Abstract
Bartonella bacilliformis is the causative agent of the biphasic human disease, Oroya fever. During the primary disease phase, up to 100% of the circulating erythrocytes can be parasitized and 80% lysed. During the secondary phase of this disease, bacterial invasion shifts to endothelial cells lining the vasculature. B. bacilliformis is transferred between human hosts by the sandfly, Lutzomyia verrucarum. To investigate the regulation of ialB by environmental cues signaling vector-to-host transmission; nuclease protection assays were performed to compare the amount of ialB mRNA in bacteria subjected to temperature shift, pH change, oxidative stress, or hemin limitation. The amount of ialB mRNA increased by 223–310% in acid-treated samples and decreased by 28–39% in base-treated samples as compared to bacteria kept at pH 7.2. B. bacilliformis samples showed a 56–63% and 74–80% decrease in ialB mRNA when shifted to 37 °C from growth temperatures of 20 and 30 °C, respectively. Oxidative stress (1 mM H2O2) and hemin limitation had no significant effect on mRNA levels. Determination of ialB protein amounts using SDS–PAGE and immunoblotting showed the greatest amounts of ialB under acidic conditions or at 20 °C. The least amount of ialB was synthesized under basic conditions or at 37 °C. The viability of wild-type B. bacilliformis under the various experimental culture conditions was determined and found not to affect ialB mRNA amounts in these experiments. Finally, we compared the survival of wild-type and ialB mutant B. bacilliformis and found no difference in the viability of these two strains, demonstrating that ialB does not aid bacterial survival under these conditions.
Keywords: Bartonella bacilliformis, Invasion-associated locus B (ialB) gene, Erythrocyte adherence and invasion, Bacterial pathogenesis
1. Introduction
Bartonella bacilliformis is unparalled in its ability to parasitize human erythrocytes, invading on average 61% of circulating erythrocytes during the primary phase of Oroya fever [1]. B. quintana, the causative agent of trench fever, also invades human erythrocytes but parasitizes less than 1% of circulating erythrocytes [2]. Other bacteria are known to parasitize mammalian erythrocytes (e.g. Anaplasma, Haemobartonella, and other Bartonella species), but B. bacilliformis is unsurpassed among bacteria in its efficiency as an erythrocyte parasite.
The B. bacilliformis invasion-associated locus B gene (ialB) was shown to have a direct role in human erythrocyte parasitism by our lab [3]. Insertional mutagenesis of ialB resulted in a 47 to 53% decrease in human erythrocyte adherence and invasion compared to the parental strain. Trans-complementation of the mutant with wild-type ialB restored erythrocyte adherence and invasion to parental levels.
ialB homologues are present in the other two Bartonella species that cause human disease. B. quintana and B. henselae cause trench fever and cat-scratch disease (CSD), respectively. All three Bartonella species share phenotypic similarities: they are transmitted by an arthropod vector, are facultative intracellular parasites, and have an absolute growth requirement for hemin. All three species can attach to or invade erythrocytes [2,4,5] and endothelial cells during the course of disease [6,7]. Given these phenotypic similarities, ialB may share a similar function contributing to the virulence of all three species, and investigation into ialB regulation may provide insights into the disease process of all three pathogens.
The pivotal role played by ialB in erythrocyte parasitism led us to hypothesize that ialB is upregulated in response to environmental cues signaling vector-to-host transmission. Such environmental cues would include, but not be limited to, temperature, pH, oxidative stress, and hemin limitation. We also hypothesized that ialB would aide B. bacilliformis survival under stress-inducing environmental conditions. This study was undertaken to test these hypotheses and to gain insight into the function of ialB in erythrocyte parasitism.
2. Results
2.1. Nuclease protection assays (NPA)
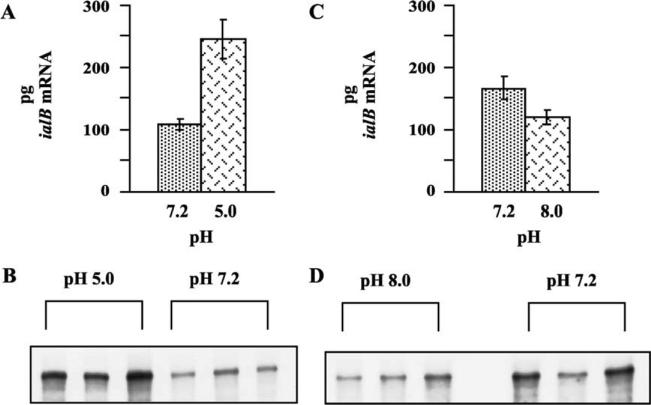
NPA's with sample RNA isolated from B. bacilliformis strain JB584 were performed several times (n = 2 or 3 with 3 replicates each). While the amount of ialB mRNA varied slightly between experiments, the data trends remained consistent. There was a very significant difference (P < 0:01) in the amount of ialB mRNA in JB584 subjected to acidic pH (pH 5.0) for 30 min as compared to JB584 kept at pH 7.2. The amount of ialB mRNA in acid-treated B. bacilliformis increased by 228–310% over bacteria kept at near-neutral pH. In a representative experiment, there was a 231% increase in ialB mRNA for acid-treated B. bacilliformis compared to bacteria kept at pH 7.2 (245 ± 32 pg vs 106 ± 9 pg, respectively) (Fig. 1(A) and (B)). In NPA's with mRNA isolated from base-treated (pH 8.0) bacteria and bacteria kept at pH 7.2, there was a significant decrease in ialB mRNA at basic pH (P < 0:05). In this set of experiments, ialB mRNA decreased by 28–39% with a 30 min incubation in pH 8.0 HIB. In a representative experiment, there was a 29% decrease in ialB mRNA when bacteria were incubated at pH 8.0 as compared to pH 7.2 (118 ± 11 vs 165 ± 19 pg, respectively) (Fig. 1(C) and (D)). B. bacilliformis JB584 survival under these experimental conditions was investigated and the results are presented in a later section.
Fig. 1.
Effect of pH on B. bacilliformis ialB mRNA levels. (A) The amount of ialB mRNA increased 231% in acid-treated bacteria (pH 5.0) as compared to bacteria kept at pH 7.2. Data are from a representative experiment comparing the amount of ialB mRNA in 4 μg of total RNA isolated from pH-treated B. bacilliformis samples. (B) NPA shows the amount of ialB mRNA increases at pH 5.0 (3 lanes on left) as compared to samples kept at pH 7.2 (3 lanes on right). NPA's were done in triplicate. (C) In a representative experiment comparing the amount of ialB mRNA in B. bailliformis samples kept at pH 7.2 or shifted to pH 8.0, there was a 29% decrease in ialB mRNA in pH 8.0 samples. (D) The amount of ialB mRNA decreased in base-treated samples (3 lanes on the left) as compared to samples kept at pH 7.2 (3 lanes on the right).
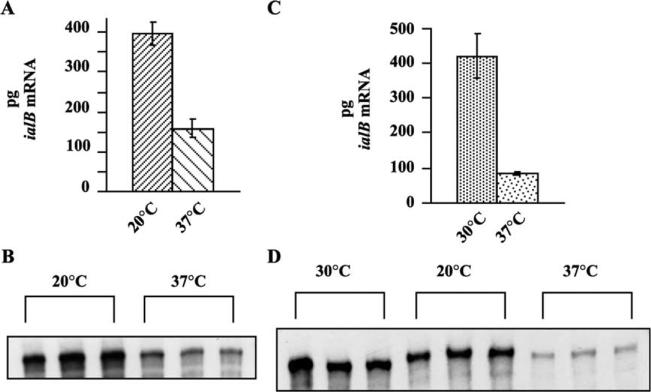
Two sets of experiments were conducted to determine the effect of temperature shift on ialB mRNA levels. In one set of experiments, B. bacilliformis, strain JB584, was grown at 20 °C and then upshifted to 37 °C for 30 min. This experiment showed a very significant decrease (P < 0:01) in the amount of ialB mRNA in upshifted bacteria as compared to bacteria maintained at 20 °C. In these experiments, ialB mRNA decreased 56–75% with temperature upshift. In a representative experiment, there was a decrease of 60% in ialB mRNA in upshifted bacteria as compared to control bacteria (158 ± 23 vs 396 ± 27 pg, respectively) (Fig. 2(A) and (B)).
Fig. 2.
Effect of temperature shift on B. bacilliformis ialB mRNA levels. (A) In a representative experiment, the amount of ialB mRNA decreased 60% when B. bacilliformis grown at 20 °C were temperature upshifted to 37 °C. (B) NPA shows the amount of ialB mRNA decreases when bacteria grown at 20 °C (3 lanes on the left) are temperature shifted to 37 °C (3 lanes on the right). (C) Temperature upshift of 30 °C-grown B. bacilliformis to 37 °C resulted in an 80% decrease in the amount of ialB mRNA in a representative experiment. (D) No significant differences were seen between the amounts of ialB mRNA in 30 °C-grown B. bacilliformis (left 3 lanes) and bacteria temperature shifted to 20 °C (center 3 lanes); however, mRNA decreased significantly in bacteria shifted to 37 °C (right 3 lanes).
In the second set of experiments, JB584 grown at 30 °C was either upshifted to 37 °C, kept at 30 °C, or down-shifted to 20 °C for 30 min. While there was a significant decrease (P < 0:05) in the amount of ialB mRNA in upshifted samples when compared to either bacteria kept at 30 °C or shifted to 20 °C; there was no significant difference between ialB mRNA amounts in the 30 and 20 °C samples (P > 0:05). For upshifted bacteria, ialB mRNA amounts decreased 74–80% from mRNA amounts for bacteria kept at 30 °C. In one representative experiment, ialB mRNA decreased 80% in the upshifted B. bacilliformis from control levels (83 ± 4 at 37 °C vs 420 ± 64 pg at 30 °C) (Fig. 2(C) and (D)). Both sets of temperature experiments showed a significant decrease in the amount of ialB mRNA for bacterial samples upshifted to 37 °C for 30 min, regardless of growth temperature.
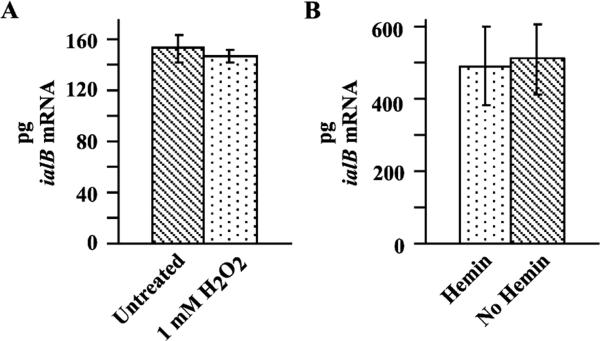
To determine if ialB mRNA levels are affected by oxidative stress, suspensions of B. bacilliformis JB584 were exposed to 1 mM H2O2 for 30 min. Comparison of ialB mRNA in oxidatively stressed samples and control samples showed no statistically significant difference (P > 0:05) (Fig. 3(A)).
Fig. 3.
Effect of 1 mM H2O2 and hemin limitation on ialB mRNA levels. (A) There is no significant difference in the amount of ialB mRNA produced by 1 mM H2O2-treated and untreated B. bacilliformis (146 ± 5 and 152 ± 11 pg, respectively). (B) The amount of ialB mRNA in hemin-limited and control bacterial samples is not significantly different (511 ± 97 pg vs 490 ± 107 pg, respectively).
Finally, the effect of hemin limitation on ialB mRNA levels was determined. Bacteria were harvested and resuspended in media with or without a source of hemin (RB and HIB, respectively) and incubated for 30 min at 30 °C. There was no significant difference in the amount of ialB mRNA under these experimental conditions (P > 0:05) (Fig. 3(B)).
2.2. B. bacilliformis JB584 survival in varying culture conditions
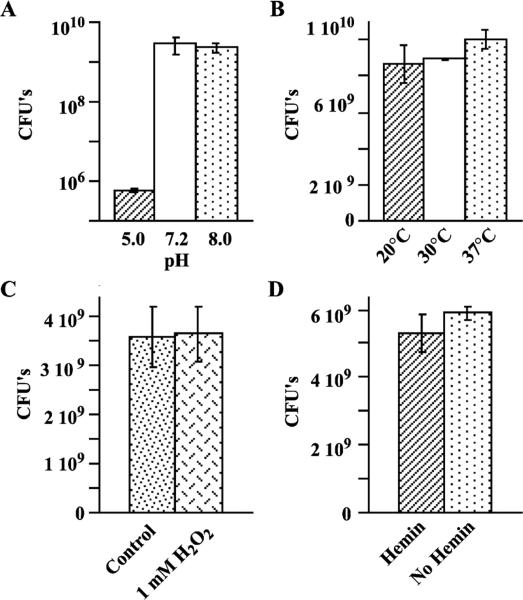
To determine if changes in ialB mRNA amounts were caused by bactericidal experimental conditions, plate counts were done. B. bacilliformis strain JB584 suspensions were subjected to acidic or basic conditions, temperature shifts, oxidative stress, or hemin limitation for 30 min. Bacteria were serially diluted, plated, and CFU's counted after 12 days.
The survival of acid-shocked B. bacilliformis JB584 was significantly reduced (P < 0:05) as compared to bacteria kept at pH 7.2 for 30 min, but there was no significant difference (P > 0:05) in bacterial survival at pH 7.2 vs pH 8.0 (Fig. 4(A)). In a representative experiment, 0.021% of bacteria survived at pH 5.0 as compared to pH 7.2 (Fig. 4(A)).
Fig. 4.
Effect of pH, temperature, oxidative stress, and hemin limitation on B. bacilliformis JB584 survival. (A) Less than 1% of bacteria survived after 30 min in pH 5.0 RB but there was no statistically significant difference in bacterial survival at pH 7.2 and pH 8.0. (B) B. bacilliformis JB584 grown at 30 °C then shifted to 20 or 37 °C has the same survival rate as bacteria kept at 30 °C. (C) There is no decrease in viability of B. bacilliformis after treatment with 1 mM H2O2. (D) Survival of bacteria under hemin-limiting conditions is unaffected.
For B. bacilliformis that was grown at 30 °C and then incubated at 20, 30 or 37 °C for 30 min, there was no significant difference (P > 0:05) in bacterial survival at the various temperatures after 30 min of incubation (Fig. 4(B)). Treatment with 1 mM H2O2 and hemin limitation did not significantly affect bacterial survival (P > 0:05) (Fig. 4(C) and (D), respectively).
2.3. ialB protein
Experiments were performed to determine if the amount of ialB protein changed under culture conditions that affected ialB mRNA levels, i.e. pH and temperature shift. B. bacilliformis JB584 was incubated for 4 h in RB at pH 5.0, 7.2, or 8.0 and 5 μg of the resulting protein analyzed by SDS–PAGE. Western blots showed an apparent increase in the amount of ialB for bacteria incubated at pH 5.0 RB as compared to bacteria incubated at pH 7.2. In addition, an apparent decrease in the amount of ialB was observed for bacteria incubated at pH 8.0 (Fig. 5(A)).
Fig. 5.
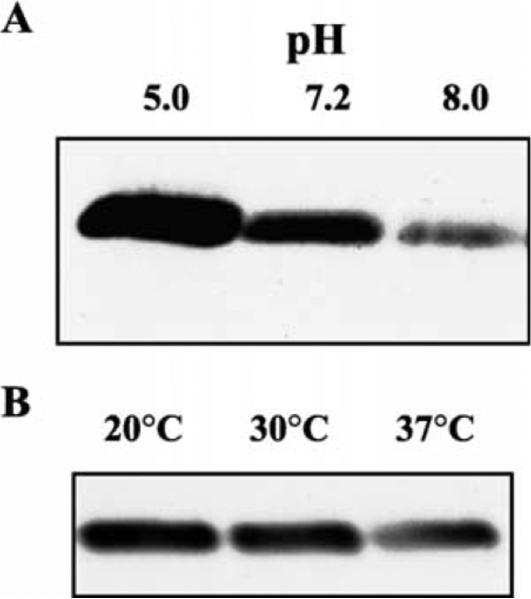
ialB protein levels are responsive to pH and temperature. (A) Bacterial cultures were incubated at pH 5.0, 7.2, or 8.0 for 4 h; cell lysates were separated by SDS–PAGE; and immunoblots reacted with polyclonal anti-ialB antibodies. The amount of ialB increases as pH decreases. (B) Immunoblot of bacterial samples grown at 30 °C (lane 2) and temperature shifted to 20 or 37 °C (lanes 1 and 3, respectively). The amount of ialB decreases at 30 and 37 °C as compared to 20 °C.
For experiments examining the effect of temperature shift on ialB, B. bacilliformis JB584 was grown in a 30 °C water-saturated incubator, harvested and then temperature shifted for 4 h. Protein samples analyzed by Western blot showed an apparent decrease in ialB when bacteria were upshifted to 37 °C (Fig. 5(B)). Although the amount of ialB in samples shifted to 20 °C and samples kept at 30 °C, was similar by visual inspection, densitometry of Western blots revealed a slight increase in the amount of ialB protein in bacterial samples shifted to 20 °C.
2.4. Comparison of B. bacilliformis strain survival under culture conditions
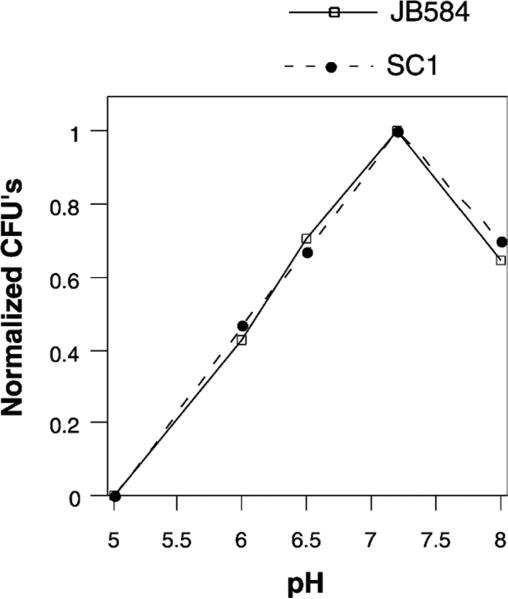
To determine if ialB confers a survival advantage to bacteria, wild-type and ialB mutant B. bacilliformis strains were compared for the ability to survive the stressful environmental conditions under investigation. Since the number of JB584 bacteria surviving a 30 min incubation at pH 5.0 was so low, bacterial survival for the two strains was compared at pH 5.0–8.0. Survival experiments over this pH range were done four times with each experiment being plated in triplicate. In order to compensate for the differing number of bacteria used in these experiments, CFU's for each pH were normalized using the surviving CFU's for that strain at pH 7.2. The experimental results showed no difference in survival between JB584 and SC1 (Fig. 6), indicating that ialB does not confer a survival advantage at acidic or basic pH's.
Fig. 6.
Viability comparison of wild-type B. bacilliformis and an ialB mutant strain from pH 5.0 to pH 8.0. The graph represents the averaged results from four experiments and shows that ialB does not confer a survival advantage under these experimental conditions. To compensate for the differing number of bacteria used in each experiment, CFU's for each experiment were normalized using that experiment's CFU count at pH 7.2.
Additional CFU experiments comparing wild-type and ialB mutant strains (JB584 and SC1, respectively) showed no significant differences in bacterial survival of temperature shift, oxidative stress, or hemin limitation (data not shown).
3. Discussion
Nuclease protection assays revealed that the greatest increases in ialB mRNA levels occur in acid-shocked bacteria (pH 5.0), bacteria grown at 20 °C or temperature shifted to 20 °C. The lowest amount of ialB mRNA was observed in response to basic pH (pH 8.0) or temperature upshift to 37 °C. The amount of ialB mRNA did not significantly change under conditions of oxidative stress (1 mM H2O2) or hemin limitation.
Changes in the amount of ialB mRNA were not due to differences in B. bacilliformis survival under the experimental conditions of this study. After 30 min of acid shock at pH 5.0, less than 1% of the bacteria survived, yet the amount of ialB mRNA increased dramatically compared to bacteria kept at pH 7.2. Although there was no significant difference in bacterial survival at neutral and basic pH, ialB mRNA levels significantly decreased under basic conditions. For temperature experiments, there was no significant difference in bacterial survival at 20, 30, and 37 °C; however, ialB mRNA amounts decreased with increasing temperature. In short, the different quantities of ialB mRNA in our samples were not a result of differences in bacterial survival.
Experiments to determine the amount of ialB showed that protein levels were correlated with changes in ialB mRNA quantities. The greatest amount of ialB was present in B. bacilliformis that had been acid-shocked, grown at 20 °C, or temperature shifted to 20 °C. As with ialB mRNA levels, ialB protein amounts decreased in response to basic pH (pH 8.0) or temperature shift to 37 °C. That ialB protein and ialB mRNA levels have the same fluctuation pattern in response to pH and temperature suggests that regulation mainly occurs at the mRNA level. Regulation of ialB expression could occur at the transcriptional level or by regulation of mRNA half-life, or both.
Based on epidemiological data, humans are presumed to be the reservoir for B. bacilliformis. Bacteria are transmitted between human hosts by the sandfly vector, Lutzomyia verrucarum. The hematophagous female sandfly presumably acquires B. bacilliformis while feeding on bacteremic humans and incidentally transfers bacteria with subsequent feedings. Upon ingestion by the sandfly, bacteria undergo a temperature shift from 37 to 20 °C as the blood meal in the insect cools to ambient temperature. Concurrent with this temperature shift, bacterial ialB expression would presumably be upregulated, as we demonstrated. If for some reason sandfly feeding is interrupted, ingested B. bacilliformis would have upregulated ialB expression and be ‘primed’ for erythrocyte adherence and invasion following transfer to another human host when the insect next feeds.
Little research has been done on the fate of B. bacilliformis ingested by its sandfly vector, but presumably bacteria ingested with a blood meal would have to either escape from the insect midgut or survive the digestive process in order to be transferred to a new host. Reported pH values for the sandfly midgut differ depending on experimental methods [8,9]. However, experiments with sandflies being offered a blood meal and then dissected at 24 h intervals most closely recapitulate natural feeding behavior; and those values are used here [9]. In unfed sandflies, the midgut has a near neutral pH (pH 7.0–7.3). After ingesting a blood meal, the midgut pH increases slightly to pH 7.4 (the pH of human blood) and 36–48 h later becomes acidic (pH 6.8 or lower). Around 3 days post-ingestion as the insect midgut becomes acidified, ialB expression by ingested B. bacilliformis would presumably be upregulated as it was experimentally. With female sandflies needing to ingest a blood meal every 4–5 days for egg development, B. bacilliformis in the insect midgut would be ‘primed’ for erythrocyte invasion just prior to the time when the sandfly is likely to feed again. ‘Priming’ of B. bacilliformis bacteria in its arthropod vector could expedite bacterial invasion of erythrocytes thus minimizing bacterial exposure to the host immune response.
Bacterial transmission from arthropod vector to human host would be signalled by a rapid upshift in temperature to 37 °C and an increase in pH to 7.4. In this study, we showed ialB expression is downregulated but not abrogated under these conditions. As previously demonstrated by our lab, mutagenesis of ialB reduces human erythrocyte parasitism by 47–53% [3]. That ialB mutagenesis reduces but does not abolish erythrocyte parasitism by B. bacilliformis suggests that other bacterial factors are involved in erythrocyte adherence and invasion. Even though ialB expression is apparently downregulated by environmental cues signaling vector-to-host transmission, it is possible that the basal level of ialB expression and/or other bacterial factors are responsible for erythrocyte parasitism during the primary phase of Oroya fever.
During the secondary phase of Oroya fever, bacterial invasion shifts from erythrocytes to vascular endothelial cells. This phase of the disease is characterized by neovascularization of tissues on the head, neck and extremities of the body resulting in hemangiomas, nicknamed verruga peruana. The predilection for hemangioma formation on those parts of the human body with a lower temperature might be a result of increased ialB expression by B. bacilliformis at lower temperatures. The possible role of ialB in human endothelial cell invasion has yet to be explored and would be a productive area for future research.
The occurrence of Oroya fever is limited (for the most part) to the Andean mountain valleys of South America, presumably due to the geographical restriction of its sandfly vector. Within endemic areas up to 60% of the human population is seropositive for B. bacilliformis antibodies [10], and the carrier rate for seropositive and convalescent individuals is significant. In one study of 75 seropositive individuals, 11% were culture positive for B. bacilliformis. Fourteen percent of convalescent patients who had presented with characteristic severe hemolytic anemia and received antibiotic treatment were bacteremic. Additionally, 23% of patients with chronic rash were also bacteremic (L. Laughlin, personal communication). Besides providing additional evidence for humans being the reservoir for B. bacilliformis, these statistics highlight the chronic nature of B. bacilliformis infection. Over half of the population in areas endemic for Bartonellosis have been infected and after antibiotic treatment a significant percentage remain bacteremic. The regulatory mechanisms governing ialB expression may contribute to the chronic nature of this disease. By downregulating ialB expression at 37 °C and near neutral pH, B. bacilliformis would presumably be less efficient at parasitizing circulating erythrocytes. If this were the case, subacute bacteremia could develop in infected individuals and B. bacilliformis infection could be prolonged in the human host, increasing the chance of bacterial transmission via sandflies to other human hosts.
To date, database searches for proteins with homology to ialB have not revealed clues to its mechanism or function. We hoped that experiments comparing the survival of JB584 (wild-type) to SC1 (ialB mutant) under stressful culture conditions would reveal a survival advantage conferred by ialB, but this was not the case.
The ialB gene is highly conserved among Bartonella species that are human pathogens [11] and ialB homologues are found in other bacteria that interact with eukaryotic cells in a parasitic or mutualistic association, such as Brucella melitensis [12]. In an article characterizing the heat, oxidative, and acid stress response of B. melitensis; it was reported that synthesis of an ialB homologue was significantly reduced by heat shock (i.e. 37–42 °C) but was unaffected by oxidative stress (50 mM H2O2). These results agree with our data on ialB regulation in B. bacilliformis. However, the amount of the B. melitensis ialB homologue was reported to be unchanged after acid stress (pH 5.5). Our data showed a dramatic increase in B. bacilliformis ialB in response to acid shock. Differences in ialB synthesis under acid conditions may be due to the unique regulatory mechanisms of the two organisms.
Although ialB has been shown to have a significant effect on erythrocyte association/invasion, it is an inner membrane protein and unlikely to directly interact with erythrocytes [3]. Future research will focus on identifying proteins that interact with ialB that might be more directly involved in human erythrocyte invasion.
4. Materials and methods
4.1. Bacterial strains
B. bacilliformis strain JB584 (a transformation-competent strain [13]) was cultured on heart infusion agar blood (HIAB) plates (heart infusion agar supplemented with 4% sheep erythrocytes and 2% sheep serum) in a water-saturated incubator at 30 °C. B. bacilliformis SC1, an ialB mutant strain [3], was cultured in the presence of kanamycin (25 μg/ml). E. coli strain DH5a was cultured in Luria– Bertani (LB) broth at 37 °C in the presence of antibiotics as needed.
4.2. Culture conditions
Three HIAB plates of 3-day-old B. bacilliformis strain JB584 were harvested into 3 ml of 30 °C heart infusion broth (HIB) or recovery broth (RB; i.e. HIB supplemented with 5% sheep erythrocyte lysate and 50 mg/ml bovine serum albumin). For pH, temperature, and hemin limitation experiments; 0.5 ml of bacterial culture was centrifuged 1 min. at 16,000g and the supernatant discarded. Bacterial pellets were resuspended in RB pre-equilibrated to either 20, 30, or 37 °C for the temperature experiments; in 30 °C prewarmed RB at pH 5.0, 7.2, or 8.0 for the pH experiments, and in either HIB or RB for the hemin-limiting experiments. For oxidative stress experiments, H2O2 was added to the final concentration of 1 mM. Hemin-limited, H2O2 and pH treated samples were placed in a 30 °C, humidified incubator for 30 min. For temperature experiments, samples were incubated in a 20, 30, or 37 °C water bath for 30 min.
4.3. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotting
B. bacilliformis JB584 was subjected to culture conditions as described above but with a 4 h incubation time. After incubation, bacterial cultures were centrifuged at 16,000g for 1 min and the supernatant discarded. Bacterial pellets were flash frozen in −70 °C ethanol, and stored at −70 °C until used. Frozen bacteria were vigorously resuspended in 500 ml distilled H2O and protein concentrations determined using a bicinchoninic acid protein kit as per the manufacturer's instructions (Pierce Biotechnology, Inc., Rockford, IL, USA).
SDS–PAGE was done following the general procedures of Laemmli [14] with 12.5% polyacrylamide (wt/vol) gels and 5 μg of protein added per lane. Gels were either silver-stained [15] or electrophoretically transferred to a supported nitrocellulose membrane (pore size 0.45 μM; Schleicher and Schuell, Keene, N.H.) for Western blotting. Chemiluminescent immunodetection was done using ECL™ Western blotting detection reagents following the manufacturer's instructions (Amersham Pharmacia Biotech Inc, Piscataway, NJ, USA). Antibodies against ialB were generated as previously described [3] and used at a 1:1000 dilution.
4.4. Nuclease protection assay
B. bacilliformis JB584 was cultured under conditions as described above with a 30 min incubation time. Samples were centrifuged at 16,000g for 1 min, the supernatant removed, and the bacterial pellet flash frozen in 100% ethanol at −70 °C. Samples were stored at −70 °C until used. RNA was extracted from samples and DNase treated using a RNeasy kit (Qiagen Inc., Chatsworth, CA, USA). RNA was quantified by spectrophotometry at 260 nm.
For in vitro transcription of probe and standard ialB mRNA, the 505-bp, Ssp I–Pst I fragment encoding the 3′ terminus of ialB was cloned into pBluescript SK+ digested with the restriction enzymes EcoRV and Pst I. The resulting phagemid, pBSialB was digested with Hpa I and electro-phoresed on a 1% agarose gel. Strips cut from each side of the gel were stained with ethidium bromide, realigned with the unstained portion and the linearized pBSialB excised. Linearized pBSialB was purified using a GeneClean II kit (Bio 101, La Jolla, CA, USA), then used as the template in an in vitro transcription reaction using T7 RNA polymerase and an Ampliscribe Transcription kit (Epicentre Tech., Madison, WI, USA). The resulting single-stranded ialB mRNA was labeled using a BrightStar™ Psoralen-Biotin nonisotopic labeling kit (Ambion, Austin, TX, USA). Labeled ialB mRNA probe was gel purified and UV shadowed as per Ambion's Technical Bulletin #171. Standard ialB mRNA was generated in vitro by T3 RNA polymerase transcription of pBSialB linearized with Mfe I. Both probe and standard mRNA were quantified by spectrophotometry at 260 nm.
Nuclease protection assays were performed as per the manufacturer's instructions for the S1-Assay™ kit (Ambion, Austin, TX, USA) with elevation of the recommended hybridization temperature to 48 °C and the S1 nuclease digestion temperature to 42 °C. Briefly, sample or standard mRNA was added to psoralen-biotin labeled ialB probe and concentrated by ethanol precipitation. Hybridization was allowed to proceed overnight at 48 °C in hybridization buffer. RNA was digested with S1 nuclease at 42 °C for 45 min then 42 μl of stop buffer was added (185 μg/ml tRNA, 28.5 mM EDTA (pH 8.0), 4.1 M NH4OAc, and 713 μg/ml GlycoBlue™ coprecipitent (Ambion)). Hybridized RNA was precipitated with ethanol, resuspended in 10 μl Gel Loading Buffer II (Ambion, Austin, TX, USA), separated on a 5% polyacrylamide gel, and transferred to nylon membrane using a semi-dry electroblotting system (The Panther™, Owl Separation Systems, Portsmouth, NH, USA). RNA was immobilized on membranes by UV crosslinking at 125 mJ and ialB mRNA detected with a BrightStare BioDetect™ Nonisotopic Detection Kit (Ambion, Austin, TX, USA).
Known amounts of standard ialB mRNA were used to generate a concentration curve (range: 0–600 pg and r2 > 0:92) from which sample mRNA amounts were extrapolated using Scanalytics’ OneDScan program (CSPI, Billerica, MA, USA).
4.5. Bacterial survival in varying culture conditions
Bacterial survival for B. bacilliformis strains JB584 and SC1 was determined under the various culture conditions described above. In short, bacteria were harvested, centrifuged and then subjected to either temperature shift, pH shift, oxidative stress or hemin limitation for 30 min. Cultures were serially diluted then plated onto HIAB plates. Plates were incubated in a 30 °C water-saturated incubator for 12 days and then counted.
4.6. Densitometry
X-ray films of protein and mRNA data were scanned with a UMAX Astra 1200S scanner (UMAX, Technologies, Inc., Dallas, TX, USA) and images analyzed with Scanalytics’ OneDScan program (CSPI, Billerica, MA, USA).
4.7. Statistical analysis
Statistical significance of data was determined using the Student's t test. A value of P of <0.01 was considered very significant and P < 0:05 was considered significant. For mRNA and CFU experiments, results are reported as mean values ± the standard error of the mean.
Acknowledgements
MFM was supported by Public Health Service grant AI 45534 from the National Institutes of Health and American Heart Association Established Investigator Grant 9940002N. SAC was supported by a Predoctoral Honors Fellowship from the University of Montana.
References
- 1.Maguina C, Garcia PJ, Gotuzzo E, Cordero L, Spach DH. Bartonellosis (Carrion's disease) in the modern era. Clin Infect Dis. 2001;36(6):772–9. doi: 10.1086/322614. [DOI] [PubMed] [Google Scholar]
- 2.Rolain JM, Foucault C, Guieu R, La Scola B, Brouqui P, Raoult D. Bartonella quintana in human erythrocytes. Lancet. 2002;360:226–8. doi: 10.1016/s0140-6736(02)09462-x. [DOI] [PubMed] [Google Scholar]
- 3.Coleman SA, Minnick MF. Establishing a direct role for the Bartonella bacilliformis invasion-associated locus B (IalB) protein in human erythrocyte parasitism. Infect Immun. 2001;69(7):4373–81. doi: 10.1128/IAI.69.7.4373-4381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kordick DL, Breitschwerdt EB. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol. 1995;33:1655–6. doi: 10.1128/jcm.33.6.1655-1656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrell BR, Weiss E, Dasch GA. Morphological and cell association characteristics of Rochalimaea quintana: comparison of the Vole and Fuller strains. J Bacteriol. 1978;135:633–40. doi: 10.1128/jb.135.2.633-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batterman HJ, Peek JA, Loutit JS, Falkow S, Tompkins LS. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect Immun. 1995;63:4553–6. doi: 10.1128/iai.63.11.4553-4556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci. 1997;110:2141–54. doi: 10.1242/jcs.110.18.2141. [DOI] [PubMed] [Google Scholar]
- 8.Gontijo NF, Almeida-Silva S, Costa FF, Mares-Guia ML, Williams P, Melo MN. Lutzomyia longipalpis: pH in the gut, digestive glycosidases, and some speculations upon Leishmania development. Exp Parasitol. 1998;90(3):212–9. doi: 10.1006/expr.1998.4336. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Anez N. Phenol red method for measuring the pH of the gut contents in Lutzomyia longipalpis (Psychodidae: Diptera). Chin J Parasitol Parasitic Dis. 1998;16(1):62–6. [PubMed] [Google Scholar]
- 10.Knobloch J, Solano L, Alvarez O, Delgado E. Antibodies to Bartonella bacilliformis as determined by fluorescence antibody test, indirect hemagglutination and ELISA. Trop Med Parasitol. 1985;36:183–5. [PubMed] [Google Scholar]
- 11.Mitchell SJ, Minnick MF. A carboxy-terminal processing protease gene is located immediately upstream of the invasion-associated locus from Bartonella bacilliformis. Microbiology. 1997;143:1221–33. doi: 10.1099/00221287-143-4-1221. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira-Gomes AP, Cloeckaert A, Zygmunt MS. Characterization of heat, oxidative, and acid stress responses in Brucella melitensis. Infect Immun. 2000;68:2954–61. doi: 10.1128/iai.68.5.2954-2961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battisti JM, Minnick MF. Development of a system for genetic manipulation of Bartonella bacilliformis. Appl Environ Microbiol. 1999;65(8):3441–8. doi: 10.1128/aem.65.8.3441-3448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Wray W, Boulikas T, Wray VP, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]