Abstract
Background
This retrospective study evaluated and assigned scores to six prognostic factors and derived a quantitative scoring system used to determine the periodontal prognosis on molar teeth.
Methods
Data were gathered on 816 molars in 102 patients with moderate to severe periodontitis. The six factors evaluated, age, probing depth, mobility, furcation involvement, smoking, and molar type, were assigned a numerical score based on statistical analysis. The sum of the scores for all factors was used to determine the prognosis score for each molar. Only patients with all first and second molars at the initial examination qualified for the study. All patients were a minimum of 15 years post treatment.
Results
The post treatment time ranged from 15 to 40 years and averaged 24 years. When the study was completed, 639 molars survived (78%), and of those surviving molars, 566 survived in health (89%). In molars with lower scores (1,2,and 3) the 15-year survival rates ranged from 99% to 96%. For scores 4, 5, 6 the 15 year survival rates ranged was 95% to 90% and for molars with scores of 7, 8, 9, and 10 the survival rates ranged from 86% to 67%.
Conclusions
Our results indicate that the periodontal prognosis on molars diagnosed with moderate to severe periodontitis can be calculated using an evidence-based scoring system.
Key words/phrases: Prognosis, Smoking, Tooth Mobility, Periodontitis, Long-Term Care, Molar
Introduction
Determining prognosis is one of the most important functions undertaken in clinical practice. In medicine, determining treatment and prognosis is often assisted by quantitative methods, including combinations of algorithms, decision trees, and/or clinical balance sheets.1,2 Although there are many systems for determining prognosis in periodontal disease3–8, there is a need for an objective, evidenced-based scoring system which will provide a prognosis score for each individual tooth.9,10 Such a scoring system would be beneficial because determining an accurate prognosis on periodontally involved teeth is crucial to the development of an appropriate treatment plan.11 One clinician described assigning periodontal prognosis as an “art based on a science.”12 Another stated, “that a coin toss would be an easier and more accurate way for a clinician to assign a prognosis under traditional guidelines”.13,14
Periodontal disease is multi-factorial and includes both risk factors (factors that cause disease) and prognostic factors (factors that focus on disease out come once disease is present.15–17 Abundant evidence exists in the periodontal literature regarding the association between prognostic factors and tooth loss in periodontally maintained patients.6,13,18–28
While some prognostic factors can be altered by treatment others cannot. Prognostic factors can be categorized into those that can be controlled by the patient (daily plaque removal, smoking cessation, compliance with wearing occlusal guards, compliance with the recommended preventive maintenance schedule); those impacted by periodontal treatment (probing depth, mobility, furcation involvement, trauma from occlusion, bruxism, other parafunctional habits); those associated with systemic disease (diabetes mellitus, immunological disorders, hypothyroidism); and those that are uncontrollable (poor root form, poor crown-root ratio, tooth type, age, genetics).29
Traditionally prognosis of periodontally involved teeth has been evaluated using the terms “good,” “fair,” “poor,” “questionable,” and “hopeless.”7,14 Additionally, “short-term” and “long-term” have been used to signify the future. These arbitrary terms do not offer clinicians a reliable method for assigning prognosis. McGuire and Nunn13 concluded that the ability to predict tooth survival accurately is the ultimate test for any system devised to determine prognosis. The current concept of assigning periodontal prognosis is often based on clinical opinion. The clinician typically considers many factors, including disease severity. While clinical experience, therapeutic skill, and patient compliance can impact prognosis, an objective way of determining prognosis is needed. The purpose of this study was to develop and test a practical, evidence-based scoring system to objectively determine the prognosis on periodontally involved molars. This study has four significant features including: (1) the use of a long-term cohort study, (2) scoring all molars at the initial examination, even those planned for extraction, (3) scoring molars only, the most difficult teeth to treat and maintain, (4) evaluation of the periodontal health of the surviving molars. The scoring system must be simple to score as well as easily understood by both dentist and patient. It should be designed so a dental assistant can calculate the score from examination data. Ultimately a software could be developed that would calculate the score electronically.
Materials and Methods
The study received Institutional Review Board (IRB) approval at the Medical University of South Carolina. Over 800 recall patients treated in a private periodontal practice between 1969 and 1994, were evaluated (PDM). Criteria for inclusion were as follows: 1) all first and second molars present at the initial examination, 2) a diagnosis of moderate to severe chronic periodontal disease30 and 3) periodontal maintenance (PM) for at least 15 years. For patients meeting these criteria a data collection sheet was completed.
Only 106 patients met the inclusion criteria as most patients referred for periodontal treatment were already missing at least one molar. The dates of molar extraction could not be found on four patients, and they were eliminated from the study. Thus our study consisted of 102 patients with 816 molars. Dates of molar extraction(s), final charting (including the health status of surviving teeth), were recorded at the exit examination (PDM). Active treatment began with the initial examination and ended at the first periodontal maintenance appointment, where oral hygiene instructions were reinforced and scaling and polishing were performed. Periodontal maintenance lasted for as long as the patient continued to be seen and included periodontal health and oral hygiene assessment, retreatment when necessary, and surgery when periodontal health could not be maintained by non-surgical therapy.
Six prognostic factors that could be quantitatively evaluated were selected to be scored: age, probing depths, furcation involvement, mobility, molar type, and smoking. A statistically derived score was determined for each factor. The sum of these scores became the score for that tooth.
Originally diabetes mellitus (DM) was to be a scored factor. However, at the initial examination only two patients had a documented diagnosis of DM and both reported their diabetes was well controlled. This low incidence of DM in our patient pool prevented us from evaluating statistically DM as a factor. Plaque and bleeding scores were not included because they were not recorded in patient records. After the preliminary data analysis, scores were assigned for each factor, as follows:
Age: Based on the statistical analysis of the patient pool, molars from patients aged <40 years were assigned a score = 0, while those from patients age ≥40 years were assigned a score = 1.
Probing Depth (PD): Probing depth, not clinical attachment loss (CAL), was scored since clinical attachment level (CAL) was not a commonly recorded examination finding when the study began in 1969.The deepest probing depth of 6 probing sites on a molar was used to determine the PD score. <5mm=0, 5–7mm=1, 8–10mm =2, and >10mm =3
- Mobility:Class 1 mobility = 0; Class 2 = 1; Class 3 = 2. A new and simplified mobility classification was used to determine mobility and was defined as follows:
- Class 1: A tooth is mobile, but in the opinion of the clinician the mobility is not impacting prognosis.
- Class 2: A tooth is mobile, and in the opinion of the clinician the level of the mobility is impacting prognosis.
- Class 3: A tooth is mobile and while perhaps considered hopeless, may be treated under certain circumstances and maintained.
Furcation involvement: The severity of a furcation was not assessed, just the presence of a furcation involvement was used for scoring. In other words, if the concavity of a furcation was detected it was scored as a furcation involvement. No furcation involvement= 0; one furcation = 1; two furcations = 2; three furcations or thru and thru furcations on mandibular molars = 3.
Molar type: Both mandibular first and second molars were assigned a score = 0; maxillary first molars score = 1; and maxillary second molar = 2.
Smoking: Smoking was assessed only at the initial examination. Non-smokers were assigned a score = 0; while smokers were assigned a score = 4.
A summary of the scoring system can be found in Table 1.
Table 1.
Determining the Miller-McEntire Score for Each Tooth
| Age | Number of Furcations/Tooth | Smoking |
|---|---|---|
| 0 – 39 = 0 | 0 furcations = 0 | Non-smoking = 0 |
| 40+ = 1 | 1 furcation = 1 | Smoking = 4 |
| 2 furcations = 2 | ||
| 3 furcations = 3 | ||
| Thru-Thru = 3 (Mandibular molars) | ||
| Pockets (mm) | Mobility | Molar Type |
|---|---|---|
| < 5 = 0 | 0 – 1 = 0 | Mandibular = 0 |
| 5 – 7 = 1 | 2 = 1 | Maxillary 1st = 1 |
| 8 – 10 = 2 | 3 = 2 | Maxillary 2nd = 2 |
| 10+ = 3 | ||
| Miller-McEntire Score = Age + Pockets + # of Furcations/Tooth + Mobility + Smoking + Molar Type | ||
At the exit examination identical data as those taken at the initial examination were recorded. At the exit examination the periodontal health of the surviving molars was assessed using criteria established by the American Academy of Periodontology which defines health as “the absence of inflammation which may appear clinically as redness, suppuration, and bleeding on probing”.31
Data were imported into statistical software program* for all statistical analyses. Scoring assignments for each prognostic factor were determined by exploratory visual examinations of plots for unadjusted Kaplan-Meier survival analysis. Molars extracted for any reason were treated as failures. They were censored at the time of extraction if performed in our practice. However, if the patient’s general dentist extracted a tooth the date used for statistical analysis was the last PM appointment in our office. Molars never extracted were treated as successes, and they were censored at the exit exam. An iterative series of Kaplan-Meier procedures was used to determine applied clinically meaningful classifications for each factor until the survival distribution functions appeared proportional over the selected strata groups for all factors. Finally, we constructed Harrell’s C-index to examine the predictive accuracy of our survival analysis model, with a result of 67.1% (95% CI: 49.7–82.6%). Harrell’s C-index statistic can be interpreted as the probability that a subject from the molar extraction group has a higher probability of having an extraction than a subject from the molar survival group. Future studies are needed to test the periodontal prognostic reliability of the Miller-McEntire score, while also considering differing subjective factors, including patient compliance and the clinician’s philosophy.
The individual scores for each factor was statistically determined only using surviving molars, hence the 32 molars extracted in active treatment were excluded from the analysis. Including healthy molars would necessarily skew the data and build in a bias since the scoring was based on diseased molars. An additional 40 healthy molars survived, each of which had a zero-score for smoking, probing depth, mobility, and furcation involvement were not included in the statistical analysis, since the analysis was based on diseased molars only. To assure the stability of regression models, each score level had to include at least 10 molars. Therefore, 7 surviving molars with a score of 12 and 2 surviving molars with a score of 13 were excluded. This left 735 molars to be analyzed statistically. After the scoring levels for each factor was established then all 816 molars were scored (Table 2).
Table 2.
Descriptive statistics of the periodontally involved molar study population (N=816).
| Analytical molars* (N=735) | All molars (N=816) | |||
|---|---|---|---|---|
| Extracted | Not Extracted | Extracted | Not Extracted | |
| Variable | N (%) | N (%) | N (%) | N (%) |
| ≥ 40 years of age | 352 (58.76) | 82 (60.29) | 110 (62.15) | 370 (57.90) |
| < 40 years of age | 247 (41.24) | 54 (39.71) | 67 (37.85) | 269 (42.10) |
| PPD < 5mm | 1 (0.74) | 20 (3.34) | 5 (2.82) | 56 (8.76) |
| PPD 5–7 mm | 71 (52.21) | 428 (71.45) | 75 (42.37) | 428 (66.98) |
| PPD 8–10 mm | 54 (39.71) | 146 (24.37) | 68 (38.42) | 150 (23.47) |
| PPD 11+ | 10 (7.35) | 5 (0.83) | 29 (16.38) | 5 (0.78) |
| No mobility or Cl I | 108 (79.41) | 566 (94.49) | 120 (67.80) | 603 (94.37) |
| Cl II | 20 (14.71) | 23 (3.84) | 29 (16.38) | 24 (3.76) |
| Cl III | 8 (5.88) | 10 (1.67) | 28 (15.82) | 12 (1.88) |
| Non-smoker | 424 (70.78) | 63 (46.32) | 92 (51.98) | 460 (71.99) |
| Smoker | 175 (29.22) | 73 (53.68) | 85 (48.02) | 179 (28.01) |
| No Furcation | 44 (32.35) | 311 (51.92) | 50 (28.25) | 347 (54.30) |
| 1 Furcations | 51 (37.50) | 185 (30.88) | 54 (30.51) | 185 (28.95) |
| 2 Furcations | 26 (19.12) | 75 (12.52) | 39 (22.03) | 77 (12.05) |
| 3 Furcations or “thru-thru” | 15 (11.03) | 28 (4.67) | 34 (19.21) | 30 (4.69) |
| Mandibular molar | 59 (43.38) | 310 (51.75) | 74 (41.81) | 334 (52.27) |
| First maxillary molar | 37 (27.21) | 152 (25.38) | 46 (25.99) | 158 (24.73) |
| Second maxillary molar | 40 (29.41) | 137 (22.87) | 57 (32.20) | 147 (23.00) |
| Miller-McEntire Score = 13 | N/A | N/A | 1 (0.56) | 2 (0.31) |
| Miller-McEntire Score = 12 | N/A | N/A | 7 (3.95) | 2 (0.31) |
| Miller-McEntire Score = 11 | 10 (7.35) | 4 (0.67) | 16 (9.04) | 4 (0.63) |
| Miller-McEntire Score = 10 | 9 (6.62) | 13 (2.17) | 12 (6.78) | 13 (2.03) |
| Miller-McEntire Score = 9 | 15 (11.03) | 21 (3.51) | 21 (11.86) | 21 (3.29) |
| Miller-McEntire Score = 8 | 15 (11.03) | 45 (7.51) | 20 (11.30) | 38 (5.95) |
| Miller-McEntire Score = 7 | 16 (11.76) | 38 (6.34) | 20 (11.30) | 45 (7.04) |
| Miller-McEntire Score = 6 | 15 (11.03) | 45 (7.51) | 27 (15.25) | 80 (12.52) |
| Miller-McEntire Score = 5 | 15 (11.03) | 80 (13.36) | 16 (9.04) | 80 (12.52) |
| Miller-McEntire Score = 4 | 10 (7.35) | 69 (11.52) | 11 (6.21) | 69 (10.80) |
| Miller-McEntire Score = 3 | 13 (9.56) | 94 (15.69) | 14 (7.91) | 97 (15.18) |
| Miller-McEntire Score = 2 | 4 (2.94) | 100 (16.69) | 6 (3.39) | 105 (16.43) |
| Miller-McEntire Score = 1 | 4 (2.94) | 55 (9.18) | 5 (2.82) | 69 (10.80) |
| Miller-McEntire Score = 0 | N/A | N/A | 1 (0.56) | 14 (2.19) |
| Miller-McEntire score for all molars | 6.93±2.89 (0–13) | 4.32±2.56 (0–13) | 6.54±2.86 (0–12) | 4.32±2.56 (0–13) |
| Miller-McEntire score molars with PPD <5 mm |
1.20±0.84 (0–2) | 2.39±2.22 (0–7) | 1.20±0.84 (0–2) | 2.39±2.22 (0–7) |
| Miller-McEntire score molars with PPD ≥5 mm | 7.10±2.76 (1–13) | 4.50±2.52 (1–13) | 6.74±2.71 (1–12) | 4.50±2.52 (1–13) |
Molars used for determining scoring levels.
Cox proportional hazards regression models that applied both the derived prognosis score and the simultaneous impact of each individual factor score were assessed to estimate associations with molar survival. Given the clustered nature of molars within the same patient, the robust sandwich variance estimate of Lin and Wei was applied for statistical inference of correlated survival data.32 The proportionality of hazards assumption was tested using by score by time interaction and was satisfied for all of our models.
Results
Our study population at the initial examination included 58 females and 44 males. The majority (99) were non-Hispanic whites, 1 was non-Hispanic black and 2 were of other ethnicities. The mean age was 42 (SD=9.47), ranging from 23–71, and 42 were less than age 40 while 60 were 40 or older; 34 (33.3%) were smokers.
At the initial examination of the 816 molars, 7.5% had PD <5 mm; 61.6%, PD 5–7 mm; 26.7%, PD 8–10 mm; and 4.2%, PPD >10. A mobility score of 0 to 1 was noted in 88.6%; 2 was noted in 6.5%; and 3 in 4.9%. No furcation involvement was found in 48.7%; 29.3% had 1 furcation involvement; 14.2% had 2 furcation involvements; and 7.8% had 3 or “thru-thru” involvements.
Overall, 177 total molars were extracted-32 molars (3.9%) were extracted during the active phase of treatment, i.e., before patients entered the maintenance phase of treatment while an additional 145 (17.7%) were extracted during the PM phase of the study. This left 639 molars (78.4%) that survived the duration of the study. Of the 639 molars that survived 588 (87.3%) survived in health and 512 (79.4%) of those had a probing depth < 5mm.
Each patient lost an average of 1.7 molars. The average number of extractions per patient during PM was 1.4. The 32 molars extracted during active treatment had an average initial prognosis score of 8.68. The 145 molars extracted during PM had an average initial score of 6.54 and survived an average of 15.4 years. The surviving 639 molars had an average initial score of 4.32 and survived an average of 24.2 years. Demographic patient characteristics, survival scores and survival in years are provided. (Table 3)
Table 3.
Demographic patient characteristics (N=102), survival scores of the molars (N=816) and survival in years.
| Mean±SD (Range) or N (%) | |
|---|---|
| Females | 58 (56.86%) |
| Non-Hispanic white | 99 (97.06%) |
| Age (years) | 42±9.47 (23–71) |
| Age <40 years | 42 (41.18%) |
| Smokers | 34 (33.33%) |
| Total molars per patient | 8 (100%) |
| Molars extracted per patient | 1.7 ±2.01 (0–8) |
| PPD among molars per patient | 6.57±1.55 (2.75–10.5) |
| Miller-McEntire Score per patient | 4.54±2.21 (1.13–9.63) |
| Average score of 32 molars extracted during active treatment | 8.68 ±2.39 (9–13) |
| Average score of 145 molars extracted during periodontal maintenance | 6.54 ±2.86 (0–12) |
| Average score of the 639 surviving molars | 4.32 ±2.56 (0–13) |
| Average years of survival the 32 molars extracted during active treatment | 0.54 ±0.63 (0–2.33) |
| Average years of survival the 145 molars extracted during periodontal maintenance | 15.41 ±8.46 (1.42–35.02) |
| Average years of survival for the 639 surviving molars | 24.20 ±6.46 (14.33–40.66) |
Risk of Molar Extraction by Clinical Factors and Miller-McEntire Prognosis Score
Multivariable Cox proportional hazards regression models for each individual score component among the analytical molars (N=735) found smoking had the largest effect with a hazards ratio [HR] = 3.46, 95% CI = 2.04–5.88. Second was probing depth (HR = 2.20, 95% CI = 1.69–2.88), followed by mobility (HR = 2.08, 95% CI = 1.45–2.99), and furcation involvement (HR = 1.21, 95% CI = 1.01–1.45). Molar-type score showed marginally increased effects (HR = 1.20, 95% CI = 0.99–1.46), while the age score was (HR = 1.27, 95% CI = 0.96–1.64) (Table 4).
Table 4.
Multivariable Cox proportional hazards model results for molar extraction (N=735).
| Prognostic factor | Parameter Estimate |
Standard Error |
P-value | Hazard Ratio |
95% Hazard Ratio Confidence Limits |
|
|---|---|---|---|---|---|---|
| Age | 0.24 | 0.25 | 0.3271 | 1.27 | 0.79 | 2.06 |
| Smoking | 1.24 | 0.27 | <.0001 | 3.46 | 2.04 | 5.88 |
| PPD | 0.79 | 0.14 | <.0001 | 2.20 | 1.69 | 2.88 |
| Mobility | 0.73 | 0.19 | <.0001 | 2.08 | 1.45 | 2.99 |
| Furcation | 0.19 | 0.09 | 0.0446 | 1.21 | 1.01 | 1.45 |
| Molar-type | 0.19 | 0.10 | 0.0574 | 1.20 | 0.99 | 1.46 |
Time dependent covariates (interaction of each scored factor with loge* follow-up-time) were added to the model to test and verify the proportionality of hazards for our regression method and that assumption was satisfied. The constructed Kaplan-Meier curves and plots for the factor scoring values were proportional and approximately parallel.
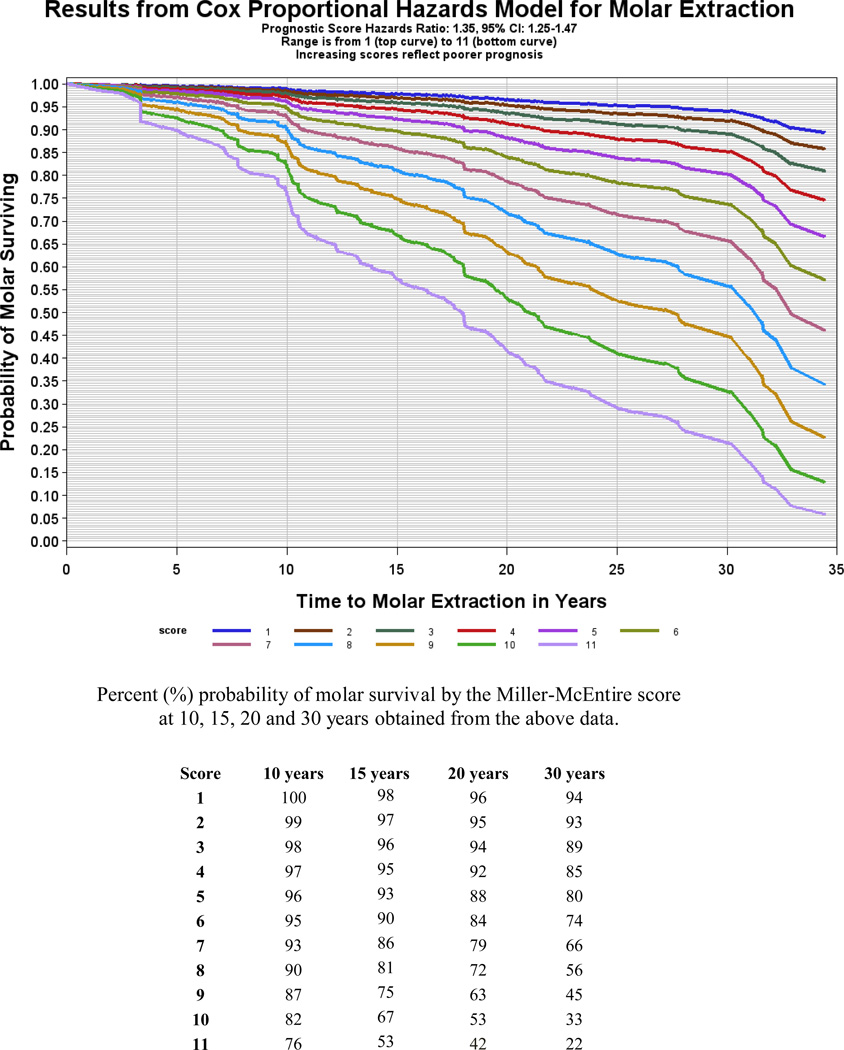
Models for the Miller-McEntire score for all molars showed an increase risk of 38% for molar extraction with every unit increase in score (HR = 1.38, 95% CI = 1.34–1.61). A time dependent covariate was added to test and verify the proportionality of hazards for our regression method, and results showed that this assumption was satisfied. Results from this Cox proportional hazards regression model were also used to produce estimated survival probability curves for each prognosis score level and then converted into percentages (Figure 1).
Figure 1.
Discussion
We elected to use a multivariable approach for statistical analysis rather than a univariable approach to assign scores and evaluate prognostic factors. Multivariable analyses is considered superior to univariable models of prognostic factors, we elected to use this method for our statistical analysis.33,34 Using the Cox hazards regression model, McGuire and Nunn found probing depth (RR=1.39), furcation involvement (RR=1.29), mobility (RR=2.05), percentage bone loss (RR=1.04), parafunctional habit without a biteguard (RR=2.17,) and smoking (RR=2.06) significantly associated with tooth loss in periodontal maintenance patients.13
Using a multivariable logistic regression analysis, Fardal et al. in a group of 100 periodontally maintained patients, identified gender (OR=2.84), age (OR=4.02), and smoking (OR=4.18) as significant predictors of tooth loss.19 Like the present study, Dannewitz et al. used a multi-level proportional hazards model for analyzing only molars. They identified Class III furcation involvements (HR=3.25), baseline bone loss (HR=2.55), smoking (pack-years) – i.e., number of cigarettes/day for 20 years - (HR=1.40), and number of molars left (HR=0.77) significantly related to the retention time.20 Using a logistic regression model, Faggion et al. identified diabetes mellitus (OR=4.17), alveolar bone level (OR=1.04), tooth mobility (OR=5.52), root type (OR=1.82), and a non-vital pulp (OR=2.24) as significant factors.9 The present study found that that smoking (HR=3.38), probing depth (HR=1.33), and mobility (1.45) were the most significant prognostic factors. A comparison between our study and these other prognosis studies can be found in (Table 5).
Table 5.
Comparative Studies
| Hirschfield - Wasserman (1978) | McFall (1982) | Wood et al. (1989) | Tonetti et al. (2000) | Konig et al. (2002) | Fardal et al. (2004) | Dannewitz et al. (2006) | Faggion et al. (2007) | Miyamoto et al. (2010) | Present study (2012) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 600 | 100 | 63 | 273 | 142 | 100 | 71 | 195 | 295 | 102 |
| Mean age | 42 | 44 | 45 | 52 | 46 | 46 | 46 | 48 | 42 | 42 |
| Mean observation period (years) | 22 | 19 | 13.6 | 5.6 | 10.5 | 9.8 | 5 | 11.8 | 20 | 24.2 |
| Teeth evaluated | All Teeth | All Teeth | All Teeth | All Teeth | All Teeth | All Teeth | Molars Only | All Teeth | All Teeth | Molars Only |
| Percentage of teeth extracted during active therapy | N/A | N/A | N/A | 4.8% | N/A | 4.98% | 6.5% | 3.6% | 5 | 3.9% |
| Percentage of teeth extracted during maintenance | 8.4% | 11.4% | 7.1% | 4.2% | 3.0% | N/A | 7.5% | 5.5% | 8.6% | 17.7% |
| Mean annual tooth-loss rate | 0.08 | 0.14 | 0.10 | 0.40 | 0.07 | 0.04 | N/A | 0.11 | N/A | 0.07 |
Table adapted from Faggion, CM: Prognostic model for tooth survival in patients treated for periodontitis, J Clin Periodontol 2007; 34: 226–231.
Multivariable models showed that the patient’s age was not a significant factor for tooth loss. These results are consistent with those of Dannewitz et al. and Muzzi et al. regarding age as a non-significant prognostic factor of molar tooth loss.20,33 Others have reported age as a significant factor; yet, these studies found age group >60 years were to be significant.25,26 However, using Kaplan-Meier survival curves for age, we found appropriate generally proportional survival curves for <40 versus ≥40, whereas curves for <60 versus ≥60 years were not.
Even though age was the least significant factor statistically in our study, we elected to include it as a prognostic factor. Whether age should be included as a prognostic factor is debatable because the literature is ambivalent.14, 27 Additionally scoring uncontrollable factors such as age and molar type does not necessarily depict the impact that periodontal treatment can have on lowering the overall score. Some clinicians are reluctant to provide in-depth surgical treatment to younger patients (age 20 to 30) with severe periodontal disease because the long-term prognosis is considered poor. Our findings, however, suggest these patients can have a favorable prognosis when they receive comprehensive periodontal therapy.
In our study the presence of furcation involvements was less significant when compared with other studies.14,18,22,25,35,36 McGuire and Nunn, Dannewitz et al. and Konig et al. conclude that increased furcation involvement significantly reduces molar survivorship.13,20,35
The difference in our findings could be accounted for by the way furcations were scored. Only the presence of furcation involvements was scored, not the severity. Therefore the impact of their severity could not be analyzed in our statistical models.
Others have reported the negative influence of smoking on periodontal prognosis.37,38 This finding is supported by the dramatic impact that smoking had in our study. Future studies should include more detailed data on smoking, including amount of smoking, as well as changes in smoking habits. In an effort to provide more clinically meaningful smoking scores the clinician may consider the following proposed scores for smoking per day: 1 = occasional smoker, 2 = ≤ ½ pack, 3 = > ½ to 1 pack, and 4 = > 1 pack.
Adherence to preventive maintenance therapy (SPT) is a key factor in maintaining periodontal health as well as determining prognosis.23,24,39 Compliance with the recommended PM interval is variable and can range as low as 16%.27,40 Lang and Tonetti stated under optimal circumstances SPT will be able to maintain stable clinic attachment levels for years.4 In the present study compliance in keeping PM appointments improved over time confirming an observation made by Miyamoto that older patients were more compliant.24 Additionally Costa et al. found that personality type played a role in compliance and that neurotic patients were more compliant.5,41–43 While our study did not evaluate retreatment, Fardal44 found (1) 50% of patients required retreatment, (2) need for retreatment occurred every 6.7 years, and (3) 40% of maintenance patients required additional surgery.
Early in our study PM was done by alternating appointments with the general dentist. Over time, as general practitioners retired, many of these patients had all PM done by the hygienist in the periodontist’s office.
The mean annual tooth loss rate of 0.07 in the present study is comparable to findings in previous retrospective studies. Hirschfeld and Wasserman18; Konig et al.35; and Fardal et al.19 reported mean annual tooth loss rates of 0.08, 0.07 and 0.04, respective. However, these studies included both multi-rooted and single-rooted teeth as well as teeth with minimal or no periodontal involvement. Multiple studies have shown that molar teeth are at greatest risk for disease and tooth loss in periodontally involved patients.18,22,24,33,35 Dannewitz et al. evaluated molars and reported a mean tooth loss rate of 0.06, over a minimum of 5 years PM, providing evidence that molar teeth can be well-maintained in the periodontally compromised patients.20 Our findings confirm their results but over a much longer period of time.
As in any retrospective study our study has limitations. It is well recognized that diabetes mellitus negatively impacts the progression of periodontal disease, yet this factor could not be scored statistically since only 2 of the 102 patients had a diagnosis of DM at the initial examination.
Over the duration of the study only 2 additional patients were diagnosed with DM. This is a puzzling finding given the recent rise in patients diagnosed with DM. This raises an interesting question, "Does controlling periodontal disease, by reducing the overall inflammatory load, play a significant role in preventing the onset of DM?" Although our data did not enable us to score DM, future studies could assign scores based on hemoglobin A1C levels. An example of how hemoglobin A1C levels might be incorporated in a future scoring system could be as follows: 1 = 6.5 – 7.0, 2 = 7.1 – 8.0, 3 = 8.1 – 9.0, and 4 = 9.1 or greater.
Additionally our study did not score the severity of furcation involvement, and future research should evaluate their severity as well as provide more comprehensive data on smoking history (amount, duration and impact of cessation, etc.). Finally, for completeness, all teeth need to be scored not just molars.
Because of our impressive long-term results clinicians might ask: “What type of periodontal treatment was rendered?” From a treatment philosophy any non-mobile molar, regardless of probing depth or multiple furcation involvement was treated. This could account for the low percentage (3.9%) of molars extracted during active treatment. Since the mean probing depth (6.7mm) exceeded the depth of effective root planing (5mm) surgery was done on most molars45,46 with emphasis on thorough root planing. Conservative osseous surgery was done to remove thickened bone and to enhance flap adaptation. Before suturing the roots were briefly scrubbed with a cotton pledget soaked in saturated citric acid to further decontaminate the root surface. Flaps were sutured (4-0 chromic gut) and positioned to cover the bone at the osseous crest or positioned slightly coronal. A periodontal dressing was placed with emphasis on obliterating the interproximal space to help prevent tissue proliferation during primary healing. No attempt was made to obtain a highly scalloped osseous architecture.
Later when freeze dried bone allograft (FDBA) became available osseous graft material was placed in craters; even later strips of polygalactin 910 tight weave woven mesh† were placed under the flap and over the bone graft material in the interproximal space to stabilize the clot and prevent loss of the graft material. This procedure could be considered an early attempt at excluding epithelium and enhancing regeneration i.e., a precursor to guided bone regeneration.47
Greenstein et al.48 concluded that you can have periodontal stability on compromised molars despite less than ideal probing depths. They referred to this as “clinical periodontal health”. In this scenario probing depths remain stable over time, with no additional bone loss, nor clinical attachment loss. Based on a meta analysis of the literature they found that bleeding on probing was a poor forecaster of disease activity and was not a reliable indicator of the demise of teeth. Nevertheless the absence of bleeding on probing is an excellent predictor of no future clinical attachment loss.
In our study 79.4% of surviving molars had a probing depth 5mm or less and 87.3% of those survived in health.31 Currently the treatment of teeth with continued clinical attachment loss is easier and more predictable than treating peri-implantitis on a failing implant. Thus a reason for maintaining periodontally healthy but compromised teeth. In our study when increasing probing depths were noted these areas were generally treated with root planing or by localized gingivectomy using radiosurgery‡ especially on the palatal interproximals of maxillary molars.
Within the parameters of this study, we propose that a more accurate periodontal prognosis can be determined provided the following criteria are met by the patient: (1) They complete the recommended periodontal therapy; (2) follow the recommended maintenance regimen; (3) practice adequate daily plaque removal, and (4) refrain from smoking. While smoking cessation is desired even a marked reduction in smoking should improve the prognosis. Although diabetes mellitus was not a scored factor, control of blood sugar in patients with diabetes mellitus must be a considered criterion.
Finally, active treatment for all patients in this study was completed by the end of 1994 before newer regenerative products and materials were available. As periodontal regenerative techniques evolve, the prognosis on all teeth, not only molars, will improve.49 This alone places a greater emphasis on conveying a more accurate prognosis to the patient.
Conclusions
In this study, 78.3% of the molars treated were never extracted and survived for an average of 24.2 years. They had an initial prognosis score of 4.32. Molars extracted during active treatment (3.9%) had a score of 8.34 while molars extracted during preventive maintenance (17.7%) had an initial score of 6.54 and were maintained on average for 15.4 years before they were extracted. Periodontal health, not simply retention of teeth, should be the goal of periodontal therapy. While 78.3% molars survived it is important to note that 87.3% of those survived in health.
Our statistically derived prognosis scoring system allows clinicians and their patients to make more informed prognostic assessments of periodontally compromised molars. This should substantially improving treatment planning decisions and increase the number of patients accepting periodontal treatment. Of all the prognostic factors evaluated smoking had the most negative impact, far exceeding the impact of probing depth, mobility, or furcation involvement. Molar type had a lesser impact while age had the least impact. Finally, treating moderate to severe periodontal disease can result in an excellent long-term prognosis regardless of the patient’s age.
Acknowledgements
This investigation was supported by a research grant from NIH/NCRR grant P20 RR017696, Medical University of South Carolina (MUSC), Division of Biostatistics and Epidemiology, Center for Oral Health Research. The authors greatly acknowledge the contributions of Dr. James R. Ross (private practice, Memphis, TN) and Dr. Lesley H. Binkley (private practice, Memphis, TN) and the technical editing assistance of Dr. Jennie Ariail (MUSC).
Footnotes
SAS Version 9.2, Cary, NC
Vicryl Product #VWMM, Ethicon Inc., Somerville, NJ
Dento-Surg 90, Ellman International, Oceanside, NY
The authors report no conflict of interest related to this study.
REFERENCES
- 1.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111(1):58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 2.Martin KE, Helvie MA, Zhou C, et al. Mammographic density measured with quantitative computer-aided method: comparison with radiologists' estimates and BI-RADS categories. Radiology. 2006;240(3):656–665. doi: 10.1148/radiol.2402041947. [DOI] [PubMed] [Google Scholar]
- 3.Heitz-Mayfield LJ. Disease progression: identification of high-risk groups and individuals for periodontitis. J Clin Periodontol. 2005;32(Suppl 6):196–209. doi: 10.1111/j.1600-051X.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Lang NP, Tonetti MS. Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT) Oral health & preventive dentistry. 2003;1(1):7–16. [PubMed] [Google Scholar]
- 5.Leininger M, Tenenbaum H, Davideau JL. Modified periodontal risk assessment score: long-term predictive value of treatment outcomes. A retrospective study. J Clin Periodontol. 2010;37(5):427–435. doi: 10.1111/j.1600-051X.2010.01553.x. [DOI] [PubMed] [Google Scholar]
- 6.Matuliene G, Pjetursson BE, Salvi GE, et al. Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol. 2008;35(8):685–695. doi: 10.1111/j.1600-051X.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- 7.Kwok V, Caton JG. Commentary: prognosis revisited: a system for assigning periodontal prognosis. J Periodontol. 2007;78(11):2063–2071. doi: 10.1902/jop.2007.070210. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Page RC, Loeb CF, Levi PA., Jr Tooth loss in 776 treated periodontal patients. J Periodontol. 2010;81(2):244–250. doi: 10.1902/jop.2009.090184. [DOI] [PubMed] [Google Scholar]
- 9.Faggion CM, Jr, Petersilka G, Lange DE, Gerss J, Flemmig TF. Prognostic model for tooth survival in patients treated for periodontitis. J Clin Periodontol. 2007;34(3):226–231. doi: 10.1111/j.1600-051X.2006.01045.x. [DOI] [PubMed] [Google Scholar]
- 10.Hujoel PP. Endpoints in periodontal trials: the need for an evidence-based research approach. Periodontol 2000. 2004;36:196–204. doi: 10.1111/j.1600-0757.2004.03681.x. [DOI] [PubMed] [Google Scholar]
- 11.Mordohai N, Reshad M, Jivraj S, Chee W. Factors that affect individual tooth prognosis and choices in contemporary treatment planning. Br Dent J. 2007;202(2):63–72. doi: 10.1038/bdj.2007.23. [DOI] [PubMed] [Google Scholar]
- 12.Prichard JF. Advanced periodontal disease: surgical and prosthetic management. 2nd ed. Philadelphia: WB: Saunders; 1972. [Google Scholar]
- 13.McGuire MK, Nunn ME. Prognosis versus actual outcome. III. The effectiveness of clinical parameters in accurately predicting tooth survival. J Periodontol. 1996;67(7):666–674. doi: 10.1902/jop.1996.67.7.666. [DOI] [PubMed] [Google Scholar]
- 14.McGuire MK, Nunn ME. Prognosis versus actual outcome. II. The effectiveness of clinical parameters in developing an accurate prognosis. J Periodontol. 1996;67(7):658–665. doi: 10.1902/jop.1996.67.7.658. [DOI] [PubMed] [Google Scholar]
- 15.Laupacis A, Wells G, Richardson WS, Tugwell P. Users' guides to the medical literature. V. How to use an article about prognosis. Evidence-Based Medicine Working Group. Jama. 1994;272(3):234–237. doi: 10.1001/jama.272.3.234. [DOI] [PubMed] [Google Scholar]
- 16.Beck JD. Methods of assessing risk for periodontitis and developing multifactorial models. J Periodontol. 1994;65(5 Suppl):468–478. doi: 10.1902/jop.1994.65.5s.468. [DOI] [PubMed] [Google Scholar]
- 17.Beck JD. Risk revisited. Community Dent Oral Epidemiol. 1998;26(4):220–225. doi: 10.1111/j.1600-0528.1998.tb01954.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirschfeld L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49(5):225–237. doi: 10.1902/jop.1978.49.5.225. [DOI] [PubMed] [Google Scholar]
- 19.Fardal O, Johannessen AC, Linden GJ. Tooth loss during maintenance following periodontal treatment in a periodontal practice in Norway. J Clin Periodontol. 2004;31(7):550–555. doi: 10.1111/j.1600-051X.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 20.Dannewitz B, Krieger JK, Husing J, Eickholz P. Loss of molars in periodontally treated patients: a retrospective analysis five years or more after active periodontal treatment. J Clin Periodontol. 2006;33(1):53–61. doi: 10.1111/j.1600-051X.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- 21.Chace R, Sr, Low SB. Survival characteristics of periodontally-involved teeth: a 40-year study. J Periodontol. 1993;64(8):701–705. doi: 10.1902/jop.1993.64.8.701. [DOI] [PubMed] [Google Scholar]
- 22.McFall WT., Jr Tooth loss in 100 treated patients with periodontal disease. A long-term study. J Periodontol. 1982;53(9):539–549. doi: 10.1902/jop.1982.53.9.539. [DOI] [PubMed] [Google Scholar]
- 23.Becker W, Becker BE, Berg LE. Periodontal treatment without maintenance. A retrospective study in 44 patients. J Periodontol. 1984;55(9):505–509. doi: 10.1902/jop.1984.55.9.505. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto T, Kumagai T, Lang MS, Nunn ME. Compliance as a prognostic indicator. II. Impact of patient's compliance to the individual tooth survival. J Periodontol. 2010;81(9):1280–1288. doi: 10.1902/jop.2010.100039. [DOI] [PubMed] [Google Scholar]
- 25.Goldman MJ, Ross IF, Goteiner D. Effect of periodontal therapy on patients maintained for 15 years or longer. A retrospective study. J Periodontol. 1986;57(6):347–353. doi: 10.1902/jop.1986.57.6.347. [DOI] [PubMed] [Google Scholar]
- 26.Chambrone LA, Chambrone L. Tooth loss in well-maintained patients with chronic periodontitis during long-term supportive therapy in Brazil. J Clin Periodontol. 2006;33(10):759–764. doi: 10.1111/j.1600-051X.2006.00972.x. [DOI] [PubMed] [Google Scholar]
- 27.Wood WR, Greco GW, McFall WT., Jr Tooth loss in patients with moderate periodontitis after treatment and long-term maintenance care. J Periodontol. 1989;60(9):516–520. doi: 10.1902/jop.1989.60.9.516. [DOI] [PubMed] [Google Scholar]
- 28.Tonetti MS, Steffen P, Muller-Campanile V, Suvan J, Lang NP. Initial extractions and tooth loss during supportive care in a periodontal population seeking comprehensive care. J Clin Periodontol. 2000;27(11):824–831. doi: 10.1034/j.1600-051x.2000.027011824.x. [DOI] [PubMed] [Google Scholar]
- 29.Newman MG, Takei HH, Klokkevold PR, Carranza FA. Carranza's Clinical Periodontology. Elsevier Saunders; 2012. Clinical Risk Assessment; pp. 370–372. [Google Scholar]
- 30.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Comprehensive periodontal therapy: A statment by the American Academy of Periodontology. J Periodontol. 2011;82(7):943–949. doi: 10.1902/jop.2011.117001. [DOI] [PubMed] [Google Scholar]
- 32.Wei LJ, Lin DY, Weissfeld L. Regression analysis of mulitvariate incomplete failure time data by using the marginal distributions. Journal of American Statistical Association. 1989;84:1065–1073. [Google Scholar]
- 33.Muzzi L, Nieri M, Cattabriga M, Rotundo R, Cairo F, Pini Prato GP. The potential prognostic value of some periodontal factors for tooth loss: a retrospective multilevel analysis on periodontal patients treated and maintained over 10 years. J Periodontol. 2006;77(12):2084–2089. doi: 10.1902/jop.2006.050227. [DOI] [PubMed] [Google Scholar]
- 34.Albandar JM, Goldstein H. Multi-level statistical models in studies of periodontal diseases. J Periodontol. 1992;63(8):690–695. doi: 10.1902/jop.1992.63.8.690. [DOI] [PubMed] [Google Scholar]
- 35.Konig J, Plagmann HC, Ruhling A, Kocher T. Tooth loss and pocket probing depths in compliant periodontally treated patients: a retrospective analysis. J Clin Periodontol. 2002;29(12):1092–1100. doi: 10.1034/j.1600-051x.2002.291208.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang HL, Burgett FG, Shyr Y, Ramfjord S. The influence of molar furcation involvement and mobility on future clinical periodontal attachment loss. J Periodontol. 1994;65(1):25–29. doi: 10.1902/jop.1994.65.1.25. [DOI] [PubMed] [Google Scholar]
- 37.Johnson GK, Guthmiller JM. The impact of cigarette smoking on periodontal disease and treatment. Periodontol 2000. 2007;44:178–194. doi: 10.1111/j.1600-0757.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 38.Patel RA, Wilson RF, Palmer RM. The effect of smoking on periodontal bone regeneration: a systematic review and meta-analysis. J Periodontol. 2012;83(2):143–155. doi: 10.1902/jop.2011.110130. [DOI] [PubMed] [Google Scholar]
- 39.McGuire MK. Prognosis versus actual outcome: a long-term survey of 100 treated periodontal patients under maintenance care. J Periodontol. 1991;62(1):51–58. doi: 10.1902/jop.1991.62.1.51. [DOI] [PubMed] [Google Scholar]
- 40.Wilson TG, Jr, Glover ME, Malik AK, Schoen JA, Dorsett D. Tooth loss in maintenance patients in a private periodontal practice. J Periodontol. 1987;58(4):231–235. doi: 10.1902/jop.1987.58.4.231. [DOI] [PubMed] [Google Scholar]
- 41.Costa FO, Cota LO, Lages EJ, et al. Periodontal risk assessment model in a sample of regular and irregular compliers under maintenance therapy: a 3-year prospective study. Journal of periodontology. 2012;83(3):292–300. doi: 10.1902/jop.2011.110187. [DOI] [PubMed] [Google Scholar]
- 42.Costa FO, Santuchi CC, Lages EJ, et al. Prospective study in periodontal maintenance therapy: comparative analysis between academic and private practices. Journal of periodontology. 2012;83(3):301–311. doi: 10.1902/jop.2011.110101. [DOI] [PubMed] [Google Scholar]
- 43.Costa FO, Miranda Cota LO, Pereira Lages EJ, et al. Oral impact on daily performance, personality traits, and compliance in periodontal maintenance therapy. Journal of periodontology. 2011;82(8):1146–1154. doi: 10.1902/jop.2011.100515. [DOI] [PubMed] [Google Scholar]
- 44.Fardal O, O'Neill C, Gjermo P, et al. The lifetime direct cost of periodontal treatment: a case study from a Norwegian specialist practice. Journal of periodontology. 2012;83(12):1455–1462. doi: 10.1902/jop.2012.110689. [DOI] [PubMed] [Google Scholar]
- 45.Gellin RG, Miller MC, Javed T, Engler WO, Mishkin DJ. The effectiveness of the Titan-S sonic scaler versus curettes in the removal of subgingival calculus. A human surgical evaluation. J Periodontol. 1986;57(11):672–680. doi: 10.1902/jop.1986.57.11.672. [DOI] [PubMed] [Google Scholar]
- 46.Waerhaug J. Healing of the dento-epithelial junction following subgingival plaque control. II: As observed on extracted teeth. J Periodontol. 1978;49(3):119–134. doi: 10.1902/jop.1978.49.3.119. [DOI] [PubMed] [Google Scholar]
- 47.Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984;11(8):494–503. doi: 10.1111/j.1600-051x.1984.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 48.Greenstein G, Greenstein B, Cavallaro J. Prerequisite for treatment planning implant dentistry: periodontal prognostication of compromised teeth. Compend Contin Educ Dent. 2007;28(8):436–446. quiz: 447, 470. [PubMed] [Google Scholar]
- 49.Cortellini P, Stalpers G, Mollo A, Tonetti MS. Periodontal regeneration versus extraction and prosthetic replacement of teeth severely compromised by attachment loss to the apex: 5-year results of an ongoing randomized clinical trial. J Clin Periodontol. 2011;38(10):915–924. doi: 10.1111/j.1600-051X.2011.01768.x. [DOI] [PubMed] [Google Scholar]