Abstract
Genes constitute ~3% of the human genome, whereas human endogenous retroviruses (HERVs) represent ~8%. We examined post-burn HERV expression in patients’ blood cells, and the inflammatory potentials of the burn-associated HERVs were evaluated. Buffy coat cells, collected at various time points from 11 patients, were screened for the expression of eight HERV families, and we identified their divergent expression profiles depending on patient, HERV, and time point. The population of expressed HERV sequences was patient-specific, suggesting HERVs’ inherent genomic polymorphisms and/or differential expression potentials depending on characteristics of patients and courses of injury response. Some HERVs were shared among the patients, while the others were divergent. Interestingly, one burn-associated HERV gag gene from a patient’s genome induced IL-6, IL-1β, Ptgs-2, and iNOS. These findings demonstrate that injury stressors initiate divergent HERV responses depending on patient, HERV, and disease course and implicate HERVs as genetic elements contributing to polymorphic injury pathophysiology.
Keywords: HERV polymorphism, burn patient, divergent injury response
Introduction
The stress signals originating from burn injury sites are often transmitted to distant organs through various layers of both characterized and uncharacterized pathways, leading to divergent and often unpredictable clinical manifestations such as inflammatory disorder and organ failure (Fayazov et al., 2009; Kallinen et al., 2012). The mechanisms underlying the complex and polymorphic network of post-burn pathologic events have been investigated primarily by studying the relationship between burn-incited phenotypes and altered functions of genes, focusing on differential expression profiles and non-synonymous single nucleotide polymorphisms (SNPs) (Barber et al., 2004; Barber et al., 2006). Although substantial progress has been made in understanding the basics of local and distant response to burn injury, the vast majority of the multifactorial characteristics of the disease courses and clinical outcomes occurring in a heterogeneous population of burn patients are far from being fully grasped.
Human endogenous retroviruses (HERVs) occupy ~8 % of the human genome while the entire set of protein coding genes consists of only ~3 % (Lander et al., 2001; Venter et al., 2001). HERVs are reported to participate in a range of disease processes such as degeneration of oligodendrocytes, type-I diabetes mellitus, rheumatoid arthritis, and breast cancer (Conrad et al., 1997; Contreras-Galindo et al., 2008; Frank et al., 2005; Freimanis et al., 2010). In addition, the envelope (env) polypeptides of certain murine endogenous retroviruses (ERVs) are capable of inducing pro-inflammatory cytokines (e.g., IL-6) in macrophages (Lee et al., 2011). Burn-elicited stress signals have been found to differentially alter the expression of murine ERVs, some of which retain intact coding potentials for virion assembly, in a tissue/cell type- and time after injury-specific manner (Cho et al., 2008; Kwon et al., 2009; Lee et al., 2008), unpublished data). The ERVs, which are activated in response to burn-incited stress signals, may exert their biologic activity via their gene products and/or replication/infection (Boller et al., 2008; Holder et al., 2012; Weis et al., 2007). Alternatively, ERVs, which are integrated into genes, may affect their neighboring genes through their transcription regulatory activity and post-transcriptional modifications, including alternative splicing that leads to the generation of fusion transcripts (Feuchter-Murthy et al., 1993; Medstrand et al., 2001; Ting et al., 1992).
The human population, regardless of genetic background, is presumed to share a substantial number of HERV loci in their genomes; however, at the same time, it is anticipated that each individual has a unique genomic HERV profile. We postulate that the polymorphic HERV profiles in the genomes of a heterogeneous population of burn patients are closely linked to the divergent and often unpredictable disease courses and outcomes. In this study, post-burn changes in the HERV expression profiles were examined in a heterogeneous patient population, and pathologic properties of the gene products of the burn-associated HERVs were examined.
Materials and Methods
Patient population and blood collection
This study has been reviewed and approved by the Institutional Review Boards Administration of the University of California, Davis and University of Michigan, Ann Arbor in accordance with the common rule and any other governing regulations. Participants or the next of kin, caretakers, or guardians on the behalf of the minors/children provide their written informed consent to participate in this study. Subjects enrolled in this study had a minimum of a 30% total body surface area burn. Detailed information regarding the patients and schedules for the blood sample collection is summarized in Table 1. Approximately 4–8 ml of blood samples were collected at several time points up to 270 days post-admission.
Table 1.
Patient demographics and sample collection time points.
| Patients | Age | Sex | Race | Sample collection: Post-burn time points (hour)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||
| Patient-1 | 6 | F | C | 48 | 63 | 135 | 303 | 643 | 6473 | |||||
| Patient-2 | 13 | M | C | 13 | 25 | 48 | 61 | 157 | 325 | 669 | 2156 | |||
| Patient-3 | 20 | M | C | 74 | 141 | 316 | 645 | |||||||
| Patient-4 | 57 | M | C | 19 | 24 | 49 | 73 | 210 | 354 | 657 | 1261 | 1504 | 1625 | 3953 |
| Patient-4:D1 | N/A | F | N/A | |||||||||||
| Patient-4:D2 | N/A | F | N/A | |||||||||||
| Patient-5 | 44 | M | C | 24 | 30 | 45 | 69 | 165 | 373 | 679 | 1665 | |||
| Patient-6 | 3 | M | H | 24 | 34 | 49 | 72 | 196 | 364 | 633 | 2360 | |||
| Patient-7 | 2 | M | H | 25 | 28 | 45 | 70 | 193 | 362 | 696 | 1416 | |||
| Patient-8 | 17 | F | H | 1 | 9 | 54 | 79 | 178 | 342 | 703 | ||||
| Patient-9 | 12 | M | H | 16 | 26 | 46 | 68 | 165 | 333 | |||||
| Patient-10 | 5 | F | H | 4 | 12 | 61 | 84 | 200 | 368 | |||||
| Patient-11 | 53 | F | C | 96 | 192 | 360 | 432 | 936 | 1200 | 1272 | ||||
F (female); M (male); C (Caucasian); H (Hispanic).
Blood samples from the two daughters (D1 and D2) of patient-4 were obtained as a no-burn control.
Semi-quantitative RT-PCR analyses of HERV expression
Buffy coat was isolated from each blood sample by centrifugation at 2,000 xg for 10 minutes at room temperature. Total RNA was isolated from the buffy coat using the RNeasy Mini kit (Qiagen, Valencia, CA) with modifications, including treatment with TRIzol (Invitrogen, Carlsbad, CA) and DNase I (to remove any genomic DNA contamination). cDNA was synthesized using 100 ng of total RNA from each sample, Sensiscript reverse transcriptase (Qiagen), RNase inhibitor (Promega, Madison, WI) and an oligo-dT primer (5′-GGC CAC GCG TCG ACT AGT ACT TTT TTT TTT TTT TTT T- 3′). The absence of genomic DNA contamination in the cDNA preparations was verified using the control samples without reverse transcriptase treatment. The primer sets, which were used to amplify the 3′ long terminal repeat (LTR) regions of eight different HERV families, are listed in Table 2. β-actin was amplified as a normalization control using the primer set: 5′-CCA ACT GGG ACG ACA TGG AG-3′ and 5′-GTA GAT GGG CAC AGT GTG GG-3′. Densitometric quantitation was performed for the individual HERV amplicons using the Kodak MI system (Carestream Health, Rochester, NY). The intensity of each HERV amplicon was normalized with the matching β-actin.
Table 2.
(top) primers for HERVs, (middle) primers for gag genes, and (bottom) primers for inflammatory mediators
| HERV family | Primer name | Sequence | Reference | |
|---|---|---|---|---|
| HERV | E | HERV-E(F) HERV-E(R) |
CACTTCTCCTGTTGTCCTT TACAACTCTAAGGGGTCT |
M10976 and AB062274 |
| FRD | HERV-Frd(F) HERV-Frd(R) |
CAGG/TCTCCCAAC/TAGATAGA AGAGCAGGAG/ATGAAAGGAA |
AC004022 | |
| H | HERV-H(F) HERV-H(R) |
CTGATGAC/TATTCCACCA AGACCACCAAACAGGC/GTT |
D11078, AJ289709, AJ289711, M18048 | |
| L | HERV-L(F) HERV-L(R) |
GATTGG/TATG/TGAAGGATGC CTGAGTCTGAGTCCCAAAGC |
X89211 | |
| R | HERV-R(F) HERV-R(R) |
AGGAAAAACAAGTAAAGGG CCGTGACAGGTTTTACAA |
M12410 | |
| K1 | HERV-K(HML1)(F) HERV-K(HML1)(R) |
CTGCTCTCCATTATCTCAAGT AAGGAATGAGAAAAGACAGT |
Repbase version 8.2.0 consensus sequences | |
| K2 | HERV-K(HML2)(F) HERV-K(HML2)(R) |
GTCATCACCACTCCCTAATCT GGAAAAGAAAAAGACACAGAG |
AF074086, AF164609, AF164610 | |
| K4 | HERV-K(HML4)(F) HERV-K(HML4)(R) |
TGTGGGCGAAGGATTACC AATGTCCCTTCAGCACCT |
AF020092 | |
| K5 | HERV-K(HML5)(F) HERV-K(HML5)(R) |
GCAGAGGGCAAGGAGTAG GCGATGGATGAAAGGAGTA |
Repbase version 8.2.0 consensus sequences | |
| K6 | HERV-K(HML6)(F) HERV-K(HML6)(R) |
CAGAATGTGGGCAAACTC TGAACAAAGGAGGACGAA |
AF079797 | |
| W | HERV-W(F) HERV-W(R) |
AGAGCACAGCAGGAGGGA GGGGTCCTTGCTCACAGA |
AY101582, AY101583, AY101584 AY101585 | |
| RRI | RRHERV-I(F) RRHERV-I(R) |
TATCTCTCCCTTTCCCAG AGGAATCAGAGAGACCA/GG/ATG |
M64936 | |
| HERV | Region | Primer name | Sequence | Reference |
|---|---|---|---|---|
| HERV-K109 (AF164615.1) | LTR-gag | 6D01_LTR-1A 3D02_Sgag-2A |
CAGTCTCAGGTGTTTGGATCTTCCAC CGCGgcggccgcCTACTGCTGCACTGCCGCTTGT |
NT_007299.13 (16546496-16555953) |
| gag | 3D02_Sgag-1A 3D02_Sgag-2A |
CGCGgcggccgcATGGGGCAAACTAAAAGTAAAG CGCGgcggccgcCTACTGCTGCACTGCCGCTTGT |
||
| HERV-K115 (AY037929.1) | LTR-gag | 8D01_LTR-1A 3D02_Lgag-2A |
TGATCAATATAAAAGGTGTAGGGGTGG CGCGgcggccgcGCTTATTCCCTGAAACACTTGGGAC |
NT_023736.17 (7345381-7354859) |
| gag | 3D02_Lgag-1B 3D02_Lgag-2A |
CGCGgcggccgcGCTAGGGTGATAATGGGGCAAAC CGCGgcggccgcGCTTATTCCCTGAAACACTTGGGAC |
| Cytokines | Primer name | Sequence | Reference |
|---|---|---|---|
| Ptgs2 | COX2_1B COX2_2A |
ACACAGTGCACTACATCCTGAC ATCATCTCTACCTGAGTGTC |
NM_011198.3 |
| ICAM1 | ICAM1_ 1A ICAM1_ 2A |
AGCTGTTTGAGCTGAGCGAGA CTGTCGAACTCCTCAGTCA |
NM_010493.2 |
| Il1b | Il1b_ 1A Il1b_ 2A |
GACAGTGATGAGAATGACCTG GAACTCTGCAGACTCAAACTCCA |
NM_008361.3 |
| Il6 | Il6_1E Il6_2G |
GCCTTCCCTACTTCACAAGTCC CACTAGGTTTGCCGAGTAGATCTC |
NM_031168.1 |
| Nos2 | Nos2_1B Nos2_2B |
ACAAGCTGCATGTGACATCGA CAGAGCCTGAAGTCATGTTTGC |
NM_010927.3 |
| Tnf | Tnf_1A Tnf_2A |
GCATGATCCGCGACGTGGAA AGATCCATGCCGTTGGCCAG |
NM_013693.2 |
| b-actin | b-actin-1A b-actin-2A |
CCAACTGGGACGTGGAA GTAGATGGGCACAGTGTGGG |
NM_007393.3 NM_001101.3 |
bold font (degenerate nucleotides); italic font (NotI restriction enzyme site)
Cloning and sequencing
A total of 344 HERV amplicons (from patient-1, patient-2, patient-4, and patient-11) were purified using the QIAquick Gel Extraction kit (Qiagen) and then cloned into the pGEM-T Easy vector (Promega). Three clones were picked for each amplicon, and plasmid DNAs were prepared using the QIAprep Miniprep kit (Qiagen) for sequencing analysis. Sequencing was performed at Functional Biosciences (Madison, WI). DNA sequences were analyzed using the EditSeq and MegAlign programs (DNASTAR, Madison, WI).
Multiple alignment and phylogenetic analyses of expressed HERV sequences within each HERV family
A total of 1,026 3′ LTR region sequences were obtained from the 344 HERV amplicons. To evaluate whether the expressed HERV sequences are shared among the four patients (patient-1, patient-2, patient-4, and patient-11), the LTR region sequences were subjected to alignment analyses within each HERV family using the ClustalW protocol, and phylogenetic trees were generated using the MEGA4 program (Tamura et al., 2007).
In silico mapping of HERV loci
Among the 137 and 202 unique 3′ LTR region sequences which were identified from patient-1 and 2, respectively, only 37 sequences were shared by both patients. The reference human genome database (Build 37.1) from the National Center for Biotechnology Information (NCBI) was surveyed for putative HERVs which share greater than 98 % identity using each unique 3′ LTR region sequence as a mining probe and the Advanced Blast program. The percent identity was reduced to 95 % or 90 % step-wise if no hits were retrieved with the 98 % identity threshold. The regions, which span 12 Kb upstream and downstream from the individual LTR hits, were surveyed to identify putative HERV loci. For each putative HERV locus, the coding potentials for three genes (gag, pol, and env) were examined using the SeqBuilder program (DNASTAR). The open reading frames, which encode greater than 100 amino acids, were recorded and the others were denoted as defective.
Cloning of gag polypeptide coding sequences from a patient’s genomic DNA
The gag polypeptide coding regions of two different HERVs were amplified from patient-1’s genomic DNA by a two-step PCR protocol using a combination of two primer sets for each HERV to obtain locus-specificity (primer sequences are listed in Table 2). First, the 5′ LTR-gag regions were amplified (30 cycles) using a set of primers that span the 5′-proviral junction to the end of the gag coding sequence. During the second round of PCR (20 cycles), the specific gag coding regions (start to end) were amplified from the 5′ LTR-gag amplicon from the first PCR, followed by cloning into the pGEM-T Easy vector (Promega) and subcloning into the pcDNA4/HisMax expression vector (Invitrogen). All constructs were sequenced to confirm the inserts.
Real-time RT-PCR measurement of inflammatory mediators in RAW264.7 cells
RAW264.7 cells were transfected with individual pcDNA4/HisMax expression constructs (two gag coding sequences, one gag in reverse coding orientation, and vector only). Transfected cells were harvested at day 1 to examine changes in the expression (mRNA) of a set of six inflammatory mediators by real-time RT-PCR (primer sequences for each mediator are listed in Table 2). Total RNA was isolated using the RNeasy Mini kit (Qiagen), and real-time RT-PCR was performed using the QuantiTect RT kit (Qiagen) and Brilliant III SYBR green QPCR master mix in the Mx3005P cycler (Agilent Technologies, Santa Clara, CA).
Statistical analysis
Statistical analysis was performed using a one-way ANOVA and Tukey’s HSD test, and statistical significance was determined as *P < 0.05 and **P < 0.01.
Results
Divergent and dynamic HERV expression profiles among burn patients
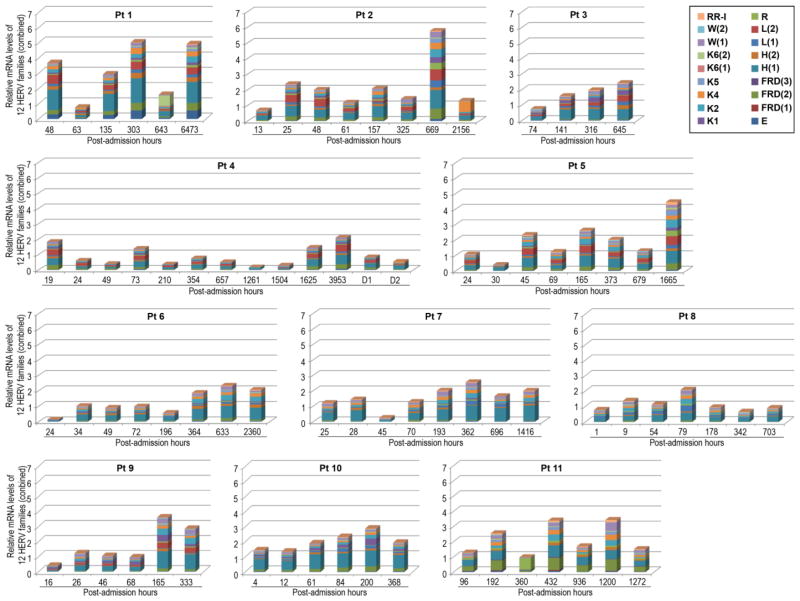
The blood samples of the 11 patients (Table 1), which were collected at various post-admission time points, were subjected to semi-quantitative RT-PCR analyses to determine whether burn-elicited stress signals and accompanying clinical courses alter the expression of 12 HERV families (Table 2). Within each patient, there were dynamic changes in the expression patterns of the individual HERV families in a time after injury-dependent manner (Figure 1). The patient-specific temporal HERV expression profile may be directly linked to the inherent genomic HERV polymorphisms among patients and/or differential expression depending on age, gender, clinical courses, and/or injury severity. Some HERV families (e.g., HERV-E, HERV-K(HML-1) [HERV-K1], RRHERV-I) were expressed only in certain patients, but not in others. In another analysis for individual patients, at each time point, the post-injury fold-changes in all HERV amplicons derived from the 12 families were compiled in order to examine accumulative temporal changes in HERV expression (Figure 2). There were substantial differences in the accumulative levels of HERV expression over the various post-injury time points in certain patients (e.g., patient-1, patient-2) compared to the others (e.g., patient-4, patient-8, patient-10). Since only a limited number of patients were enrolled in this study, it may not be practical to correlate the HERV expression data with any specific disease courses (e.g., septic episodes) and/or treatment protocols (e.g., transfusion, surgery).
Figure 1. Post-burn alterations in HERV expression: patient-, HERV-, and time after injury-specific patterns.
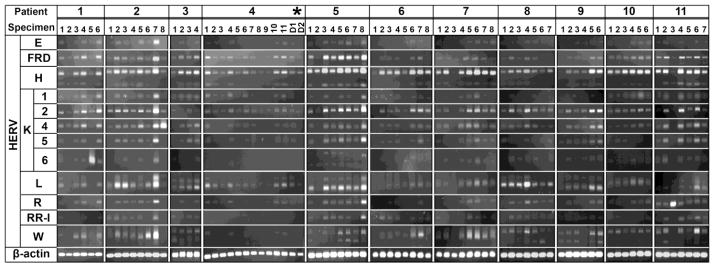
Post-burn changes in the expression of 12 different HERV families were analyzed by semi-quantitative RT-PCR. The post-burn HERV expression profiles were specific for patient, HERV family, and time point (Please refer to Table 1 for detailed time point information). *Blood samples from the two daughters of patient-4 (D1 and D2) were included as no-injury controls. β-actin serves as a normalization control. RR-I (RRHERV-I).
Figure 2. Dynamic and patient-specific changes in the overall HERV expression at different post-burn time points.
The post-burn changes in the levels of the individual amplicons from 12 HERV families were combined at each time point (represented as a single mosaic bar) for each patient. Five HERV families (FRD, H, L, K(HML-6) [K6] and W) had multiple amplicons, which are indicated by sequential numbers in parenthesis in the legend. There were dynamic and patient-specific changes in the overall expression of HERVs at various post-burn time points. Pt (patient); RR-I (RRHERV-I).
Prevalence of uncommon and patient-specific expressed HERV sequences
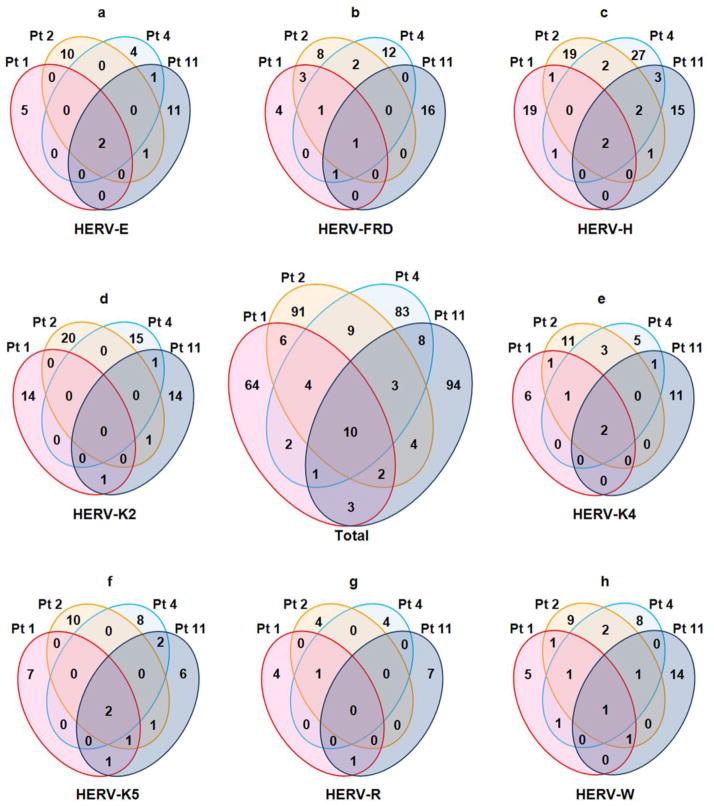
The entire set of HERV RT-PCR amplicons from four patients (patient-1, patient-2, patient-4, and patient-11) was subjected to sequence analyses. One to three 3′ long terminal repeat (LTR) sequences were obtained from each visible amplicon: 211 3′ LTR sequences from 67 amplicons in patient-1; 276 sequences from 92 amplicons in patient-2; 297 sequences from 99 amplicons in patient-4; and 242 sequences from 86 amplicons in patient-11. For each patient, the population of HERV LTR sequences derived from the entire set of amplicons was sorted by multiple alignment and phylogenetic analysis to identify unique LTR sequences within each HERV family as well as among all the HERV families combined. There were 137, 202, 154, and 159 unique sequences from patient-1, patient-2, patient-4, and patient-11, respectively, when all the HERV amplicons were combined. It needs to be noted that certain HERV families were not expressed in all four patients. Among the 3′ LTR sequences of HERV-H (both H1 and H2 amplicons) (23 from patient-1, 27 from patient-2, 37 from patient-4, and 23 from patient-11), only two were shared by all four patients, and the vast majority were unique for each patient (Figure 3-panel c). Similarly, none of the HERV-K(HML-2) (HERV-K2) LTR sequences (15 from patient-1, 21 from patient-2, 16 from patient-4, and 17 from patient-11) were shared among the four patients and only two sequences, one from the HERV-K(HML-4) (HERV-K4) family and the other HERV-K(HML-5) (HERV-K5) family, were found in all four patients (Figure 3-panel d). Among the LTR sequences from all HERV families, only 10 sequences were shared by all four patients while each patient had a number of unique sequences ranging from 64 (patient-1) to 94 (patient-11) (Figure 3-center panel). These findings suggest that there is a high prevalence of uncommon expressed HERV sequences among the four patients examined in this study.
Figure 3. High degree of polymorphisms in post-burn expressed HERVs among patients.
The HERV 3′ LTR sequences, which were isolated from RT-PCR amplicons of HERV-E, HERV-FRD, HERV-H, HERV-K2, HERV-K4, HERV-K5, HERV-R, and HERV-W from each patient, were compared among four patients (patient-1, patient-2, patient-4, and patient-11) by multiple alignment analyses. A Venn diagram, which shows the numbers of shared and unique HERV LTR sequences, was drawn for each HERV family to show the polymorphisms of expressed HERV sequences among the four patients. The center Venn diagram (labeled “Total”) combines the results from all eight HERV families. The profiles of post-burn expressed HERVs were highly polymorphic among the four patients in all eight HERV families.
HERV family-specific differential population diversity of expressed 3′ LTR sequences among the patients
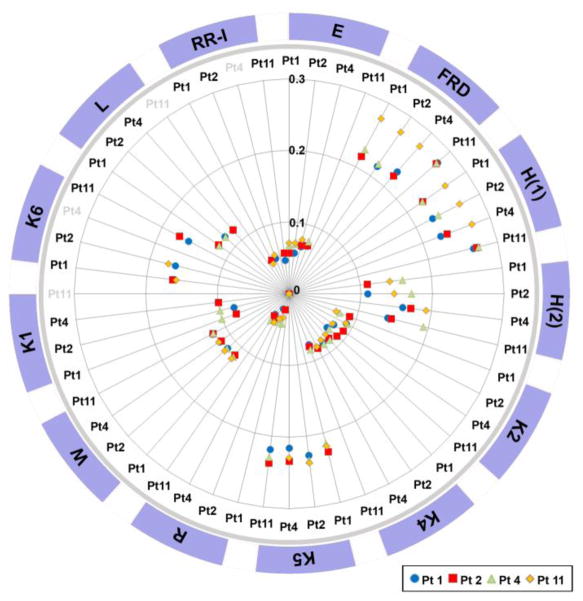
In a separate study, the relatedness of the expressed HERV sequences among the four patients was measured for individual HERV families. In Figure 4, the degree of relatedness of the 3′ LTR sequences of each HERV family among the four patients (patient-1, patient-2, patient-4, and patient-11) is indicated by a spoke on the graph, which represents each patient in comparison to the other three or two patients. The distance from the center of the circle indicates a decrease in the relatedness between the patients being compared. The 3′ LTR sequences of three HERV families (FRD, H(1), and K5) were relatively divergent among the patients whereas the HERV-R LTR sequences were highly homologous. It was interesting to note that the 3′ LTR sequences of HERV-FRD and HERV-H(1) from patient-11 were most distantly related to the other three patients. The data obtained from this study indicates that the populations of the post-burn expressed 3′ LTR sequences of certain HERV families are more individual patient-specific than the others. It is likely that these differential population diversities are associated with the patients’ genomic HERV profiles as well as the evolutionary phylogenic characteristics of the individual HERV families.
Figure 4. HERV family-specific population diversity of post-burn expressed HERVs among four patients.
Within each HERV family, the diversity of post-burn expressed HERV populations among four patients was measured. Each patient’s HERV population was compared to the HERV populations of the other three or two patients within each HERV family. Individual patients are identified with various symbols. As the distance from the center increases, the relatedness of the indicated HERV populations to the reference HERV population decreases (e.g., Pt1 [patient-1] vs. Pt2, Pt4, and Pt11). Within the HERV-FRD, HERV-H(1), and HERV-K5 families, the expressed HERV populations were relatively diverse among the four patients in comparison to the other families, such as HERV-E, HERV-K2, and HERV-K4. HERV-H(1) and HERV-H(2) were derived from two RT-PCR amplicons of HERV-H. HERV-K1, HERV-K6, HERV-L and RRHERV-I were examined only in three patient groups. RR-I (RRHERV-I).
In silico mapping and characterization of burn-associated HERVs
Using the entire set of the uniquely expressed HERV LTR sequences obtained from patient-1 and patient-2 as probes, the reference human genome database from the National Center for Biotechnology Information (NCBI) was surveyed to identify putative HERVs which retain substantial coding potentials of at least 500 amino acids for three retroviral polypeptides (gag, pol, and/or env). The identity threshold was set to “at least 98 %” for both LTR hits during the survey. A total of 18 putative HERV loci, which are capable of coding at least one of the three polypeptides with a minimum coding potential of 500 amino acids (Table 3), were mapped; 15 of them were identified using the probes derived from both patients. The sizes of the putative HERV proviral sequences ranged from 6,373 to 10,222 nucleotides. The lengths of putative coding sequences for the individual polypeptides were somewhat variable: gag polypeptide (647 to 756 amino acids), pol polypeptide (954 to 1,014 amino acids), and env polypeptide (538 to 699 amino acids). Only one putative HERV locus was determined to retain coding potentials for all three polypeptides (Table 3).
Table 3. Putative HERVs mined from the NCBI reference genome using the burn-associated HERVs as a probe.
Among the putative HERVs mined from this study, only the ones which retain at least one 500 amino acid coding potential for gag, pol, or env polypeptides are listed.
| HERV locus | Contig | Chr | +/− | Chr location | Size | gag | pol | env | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||
| from | to | start | end | size (a.a.) | start | end | size (a.a.) | start | end | size (a.a.) | ||||||
|
| ||||||||||||||||
| Pt 1 | 01-H-6A-02_2D01 (HERV-H/env62)* | NT_005403.17 | 2 | − | 166564060 | 166572708 | 8649 | 1227 | 1874 | 215 | (−) | 6255 | 8009 | 584 | ||
|
| ||||||||||||||||
| Pt 1 and Pt 2 | 12-K2-2A-04_1D02 | NT_032977.9 | 1 | + | 75842771 | 75849143 | 6373 | (−) | (−) | 3685 | 5451 | 588 | ||||
| 12-K2-2A-04_1D01 (HERV-K50A) | NT_004487.19 | 1 | − | 155596457 | 155605636 | 9180 | 1112 | 1648 | 178 | 4131 | 6434 | 767 | 6492 | 8231 | 579 | |
| 12-K2-2A-04_3D01 | NT_005612.16 | 3 | − | 112693124 | 112702282 | 9159 | 1104 | 3188 | 694 | 3870 | 6014 | 714 | 6478 | 7728 | 416 | |
| 12-K2-2A-04_3D02 (HERV-K50B) | NT_005612.16 | 3 | − | 185230336 | 185239436 | 9101 | 1032 | 3038 | 668 | 3800 | 6709 | 969 | (−) | |||
| 12-K2-2A-04_5D01 (JN675037.1)* | NT_023133.13 | 5 | − | 156084717 | 156093896 | 9180 | 1112 | 3112 | 666 | 3879 | 6923 | 1014 | (−) | |||
| 12-K2-2A-04_6D01 (HERV-K109) | NT_007299.13 | 6 | − | 78426663 | 78436083 | 9421 | 1104 | 3104 | 666** | 3871 | 4455 | 195 | 6412 | 8508 | 698 | |
| 12-K2-2A-04_7D01 (HML-2.HOM)* | NT_007819.17 | 7 | − | 4622057 | 4631528 | 9472 | 1865 | 2401 | 178 | 4632 | 7502 | 956 | 7204 | 9303 | 699 | |
| 12-K2-2A-04_7D02 (HML-2.HOM)* | NT_007819.17 | 7 | − | 4630560 | 4640030 | 9471 | 1111 | 2205 | 364 | 3877 | 6747 | 956 | 7203 | 9302 | 699 | |
| 12-K2-2A-04_8D01 (HERV-K115) | NT_023736.17 | 8 | − | 7355397 | 7364859 | 9463 | 1104 | 3047 | 647** | 3870 | 6740 | 956 | 6442 | 8541 | 699 | |
| 12-K2-2A-04_11D01 (JN675064.1)* | NT_033899.8 | 11 | + | 101565794 | 101575259 | 9466 | 926 | 1648 | 240 | 3879 | 6743 | 954 | 7198 | 9183 | 661 | |
| 12-K2-2A-04_12D01 (JN675068.1)* | NT_029419.12 | 12 | − | 58721242 | 58730698 | 9457 | 1113 | 3113 | 666 | (−) | 6440 | 8536 | 698 | |||
| 12-K2-2A-04_22D01 (JN675087.1)* | NT_011519.10 | 22 | + | 18926187 | 18935361 | 9175 | 1112 | 3112 | 666 | (−) | 6491 | 8248 | 585 | |||
| 12-K2-4A-01_3D01 (JN675021.1)* | NT_005612.16 | 3 | + | 101360737 | 101369859 | 9123 | 1113 | 3116 | 667 | (−) | (−) | |||||
| 12-R-3A-05_7D01 (ERV3-1) | NT_007933.15 | 7 | − | 64450702 | 64460320 | 9619 | (−) | (−) | 7893 | 9707 | 604 | |||||
| 12-W-5A-01_7D01 (ERVW-1) | NT_007933.15 | 7 | − | 92097285 | 92107506 | 10222 | (−) | 4564 | 4974 | 136 | 7812 | 9428 | 538 | |||
|
| ||||||||||||||||
| Pt 2 | 02-E-7A-01-17D01 (AB062274.1)* | NT_010799.15 | 17 | − | 26557545 | 26566365 | 8821 | 1462 | 2655 | 397 | 2689 | 4371 | 560 | 6264 | 6923 | 219 |
| 02-K2-7A-09-1D01 (JN675013.1)* | NT_004487.19 | 1 | + | 160660575 | 160669807 | 9233 | 1113 | 1874 | 253 | 4188 | 5777 | 529 | 6544 | 8226 | 560 | |
Pt (patient); Chr (chromosome); +/− (strand orientation); underline (putative full coding potential);
HERV references with an incomplete match to the corresponding putative HERV loci identified in this study;
two gag polypeptides which were subjected to functional analysis.
Differential induction of inflammatory mediators by gag polypeptides of two burn-associated HERVs
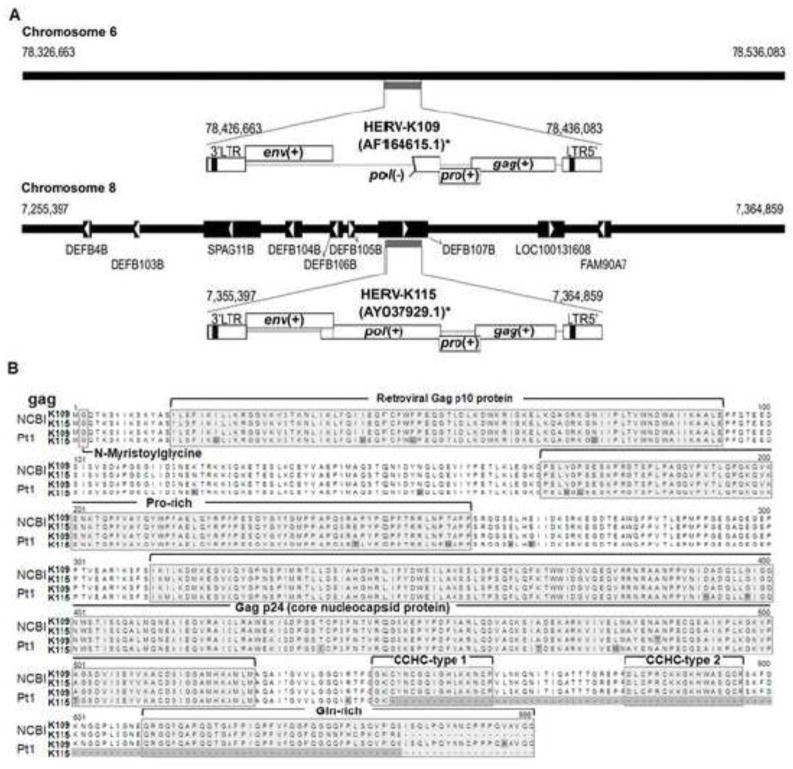
It has been reported that a sub-genomic murine ERV, which retains a full coding potential only for the gag polypeptide, participates in inflammatory disorders in mice (Cho et al., 2002; Jolicoeur, 1991). In addition, our previous study demonstrated that burn-elicited stress signals rapidly and transiently induce expression of a sub-genomic murine ERV in the liver of mice (Cho et al., 2002). In this study, the inflammatory potentials of the gag polypeptides of two burn-associated HERVs were evaluated. Two putative HERV loci (HERV-K109 [chromosome 6] and HERV-K115 [chromosome 8]), which were mapped on the NCBI reference genome with the 3′ LTR sequences from patient-1 and patient-2, were selected to clone the putative gag polypeptide coding sequences. The sizes of the two gag polypeptides on the reference loci were 666 (HERV-K109) and 648 (HERV-K115) amino acids (Figure 5). A two-step PCR scheme was developed to target the specific gag coding sequences of the individual HERV loci using the genomic DNA of patient-1. Sequence analyses revealed that the two gag coding regions isolated from patient-1’s genomic DNA harbor significant mismatches (e.g., missense mutation, C-terminus variation) in comparison to the respective reference loci (Figure 5). The gag coding region obtained from the putative HERV-K109 locus was 666 amino acids (same size as reference) and the second gag coding sequence (from the putative HERV-K115 locus) was 545 amino acids (648 amino acids for the reference locus). This finding confirms the existence of HERV polymorphisms between the genomes of NCBI reference and patient-1.
Figure 5. Genomic map of two burn-associated HERV loci and comparison of their putative gag polypeptide sequences.
A. HERV-K109 and HERV-K115 were mapped in silico on the NCBI human reference genome using the post-burn expressed HERV LTR sequences as a mining probe. The proviral structures of putative HERV-K109 and HERV-K115, which were mapped on chromosome 6 and 8, respectively, were drawn with their neighboring annotated genes. Horizontal thick line (chromosome); black box with arrow (gene); +/− (intact/defective open reading frame) B. The putative gag polypeptide sequences of HERV-K109 and HERV-K115 from the NCBI human reference genome and patient-1 genome were compared. Mismatched amino acid positions and a C-terminus truncation are highlighted in grey. Processed peptides and key motifs are also indicated. NCBI (reference human genome); Pt1 (patient-1).
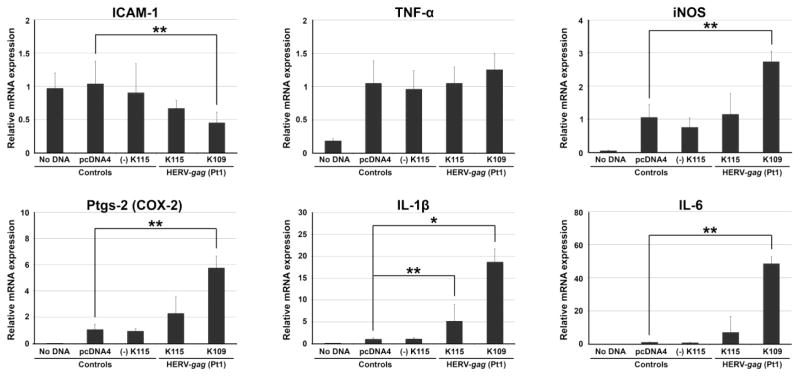
To evaluate the inflammatory potentials of the two HERV gag polypeptides, which were derived from the putative HERV-K109 and HERV-K115 loci of patient-1’s genome, individual gag polypeptides were overexpressed in RAW264.7 macrophage cells followed by the measurement of changes in mRNA production of six inflammatory mediators: IL-6, IL-1β, iNOS, Ptgs-2 (COX-2), TNF-α, and ICAM-1 (Figure 6). The expression of IL-6, IL-1β, iNOS, and Ptgs-2 was substantially increased following over-expression of the gag polypeptide of HERV-K109Pt1, which was presumed to be derived from the putative HERV-K109 locus on chromosome 6 of the genomic DNA of patient-1. In contrast, over-expression of the gag polypeptide of HERV-K115Pt1, presumed to be cloned from the putative HERV-K115 locus (chromosome 8), resulted in an increase of only IL-1β expression (~ 5 fold compared to over 40 fold by HERV-K115Pt1 gag polypeptide). Interestingly, the expression of ICAM-1 was reduced by the gag polypeptide of HERV-K109Pt1. The difference in inflammatory properties between the gag polypeptides of HERV-K109Pt1 and HERV-K115Pt1 may be explained by: 1) C-terminus polymorphisms, in particular, a C-terminus truncation of 121 amino acids in the HERV-K115Pt1 gag polypeptide and 2) 25 mismatched amino acids distributed throughout the polypeptides. It needs to be noted that the C-terminus 121 amino acid region includes various functional motifs, such as CCHC-type 1, CCHC-type 2, and a glutamine-rich region (Figure 5) (Bayer et al., 1995; Dorfman et al., 1993).
Figure 6. HERV-gag isoform-specific induction of inflammatory mediators in macrophage cells.
The two gag isoforms from putative HERV-K109Pt1 and HERV-K115Pt1, which were isolated from patient-1’s genomic DNA, were examined for their effects on the expression of inflammatory mediators upon overexpression in RAW264.7 cells. Overexpression of HERV-K109Pt1-gag substantially induced various inflammatory mediators, such as IL-1β and IL-6, iNOS, and Ptgs-2 (COX-2) while only a slight increase in IL-1β expression was observed with HERV-K115Pt1-gag. There are three different negative controls: No DNA, pcDNA4 (empty pcDNA4/HisMax plasmid), and (−)K115Pt1-gag (reverse oriented HERV-K115Pt1-gag insert in pcDNA4). K109Pt1-gag (HERV-K109Pt1-gag); K115Pt1-gag (HERV-K115Pt1-gag). *P < 0.05; **P < 0.01; error bar (standard deviation).
Discussion
The complex network of post-burn pathogenesis has been investigated primarily by focusing on common genetic polymorphisms and expression profiles of select genes which are reported to be responsible for a host of pathologic phenotypes, such as inflammation, cytotoxicity, and apoptosis (Barber et al., 2006; Feezor et al., 2005; Schwacha et al., 2005). However, a comprehensive knowledge in regard to the proteins, genetic elements, and cells, which control the divergent and often unpredictable pathologic episodes occurring in burn patients, has not yet been formulated. Genomic sequences dedicated to conventional protein-coding genes only comprise ~3 % of the human genome while HERVs and related elements make up ~8 % (Lander et al., 2001; Venter et al., 2001). Our recent findings that burn-incited stressors differentially activate ERVs in mice and that some murine ERV gene products harbor pro-inflammatory potential led us to investigate HERV responses in burn patients (Kwon et al., 2009; Lee et al., 2007; Lee et al., 2011; Lee et al., 2008). It is possible that HERVs and other human genomic elements, such as small interspersed nuclear elements (SINEs) and long interspersed nuclear elements (LINEs), could also participate in burn-elicited pathologic events.
The patient-specific and highly polymorphic post-burn HERV response patterns observed in this study could be attributed to several factors. These patterns may be directly linked to a series of specific pathologic episodes and/or treatment regimens which are unique for individual patients with divergent genetic backgrounds. On the other hand, it is likely that the diversity in genomic HERV profiles among the patient population, which account for uncommon genetic variations, contributed to the divergent HERV responses and variable responses to injury. Specifically, patient-specific genomic HERVs may play a role, at least in part, in the variable and uncommon post-burn pathologic episodes. The expression of certain HERV loci (e.g., HERV-K109Pt1), which reside only on the genomes of certain patients, may be differentially induced post-burn in conjunction with their unique transcription regulatory profiles and inherent epigenetic status (Chiu et al., 2010; Conley and Jordan, 2012; Rebollo et al., 2012).
The gene products of the HERVs, which were induced in response to burn-elicited stress signals, may participate in patient-specific pathogenesis. In fact, the finding from this study that the gag polypeptide of putative HERV-K109Pt1 retains substantial potential to induce pro-inflammatory mediators in comparison to the other gag polypeptide (HERV-K115Pt1) implies that uncommon variations in genomic HERV profiles may be directly linked to the divergent post-burn pathologic courses observed among patient populations. Further studies focusing on the biological significance of the 25 mismatched amino acids between the two gag polypeptides as well as a C-terminus truncation of 121 amino acids in the gag polypeptide of HERV-K115Pt1 will shed fresh insights into the impacts of genomes’ uncommon variations on divergent post-burn pathogeneses.
Conclusions
The findings from this study provide some evidence that certain HERVs contribute to divergent, often unpredictable, disease courses of a heterogeneous population of burn patients. In addition, the uncommon variant loci of the genomes of the human population, such as HERV-K109pt1, may serve as critical genomic markers for the development of tailored treatment regimens for different individuals.
Highlights.
Divergent and dynamic post-burn HERV expression profiles in blood cells of patients
Patient-specific population of post-burn expressed HERV sequences
Induction of IL-6, IL-1β, COX-2, and iNOS by gag gene of a burn-associated HERV
Implication of HERVs as genetic elements contributing to divergent injury pathology
Acknowledgments
This study was supported, in part, by grants from Shriners of North America (No. 86800 to KC, No. 84302 to KHL [postdoctoral fellowship], and No. 84308 to YKL [postdoctoral fellowship]), National Institutes of Health (R01 GM071360 to KC), and Michigan Institute for Clinical and Health Research pilot grant (JN, WW, and KC). This study was not possible without the contributions from Mary Beth Lawless, Katrina Falwell, and Terese Curri of the Burn Division clinical research group at the Department of Surgery, University of California, Davis.
Abbreviations
- ERVs
endogenous retroviruses
- HERVs
human endogenous retroviruses
- HERV-K1
HERV-K(HML-1)
- HERV-K2
HERV-K(HML-2)
- HERV-K4
HERV-K(HML-4)
- HERV-K5
HERV-K(HML-5)
- HERV-K6
HERV-K(HML-6)
- LTRs
long terminal repeats
- NCBI
national center for biotechnology information
- SINEs
short interspersed nuclear elements
- LINEs
long interspersed nuclear elements
- SNPs
single nucleotide polymorphisms
- Pt
patient
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barber RC, et al. TLR4 and TNF-alpha polymorphisms are associated with an increased risk for severe sepsis following burn injury. J Med Genet. 2004;41:808–13. doi: 10.1136/jmg.2004.021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RC, et al. Innate immunity SNPs are associated with risk for severe sepsis after burn injury. Clin Med Res. 2006;4:250–5. doi: 10.3121/cmr.4.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer P, et al. Structural studies of HIV-1 Tat protein. J Mol Biol. 1995;247:529–35. doi: 10.1006/jmbi.1995.0158. [DOI] [PubMed] [Google Scholar]
- Boller K, et al. Human endogenous retrovirus HERV-K113 is capable of producing intact viral particles. J Gen Virol. 2008;89:567–72. doi: 10.1099/vir.0.83534-0. [DOI] [PubMed] [Google Scholar]
- Chiu S, et al. Post-injury stress signals alter epigenetic profile of cytosine methylation in the proviral genome of endogenous retroviruses. Exp Mol Pathol. 2010;89:291–300. doi: 10.1016/j.yexmp.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Cho K, et al. Induction of murine AIDS virus-related sequences after burn injury. J Surg Res. 2002;104:53–62. doi: 10.1006/jsre.2002.6410. [DOI] [PubMed] [Google Scholar]
- Cho K, et al. Endogenous retroviruses in systemic response to stress signals. Shock. 2008;30:105–16. doi: 10.1097/SHK.0b013e31816a363f. [DOI] [PubMed] [Google Scholar]
- Conley AB, Jordan IK. Endogenous Retroviruses and the Epigenome. Viruses: Essential Agents of Life. 2012:309–323. [Google Scholar]
- Conrad B, et al. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell. 1997;90:303–13. doi: 10.1016/s0092-8674(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Contreras-Galindo R, et al. Human endogenous retrovirus K (HML-2) elements in the plasma of people with lymphoma and breast cancer. J Virol. 2008;82:9329–36. doi: 10.1128/JVI.00646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman T, et al. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993;67:6159–69. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayazov AD, et al. Disorders of the immune system in severely burned patients. Ann Burns Fire Disasters. 2009;22:121–30. [PMC free article] [PubMed] [Google Scholar]
- Feezor RJ, et al. Functional genomics and gene expression profiling in sepsis: beyond class prediction. Clin Infect Dis. 2005;41(Suppl 7):S427–35. doi: 10.1086/431993. [DOI] [PubMed] [Google Scholar]
- Feuchter-Murthy AE, et al. Splicing of a human endogenous retrovirus to a novel phospholipase A2 related gene. Nucleic Acids Res. 1993;21:135–43. doi: 10.1093/nar/21.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank O, et al. Human endogenous retrovirus expression profiles in samples from brains of patients with schizophrenia and bipolar disorders. J Virol. 2005;79:10890–901. doi: 10.1128/JVI.79.17.10890-10901.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimanis G, et al. A role for human endogenous retrovirus-K (HML-2) in rheumatoid arthritis: investigating mechanisms of pathogenesis. Clin Exp Immunol. 2010;160:340–7. doi: 10.1111/j.1365-2249.2010.04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder BS, et al. Syncytin 1 in the human placenta. Placenta. 2012;33:460–6. doi: 10.1016/j.placenta.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. FASEB J. 1991;5:2398–405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- Kallinen O, et al. Multiple organ failure as a cause of death in patients with severe burns. J Burn Care Res. 2012;33:206–11. doi: 10.1097/BCR.0b013e3182331e73. [DOI] [PubMed] [Google Scholar]
- Kwon DN, et al. Cloning and characterization of endogenous retroviruses associated with postinjury stress signals in lymphoid tissues. Shock. 2009;32:80–8. doi: 10.1097/SHK.0b013e31818bc193. [DOI] [PubMed] [Google Scholar]
- Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lee YK, et al. Genome-wide changes in expression profile of murine endogenous retroviruses (MuERVs) in distant organs after burn injury. BMC Genomics. 2007;8:440. doi: 10.1186/1471-2164-8-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, et al. Tropism, cytotoxicity, and inflammatory properties of two envelope genes of murine leukemia virus type-endogenous retroviruses of C57BL/6J mice. Mediators Inflamm. 2011;2011:509604. doi: 10.1155/2011/509604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, et al. Genome-wide expression profiles of endogenous retroviruses in lymphoid tissues and their biological properties. Virology. 2008;373:263–73. doi: 10.1016/j.virol.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medstrand P, et al. Long terminal repeats are used as alternative promoters for the endothelin B receptor and apolipoprotein C-I genes in humans. J Biol Chem. 2001;276:1896–903. doi: 10.1074/jbc.M006557200. [DOI] [PubMed] [Google Scholar]
- Rebollo R, et al. Epigenetic interplay between mouse endogenous retroviruses and host genes. Genome Biol. 2012;13:R89. doi: 10.1186/gb-2012-13-10-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacha MG, et al. Genetic variability in the immune-inflammatory response after major burn injury. Shock. 2005;23:123–8. doi: 10.1097/01.shk.0000148073.19717.a9. [DOI] [PubMed] [Google Scholar]
- Tamura K, et al. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Ting CN, et al. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev. 1992;6:1457–65. doi: 10.1101/gad.6.8.1457. [DOI] [PubMed] [Google Scholar]
- Venter JC, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Weis S, et al. Reduced expression of human endogenous retrovirus (HERV)-W GAG protein in the cingulate gyrus and hippocampus in schizophrenia, bipolar disorder, and depression. J Neural Transm. 2007;114:645–55. doi: 10.1007/s00702-006-0599-y. [DOI] [PubMed] [Google Scholar]