antidepressants, anxiety, depression, hallucinogen, LSD, psilocybin
Introduction
Hallucinogens have been employed entheogenically in human spiritual practice for several millennia, often in shamanistic approaches to healing or as a gateway for communication with deities. Despite, or perhaps in reaction to, this rich tradition, judicial processes in the 1960s and 1970s criminalized these substances without a clear scientific rationale, but more as a societal and political response to an emerged counter-culture. A historical legacy of the United Nations Convention on Psychotropic Substances [United Nations, 1971] has been significant obstacles and hurdles for scientific research using and investigating hallucinogens, whose neuropharmacology have remained incompletely understood [Vollenweider and Kometer, 2010]. Recently, it has been argued that the scientific community itself must lead the case for change in policies, as current drug laws severely limit scientific exploration of both neurobiological mechanisms and clinical effects that may prove valuable in the understanding of consciousness and mental illness, and offer novel therapies [Nutt et al. 2013].
Evaluation of acute drug effects demonstrate that the term ‘hallucinogen’ is actually a misnomer, as subjective effects do not include true hallucinations, but rather perceptual alterations of real stimuli. However, although other names have been suggested over the decades, ‘hallucinogens’ remains the most commonly used name for this class of substances [Nichols, 2004] and will be adhered to in this review. Although several other agents that may induce altered states of consciousness have been sometimes referred to as hallucinogens, such as the psychotomimetic N-methyl-D-aspartate (NMDA)-receptor antagonist ketamine, the stimulant 3,4-methylenedioxy-N-methylamphetamine (MDMA) or the dissociative opioid-κ agonist salvinorin A, these appear to be mediated by different receptor systems, produce different neuropharmacological and subjective psychological effects, and are beyond the scope of the present review: we refer the interested reader to our recent meta-analysis on ketamine [Caddy et al. 2014].
Classical hallucinogens are typically divided in three groups (see Table 1): tryptamines, such as psilocin (the psychoactive metabolite of psilocybin, contained in ‘magic’ mushrooms) and N,N-dimethyltryptamine (DMT, the psychoactive compound of ayahuasca); lysergamines (a subgroup of tryptamines), prominently lysergic acid diethylamide (LSD); and phenethylamines, such as mescaline (the psychoactive compound of the peyote cactus) and 2,5-dimethoxy-4-iodoamphetamine (DOI, one of many synthetic hallucinogens discovered in the last decades) [Geyer et al. 2009]. They appear to exert their effects through the serotonergic system, and there is a small but growing body of research indicating that they may have therapeutic effects in treating depressive and anxiety disorders. This present paper will review recent findings on neuropharmacological actions of these drugs, consider how such pharmacodynamics might have clinical potential, and thereafter review the current literature on trials in both healthy human participants and clinical populations. The paucity of recent research rendered a systematic review of the topic not feasible, and a narrative description of the topic has been utilized.
Table 1.
Classical hallucinogens divided into the three biochemical classes of tryptamines, lysergamines and phenethylamines. Psilocin, DMT and mescaline are naturally occurring, the rest are synthetic: this table is illustrative, and other compounds have been discovered.
| Tryptamines | Lysergamines | Phenethylamines |
|---|---|---|
| Psilocin | LSD (lysergic acid diethylamide) | Mescaline |
| DMT (N,N-dimethyltryptamine) | LSP (lysergic acid 3-pentylamide) | DOI (2,5-dimethoxy-4-iodoamphetamine) |
| 5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) | ETH-LAD (6-ethyl-6-nor-lysergic acid diethylamide) |
DOB (dimethoxybromoamphetamine) 2C-B (4-bromo-2,5-dimethoxyphenethylamine) |
| DET (N,N-diethyltryptamine) | AL-LAD (6-allyl-6-nor-lysergic acid diethylamide) | 2C-E (2,5-dimethoxy-4-ethylphenethylamine) |
| 5-MeO-DALT (N,N-diallyl-5-methoxytryptamine) | ALD-52 (N-acetyl-LSD) | 25I-NBOMe (4-iodo-2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine) |
Pharmacodynamics and molecular biology
Despite the biochemical delineation of classical hallucinogens, there is extensive evidence that most of these substances, for example psilocybin and LSD, produce cross-tolerance [Nichols, 2004]: pharmacodynamic research strongly indicates that the common psychedelic mechanism of action of these chemicals is through agonism on the serotonin 2A (5-HT2A) receptor [Presti and Nichols, 2004] (see Figure 1) and the selective 5-HT2A antagonist ketanserin and the partial antagonist risperidone have been shown to block subjective psychedelic effects of psilocybin [Vollenweider et al. 1998]. Classical hallucinogens generally display a similar potency as agonists for 5-HT2C and 5-HT1A receptors, although phenethylamines do not have high affinity for the latter, as well as some degree of action on most other serotonin receptor classes [Nichols, 2004]. Nonetheless, LSD, despite being one of the most potent of these agents, is only a relatively weak partial agonist on 5-HT2A [Nichols, 2004] and further the partial 5-HT2A agonist lisuride has a higher affinity for both 5-HT2A and 5-HT2C receptors than LSD but nonetheless produces no hallucinogenic effect [Egan et al. 1998], suggesting that whilst activation of 5-HT2A may be a crucial mediator of hallucinogenic effects it is not sufficient for the emergence of psychedelic phenomena. Classical hallucinogens are also associated with a rapid induction of tolerance upon repeated administration that is hypothesized to be a result of 5-HT2A receptor downregulation, and it has been argued that this property makes dependence and frequent use less likely than other illicit drugs, as the psychoactive effects of, for example, LSD are abolished after 4 days of daily administration [Nichols, 2004].
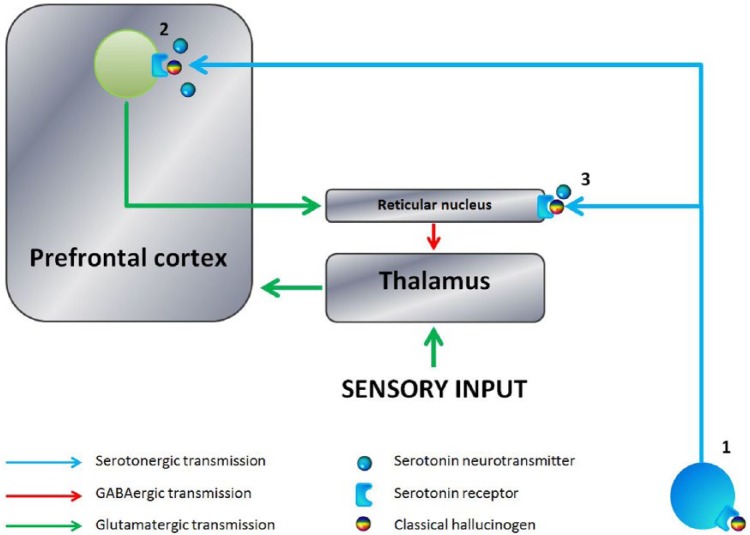
Figure 1.
Schematic illustration of the psychedelic effects of classical hallucinogens. 1. Normal serotonergic tone in the brain is maintained by the Raphe nuclei that have numerous projections to the prefrontal cortex and subcortical structures. Classical hallucinogens may disrupt this through several mechanisms, including binding with Raphe nuclei presynaptic 5HT1A receptors, altering serotonergic output to the rest of the brain. 2. Hallucinogen binding with postsynaptic 5HT2A receptors in cortical neurons, particularly at cortical layer V, may alter prefrontal glutamatergic signalling, and change cellular functioning through alteration of protein expression, with complex manifestations in consciousness. 3. The prefrontal cortex ordinarily limits sensory input through activation of intermediary GABAergic neurons in the reticular nucleus. Hallucinogen binding disrupts this process through binding with reticular postsynaptic 5HT2A receptors, altering sensory information to the cortex and the intrinsic activity of the sensory cortices.
Serotonin receptors are a phylogenetically ancient, heterotrimeric G-protein coupled receptor (GPCR) class that is preserved in a very wide range of species. Its activation alters several different intracellular secondary cascades [Wang et al. 2013]. Receptors are divided into ‘families’ depending upon their primary signalling pathway(s), although the broader biochemical principle of signalling divergence and convergence mean there is significant crossover between subtypes, and no one receptor utilizes a single transduction mechanism [Millan et al. 2008]. The so-called ‘5HT2 family’ all use phospholipase C (PLC) via Gq/11 as their major signalling route, and it consists of A, B and C subtypes. Activation of the 5-HT2A receptor subtype stimulates phospholipase C (PLC), which leads to downstream activation of protein kinase C (PKC) as well as increased release of Ca2+ from intracellular stores [Brown and Tracy, 2013].
Serotonin receptors contain sites that can induce post-translational modification and thence the alteration of signal transduction. Noteworthy, PKC isozymes alpha and/or epsilon appear to mediate the desensitization of 5-HT2A receptors during intermediate (2–6 hours) but not later (>24 hours) phases of DOI exposure [Roth et al. 1995]. PKC inhibition in the presence of ligand-binding prevents internalization, whereas specific activation of PKC in the absence of ligand-binding leads to internalization and subsequent recycling of 5-HT2A receptors to the membrane surface, suggesting endocytosis to be mediated by PKC [Bhattacharyya et al. 2002]. Interestingly, whilst serotonin-induced endocytosis has been shown to lead to recycling of 5-HT2A receptors to the cell surface within 2.5 hours, activation of the receptors by agents such as DOI prolongs the recycling process to 7.5 hours, potentially dependent on receptor dephosphorylation by protein phosphatase 2A [Raote et al. 2013]. Although the PLC pathway was originally assumed to be the main signalling pathway activated by hallucinogens, increasing evidence has emerged there is also independent stimulation of phospholipase A2 (PLA2), which leads to the formation of arachidonic acid [Nichols, 2004].
Further, long-term changes to intracellular signalling pathways may occur due to drug-induced alterations in gene expression, with evidence suggesting DOI may increase expression of several genes including c-fos, arc, sgk, ania3 and egr-2 [Leslie et al. 1993; Pei et al. 2000; Nichols and Sanders-Bush, 2002]. In mice, Gonzalez-Maeso and colleagues obtained evidence that gene expression differs depending on the hallucinogenic properties of 5-HT2A agonist effects, with data suggesting that whilst both LSD and lisuride induce expression of c-fos in several brain regions, including the prefrontal cortex (PFC), cingulate cortex and somatosensory cortex, egr-1 and egr-2 signalling in the same regions is activated only by LSD [Gonzalez-Maeso et al. 2007]. Pretreatment of cell cultures with pertussis toxin (PTX), which prevents the ability of PTX-sensitive Gi/o proteins to interact with GPCRs, affected only gene expression pathways elicited by LSD, but not lisuride. Downstream effects of Gi/o proteins include activation of Src, inhibition of which also led to attenuation of LSD-specific gene response patterns [Gonzalez-Maeso et al. 2007]. Moreno and colleagues elaborated on this showing that a complex of 5-HT2A with the metabotropic glutamate receptor mGluR2 may underlie the hallucinogen-specific signalling cascades and some of the cellular and behavioural effects [Moreno et al. 2011]: when compared with wild-type mice mGluR2-knockout (KO) mice injected with LSD and DOI did not show the head-twitch response, a rapid side-to-side head movement rodents typically exhibit in response to classic hallucinogens, and DOI injection led to expression of c-fos in both mGluR2-KO and wild-type mice, however egr-2 expression was abolished in mGluR2-KO mice only. These findings suggest hallucinogenic effects are mediated by co-activation of 5-HT2A and mGluR2, as well as Gi/o proteins and their downstream cascades.
5-HT2A receptors are located throughout several brain areas, most notably on the apical dendrites of pyramidal neurons of Layer V projecting into Layer I of the cortex, as well as in the thalamus, specifically in the reticular nucleus, which regulates processing of signals from the thalamus to the cortex [Nichols, 2004]. The reticular nucleus sends GABAergic projections to the thalamus, inhibiting its activity and potentially allowing for more sensory information to pass through to cortical areas. Psilocybin decreases metabolic activity of parts of the thalamus in human subjects [Gouzoulis-Mayfrank et al. 1999; Carhart-Harris et al. 2012], and may thus underlie the sensory alterations associated with hallucinogens: it appears feasible to suggest that as the thalamic filtering of information decreases, the infamous ‘doors of perception’[Huxley, 1954] open wider.
Despite the common shared mechanisms, individual agents vary in their exact pharmacodynamic activities regarding both receptor affinity as well as the degree of subsequent activation of intracellular signalling pathways, commonly termed functional selectivity [Nichols, 2004; Wacker et al. 2013]: the importance of these variations is incompletely understood, although drug consumers have long anecdotally recognized and reported differences in their effects. Further, the half-life of the substances also differs slightly, with psilocybin staying psychoactive for 4–6 hours in human participants, whereas LSD can last for up to 12 hours [Nichols, 2004].
Electrophysiological and neuroimaging data
Recent electroencephalography (EEG) data [Kometer et al. 2013] evaluated cortical excitability and early visual-evoked P1 and N170 potentials in 17 healthy participants administered either psilocybin (215 µg/kg) or placebo, having been pretreated with either the 5HT2A antagonist ketanserin or placebo. The electrophysiological results demonstrated that psilocybin significantly altered visual processing, and the authors argued this may demonstrate that 5HT2A agonism leads a large increase in spontaneous neuronal excitement modulated by increased α oscillations, which overwhelms cortical excitation from external stimuli.
With the shift towards sensory awareness, self-referential processes may decrease. Evidence recently obtained through functional magnetic resonance imaging (fMRI) in 15 healthy volunteers showed significant decreases in cerebral blood flow in the thalamus, hypothalamus, the anterior and posterior cingulate cortices and large parts of the PFC including the medial PFC (mPFC), lateral orbitofrontal cortex and the frontal gyri following intravenous injection of 2 mg of psilocybin [Carhart-Harris et al. 2012]. Further analysis of the sample revealed increased functional connectivity of the so-called default-mode network (DMN), comprised of the posterior cingulate cortex, mPFC and lateral inferior parietal cortex, and the task-positive network (TPN), comprised prefrontal and parietal structures [Carhart-Harris et al. 2013]. These anticorrelated networks are associated with non-goal-orientated introspection and goal-directed attentional tasks, respectively [Tracy and Shergill, 2013].
The single positron emission tomography (PET) study on cortical changes induced by psilocybin [Vollenweider et al. 1997] demonstrated a global increase in cerebral activity, markedly so in frontomedial, frontolateral, anterior cingulate and temporomedial cortices, as well as the basal ganglia and thalamus. Notably, current research on neurological effects induced by classical hallucinogens in healthy volunteers is too sparse to be able to draw more definite conclusions about the effects of classical hallucinogens on neural activity.
Pharmacotherapeutic models for classical hallucinogens
The Deakin/Graeff hypothesis
A role for the serotonergic system in anxiety and depressive disorders is long established though the precise physiological roles of the subsystems, their varying dysfunction in mental illnesses, and the exact therapeutic mechanisms of action of actions of even very well studied drugs such as selective serotonin reuptake inhibitors (SSRIs) remain incompletely understood. There are (at least) 14 5HT receptor subtypes, with differential serotonin affinity, varying localization presynaptically and postsynaptically and topographically through the brain, although all, except for the 5HT3 receptor, are GPCRs [Millan et al. 2008; Vitalis et al. 2013]. An influential model, though not without criticism, is the so-called Deakin/Graeff hypothesis [Deakin and Graeff, 1991] that posits that specific serotonergic networks have a role in coordinating physiologically complex evolved responses to stress, which, depending upon environmental circumstances and pathway activation, may be either anxiogenic or anxiolytic. Further, differential dysfunction of these pathways may lead to mental illnesses. A refined version of this model, based upon the recent review by Paul and Lowry [Paul and Lowry, 2013], states the following.
The median dorsal raphe forebrain bundle projects to the hippocampus and limbic system, and released serotonin binds primarily with postsynaptic 5HT1A receptors in these brain regions. This pathway has putative roles in developing tolerance or resilience to chronic stressors, allowing hippocampally mediated behavioural adaption. Dysfunction is hypothesized to be associated with depression.
The lateral dorsal raphe nuclei act as an inhibitory restraint, diminishing ‘fight or flight’ reactions to stressors, primarily via 5HT1A and 5HT2A receptors. Such a reaction might afford more nuanced anticipatory anxiety response to more distal threats. Dysfunction, and thus failure to inhibit such responses, may be linked with panic attacks.
The caudal aspects of the dorsal raphe nuclei that project to the limbic system, hippocampus and prefrontal cortex enhance stress-related processes in reaction to stressors, primarily via 5HT2A/2C and 5HT3 receptors. Such a response might be most appropriate when faced with imminent dangers. Dysfunction may be associated with anxiety disorders.
Whilst there is good empirical evidence to support this physiological model, linkage with mental illness, and the precise effects of medications remain only partially understood. We refer the interested reader to Deakin’s reappraisal of his hypothesis [Deakin, 2013].
Post-mortem studies
There is evidence for increased cortical 5-HT2A receptor expression in post-mortem samples of depressed and suicidal patients [Pandey et al. 2002; Mendelson, 2000; Shelton et al. 2009], but also decreased 5-HT2A binding in hippocampal areas of depressed patients [Sheline et al. 2004]. The 5-HT2A receptor may thus play a role in the regulation of mood state, particularly evidenced by modulation of anxiety behaviours in animal models [Weisstaub et al. 2006]. Notably, induction of learned helplessness in rats is associated with 5-HT2A upregulation in cortical areas [Dwivedi et al. 2005]. Moreover, increased 5-HT2A receptor densities in post-mortem PFCs of depressed patients are associated with decreased activity of protein kinase A, but not PKC [Shelton et al. 2009]. Although the psychedelic effects of classical hallucinogens appear likely to be due to agonist action on the 5-HT2A receptor, binding to 5-HT2C and 5-HT1A receptors [Nichols, 2004] have also been implicated in depressive and anxiety disorders and treatment thereof.
Animal models: 5HT2C
Increased expression of 5-HT2C receptors in the forebrain is associated with anxiety-like behaviours and reduced activity in animal models, whilst knockout mice show a blunted response of the amygdala in response to anxiety stimuli [Kimura et al. 2009]. Conversely, post-mortem investigation in depressed patients has found decreased 5-HT2C densities in prefrontal cortex [Pandey et al. 2006]. Activation of 5-HT2C receptors in the paraventricular nucleus of the hypothalamus increases secretion of corticotropin-releasing hormone and may thus form a link between serotonergic systems and activation of the HPA axis, based on observations in knockout mice [Heisler et al. 2007]. However, there is also evidence that agonist action on 5-HT2C may have antidepressant-like effects in animal models, as reported by Rosenzweig-Lipson and colleagues acute administration of the selective agonist WAY-163909 decreased immobility in the forced swim test as well as aggression in resident-intruder settings, suggesting a rapid onset of effects [Rosenzweig-Lipson et al. 2007]. Interestingly, activation of 5-HT2C receptors inhibits dopaminergic neurons in the ventral tegmental area [De Deurwaerdere et al. 2004], which, next to issues of tolerance, may contribute to the low addictive potential of classical hallucinogens.
Animal models: 5HT1A
5-HT1A receptors can have roles as presynaptic autoreceptors on serotonergic neurons and thus inhibit neuronal firing and release of vesicular serotonin. However, the receptor type is also expressed postsynaptically, with high densities in the hippocampus, hypothalamus, amygdala and cingulate and entorhinal cortices [Celada et al. 2013]. PET has consistently shown reduced postsynaptic binding potential in the amygdala, hippocampus and medial PFC of depressed patients, receptor agonists such as buspirone have been shown to exert antidepressant and anxiolytic effects, and further 5-HT1A KO mice show increased anxiety resistant to antidepressant treatment [Savitz et al. 2009]. Further, activation of the 5-HT1A receptor may have analgesic effects on acute, tonic and chronic nociceptive pain in animal models similar to those of opiate-based agents [Colpaert et al. 2006]. The modification of the various serotonin receptor activities by classical hallucinogens may thus hypothetically impact on mood and exert potential therapeutic effects through these mechanisms, although this issue remains speculative.
Modulation of glutamatergic functioning: altering BDNF and neurogenesis
Several studies have presented evidence that there is an increase of glutamate-dependent activity in prefrontal areas, induced by agonism of 5-HT2A receptors by classical hallucinogens [Aghajanian and Marek, 1997, 1999; Beique et al. 2007]. Based on this finding, Vollenweider and Kometer have suggested that the indirect activation of glutamate networks by classical hallucinogens enhances neuroplasticity, specifically so via the AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid) receptor, with subsequent downstream increases in the cellular protein brain-derived neurotrophic factor (BDNF) [Vollenweider and Kometer, 2010]. This is supported by the finding that agonist action on the 5-HT2A receptor may lead to increased expression of BDNF in prefrontal areas [Kometer and Vollenweider, 2010].
BDNF, amongst other proteins that may enhance neuroplasticity, has been implicated in neurotrophic theories of depression and treatment thereof [Penn and Tracy, 2012; Caddy et al. 2013], with robust evidence that serum BDNF levels are decreased in depressed individuals and that treatment with antidepressants normalizes levels of BDNF [Sen et al. 2008]. BDNF may play a vital role in adult neurogenesis [Bath et al. 2012] and there is evidence that depression is partially linked to insufficient neurogenesis and neurotrophic activity [Krishnan and Nestler, 2008].
Interestingly, Catlow and colleagues have investigated the effects of psilocybin, 25I-NBOMe (a synthetic phenethylamine) and ketanserin administrations on hippocampal neurogenesis and extinction of fear conditioning in adult rats [Catlow et al. 2013]. Low single doses of psilocybin (once per week over the course of 1 month) led to more rapid extinction of cued fear conditioning and increased neurogenesis when compared to saline control. However, administration of high doses of psilocybin, 25I-NBOMe and ketanserin all led to decreased neurogenesis, suggesting a biphasic, dose-dependent relationship that needs to be researched further. Nonetheless these findings support the assertion that hallucinogens may have some potentially similar effects to that of antidepressants, as there is evidence that SSRIs increase neurogenesis [Ohira et al. 2013] and that behavioural effects of antidepressants can be blocked by directly disrupting neurogenesis [Santarelli et al. 2003].
Modulation of the immune system
Messengers of the innate immune system, notably interleukin (IL)-6, IL-1β, interferon-α and other cytokines, may bring on symptoms associated with both depression and sickness, such as lethargy, low mood, irritability, loss of appetite, fragmented sleep and mild impairments of attention and concentration [Dantzer et al. 2008], and it has thus been suggested that dysregulation of cytokines can contribute towards depressive illness. Indeed, there is robust evidence for increased levels of cytokines such as tumour necrosis factor-α (TNF-α) and IL-6 in major depression [Dowlati et al. 2010] and it has been shown that in patients receiving amitriptyline, a tricyclic antidepressant, treatment response is associated with a significant decrease in TNF-α [Lanquillon et al. 2000]. Furthermore, TNF-α can stimulate indoleamine 2,3 dioxygenase (IDO) to metabolize tryptophan, the precursor of serotonin, along the kynurenine pathway. This competing pathway has been implicated in regulation of the innate immune system as well as potential neurotoxicity through NMDA receptor activation by the downstream metabolite quinolinic acid [Dantzer et al. 2008], which mediates cytokine-induced inhibition of neurogenesis in human hippocampal progenitor cell lines [Zunszain et al. 2012]. Interestingly, activation of the 5-HT2A receptor by DOI rapidly suppresses the mediation of inflammatory processes by tumour necrosis factor α (TNF-α), including the transcription of IL-6, thus leading to a reduced inflammatory response [Yu et al. 2008]. Thus, 5-HT2A-mediated inhibition of TNF-α may lead to neuroprotective effects as well as increased bioavailability of tryptophan, allowing for augmented serotonergic activity. However, agonist action on the 5-HT2A receptor also causes the serotonergic neurons of the raphe nuclei to cease firing [Nichols, 2004], which may cause a temporary paucity of serotonin in the brain; a finding not easily coalesced with the increased serotonergic functioning brought on by antidepressants.
Alteration of cortical networks
Therapeutic actions might also occur at a broader level of cortical connectivity. Failure to inhibit activity of the DMN and broader dysconnectivity between this and the extrinsic networks has been robustly associated with mental illness, most notably so the self-referential processes in depression that may be difficult to disengage, such as rumination [Whitfield-Gabrieli and Ford, 2012; Caddy et al. 2013]. Mindfulness-based therapies, which intend to foster nonjudgmental awareness of the present moment and sensory experience thereof whilst avoiding undue thought about future or past events, have been shown effective in reducing symptoms of depression and anxiety and reducing the risk of depressive relapse [Fjorback et al. 2011; Hofmann et al. 2010]. Interestingly, dampening of DMN activity [Brewer et al. 2011] and upregulation of networks associated with processing of sensory information [Baerentsen et al. 2010] have also been observed in experienced meditators during practice. Moreover, suppressive changes to mPFC activity are associated with a list of effective treatments for depression, including SSRIs [Kennedy et al. 2001] and cognitive-behavioural therapy [Goldapple et al. 2004], raising the somewhat speculative question as to whether the pharmacologically induced similarities in neural activation bear similar potential.
Clinical effects
Studies on healthy populations: turn on, tune in, drop out?
There are data to support the safety and therapeutic effects of classical hallucinogens. Studerus and colleagues performed a pooled analysis of acute and long-term psychological effects of psilocybin in 110 healthy volunteers from 8 separate double-blind randomized controlled trials (RCTs) conducted by the authors [Studerus et al. 2010]. Participants received 1–4 oral doses of very low to high doses of psilocybin (45–315 μg/kg of body weight). All studies included the Altered States of Consciousness Rating Scale, which comprises several dimensions including oceanic boundlessness (a measure combining insightfulness, religious experience, experience of unity and blissful state), anxious ego dissolution (referring to a combination of anxiety, impaired control and disembodiment), visionary restructuralization (referring to a combination of elementary visual alterations, audio–visual synaesthesia, vivid imagery and changed meaning of perceptions) as well as alterations of auditory perceptions and cognitive vigilance. Psilocybin had a significant effect on all of these dimensions, however with a much smaller effect for anxious ego dissolution. Adverse psychological effects were only observed in a minority of participants, who could be all calmed down verbally rather than through emergency medication. Further, these adverse effects occurred only among those who were administered high doses. Only one participant consulted the research team after the experiment regarding adverse effects, as it became apparent that suppressed memories had resurfaced. The participant was reported to have subsequently successfully resolved this issue through psychotherapy. Follow-up questionnaires provided no evidence of any mental impairment or distress resulting from psilocybin exposure. However, as the authors excluded any participants with a personal or family history of schizophrenia, major depression, bipolar disorder, borderline personality disorder, neurological disorders or alcohol or substance abuse, the significance of these findings may not be easily transferred to broader clinical populations.
Elaborating on the spiritual aspect of hallucinogens, Griffiths and colleagues conducted a double-blind RCT evaluating the psychological effects of psilocybin [Griffiths et al. 2006, 2008]. Although acute effects on mood included some increases in anxiety, the most pronounced changes were in terms of joy/intense happiness and peace/harmony. Further, a questionnaire assessing mystical experiences showed that 22 out of 36 participants had a strong mystical type experience, suggesting that psilocybin can induce spontaneous, spiritually significant states. Indeed, both at a 2-month follow up and at 14 months, the majority of participants rated the experience among the top five of their most personally meaningful experiences, attributing improvements in mood, attitudes and behaviours to it. In a subsequent study on the same sample, MacLean and colleagues reported that these experiences led to increases in the personality trait of ‘openness’, although the clinical effectiveness of such phenomena is open to debate [MacLean et al. 2011].
Grob and colleagues investigated a sample of 15 individuals who regularly ingest the DMT-containing brew ayahuasca as part of spiritual ceremonies in organised spiritual practice [Grob et al. 1996]. Ayahuasca also contains a monoamine oxidase inhibitor (MAOI), which allows the DMT to be orally ingested. Given that MAOIs are used as antidepressants this is a confounding variable, however members of the groups typically ingest the brew twice a month, making an antidepressant-like effect of the MAOI properties unlikely. Results showed that ayahuasca users performed mildly better on cognitive measures and exhibited less harm avoidance on personality measures, when compared with 15 ayahuasca-naïve individuals. Further, whilst none of the ayahuasca users met criteria for an active psychiatric disorder, five had previously suffered from alcohol abuse disorder, two from major depressive disorder and three from anxiety disorders. Amongst the controls, only one participant had previously recovered from an alcohol abuse disorder, whilst two other met criteria for an active one. Qualitative life history interviews with ayahuasca users revealed that they unequivocally attributed improvements in their mental well-being to the religious and ceremonial practice.
Some of these results were confirmed in a larger sample of religious ayahuasca users: Bouso and colleagues provided interesting evidence regarding long-term use of classical hallucinogens by comparing 127 regular ayahuasca users to 115 actively religious controls who were followed up over 1 year [Bouso et al. 2012]. Regular hallucinogen users showed lower scores on all psychopathology scales as assessed by the Symptom Check-List-90-Revised, as well as on measures of harm avoidance and self-directedness. However, they scored higher on a measure of psychosocial well-being, and performed better on the Stroop test (an indicator of resistance to emotional interference) and the Wisconsin Card Sorting Task (a measure of working memory). These data were reconfirmed at a 1-year follow up, demonstrating that there was no deterioration of mental health in regular users.
Further evidence in line with this finding was obtained by Krebs and Johansen in a population-based analysis of a nationwide Norwegian drug-use survey, with data from 21,967 participants [Krebs and Johansen, 2013]. Whilst it is not possible to assign causality in such work, it was noteworthy that 13.4% of respondents reported lifetime use of hallucinogens, and no negative relationship was observed between lifetime substance-use documentation, of any psychedelic, and any mental health outcome. In contrast, several indicators of better mental health on items relating to psychotic symptoms, generalized anxiety disorder, rates of psychiatric medication and inpatient treatment were observed, albeit with marginal yet significant differences and in specific sample subgroups (e.g. lower rates of psychiatric medication was only apparent in older adults).
Studies on patient populations
The initial surge of clinical research that took place between the mid-1950s and 1960s in response to the synthesis of LSD and psilocybin produced more than 1000 studies, involving tens of thousands of participants [Grinspoon and Bakalar, 1981]. However, as pointed out by Vollenweider and Kometer, there were difficulties with these studies [Vollenweider and Kometer, 2010]: the substances were little understood, their effects were difficult to control and different therapeutic approaches utilized them in different manners, and therefore the present review will refrain from considering evidence obtained in these studies.
Since the criminalization of these drugs there have only been three studies that the present review could identify investigating classical hallucinogens in clinical settings, all published in the last decade. Following anecdotal early case studies on the utility of psilocybin in obsessive–compulsive disorder (OCD), Moreno and colleagues administered this compound to nine OCD patients who had failed to respond to at least one therapeutic trial of a SSRI [Moreno et al. 2006]. Doses ranging from very low to high (25–300 μg/kg of body weight) were administered on four testing days at least 1 week apart. Following administration, participants listened to standardized music in a comfortable room and wore eyeshades for 8 hours, in the presence of two sitters who ensured safety and well-being but maintained minimal interaction. Analysis of OCD symptoms showed a significant effect of psilocybin on OCD symptom scales, with all of the participants responding in one or more sessions and reductions of 23–100% in symptoms scores as assessed by the Yale–Brown Obsessive Compulsive Scale (measured immediately before administration, then at 4, 8 and 24 hours). The effect lasted for more than 24 hours and interestingly appeared independent of dose, suggesting even subpsychedelic amounts of psilocybin (determined as 25 μg/kg) may alleviate symptoms: the authors postulated that agonist activity on 5-HT1A, 5-HT2A and/or 5-HT2C underlay efficacy. Scores on the Hallucinogen Rating Scale were significantly increased at the 8-hour measurement point, and the only adverse reaction recorded was one participant showing mild and temporary hypertension at one point in time, but unrelated to anxiety. Although these results shed an optimistic light on the clinical application of hallucinogens, the available data are limited by a lack of control, adequate sample size and most critically long-term follow up. All participants, through study protocol, had at least one prior exposure to indole-based psychedelics, and two had reported previous therapeutic responses to similar drugs.
Grob and colleagues investigated administration of psilocybin to advanced-stage, mood-disordered cancer patients on measures of depression, state–trait anxiety and mood states [Grob et al. 2011]. A total of 12 patients participated in two experimental sessions similar in setting utilized by Moreno and colleagues, being administered a moderate dose of psilocybin (200 μg/kg of body weight) on one occasion and an active placebo of 250 mg of niacin, which induced a mild physiological reaction of flushing, on the other in a double-blind procedure [Moreno et al. 2006]. Reported subjective effects of psilocybin were significantly increased for several items such as positive mood, positive derealization and elementary hallucinations, but not anxious derealization and thought disorder. Neither physical nor psychological adverse effects occurred. At a 6-month follow up the study found a significant decrease in Beck Depression Inventory (BDI) scores (p = 0.03), which had declined during the months following psilocybin administration, whereas reductions in trait anxiety as measures by the State–Trait Anxiety Inventory (STAI) reached significance at a 1-month follow up (p = 0.001). These data are seemingly impressive in the context of a single dosing schedule, but the work has methodological limitations in terms of sample size, variation in the cancer types, independent control, and variations of dosage and sessions.
Most recently, Gasser and colleagues utilized LSD as an adjunct to psychotherapy for anxiety from life-threatening illness in a double-blind, randomized, active placebo-controlled design [Gasser et al. 2014]. Eight participants received 200 μg, based upon early studies from the 1970s, in two experimental sessions as part of a psychotherapeutic intervention, whilst the four control participants received 20 μg of LSD as an active placebo posited to produced detectable but minimal subjective effects. Results revealed a statistically significant reduction of state anxiety on the STAI (p = 0.02) and a trend towards reduction of trait anxiety at both 2- and 12-month follow up. However, no changes on the Hamilton Depression Rating Scale were observed. No drug-related serious adverse events occurred and all other adverse reactions, such as feeling abnormal or anxious, were self-limiting, transient and required no intervention. Despite its promising findings, the research is again marked by a lack of sample size rendering the present evidence as of preliminary quality.
Risk data on classical hallucinogens
None of the studies reviewed here that utilized classical hallucinogens reported any significant adverse effects, whether physiological or psychological. Indeed, even when not consumed in controlled settings where in an emergency ketanserin could be used to block all psychedelic effects, the risks associated with classical hallucinogens appears to be low: in an influential paper Nutt and colleagues estimated the cumulative risk of 20 drugs of abuse and found psilocybin to carry the least risk with a 12-fold difference to alcohol, the most harmful one [Nutt et al. 2010]. LSD was found to be the third least harmful, showing these hallucinogens to be less harmful than other commonly prescribed psychiatric drugs such as methadone and benzodiazepines. There have been no reported fatalities directly due to psilocybin [Jerome, 2007]. Nevertheless, existing study sample sizes are generally small, and as the drugs can produce psychosis-like symptoms and have served as a serotonergic model of psychosis [Vollenweider et al. 1998; Halberstadt and Geyer, 2013; Murray et al. 2013], questions remain about how much this might limit any potential clinical application remains uncertain.
Conclusion
Hallucinogens have been used and valued for millennia for their well-recognized mind-altering properties, but initial interest in their therapeutic potential in the 20th century was rapidly stymied by counter prevailing political and social currents. However, over the past few years a renewed interest in their neurobiology has emerged, not unconnected with the pressing need to open new avenues for treatment options in mood and anxiety disorders. The emerging neuroscientific and clinical literature on serotonergic hallucinogen-like agents shows promise, and a reasonably good side-effect profile, although the marked sparsity of data and methodological caveats mean much future work is necessary. Animal models are adding to our knowledge but the unique mind-manifesting nature of these drugs may hinder the applicability of this, and studies on humans are required.
The drug pharmacodynamics indicate that hallucinogens might not require daily ingestion: depending on the frequency required this may be potentially beneficial for adherence, although some individuals might find intermittent dosing regimens confusing. It has been argued that the unique mind-manifesting properties of these drugs might offer synergistic effects to psychological care as compared with existing antidepressants, and indeed hypothetically potentially novel mechanisms of action might make them complementary when coprescribed with current antidepressant drug classes. At the very least it seems reasonable to posit that ongoing examination of these drugs is warranted to further our insights into the neurobiology of depression.
Legislation on hallucinogens, with regards to scientific research, is outdated: LSD and psilocybin remain classified as ‘schedule I’, indicating that they have high potential for abuse, no accepted medical usage, and lack of safety when used under medical supervision. The evidence behind the proscription of classical hallucinogens to prevent harm to recreational drug consumers appears misguided at best, and frankly illogical when compared to alcohol and some legally available prescribed medications [Nutt et al. 2010]; moreover the freezing effect this has on clinical research is harmful, and with an untold cost. A recent review by Nutt and colleagues noted that three out of 7000 UK hospitals have the necessary licence to research such drugs; that the cost of a licence is expensive and only lasts 1 year; and that it necessitates police reviews [Nutt et al. 2013]. It is somewhat shocking in light of the strong (though methodologically challengeable) initial research to consider that Gasser and colleagues’ trial [Gasser et al. 2014] marked the first research utilizing LSD in a therapeutic intervention in more than 40 years
Depression causes a profound burden on society. These drugs offer at least the potential of better understanding the neurobiology of depression, and of providing novel therapeutic agents. The weight of clinical need must overcome any weight of political hesitancy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Derek Tracy has received honoraria for educational talks, and had travel and conference expenses paid, from Lilly UK and Roche UK. Giovanni Giaroli has received honoraria for serving on a speaker’s bureau for Eli Lilly, FlynnPharma and Jannsen. He has also received reimbursement for travel expenses and conference attendance from Eli Lilly, FlynnPharma and Shire.
Contributor Information
David Baumeister, Consultant Psychiatrist, Oxleas NHS Foundation Trust, Princess Royal University Hospital, Orpington, BR6 8NY, UK and Cognition, Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, King’s College London, UK.
Georgina Barnes, Stress, Psychiatry and Immunology Lab, Institute of Psychiatry, Department of Psychological Medicine, Kings College London, London, UK.
Giovanni Giaroli, Cognition, Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, King’s College London, UK.
Derek Tracy, Cognition, Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, King’s College London, UK; North East London Foundation Trust, London, UK.
References
- Aghajanian G., Marek G. (1997) Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36: 589–599 [DOI] [PubMed] [Google Scholar]
- Aghajanian G., Marek G. (1999) Serotonin, via 5-HT2A receptors, increases EPSCS in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res 825: 161–171 [DOI] [PubMed] [Google Scholar]
- Baerentsen K., Stodkilde-Jorgensen H., Sommerlund B., Hartmann T., Damsgaard-Madsen J., Fosnaes M., et al. (2010) An investigation of brain processes supporting meditation. Cogn Process 11: 57–84 [DOI] [PubMed] [Google Scholar]
- Bath K., Akins M., Lee F. (2012) BDNF control of adult SVZ neurogenesis. Dev Psychobiol 54: 578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique J., Imad M., Mladenovic L., Gingrich J., Andrade R. (2007) Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci U S A 104: 9870–9875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Puri S., Miledi R., Panicker M. (2002) Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc Natl Acad Sci U S A 99: 14470–14475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouso J., Gonzalez D., Fondevila S., Cutchet M., Fernandez X., Ribeiro Barbosa P., et al. (2012) Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: a longitudinal study. PLoS One 7: e42421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J., Worhunsky P., Gray J., Tang Y., Weber J., Kober H. (2011) Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci U S A 108: 20254–20259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K., Tracy D. (2013) Lithium: the pharmacodynamic actions of the amazing ion. Ther Adv Psychopharmacol 3: 163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy C., Giaroli G., White T., Shergill S., Tracy D. (2014) Ketamine as the prototype glutamatergic antidepressant: pharmacodynamics actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R., Erritzoe D., Williams T., Stone J., Reed L., Colasanti A., et al. (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109: 2138–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R., Leech R., Erritzoe D., Williams T., Stone J., Evans J., et al. (2013) Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull 39: 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlow B., Song S., Paredes D., Kirstein C., Sanchez-Ramos J. (2013) Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res 228: 481–491 [DOI] [PubMed] [Google Scholar]
- Celada P., Bortolozzi A., Artigas F. (2013) Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 27: 703–716 [DOI] [PubMed] [Google Scholar]
- Colpaert F., Deseure K., Stinus L., Adriaensen H. (2006) High-efficacy 5-hydroxytryptamine 1a receptor activation counteracts opioid hyperallodynia and affective conditioning. J Pharmacol Exp Ther 316: 892–899 [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J., Freund G., Johnson R., Kelley K. (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdere P., Navailles S., Berg K., Clarke W., Spampinato U. (2004) Constitutive activity of the serotonin2c receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24: 3235–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin J. (2013) The origins of ‘5-HT and mechanisms of defence’ by Deakin and Graeff: a personal perspective. J Psychopharmacol, in press. [DOI] [PubMed] [Google Scholar]
- Deakin J., Graeff F. (1991) 5-HT and mechanisms of defence. J Psychopharmacol 5: 305–315 [DOI] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E., et al. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457 [DOI] [PubMed] [Google Scholar]
- Dwivedi Y., Mondal A., Payappagoudar G., Rizavi H. (2005) Differential regulation of serotonin (5HT)2A receptor MRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology 48: 204–214 [DOI] [PubMed] [Google Scholar]
- Egan C., Herrick-Davis K., Miller K., Glennon R., Teitler M. (1998) Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology (Berl) 136: 409–414 [DOI] [PubMed] [Google Scholar]
- Fjorback L., Arendt M., Ornbol E., Fink P., Walach H. (2011) Mindfulness-based stress reduction and mindfulness-based cognitive therapy: a systematic review of randomized controlled trials. Acta Psychiatr Scand 124: 102–119 [DOI] [PubMed] [Google Scholar]
- Gasser P., Holstein D., Michel Y., Doblin R., Yazar-Klosinski B., Passie T., Brenneisen R. (2014) Safety and efficacy of LSD-assisted psychotherapy in subjects with anxiety associated with life-threatening diseases: a randomized active placebo-controlled phase 2 pilot study. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M., Nichols D., Vollenweider F. (2009) Serotonin-related psychedelic drugs. In: Squire L. (ed.), Encyclopedia of Neuroscience. Oxford: Academic Press [Google Scholar]
- Goldapple K., Segal Z., Garson C., Lau M., Bieling P., Kennedy S., et al. (2004) Modulation of cortical–limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 61: 34–41 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J., Weisstaub N., Zhou M., Chan P., Ivic L., Ang R., et al. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53: 439–452 [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E., Schreckenberger M., Sabri O., Arning C., Thelen B., Spitzer M., et al. (1999) Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and D-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology 20: 565–581 [DOI] [PubMed] [Google Scholar]
- Griffiths R., Richards W., Johnson M., McCann U., Jesse R. (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol 22: 621–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R., Richards W., McCann U., Jesse R. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187: 268–283; discussion 284–292. [DOI] [PubMed] [Google Scholar]
- Grinspoon L., Bakalar J. (1981) The psychedelic drug therapies. Curr Psychiatr Ther 20: 275–283 [PubMed] [Google Scholar]
- Grob C., Danforth A., Chopra G., Hagerty M., McKay C., Halberstadt A., et al. (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68: 71–78 [DOI] [PubMed] [Google Scholar]
- Grob C., McKenna D., Callaway J., Brito G., Neves E., Oberlaender G., et al. (1996) Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J Nervous Mental Dis 2: 86–94 [DOI] [PubMed] [Google Scholar]
- Halberstadt A., Geyer M. (2013) Serotonergic hallucinogens as translational models relevant to schizophrenia. Int J Neuropsychopharmacol 16: 2165–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler L., Pronchuk N., Nonogaki K., Zhou L., Raber J., Tung L., et al. (2007) Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci 27: 6956–6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S., Sawyer A., Witt A., Oh D. (2010) The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol 78: 169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. (1954) The Doors of Perception. 1st edn. New York: Harper [Google Scholar]
- Jerome L. (2007) Psilocybin Investigator’s Brochure. Multidisciplinary Association for Psychedelic Studies. Available at: http://www.maps.org/research/psilo/psilo_ib.pdf (accessed 3 December 2013).
- Kennedy S., Evans K., Kruger S., Mayberg H., Meyer J., McCann S., et al. (2001) Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 158: 899–905 [DOI] [PubMed] [Google Scholar]
- Kimura A., Stevenson P., Carter R., MacColl G., French K., Simons J., et al. (2009) Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. Eur J Neurosci 30: 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometer M., Schmidt A., Jancke L., Vollenweider F. (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on alpha oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci 33: 10544–10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs T., Johansen P. (2013) Psychedelics and mental health: a population study. PLoS One 8: e63972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E. (2008) The molecular neurobiology of depression. Nature 455: 894–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquillon S., Krieg J., Bening-Abu-Shach U., Vedder H. (2000) Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22: 370–379 [DOI] [PubMed] [Google Scholar]
- Leslie R., Moorman J., Coulson A., Grahame-Smith D. (1993) Serotonin2/1c receptor activation causes a localized expression of the immediate–early gene C-Fos in rat brain: evidence for involvement of dorsal Raphe nucleus projection fibres. Neuroscience 53: 457–463 [DOI] [PubMed] [Google Scholar]
- MacLean K., Johnson M., Griffiths R. (2011) Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol 25: 1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson S. (2000) The current status of the platelet 5-HT(2A) receptor in depression. J Affect Disord 57: 13–24 [DOI] [PubMed] [Google Scholar]
- Millan M., Marin P. (2008) Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci 29: 454–464 [DOI] [PubMed] [Google Scholar]
- Moreno F., Wiegand C., Taitano E., Delgado P. (2006) Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive–compulsive disorder. J Clin Psychiatry 67: 1735–1740 [DOI] [PubMed] [Google Scholar]
- Moreno J., Holloway T., Albizu L., Sealfon S., Gonzalez-Maeso J. (2011) Metabotropic glutamate MGLU2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493: 76–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R., Paparelli A., Morrison P., Marconi A., Di Forti M. (2013) What can we learn about schizophrenia from studying the human model, drug-induced psychosis? Am J Med Gen B: Neuropsych Gen 162: 661–670 [DOI] [PubMed] [Google Scholar]
- Nichols C., Sanders-Bush E. (2002) A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26: 634–642 [DOI] [PubMed] [Google Scholar]
- Nichols D. (2004) Hallucinogens. Pharmacol Ther 101: 131–181 [DOI] [PubMed] [Google Scholar]
- Nutt D., King L., Nichols D. (2013) Effects of schedule I drug laws on neuroscience research and treatment innovation. Nat Rev Neurosci 14: 577–585 [DOI] [PubMed] [Google Scholar]
- Nutt D., King L., Phillips L. (2010) Drug harms in the UK: a multicriteria decision analysis. Lancet 376: 1558–1565 [DOI] [PubMed] [Google Scholar]
- Ohira K., Takeuchi R., Shoji H., Miyakawa T. (2013) Fluoxetine-induced cortical adult neurogenesis. Neuropsychopharmacology 38: 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey G., Dwivedi Y., Rizavi H., Ren X., Pandey S., Pesold C., et al. (2002) Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry 159: 419–429 [DOI] [PubMed] [Google Scholar]
- Paul E., Lowry C. (2013) Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol 27: 1090–1106 [DOI] [PubMed] [Google Scholar]
- Pei Q., Lewis L., Sprakes M., Jones E., Grahame-Smith D., Zetterstrom T. (2000) Serotonergic regulation of MRNA expression of ARC, an immediate early gene selectively localized at neuronal dendrites. Neuropharmacology 39: 463–470 [DOI] [PubMed] [Google Scholar]
- Pandey G.N., Dwivedi Y., Ren X., Rizavi H.S., Faludi G., Sarosi A., Palkovits M. (2006) Regional distribution and relative abundance of serotonin (2c) receptors in human brain: effect of suicide. Neurochem Res 31: 167–76 [DOI] [PubMed] [Google Scholar]
- Penn E., Tracy D. (2012) The drugs don’t work? Antidepressants and the current and future pharmacological management of depression. Ther Adv Psychopharmacol 2:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti D.E., Nichols. (2004) Biochemistry and neuropharmacology of psilocybin mushrooms. In: Metzner R., Darling D.C. (eds) Teonanacatl. Four Trees, El Verano, CA, pp 89–108 [Google Scholar]
- Raote I., Bhattacharyya S., Panicker M. (2013) Functional selectivity in serotonin receptor 2A (5-HT2A) endocytosis, recycling, and phosphorylation. Mol Pharmacol 83: 42–50 [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S., Sabb A., Stack G., Mitchell P., Lucki I., Malberg J., et al. (2007) Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist way-163909 in rodents. Psychopharmacology (Berl) 192: 159–170 [DOI] [PubMed] [Google Scholar]
- Roth B., Palvimaki E., Berry S., Khan N., Sachs N., Uluer A., et al. (1995) 5-hydroxytryptamine2A (5-HT2A) receptor desensitization can occur without down-regulation. J Pharmacol Exp Ther 275: 1638–1646 [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809 [DOI] [PubMed] [Google Scholar]
- Savitz J., Lucki I., Drevets W. (2009) 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol 88: 17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S., Duman R., Sanacora G. (2008) Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 64: 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton R., Sanders-Bush E., Manier D., Lewis D. (2009) Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience 158: 1406–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E., Kometer M., Hasler F., Vollenweider F. (2011) Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol 25: 1434–1452 [DOI] [PubMed] [Google Scholar]
- Tracy D., Shergill S. (2013) Mechanisms underlying auditory hallucinations—understanding perception without stimulus. Brain Sci 3: 642–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations (1971) Convention on Psychotropic Substances. Available at: http://www.unodc.org/pdf/convention_1971_en.pdf (accessed 3 December 2013).
- Vitalis T., Ansorge M., Dayer A. (2013) Serotonin homeostasis and serotonin receptors as actors of cortical construction: special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front Cell Neurosci 7: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F., Kometer M. (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11: 642–651 [DOI] [PubMed] [Google Scholar]
- Vollenweider F., Leenders K., Scharfetter C., Maguire P., Stadelmann O., Angst J. (1997) Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 16: 357–372 [DOI] [PubMed] [Google Scholar]
- Vollenweider F., Vollenweider-Scherpenhuyzen M., Babler A., Vogel H., Hell D. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–3902 [DOI] [PubMed] [Google Scholar]
- Wacker D., Wang C., Katritch V., Han G., Huang X., Vardy E., et al. (2013) Structural features for functional selectivity at serotonin receptors. Science 340: 615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Jiang Y., Ma J., Wu H., Wacker D., Katritch V., et al. (2013) Structural basis for molecular recognition at serotonin receptors. Science 340: 610–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub N., Zhou M., Lira A., Lambe E., Gonzalez-Maeso J., Hornung J.P., et al. (2006) Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313: 536–540 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J. (2012) Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76 [DOI] [PubMed] [Google Scholar]
- Yu B., Becnel J., Zerfaoui M., Rohatgi R., Boulares A., Nichols C. (2008) Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther 327: 316–323 [DOI] [PubMed] [Google Scholar]
- Zunszain P., Anacker C., Cattaneo A., Choudhury S., Musaelyan K., Myint A., et al. (2012) Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 37: 939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]