Abstract
Purpose of review
This article reviews recent literature depicting a shift in dysphagia rehabilitation in adults. Distinguishing rehabilitation from compensation in dysphagia management, a review of basic exercise principles is followed by description of recent publications depicting exercise based therapies. Subsequently, transcutaneous electrical stimulation is reviewed as it may contribute to exercise based dysphagia rehabilitation in adults.
Recent findings
Surveys have documented extensive variability in the clinical application of dysphagia therapy techniques. Despite this variability, two trends are emerging in dysphagia rehabilitation research: 1- documentation of physiologic plus functional changes within the swallowing mechanism subsequent to therapy; and 2- prophylactic exercise based therapies. In addition, extensive efforts have emerged describing the potential application of transcutaneous electrical stimulation in dysphagia rehabilitation. Though results of these efforts are conflicted, transcutaneous electrical stimulation may serve a useful role as an adjunct to well-developed exercise based rehabilitation for dysphagia.
Summary
The focus of dysphagia rehabilitation in adults is changing. Current efforts indicate that exercise based therapies should incorporate multiple principles of exercise physiology and document physiologic change within the impaired swallowing mechanism. Transcutaneous electrical stimulation may function as an adjunctive modality; however, current practices should be evaluated to develop additional parameters of stimulation that are focused toward specific dysphagia impairments.
Keywords: Dysphagia, rehabilitation, exercise, physiology, electrical stimulation, adjunctive modality
Introduction
Dysphagia rehabilitation is experiencing a paradigm shift. Though clinicians frequently report the use of ‘exercise’ as part of dysphagia rehabilitation, a recent focus on the inclusion of specific exercise principles has resulted in development of novel ‘exercise based’ therapies. Exercise based dysphagia treatment approaches should manifest in physiologic changes within the impaired swallowing mechanism in addition to resulting in improved swallowing function. Moreover, strategies are emerging to evaluate the exercise component of dysphagia therapies. Application of exercise principles and strategies to assess the impact of novel therapies will facilitate development of new and effective approaches to dysphagia rehabilitation approaches in adults.
Transcutaneous electrical stimulation is often applied as either a direct intervention or as an adjunct to exercise approaches in dysphagia rehabilitation. Outcome data from this approach is conflicted and primarily based on relatively weak research designs or small studies. Also, electrical stimulation parameters may differ across application studies. These factors lead to confusion regarding the application of transcutaneous electrical stimulation in dysphagia rehabilitation. However, with enhanced understanding of the neuromuscular impact of transcutaneous electrical stimulation, this modality may function as a valuable adjunct to exercise based therapies for dysphagia in adults.
Goals of dysphagia intervention
Dysphagia intervention often is described in reference to two general categories: compensation and rehabilitation [1, 2]. Compensation incorporates short term adjustments to patient, food, or swallowing activity with the goal of facilitating improved swallowing function. The focus of compensations is on safe swallowing, typically meaning reduced risk of aspiration. Compensatory activities are not intended to improve the physiology of the impaired swallowing mechanism. Conversely, rehabilitation efforts do intend to improve swallowing physiology and by extension enhance swallow function. A recent paradigm shift has introduced another component to dysphagia intervention; prevention. Recent studies in the area of head/neck cancer support the benefit of pre- or peri-treatment dysphagia intervention to prevent or reduce the impact of dysphagia following medical treatment [3 – 6**]. Despite variability in design and scientific rigor, these studies advocate the application of exercise based intervention either prior to or simultaneous with chemoradiotherapy for treatment of head/neck cancer. This prophylactic approach to dysphagia in head/neck cancer is highlighted in a recent evidence based review as one of three interventions that can reduce dysphagia in this high impact population [7*]. Thus, both rehabilitation and prophylactic interventions advocate the inclusion of exercise principles to develop and apply systematic dysphagia treatment. However, though current practice appears to include application of exercise activities, clinical application of exercise is highly variable and lacks a systematic programmatic approach [8*, 9*]. These recent observations merit a brief review of how exercise principles may be applied in dysphagia treatment.
Application of exercise principles
The application of exercise to alter muscle structure and function requires a number of key concepts (Table 1). Muscle is plastic and highly responsive to exercise, adapting to the stress or load placed upon it. To increase strength, a muscle must be gradually exercised at a level above its usual load or intensity. This principle of “overload” requires that any strength or conditioning program incorporate load increases that gradually and systematically progress over time. This systematic program is often termed an “ascending progression” [10]. A related principle is “adaptation”. This concept underlies the benefit of repeatedly practicing a movement, skill, or task. Through continued practice, muscles develop efficiency and stabilize motor plans. Mass practice enables muscles to develop a “memory” of the task or skill and to improve performance [11]. As muscle adapts with increased use, it also declines with disuse. It is this “reversibility” concept that underlies the rapid deconditioning noted in patients who are unable to use a limb due to injury or in athletes who stop training and lose condition. This same disuse pattern is likely to apply to swallowing musculature. “Specificity” is another important component of exercise based training programs. The principle of specificity simply states that to improve a muscle group, movement, or skill, the exercise applied must focus on that muscle group, movement, or skill. Exercise that indirectly engages those muscles will be less efficient at generating a positive training response. Finally, the principle of "recovery" needs consideration in formulating any exercise program. Rest is often as important as the exercise activity for muscle development and strength gain. Short periods of planned rest both between repetitions of exercises and between exercise sessions facilitate muscle benefit from exercise [12, 13]. Rest supports muscle healing, fiber growth, and helps reduce fatigue associated with exercise. Planning rest periods integrated with exercise is a critical feature of a comprehensive exercise program [10].
Table 1.
Definitions and application suggestions for basic exercise principles that may be incorporated into dysphagia rehabilitation programs.
| Principle | Definition | Application |
|---|---|---|
| Overload | Exercise at sufficient intensity, time and frequency to challenge muscle and create muscle change | Increase total time or load used in training |
| Progression | Systematically increasing the intensity (load) and demands (time/frequency) spent in exercise | Continually and gradually increase the demands of the exercise activity applied-perform more repetitions, increase the load, go faster |
| Intensity | The load used in an exercise | Alter the amount pushed, pulled or lifted in exercise |
| Adaptation | Repeatedly practicing a movement, skill or task to alter muscle condition | Use continued (regular) practice of a particular exercise pattern |
| Reversibility | The effect of exercise training on muscle will be lost with lack of activity | "If you don't use it, you lose it" – a maintenance plan is needed to prevent detraining |
| Specificity | Exercise should be specific to the goal | If your goal is to be a runner then exercise should include running. |
| Recovery | Rest between repetitions of movement or sets of strength training exercises | Ensure sufficient rest between activity to reduce fatigue and stabilize muscle |
In addition to the inclusion of exercise principles into dysphagia therapy, approaches to evaluate the immediate impact of exercise may be useful. Borrowing from Bastian’s work on adaptive motor learning [14], Humbert and colleagues demonstrated adaptive motor learning effects in hyolaryngeal movements during experimentally induced swallowing exercises in healthy adults [15*]. Though additional study and refinement is likely required before systematic application of this approach in dysphagia rehabilitation, the concept of adaptive motor learning may be an important metric to assess impact of exercise based rehabilitation efforts.
Clinicians may also assess potential motor learning effects during swallowing rehabilitation. Figure 1 [unpublished data] demonstrates change in swallowing performance associated with change in bolus material or volume during an exercise based dysphagia intervention (MDTP – see below). Note the increase in the frequency of coughing with swallow attempts following bolus change (identified with arrows). However, subsequent swallowing practice with each new material results in a decrease of this abnormal response. Such patterns demonstrate a motor learning effect following interruption of the learned pattern with a novel stimulus (in this case a new material or volume to be swallowed).
Figure 1.
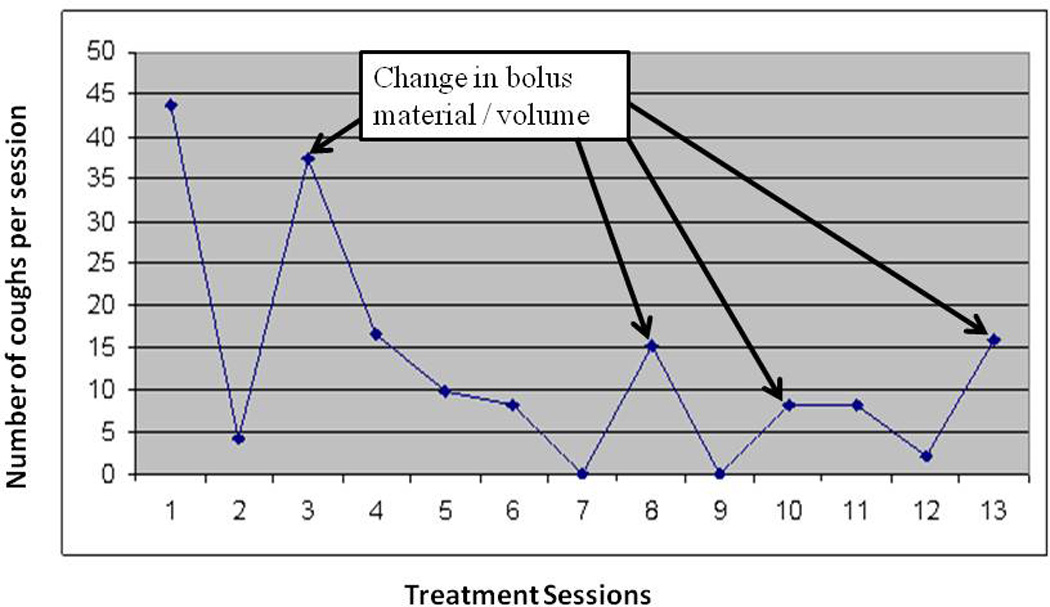
Change in cough frequency over multiple therapy (MDTP) sessions. Cough increases following change in bolus material or speed of swallowing (see arrows).
Examples of exercise based dysphagia therapies
Many recent reports have described ‘exercise based’ dysphagia interventions. Some of these reports offer novel perspectives on traditional techniques while others represent new approaches to dysphagia intervention. For example, Nekl and colleagues [16] demonstrated that the effortful swallow, a commonly used technique, has potential to increase esophageal contraction amplitudes and improve esophageal clearance. In addition, recent studies of the Mendelsohn maneuver, a technique which prolongs maximum elevation of the hyolaryngeal complex during swallowing, have reported increased opening of the upper esophageal sphincter and improved swallow coordination following treatment in stroke survivors with dysphagia [17]. Additional work incorporating healthy volunteers has documented increased tongue-palate contact pressure during application of this swallowing maneuver [18]. Finally, a simple jaw opening exercise has been shown to improve opening of the upper esophageal sphincter during swallowing [19].
Though many studies (including those identified above) have demonstrated physiologic or functional impact of ‘exercises’ on various aspects of the swallow mechanism, few have demonstrated combined physiologic and functional benefit in well-defined patient groups. Functional benefit might be defined in multiple ways, but an obvious benefit would be increased functional intake of food and liquid in the absence of dysphagia related complications. Four recently offered dysphagia therapies profess to be exercise based and have described both physiologic and functional outcomes related to clinical application in patient populations. These four therapies include a lingual resistance exercise [20], a head lift exercise [21, 22], a program of progressive swallowing exercise [23], and a program of preventative exercise [6]. Each of these approaches reports both physiologic and functional benefit in different patient populations (Table 2). However, each incorporates different exercise principles to achieve these goals (Table 3).
Table 2.
Summary of published research detailing functional and physiologic outcomes associated with four exercise based dysphagia therapies. These summaries are descriptive only and do not evaluate the scientific rigor or evidence level of each listed study. The reference number is provided for each study.
| Therapy | Sample [study reference] | Study Design | Sample Size |
Outcome Measures | Results of Therapy |
|---|---|---|---|---|---|
| Lingual Resistance | Older healthy adults [24] | Prospective cohort with intervention | 10 | Lingual pressures during swallowing and maximum tongue push task, swallow timing and bolus flow measures, lingual MRI (4 subjects only) | Lingual pressures (swallow and maximum) increased post therapy, swallow measures did not improve, lingual volume increased (MRI) |
| Stroke patients [20] | Prospective Cohort with intervention | 10 | Lingual pressures during swallowing and maximum tongue push task, swallow timing and bolus measures, lingual MRI (3 subjects only), quality of life and dietary intake | Lingual pressures (swallow and maximum) increased post therapy, mixed results regarding reduced post swallow residue and penetration/aspiration (only for some materials), few changes in swallow timing measures, two of three subjects increased lingual volume | |
| Progressive disease [25] | Case report | 1 | Lingual pressure during maximum push task, penetration/aspiration scores, residue | Posterior lingual pressure and penetration/aspiration scores maintained over course of study, no change in residue | |
| Head / Neck cancer [26] | RCT Comparing Lingual exercise plus traditional therapy vs traditional therapy along | 23 | Tongue strength (maximum pressure), OPSE, salivary flow, quality of life | No change in tongue strength or OPSE, no significant differences in quality of life | |
| Head Lift | Older healthy adults [21] | Age-matched with Random Assignment to sham vs. head lift | 31 | UES opening, Hyolaryngeal excursion, pharyngeal swallowing pressures | Increased UES opening, increased anterior laryngeal excursion, reduced pharyngeal swallowing pressures |
| Pharyngeal dysphagia [22] | Cross over design | 27 | Swallow function, UES opening, Hyolaryngeal excursion, aspiration | Improved swallow function, increased UES opening, reduced aspiration | |
| Older healthy adults [27] | Pre/Post comparison | 2 | sEMG evaluation of muscle fatigue | Initial fatigue in SCM with Subsequent strengthening, increase strength in supra/infrahyoid muscles | |
| Head/Neck cancer and stroke [28] | Pre/Post Comparison with random assignment to traditional therapy vs. head lift | 11 | Thyrohyoid shortening during swallow | Post therapy Thyrohyoid shortening was greater in head lift subgroup | |
| Older healthy adults [29] | Prospective cohort with intervention | 26 | adherence to exercise program | 50% to 70% adherence | |
| MDTP | Chronic Pharyngeal dysphagia: Head/Neck Cancer and Stroke [23,30,31, 32] | Case series [23,32] | 9 [23] 6 [32] |
Clinical and functional change in swallowing, patient perception of swallowing, hyolaryngeal excursion, lingual swallowing pressure, pharyngeal swallowing pressure, sEMG amplitude during swallowing | Clinical and Functional swallowing, and patient perception scores improved significantly, increased hyolaryngeal excursion, increased lingual pressure and sEMG amplitude for pudding swallows, after treatment |
| Parallel arm Comparison (patients with dysphagia vs. healthy controls) [31] | 42 (8 patients and 34 controls) | Timing of Physiologic pressure points during swallowing | Physiologic timing of swallow events improved following therapy becoming equivalent to healthy controls, effect most noted for thin liquids | ||
| Case-Control : MDTP vs. Traditional therapy plus sEMG biofeedback[30] | 24 (8 cases and 16 controls) | Clinical and functional change in swallowing, presence of feeding post therapy, presence of aspiration post therapy | Enhanced clinical and functional outcomes, greater feeding tube removal, greater aspiration reduction, following MDTP | ||
| Stroke (subacute rehabilitation) [33] | RCT (MDTP with sham TES, MDTP with motor level TES, traditional therapy | 53 | Clinical and Functional swallowing ability, change in body weight, dysphagia-related complications, return to pre-stroke diet, patient perception of swallow, proportion of treatment responders | Clinical and Functional measures of swallowing, proportion of treatment responders and number returning to pre-stroke diet significantly improved for the sham arm (MDTP) greater than experimental (MDTP + TES) or control arms (traditional therapy). | |
| Pharyngocise | Head/Neck cancer treated during chemoradiotherapy [6,34] | RCT: Pharyngocise vs. usual care [6] | 58 [6] | Lingual, Suprahyoid muscle size and composition – measured by MRI, Functional swallowing ability, mouth opening, taste/smell function, salivation, nutritional status, occurrence of dysphagia – related complications. | Pharyngocise Group Demonstrated superior muscle preservation, functional swallowing, mouth opening, taste and salivation. |
| RCT: Therapist Directed Pharyngocise vs. Patient directed Pharyngocise vs. usual care (control) [34] | 130 [34] | Maintenance of swallow muscle composition (MRI), functional swallowing ability, mouth opening, psycho-social adaptation, exercise compliance. | Less swallow Muscle deterioration, less functional swallow change and greater compliance identified in the therapist directed arm compared to patient directed or control | ||
MRI: magnetic resonance imaging; RCT: randomized controlled trial; OPSE: oropharyngeal swallow efficiency; UES: upper esophageal sphincter; sEMG: surface electromyography; SCM: sternocleidomastoid; MDTP: McNeill Dysphagia Therapy Program; TES: transcutaneous electrical stimulation
Table 3.
Depiction of exercise goals and incorporated exercise principles in four exercise based dysphagia rehabilitation programs.
| Program | Goal | Principles utilized |
|---|---|---|
| Lingual resistance | Development of lingual strength to improve swallowing | Progression Overload Adaptation Intensity Recovery |
| Shaker head lift | To strengthen suprahyoid musculature and improve upper esophageal sphincter opening | Adaptation Recovery |
| MDTP | Progressive development, strengthening, and refinement of the muscular components of the swallowing process | Progression Overload Adaptation Intensity Reversibility Specificity Recovery |
| Pharyngocise | Maintenance of muscle structure involved in swallow function | Adaptation Reversibility Specificity Recovery |
Robbins' work with lingual resistance was among the first to systematically utilize the concept of progressive resistance in dysphagia therapy [20]. These investigators employed "one repetition maximum" as the criterion referent used to set the target level for lingual resistance. As patients increased strength, this target was increased resulting in a program of progressive resistance over time. Results of this lingual resistance program have been shown to produce increased lingual strength and functional swallowing benefit in patients post stroke [20] and recently with progressive neuromotor disease [25]. Conversely, a recent randomized trial by Lazarus and colleagues revealed no benefit from a lingual strengthening program in patients with dysphagia following treatment for head/neck cancer [26*]. Thus, lingual resistance/strengthening exercise might be promising for some forms of dysphagia, but perhaps not all. Future research will be important in identifying patient groups that might respond favorably to this form of dysphagia intervention.
Shaker's work with a head lift exercise [21, 22, 27, 28] indicates potential of this exercise to increase upper esophageal sphincter opening by strengthening suprahyoid musculature with resulting increased hyolaryngeal excursion. This technique may invoke a degree of progressive resistance as with fatigue the head essentially becomes 'heavier' necessitating additional neuromuscular effort to continue the activity. Adherence with this technique is moderate to low [29] questioning the feasibility of this approach in clinical practice. Despite this caveat, a recent systematic review concluded that this exercise based dysphagia intervention is a promising approach requiring further study to clarify its effectiveness [35*].
The McNeill Dysphagia Therapy Program (MDTP) is described as a systematic therapy program for dysphagia in adults that utilizes swallowing as an exercise [23, 30]. By having patients swallow different materials and volumes MDTP attempts to invoke multiple exercise principles to improve swallow physiology and functional swallowing ability. MDTP studies have demonstrated improved swallow function combined with stronger lingual-palatal contact and pharyngeal constriction pressures, greater hyolaryngeal excursion, and faster timing of swallow movements [23, 31, 32]. A recent randomized clinical trial [33] indicated that MDTP produces superior outcomes to traditional therapy in stroke patients during subacute rehabilitation.
Pharyngocise is a prophylactic exercise program applied to patients with head/neck cancer simultaneous with chemoradiation treatment [6]. This program incorporates simple activities (falsetto, tongue press, hard swallow, and jaw stretching) completed twice daily in repeated cycles requiring 45 minutes to complete. Based on two randomized clinical trials [6, 34], Pharyngocise patients demonstrated less deterioration in swallow function, dietary intake, chemosensory functions (taste and smell), salivation, nutritional status, and dysphagia-related complications. Moreover, Pharyngocise resulted in less structural deterioration in key muscles of swallowing (genioglossus, hyoglossus, mylohyoid).
Though each of these exercise based dysphagia interventions is 'scientifically young', each demonstrates potential to be developed into an effective rehabilitation approach for dysphagia in adults. Each of these approaches has demonstrated physiologic change in impaired swallow mechanisms in conjunction with improved swallowing function. Though each requires further, more rigorous scientific study to claim clinical effectiveness, each of these exercise based dysphagia rehabilitation approaches has demonstrated initial evidence of clinical benefit.
Transcutaneous electrical stimulation (TES)
Transcutaneous electrical stimulation (TES) intends to enhance movement by increasing muscle contraction in stimulated muscles [36]. While ample evidence indicates that TES can enhance contraction in different muscle groups, only scant information details the direct impact of TES on muscles of swallowing. For example, Ludlow and colleagues [37] demonstrated a mild lowering of the hyoid bone upon TES in stroke patients with dysphagia. Humbert and colleagues [38] demonstrated a similar impact in healthy adults. This lowering effect of TES on resting hyoid position has been translated into a potential swallowing exercise under the premise that lowering the hyoid before swallowing induces a form of resistance into the act of swallowing (eg. TES pulls hyoid down and swallowing must overcome that downward pull to raise the hyoid bone) [11, 39*]. Beyond this demonstrated physiologic impact of TES (and resulting potential therapy application), few studies have documented a convincing physiologic impact of TES on swallowing functions. Berretin-Felix and colleagues [40] did demonstrate interactions between TES amplitude and age in measured physiologic activity in healthy adult volunteers. Specifically, older adults demonstrated a decrease in certain swallow physiologic responses with motor level stimulation (higher amplitude). Nam and colleagues [41] demonstrated that different electrode placements might have a differential impact on swallow physiology in adult patients with dysphagia. Specifically, submental electrode placement resulted in increased hyoid, but not laryngeal excursion during swallowing. A combination of submental plus infrahyoid placement resulted in increased laryngeal, but not hyoid excursion. Furuta and colleagues [42] reported an increase in spontaneous swallowing frequency in healthy adult volunteers during TES using inferential current at a sensory level (lower amplitude). Finally, Heck, Doeltgen, and Huckabee [43] reported no immediate impact of submental TES on healthy adults, but they did note a delayed impact (lasting up to one hour) manifest as decreased hypopharyngeal contraction but increased upper esophageal sphincter relaxation.
Various applications of TES are perhaps the most studied approach to dysphagia rehabilitation in recent years. In fact, in the Carnaby and Harenberg survey of dysphagia clinicians in the USA [9] TES was the most commonly reported dysphagia intervention. However, this high frequency of clinical application emerges in the presence of highly conflicted and often confusing data from treatment studies evaluating the clinical/functional impact of TES on impaired swallowing.
Though recent reports have incorporated stronger research designs than initial studies of this potential intervention, significant questions remain regarding the value of TES as a treatment for adult dysphagia [44]. For example, Baijens and colleagues [45] reported no enhanced clinical benefit of submental TES when combined with 'traditional' dysphagia therapy in patients with dysphagia secondary to Parkinson disease. Conversely, Kushner and colleagues [46] reported that TES combined with 'traditional' dysphagia produced significantly enhanced outcomes compared to traditional therapy alone in patients with dysphagia following stroke. A similar outcome was reported by Sun and colleagues [47] in a case series study of adult patients with dysphagia following stroke. Conversely, Tan et al [48*] reported results from a meta-analysis and concluded that TES was comparable to traditional dysphagia therapy following stroke, but that TES was more effective than traditional dysphagia in patients with dysphagia from variable etiologies. Perhaps the most rigorous study of TES combined with behavioral dysphagia therapy was reported by Carnaby et al [33]. This double blind, randomized clinical trial compared three treatment approaches in patients receiving dysphagia therapy in a subacute rehabilitation center: traditional dysphagia therapy, McNeill Dysphagia Therapy Program (MDTP) with sham TES, and MDTP with motor level TES. Results indicated that MDTP with either sham or motor level TES produced superior outcomes to traditional therapy. However, TES did not enhance the benefit of MDTP. Rather, MDTP plus motor level TES demonstrated inferior outcomes to MDTP with sham TES.
Many questions remain regarding the appropriate application of TES to dysphagia intervention in adults. Current literature indicates that a "one size fits all approach" for this adjunctive modality may be inappropriate. Recent studies have improved the scientific rigor with which this modality is studied, but extensive variability and limited scientific control remain. Future efforts should focus on appropriate patient selection, electrode placement, stimulation parameters, appropriate combination with exercise programs and more clinical considerations to effectively evaluate the potential benefit of this modality.
Conclusion
Dysphagia intervention in adults is experiencing new directions regarding the inclusion of exercise principles and the application of adjunctive modalities; specifically TES. Though exercise techniques have been utilized in swallowing rehabilitation, traditional approaches borrowed heavily from motor speech intervention approaches and focused primarily on oral motor activities. More recent approaches focus on inclusion of specific exercise principles (progressive resistance, intensity, specificity, recovery, and more) with resulting therapy 'programs' rather than individual techniques. Moreover, reported outcomes from these exercise-based therapy programs are promising both in reference to change in swallow physiology and enhanced functional outcomes. In addition, TES is continuing to be extensively studied both in reference to its potential physiologic impact on the swallow mechanism and potential benefit to functional outcomes of rehabilitation. However, at this juncture, many questions remain unanswered regarding the appropriate application of TES. Novel applications have recently emerged, but though interesting, additional study is warranted prior to general acceptance. Perhaps the best guidance based on available literature is to consider TES as an adjunctive modality and to use it in individual patients only when published literature provides specific application guidelines in addition to clear expectations for both physiologic and functional improvement.
Key Points.
Dysphagia rehabilitation for adults is experiencing a paradigm shift.
Though dysphagia clinicians often report inclusion of exercise for dysphagia rehabilitation, systematic incorporation of known exercise principles is only now emerging.
Exercise based dysphagia interventions apply to both rehabilitation and to prevention of dysphagia.
Transcutaneous electrical stimulation (TES) for dysphagia rehabilitation may be a useful adjunct to some exercise based interventions; but, additional details are required to effectively apply this modality.
Footnotes
Funding Disclosure: none
Contributor Information
Michael A. Crary, Swallowing Research Laboratory, Department of Speech, Language, and Hearing Sciences, University of Florida, Gainesville, FL, USA
Giselle D. Carnaby, Swallowing Research Laboratory, Department of Behavioral Science and Community Health, University of Florida, Gainesville, FL, USA
References
- 1.Groher ME, Crary MA. Dysphagia: Clinical management in adults and children. Maryland Heights, MO: Mosby/Elsevier; 2010. [Google Scholar]
- 2.Smith SK, Roddam H, Sheldrick H. Rehabilitation or compensation: time for a fresh perspective on speech and language therapy for dysphagia and Parkinson's disease? Int J Lang Commun Disord. 2012;47:351–364. doi: 10.1111/j.1460-6984.2011.00093.x. [DOI] [PubMed] [Google Scholar]
- 3.Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006;116:883–886. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 4.Carroll WR, Locher JL, Canon CL, et al. Pretreatment swallowing exercises improve swallowing function after chemoradiation. Laryngoscope. 2008;118:39–43. doi: 10.1097/MLG.0b013e31815659b0. [DOI] [PubMed] [Google Scholar]
- 5.van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemoradiotherapy: Feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155–170. doi: 10.1007/s00455-010-9288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carnaby-Mann GD, Crary MA, Schmalfuss I, Amdur R. "Pharyngocise": Randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiation Oncol Biol Phys. 2012;83:210–219. doi: 10.1016/j.ijrobp.2011.06.1954.. This article describes a prophylactic intervention which results in preservation of muscle structure, swallow function, and related oral functions in head/neck cancer patients treated with chemoradiation.
- 7. Paleri V, Roe JW, Strojan P, et al. Strategies to reduce long-term postchemoradiation dysphagia in patients with head and neck cancer: An evidence-based review. Head Neck. 2013 Jul 4; doi: 10.1002/hed.23251. [epub ahead of print]. This article summarizes available evidence regarding preventative exercise, nasogastric tube utilization, and radiation dose restriction to facilitate enhanced swallowing outcomes following treatment for head and neck cancer.
- 8. Archer SK, Wellwood I, Smith CH, Newham DJ. Dysphagia therapy in stroke: a survey of speech and language therapists. Int J Lang Commun Disord. 2013;48:283–296. doi: 10.1111/1460-6984.12006.. This article provides a good survey-based depiction of dysphagia rehabilitation practice in the UK.
- 9. Carnaby GD, Harenberg L. What is 'usual care' in dysphagia rehabilitation: a survey of USA dysphagia practice patterns. Dysphagia. 2013;28:p567–p574. doi: 10.1007/s00455-013-9467-8.. This article provides a good survey-based depiction of dysphagia rehabilitation practice in the USA. It provides a good comparison for the Archer et al reference [8].
- 10.McArdle WD, Katch FI, Katch VL. Essentials of Exercise Physiology. 3rd edition. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- 11.Lee T, Swanson L, Hall AL. What is repeated in repetition" Effects of practice conditions on motor skill acquisition. Physical Therapy. 1991;71:150–156. doi: 10.1093/ptj/71.2.150. [DOI] [PubMed] [Google Scholar]
- 12.Curtis C, Weir J. Overview of exercise responses in health and impaired states. J Neurologic Phys Therapy. 1996;20:13–19. [Google Scholar]
- 13.Rivera-Brown AM, Frontera WR. Principles of exercise physiology: responses to acute exercise and long-term adaptations to training. PMR. 2012;4:797–804. doi: 10.1016/j.pmrj.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21:628–633. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Humbert IA, Christopherson H, Lokhande R, et al. Human hyolaryngeal movements show adaptive motor learning during swallowing. Dysphagia. 2013;28:139–145. doi: 10.1007/s00455-012-9422-0.. This article demonstrates the presence of motor adaptive learning within the swallowing mechanism along with a potential method to assess adaptive learning.
- 16.Nekl CG, Lintzenich DR, Leng X, et al. Effects of effortful swallow on esophageal function in healthy adults. Neurogastroenterol Motil. 2012;24:252–e108. doi: 10.1111/j.1365-2982.2011.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough GH, Kim Y. Effects of Mendelsohn maneuver on extent of hyoid movement and UES opening post-stroke. Dysphagia. 2013;28:511–519. doi: 10.1007/s00455-013-9461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuoka T, Ono T, Hori K, et al. Effect of the effortful swallow and the Mendelsohn maneuver on tongue pressure production against the hard palate. Dysphagia. 2013;28:539–547. doi: 10.1007/s00455-013-9464-y. [DOI] [PubMed] [Google Scholar]
- 19.Wada S, Tohara H, Iida T, et al. Jaw-opening exercise for insufficient opening of upper esophageal sphincter. Arch Phys Med Rehabil. 2012;93:1995–1999. doi: 10.1016/j.apmr.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Robbins J, Kays GA, Gangnon RE, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88:150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Shaker R, Kern M, Bardan E, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol. 1997;272:G1518–G1522. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]
- 22.Shaker R, Easterling C, Kern M, et al. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122:1314–1321. doi: 10.1053/gast.2002.32999. [DOI] [PubMed] [Google Scholar]
- 23.Crary MA, Carnaby GD, LaGorio LA, Carvajal PJ. Functional and physiologic outcomes from an exercise-based dysphagia therapy: a pilot investigation of the McNeill Dysphagia Therapy Program. Arch Phys Med Rehabil. 2012;93:1173–1178. doi: 10.1016/j.apmr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Robbins J, Gangnon RE, Theis SM, et al. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 25.Malandraki GA, Kaufman A, Hind J, et al. The effects of lingual intervention in a patient with inclusion body myositis and Sjögren's syndrome: a longitudinal case study. Arch Phys Med Rehabil. 2012;93:1469–1475. doi: 10.1016/j.apmr.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 26. Lazurus CL, Husaini H, Falciglia D, et al. Effects of exercise on swallowing and tongue strength in patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. Int J Oral Maxillofac Surg. 2013 Dec 10; doi: 10.1016/j.ijom.2013.10.023. [epub ahead of print]. This article will aid clinicians in the application of lingual resistance exercises by demonstrating that certain clinical group (eg head/neck cancer) do not necessarily benefit from this exercise.
- 27.White KT, Easterling C, Roberts N, et al. Fatigue analysis before and after shaker exercise: physiologic tool for exercise design. Dysphagia. 2008;23:385–391. doi: 10.1007/s00455-008-9155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mepani R, Antonik S, Massey B, et al. Augmentation of deglutitive thyrohyoid muscle shortening by the Shaker exercise. Dysphagia. 2009;24:26–31. doi: 10.1007/s00455-008-9167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Easterling C, Grande B, Kearn M, et al. Attaining and maintaining isometric and isokinetic goals of the Shaker exercise. Dysphagia. 2005;20:133–138. doi: 10.1007/s00455-005-0004-2. [DOI] [PubMed] [Google Scholar]
- 30.Carnaby-Mann GD, Crary MA. McNeill dysphagia therapy program: a case-control study. Arch Phys Med Rehabil. 2012;91:743–749. doi: 10.1016/j.apmr.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Lan Y, Ohkubo M, Berretin-Felix G, et al. Normalization of temporal aspects of swallowing physiology after the McNeill dysphagia therapy program. Ann Otol Rhinol Laryngol. 2012;121:525–532. doi: 10.1177/000348941212100806. [DOI] [PubMed] [Google Scholar]
- 32.Carnaby-Mann GD, Crary MA. Adjunctive neuromuscular electrical stimulation for treatment-refractory dysphagia. Ann Otol Rhinol Laryngol. 2008;117:279–287. doi: 10.1177/000348940811700407. [DOI] [PubMed] [Google Scholar]
- 33.Carnaby GD, LaGorio L, Miller D, et al. A randomized double blind trial of neuromuscular electrical stimulation + McNeill Dysphagia Therapy (MDTP) after stroke (ANSRS). Presentation to the Dysphagia Research Society Annual Meeting; March, 2012; Toronto, Canada. [Google Scholar]
- 34.Carnaby GD, Crary MA, Amdur R, et al. Dysphagia prevention exercises in head neck cancer: Pharyngocise dose response study. Presentation to the Dysphagia Research Society Annual Meeting; March, 2012; Toronto, Canada. [Google Scholar]
- 35. Antunes EB, Lunet N. Effects of the head lift exercise on the swallow function: a systematic review. Gerodontology. 2012;29:247–257. doi: 10.1111/j.1741-2358.2012.00638.x.. This systematic review summarizes the vast majority of work evaluating the Shaker head lift exercise program.
- 36.Humbert IA, Michou E, MacRae PR, Crujido L. Electrical stimulation and swallowing: how much do we know? Semin Speech Lang. 2012;33:203–216. doi: 10.1055/s-0032-1320040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludlow CL, Humbert IA, Saxon K, et al. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia. 2007;22:1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humbert IA, Poletto CJ, Saxon KG, et al. The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. J Appl Physiol. 2006;101:1657–1663. doi: 10.1152/japplphysiol.00348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park JW, KIm Y, Oh JC, Lee HJ. Effortful swallowing training combined with electrical stimulation in post-stroke dysphagia: a randomized controlled study. Dysphagia. 2012;27:521–527. doi: 10.1007/s00455-012-9403-3.. This article represents one of the initial attempts to use TES as a source of resistance against which to exercise the swallowing mechanism in patients with dysphagia.
- 40.Berretin-Felix G, Carnaby-Mann GD, Sia I, Crary MA. NMES immediate effects on swallow physiology in younger vs. older healthy volunteers. Presentation to the Dysphagia Research Society Annual Meeting; March, 2011; San Antonio, TX. [Google Scholar]
- 41.Nam HS, Beom J, Oh BM, Han TR. Kinematic effects of hyolaryngeal electrical stimulation therapy on hyoid excursion and laryngeal elevation. Dysphagia. 2013;28:548–556. doi: 10.1007/s00455-013-9465-x. [DOI] [PubMed] [Google Scholar]
- 42.Furuta T, Takemura M, Tsujita J, Oku Y. Interferential electric stimulation applied to the neck tissues increases swallow frequency. Dysphagia. 2012;27:94–100. doi: 10.1007/s00455-011-9344-2. [DOI] [PubMed] [Google Scholar]
- 43.Heck FM, Doeltgen SH, Huckabee ML. Effects of submental neuromuscular electrical stimulation on pharyngeal pressure generation. Arch Phys Med Rehabil. 2012;93:2000–2007. doi: 10.1016/j.apmr.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Clark H, Lazarus C, Arvedson J, et al. Evidence-based systematic review: effects of neuromuscular electrical stimulation on swallowing and neural activation. Am J Speech Lang Pathol. 2009;18:361–375. doi: 10.1044/1058-0360(2009/08-0088). [DOI] [PubMed] [Google Scholar]
- 45.Baijens LW, Speyer R, Passos VL, et al. Surface electrical stimulation in dysphagia Parkinson patients: a randomized clinical trial. Laryngoscope. 2013;123:E38–E44. doi: 10.1002/lary.24119. [DOI] [PubMed] [Google Scholar]
- 46.Kushner DS, Peters K, Eroglu ST, et al. Neuromuscular electrical stimulation efficacy in acute stroke feeding tube-dependent dysphagia during inpatient rehabilitation. Am J Phys Med Rehabil. 2013;92:486–495. doi: 10.1097/PHM.0b013e31828762ec. [DOI] [PubMed] [Google Scholar]
- 47.Sun SF, Hsu CW, Lin HS, et al. Combined neuromuscular electrical stimulation (NMES) with fiberoptic endoscopic evaluation of swallowing (FEES) and traditional swallowing rehabilitation in the treatment of stroke-related dysphagia. Dysphagia. 2013;28:557–566. doi: 10.1007/s00455-013-9466-9. [DOI] [PubMed] [Google Scholar]
- 48. Tan C, Liu Y, Li W, et al. Transcutaneous neuromuscular electrical stimulation can improve swallowing function in patients with dysphagia caused by non-stroke diseases: a meta-analysis. J Oral Rehab. 2013;40:472–480. doi: 10.1111/joor.12057.. This article is unique in that it quantitatively compares multiple studies utilizing electrical stimulation in dysphagia intervention across various patient groups.


