Abstract
Introduction
Age associated cognitive impairment is associated with low levels of IGF-1, oxidative stress, and neuronal loss in the hippocampus. Ames dwarf mice are long-lived animals that exhibit peripheral IGF-1 deficiency. Hippocampal-based spatial memory (a homolog of cognitive function) has not been evaluated in these long-living mice.
Materials and methods
We evaluated the hippocampal-based spatial memory in 3-, 12- and 24-month-old Ames dwarf and wild type mice using the Barnes maze and the T-maze. We also examined the effect of a hippocampal-specific toxin, kainic acid (KA), on spatial memory to determine whether Ames mice were resistant to the cognitive impairment induced by this compound.
Results
We found that Ames dwarf mice exhibit enhanced learning, making fewer errors and using less time to solve both the Barnes and T-mazes. Dwarf mice also have significantly better short-term memory as compared to wild type mice. Both genotypes exhibited neuronal loss in the CA1 and CA3 areas of the hippocampus following KA, but Ames dwarf mice retained their spatial memory.
Discussion
Our results show that Ames dwarf mice retained their spatial memory despite neurodegeneration when compared to wild type mice at an “equiseizure” dose of KA.
Keywords: Ames dwarf, Hippocampus, Spatial memory, Barnes maze, Kainic acid
1. Introduction
The US Census Bureau projects that there will be 71.5 million people over the age of 65 by 2030 representing 20% of the total US population. Large numbers of elderly suffer from age-associated cognitive impairment even in the absence of any overt neurode-generative disease. Memory is one of the earliest cognitive functions to show declines during aging (Albert and Funkenstein, 1992) and this decline can have a devastating impact on individuals, families, the health care system, and society as a whole. With advancing age, humans show a 30–80% drop in performance on spatial memory tasks (Cherry and Park, 1993; Evans et al., 1984; Forster et al., 1996; Kirasic and Bernicki, 1990; Moffat et al., 2001; Moore et al., 1984; Sharps and Gollin, 1987). The hippocampus is particularly vulnerable to the effect of aging and it has been reported that a decrease in hippocampal function positively correlates with cognitive dysfunction (Mesulam, 1999; Miller and O'Callaghan, 2005; Barnes, 1979, 1988; Gallagher and Nicolle, 1993; Geinisman et al., 1986).
The gradual decline in cognition with advanced aging has been shown to be associated with the decreased activity of the growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis and oxidative stress (Aleman et al., 1999; Dik et al., 2003). It has been demonstrated that GH deficient patients exhibit reduced cognitive performance that is associated with reduced IGF-l levels in both the plasma and in the central nervous system (Trejo et al., 2004; van Dam and Aleman, 2004). Another important factor implicated in the age associated decline in learning and memory is increased oxidative damage (Agarwal and Sohal, 1996; Dubey et al., 1996; Forster et al., 1996; Sohal et al., 1994). Kainic acid (KA), an acidic pyrolidine isolated from the seaweed Digenea simplex, causes neuronal loss specifically in the CA3 and CA1 regions of the hippocampus by producing free radicals (McGeer and McGeer, 1982; Sun et al., 1992). Multiple studies have shown that KA administered by various routes affects spatial learning and memory in diverse animal models (Arkhipov et al., 2008; Brown-Croyts et al., 2000; Gayoso et al., 1994; Hashimoto et al., 1998; Holmes et al., 1988). Thus, we were interested in the effects of KA on learning and memory in a long-living GH-deficient mouse, the Ames dwarf, as this animal has been shown to resist oxidative stress.
The Ames dwarf mouse is a long-lived animal (living >50% longer than the wild type) that has a mutation in Prop 1df (Bartke et al., 2001; Brown-Borg et al., 1996). This point mutation impairs the development of the anterior pituitary resulting in a lack of circulating GH, thyroid stimulating hormone and prolactin. As a consequence of GH deficiency, these animals lack peripheral IGF-1 and are one-third the size of normal mice. Based on the literature, one would predict that with decreased plasma IGF-1 there would be cognitive impairment in these animals. However, it has been shown that old (22–29 months) Ames dwarf mice retained their memory in an inhibitory avoidance test (Kinney et al., 2001b). In addition, another study (Mattison et al., 2000) demonstrated that 18–21-month-old dwarf mice have increased retention in the inhibitory avoidance task at the 24-h and 7-day retention tests. The memory enhancement was attributed to high levels of hippocampal GH and IGF-1 observed in Ames dwarf mice compared to wild type mice (Sun et al., 2005a). This group also suggested that high IGF-1 levels may contribute to the survival of newly born neurons and subsequently delay or prevent the cognitive loss that occurs with aging (Sun and Bartke, 2007). Additionally, Ames mice have been shown to exhibit increased antioxidant capacity in peripheral tissues (Brown-Borg and Rakoczy, 2000, 2005; Brown-Borg et al., 2005; Romanick et al., 2004).
Considering the link between aging, memory, IGF-1 levels, and oxidative stress, we designed experiments to study learning and memory in long-living Ames dwarf mice. To date, hippocampal-based spatial memory has been not evaluated in this mouse and the effect of aging on spatial memory is not known. The purpose of the present study was to evaluate hippocampal-based spatial memory in Ames mice as compared to age-matched wild type siblings. To address this question we used the T-maze and Barnes maze, both behavioral paradigms known to assess spatial memory in rodents (Barnes, 1988; Bizon et al., 2007). Ames dwarf mice exhibit enhanced antioxidative enzyme activities in the periphery but the response to oxidative stress in brain has not been studied. We therefore used KA to induce oxidative stress and cause hippocampal damage, and examined the effect of this oxidative stress on spatial memory in these animals.
2. Materials and methods
2.1. Animals
Ames dwarf mice (df/df) were bred and maintained at the animal facilities of the University of North Dakota (UND) under controlled conditions of 12 h light:12 h dark cycle and temperature (22±1 °C) with ad libitum access to food (8640 Teklad 22/5 rodent diet with 22.6% crude protein, 5.2% fat, Harlan Laboratories) and water (standard laboratory conditions). The Ames dwarf mice used in this study were derived from a closed colony with a heterogeneous background (over 25 years). Homozygous (df/df) or heterozygous (df/+) dwarf males were mated with carrier females (df/+) to generate dwarf mice. All procedures involving animals followed ‘The guide for care and use of laboratory animals (National Research Council)’ and were reviewed and approved by the UND Institutional Animal Care and Use Committee. The average life span of the wild type mice in our colony is 23–24 months (Brown-Borg et al., 1996). All animals were housed in their individual home cages and moved to the Barnes maze room a day prior to the start of experiments to eliminate stress. Behavioral tests were performed in the same room by the same experimenter between 8:00 a.m. and 1:00 p.m. Animals were handled by the experimenter the day before the experiments to reduce anxiety.
Three, 12- and 24-month-old animals (n = 18 wild type/age except 24 month where n = 12; n = 12 Ames dwarf/age) were included in the study. Wild type female mice were not included to avoid potential hormonal effects on memory. Both male and female Ames dwarf mice were used in the study since female dwarfs do not exhibit estrous cycles and are therefore not influenced by sex steroid hormones. To ensure that male and female dwarf mice could be grouped together for analysis of the behavioral data, the initial analysis was performed separately for male and female mice. There were no differences between the male and female mice for errors in all 3 age groups. At the time of KA injection, wild type animals (3 and 12 months) were randomly divided into 3 groups (n = 6) to receive one of the following—normal saline (WT-SAL), KA 15 mg/kg (WT-KA 15) or KA 30 mg/kg (WTKA 30). Ames dwarf mice (3, 12 and 24 months) and wild type mice (24 months) were randomly divided to receive either normal saline (DF-SAL) or KA 15 mg/kg (DF-KA 15). The animals were weighed at the beginning of the experiment and then every week thereafter. The experimental time line for the Barnes maze study is shown in Fig. 1.
Fig. 1.
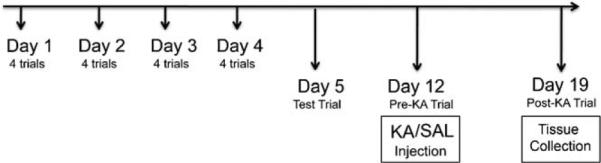
Experimental time-line for Barnes maze experiments. Mice were trained on the Barnes maze, after the initial habituation trial, for 4 days with 4 trials everyday. The inter-trial interval was at least 15 min. On day 5, a single trial was performed to assess short-term retention of memory. On day 12, another single trial was performed to assess long-term memory retention and animals were randomly divided to receive saline (SAL) or kainic acid (KA) injections. On day 19, a post-KA trial was performed on the Barnes maze. Animals were not exposed to the maze between day 5 and day 12 and between day 12 and day 19.
2.2. Behavioral study
2.2.1. Barnes maze
Spatial task performance was tested in a circular dry land maze (Barnes, 1979) which is similar to the Morris water maze but less stressful in the assessment of spatial learning (Deacon et al., 2002; Harrison et al., 2009, 2006; Pompl et al., 1999). The method was adapted from a recently published technique (Sunyer et al., 2007). The paradigm consisted of a circular platform (122 cm in diameter), at a height of 140 cm, with 40 holes (hole diameter—5 cm) along the perimeter (ENV-562-M; Med Associates). The platform was surrounded by black curtains with visual cues on them. During testing, animals received reinforcement to escape from the open platform surface to a small, dark, recessed chamber located under one of the holes called the “target box”.
In the pre-training trial, the mouse was placed in the middle of the maze under a dark colored box allowing the mice to be in random orientation before each trial. After 10 s elapsed, the chamber was lifted, and the mouse was allowed to explore the maze for 5 min. If the mouse did not find the target box, the mouse was gently guided to enter the box and was allowed to remain in the box for 1 min before returning back to the cage. The training trials were run in a similar manner. However, the trial ended when the mouse entered the target box or after 5 min had elapsed. On trials where the mouse did not enter the target box within 5 min, a time of 300 s was recorded, and the mouse was guided to the box. Immediately after the mouse entered, the box was covered and the mouse was allowed to remain in the box for 1 min. Mice were trained for four trials per day for 4 days with an inter-trial interval of at least 15 min. After each trial the entire maze was cleaned with a cleaning solution (Micro-90) and 70% alcohol. The same experimenter recorded trials each time standing in the same position. The following parameters were recorded (during acquisition and testing): errors, latency(s), and search strategy. Errors were defined as nose pokes and head deflections over any hole that did not have the target box and latency was the time taken by the mouse to enter the target box.
Mice sometimes lacked motivation and explored the maze after finding the target hole without entering into it. This resulted in an increase in the number of errors due to further exploration of the maze although the mouse learned the association between the spatial cues and the escape location. Also, mice sometimes sat near the target hole without going into the target box. Harrison et al. (2006) proposed a solution by calculating latency, path length and number of errors to the first encounter of the escape hole, called primary latency, primary path length and primary errors, respectively. We used the same method to calculate these parameters since it was important to measure both total and primary parameters for a better understanding and interpretation of data during the acquisition phase. On day 5, mice were given a single test trial on the maze to evaluate the short-term memory retention. Similarly, on day 12 and day 19, mice were again given a single test trial on the maze to evaluate long-term memory retention and the effect of KA, respectively. The position of the target box was the same as during the training period. Mice were not trained between day 5 and 12 and day 12 and 19.
Search strategies were determined by examining each trial and placing the mice into one of the three categories: (1) spatial—moving directly to target hole or to an adjacent hole before visiting the target hole (≤3 errors); (2) random—hole searches separated by crossing through the center of the maze or unorganized search; (3) serial—the first visit to the target hole was preceded by a visit to adjacent holes in serial manner, clockwise or counter clockwise in direction.
2.2.2. T-maze
A separate group of animals at 3 and 12 months (n = 12/genotype/age) were used to assess spatial memory on a T-maze designed for mice (SD Instruments, Inc.) using a method adopted from (Bizon et al., 2007). For habituation, training and probe trials, animals were food deprived to 85% of ad libitum body weight and maintained at this weight throughout the experiment (weight was recorded on the days of training as well as the probe trial).
On day 1 of habituation, animals were allowed to freely explore the maze for a 10-min period with the maze arms baited with sweetened condensed milk (diluted 1:1 with water). Arm preference was determined during habituation and the animals were trained against their preference in subsequent training trials. For training on days 2, 3 and 4, mice were given four 2-min trials with a 20 s inter-trial interval; the goal cup at the end of target arm was baited with 50 μl of milk. Entries into the unbaited arm were scored as incorrect responses, and those into the baited arm were scored as correct responses. The time required to enter the baited arm and reach the target was recorded as latency. The T-maze was swabbed with 70% ethanol between animals to eliminate odors.
In order to assess the strategy that mice were using to successfully retrieve the food reward, mice were given two probe trials (day 5 and 12). During the probe trials, the maze was rotated 180°, but all cues, including the experimenter, remained stationary. The goal arm also remained the same (e.g., if the arm to the right of the experimenter was baited before the maze was turned, then the arm to the right of the experimenter was also baited during the probe). Mice were allowed to make a single entry into either the baited or unbaited arm. Those mice entering the baited arm were designated as using a “place” strategy, which has been shown to involve the medial temporal lobe, including the hippocampus. Those mice entering the unbaited arm (i.e., making same body turn as used during training) were designated as using a “response” strategy. Between the probe trials on day 5 and 12, mice resumed ad libitum feeding for 1 week.
2.3. Kainic acid injections and scoring of seizure severity
The KA (Ascent Scientific) was dissolved in normal saline as per manufacturer's instructions. Animals were weighed and injected with 15 mg/kg i.p. In a preliminary dose–response study, we evaluated the sensitivity to seizures induced by KA (dose ranging from 5 to 40 mg/kg) in wild type and age-matched Ames dwarf animals. Based on this preliminary study, an additional group of 3- and 12-month-old wild type animals (n = 6/age) were injected with 30 mg/kg KA to match the seizure intensity displayed by Ames dwarf mice with 15 mg/kg KA. Control animals were injected with an equal volume of normal saline.
Seizure intensity after KA injection was evaluated using a modified Racine rating system (Kraft et al., 2006; Racine, 1972). Over a 2-h period following KA injection, the mice were observed for seizure activity. Every 15 min, the maximal seizure characteristic demonstrated was recorded as follows: 0, normal activity; 1, immobility, staring; 2, rigidity, tail-extension, head bobbing; 3, repetitive movements, bilateral pawing, rearing, hind limb tremors; 4, minor seizure (severe forelimb tremor) or wobbling, jumping, falling; 5, tonic clonic convulsions or multiple and/or prolonged occurrence of rating 4; 6, severe tonic–clonic seizure (observable loss of motor control); 7, death. Seven days post-KA injection, mice were tested on the Barnes maze (day 19) and then sacrificed. The brains were rapidly removed, and the hippocampal tissues dissected, rapidly frozen in liquid nitrogen and stored at−80°C until biochemical analysis.
2.4. RNA extraction and RT-PCR
Gene expression was evaluated in one-half of the hippocampus of 3-, 12- and 24-month-old Ames dwarf and age-matched wild type mice using real-time RT-PCR techniques as reported previously (Brown-Borg et al., 2008). Total RNA was extracted from tissues using Ultraspec RNA (Biotecx) based on a previously described method (Chomczynski and Sacchi, 1987). Equal amounts of RNA for the gene of interest and the reference gene β2-microglobulin (β2M; Lupberger et al., 2002) were utilized to perform one-step real-time quantitative PCR using a QuantiTect SYBR Green RT-PCR kit (Qiagen) according to the manufacturer's protocol and were assayed using a SmartCycler instrument (Cepheid). An annealing temperature of 60°C was used for IGF-1 (For 5′-CTG AGC TGG TGG ATG CTC TT-3′; Rev 5′-CAC TCA TCC ACA ATG CCT GT-3′) and IGF-1R (For 5′-ACT GAC CTC ATG CGC ATG TGC TGG-3′; Rev 5′-CTC GTT CTT GCC CCC GTT CAT-3′) and 62°C for β2M (For 5′ AAG TAT ACT CAC GCC ACC CA-3′; Rev 5′-AAG ACC AGT CCT TG-3′) primer sets. Gene expression was quantified by using the comparative threshold cycle (CT) method (Heid et al., 1996). The amount of target (in all the treatment groups) was normalized to an endogenous reference (β2M) and compared relative to the control group (wild type mice saline group).
2.5. Western blot
Proteins were extracted from the remaining one-half of the frozen hippocampal tissue by homogenization on ice in buffer (CPE buffer; Brown-Borg and Rakoczy, 2000) and the supernatant fraction was used for analysis. Protein quantification of the samples was performed using a Bradford assay (Bradford, 1976). Proteins (50 mg) were separated on 15% Criterion™ Precast Gels (Biorad) followed by transfer to PVDF membranes. Membranes were incubated overnight at 4°C with antibody to IGF-1 (1:200, Santa Cruz Biotechnology) and IGF-1R (1:200, Santa Cruz Biotechnology) to detect the protein levels using standard immunoblotting procedures and chemiluminescence (Biorad). Quantification of the results was performed by densitometry using a UVP Biomaging system (Upland). Ponceau S was used as a loading control.
2.6. Immunohistochemistry
A separate group of 3-month-old mice (n = 3/genotype/treatment) were perfused with phosphate buffered 4% paraformaldehyde pH 7.4, 7 days after saline or KA injection. Brains were removed and placed in 30% sucrose for cryoprotection. Coronal sections (20-μm thick) were obtained using a freezing sliding microtome (Leica 3000R) and collected into 0.1 M sodium phosphate buffer, pH 7.4, containing 0.9% phosphate-buffered saline. Sections were then preserved in a cryoprotectant solution consisting of 0.1 M phosphate buffer, pH 7.2 (50%, v/v), sucrose (30%, w/v), polyvinylpyrrolidone (1%, w/v) and ethylene glycol (30%, v/v) at −20°C until staining (Watson et al., 1986).
2.7. FluoroJade C staining
FluoroJade C (FJC) was used to stain for degenerating neurons in the brain using a previously described method (Schmued et al., 1997, 2005). Briefly, 3 sequential sections (~bregma –1.70 mm) were rinsed in 0.1 M Tris, pH 7.4, mounted, and air-dried at room temperature. Sections were pre-treated with alcohol and distilled water. They were then oxidized in a solution of 0.06% KMnO4, rinsed and incubated in a solution of 0.001% FJC (Chemicon) containing 0.01% of DAPI (Molecular Probes) in 0.1% acetic acid. The slides were rinsed, cleared in xylene, and coverslipped. FluoroJade C-stained cells emit a typical green color with excitation peak at 480 nm and emission peak around 525 nm. Images were captured using an Olympus BX-51 fluorescent microscope with attached DP-71 color camera.
For semi-quantification, FluoroJade C-stained degenerated neurons within the CA1 and CA3 subfields of hippocampus were counted from 20× digital images using Adobe Photoshop count tool (Adobe Photoshop CS3 extended). Two areas (measuring 240 μm2) each from CA1 and CA3 subfields of hippocampus were used (3 sections/3 animals/genotype). The data were averaged for these two areas of CA1 and two areas of CA3 to obtain one measurement each for CA1 and CA3 subfields from each section. This average was then used for statistical analysis. The counting procedure used was similar to methods described in other reports (Liu et al., 2009; Mitruskova et al., 2005).
2.8. Statistical analysis
Analysis of the behavioral data from the Barnes maze and T-maze learning curves, for the different age groups, was conducted using a two-way repeated measures (mixed models) ANOVA with genotype (wild type versus Ames dwarf) as the between-subject variable, and training day as the repeated measure. For each of 4 acquisition measures in the Barnes maze data (primary and total errors and latencies), the average of four daily trials was calculated. The slope of the learning curve comparing number of errors and time was measured using regression analysis and compared across genotypes using two-way ANOVA. Simple regression analysis was also performed to detect correlations between primary and total errors/latency. Similarly, for T-maze data, the latency and errors were calculated as an average of four trials per day. Between group comparisons for the remainder of the Barnes maze and T-maze data were performed using two-way ANOVA. Chi-square (χ2) or Fisher Exact tests were used to compare wild type and Ames dwarf mice with respect to frequencies of spatial strategy and the number of trials completed. The Bonferroni's multiple comparisons test with a p < 0.05 adjusted significance level, was used when appropriate to identify significant differences between groups. In some situations, hypothesis-testing using a Student's t-test was applied to determine the existence of significant differences between specific groups in the absence of specific genotype–treatment interactions (Wilcoxon et al., 2007). RT-PCR and western blotting data were analyzed by two-way analysis of variance (ANOVA, factors: genotype and treatment). All data are presented as mean ± SEM. GraphPad Prism (GraphPad) was used to perform all statistical analyses.
3. Results
In the present study, we examined hippocampal-based spatial memory in 3-, 12- and 24-month-old long-living Ames dwarf mice as compared to their age-matched wild type siblings using a dry-land circular Barnes maze and a T-maze. The body weights of the animals over the course of the study did not change significantly in either genotype or saline control and KA-treated groups.
3.1. Errors and latency during acquisition phase on Barnes maze
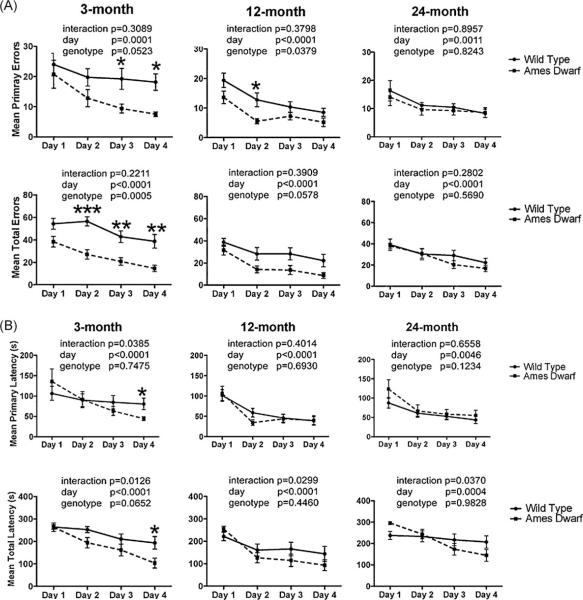
Spatial learning using a Barnes maze is hippocampus-dependent and the performance on this task is sensitive to aging (Pompl et al., 1999; Yau et al., 2007). Learning is assessed by a decrease in two major parameters, errors and latency, with each day of trial. In our study, we found that both Ames dwarf and wild type mice learned to locate the escape box during the course of the training period (days 1–4) as indicated by the progressive reduction (p < 0.0001) in error rates and escape latencies (Fig. 2) in all age groups. This reduction in errors and latency also indicates the use of more spatial strategy than random strategy to find the target box in both genotypes. However, to determine whether the learning curves were different between genotypes, regression analysis of the slope was performed for errors and latency. We found that there was a significant difference (p < 0.05) in primary and total errors and latency in 3-month-old mice, primary and total errors in 12-month-old mice and total latency in 24-month-old mice. The decrease in errors and latency along with steeper slopes suggest enhanced learning in Ames dwarf mice. We also analyzed the difference in the slope in primary and total errors or latency within the genotype to see if there was any correlation. On analysis, it was also found that there was a correlation between primary and total errors in wild type mice in all age groups but not in Ames dwarf mice. Therefore the data on primary and total, errors and latency is presented separately (Fig. 2).
Fig. 2.
Mean errors and latency(s) in Barnes maze acquisition phase. Mean number of primary (locating the escape box for the first time) and total (entry into the escape box) errors (2A) and latencies (2B) are shown for each day of the trial. Statistical comparison was performed by two-way repeated measures ANOVA. Performance improved in all groups over the course of training. Asterisk (*) indicates a significant difference (*p < 0.05, **p < 0.01, ***p < 0.001) between wild type and Ames dwarf mice on the indicated day. Ames dwarf are represented by dashed lines with closed squares and wild type mice are represented by solid lines with closed circles Data are shown as mean±SEM. In the 3- and 12-month age group n = 18 for wild type and n = 12 for Ames dwarf mice. In the 24-month age group n = 12 for both wild type and Ames dwarf mice.
During the acquisition phase, the number of errors (primary and total) was measured and analyzed using repeated measures ANOVA. Significant differences were found between genotypes during the acquisition phase in 3-month-old mice –primary errors: [F(1,28) = 4.108, p = 0.0523], total errors [F(1,28) = 15.72, p = 0.0005]; and in 12-month-old mice – primary errors [F(1,26) = 4.788, p = 0.0379] and total errors [F(1,25) = 3.954, p = 0.0578].
The latencies, both primary and total, are shown in Fig. 2B. Both wild type and Ames dwarf mice showed a decrease in primary and total latency similar to the decrease in primary and total errors from day 1 to day 4. The latency was reduced in Ames dwarf mice as compared to wild type mice at 3 months of age but no significant differences were observed between genotypes at 12 and 24 months of age. However, there were significant interactions for latencies in all age groups indicating that primary (3 months: p = 0.0385) and total latency (3 months: p = 0.0126; 12 months: p = 0.0299; 24 months: p = 0.0307) varied with both genotype and day of training and therefore the main effects were confounded.
3.2. Spatial strategy used during the acquisition phase in Barnes maze
The spatial strategies used during the acquisition phase by wild type and dwarf mice are shown in Fig. 3. During the first day training trial, all age groups in both genotypes used a more random strategy to find the target hole. An overall learning effect was observed in both genotypes by a decrease in random searching for the target box from day 1 to day 4.
Fig. 3.
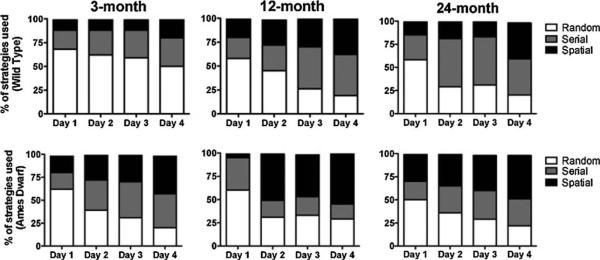
Search strategies used during the acquisition phase on the Barnes maze. Each trial was categorized into different search strategies used (1) Spatial—moving directly to target hole or to an adjacent hole before visiting the target hole (≤3 errors); (2) random—hole searches separated by crossing through the center of the maze or unorganized search; (3) serial—the first visit to the target hole was preceded by a visit to adjacent holes in serial manner, clockwise or counter clockwise in direction. Data are shown as total percentage of trials in which each strategy was used. In the 3- and 12-month age group n = 18 for wild type and n = 12 for Ames dwarf mice. In the 24-month age group n = 12 for both wild type and Ames dwarf mice.
In 3-month-old wild type mice, the use of random strategy decreased from 68.1% on day 1 to 50% on day 4, while 62.5% of young dwarf mice used a random strategy on day 1, a value that decreased to 20.8% by day 4. A similar decrease was observed in 12-month-old mice (wild type: day 1 = 58.3%, day 4 = 19.4%; dwarf: day 1 = 60.4%, day 4 = 29.2%) and 24-month-old mice (wild type: day 1 = 58.3%, day 4 = 20.8%; dwarf: day 1 = 50%, day 4 = 22.7%). On the other hand, serial strategies increased in both mouse genotypes except in 12-month-old dwarf mice where both random and serial strategy decreased and in 3-month-old wild type where there was not much change in the strategy used over 4 days.
Hippocampal-based spatial strategy (to locate the hole in ≤3 errors) increased in both strains during the training days in young 3-month-old mice (wild type: day 1 = 11.1%, day 4 = 19.4%; dwarf: day 1 = 18.8%, day 4 = 41.7%), 12-month-old mice (wild type: day 1 = 19.4%, day 4 = 37.5%; dwarf: day 1 = 4.17%, day 4 = 54.2%), 24-month-old mice (wild type: day 1 = 14.6%, day 4 = 39.6%; dwarf: day 1 = 29.5%, day 4 = 47.7%). In addition, overall, Ames mice on day 4 showed significant use of spatial strategy compared to wild type mice at 3 months of age (χ2 = 7.004, p = 0.0081) and nearly significant at 12 months of age (χ2 = 3.244, p = 0.0717) indicating a clear preference for spatial escape strategy over serial or random strategies. At 24 months of age, there was no preference for spatial strategy (χ2 = 0.6196, p = 0.5286).
3.3. Number of trials completed during acquisition phase in Barnes maze
During the acquisition phase of the Barnes maze task each mouse was given a total of 16 trials (4 trials per day for 4 days). The animals were considered to complete the trial if they located the target box within 300 s. In our study, Ames dwarf mice completed more trials as compared to wild type mice. Overall, among different age groups, the 24-month-old mice completed the fewest trials. In each age group, there was a trend for Ames dwarf mice to complete more trials from day 1 to day 2 and maintaining this increase over wild type mice on day 3 and 4, indicative of enhanced learning. The difference was statistically significant in 3-month-old (χ2 = 7.528, p = 0.0061) and 12-month-old mice (χ2 = 7.139, p = 0.0075) but not in 24-month-old animals (χ2 = 1.403, p = 0.2363).
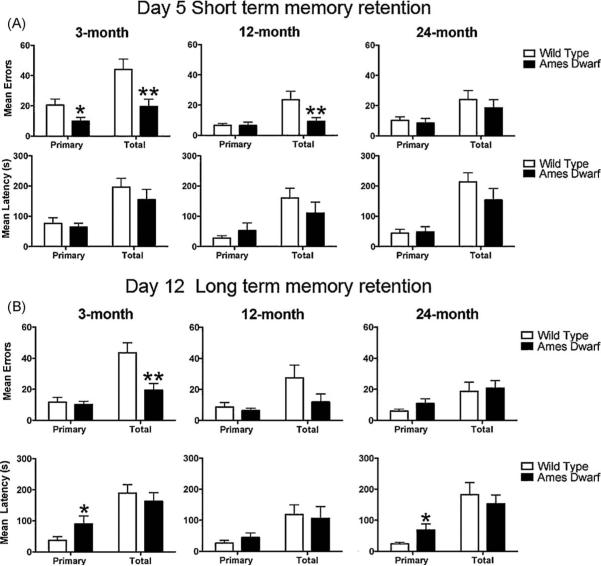
3.4. Short-term and long-term memory retention in Barnes maze
Using the Barnes maze, 24 h after the last training (acquisition) trial, the mice were run on the maze to evaluate short-term memory retention (day 5) and then again a week later, to evaluate long-term retention of memory (day 12). Primary errors and total errors were lower in 3-month-old dwarf mice as compared to age-matched wild type animals on day 5 (p = 0.0572 and p = 0.0132, respectively; Fig. 4A). Dwarf mice on day 5 also made fewer total errors at 12 months of age. Similarly, on day 12, total errors made by 3-month-old dwarf mice (19.42 ± 4.28) were significantly less (p = 0.010) as compared to wild type mice (43.50 ± 6.49) indicating better memory retention (Fig. 4B). There was no difference in the number of errors between the genotypes at 12 and 24 months of age for long-term memory retention. Similar to that observed in the acquisition phase, the primary latency to reach the target hole on day 5 was not different between the genotypes. Long term memory retention when assessed on day 12 without the period of training in-between, demonstrated that Ames dwarf mice made fewer total errors (p = 0.0101) at 3 months of age, but the primary latency (seconds) was greater in young, 3-month-old (p = 0.0441; wild type 37.17 ± 12.09 versus dwarf 90.33 ± 25.15) and in old, 24-month-old (p = 0.0363; wild type 24.09 ± 5.01 versus dwarf 68.82 ± 19.29) Ames dwarf mice. When day 5 and day 12 were compared within genotype, there was no difference between primary and total errors and latency except in 24-month-old wild type mice where primary errors were significantly less on day 12 (p = 0.0384) as compared to day 5.
Fig. 4.
Short-term and long-term memory retention on the Barnes maze. Short-term (A) and long-term memory (B) retention was assessed on day 5 and day 12 respectively. A single trial was given to each mouse on the Barnes maze and the primary and total errors and latency(s) were evaluated as in the acquisition phase. Data are presented as mean±SEM. Asterisk (*) indicates a significant difference (*p < 0.05, ** < 0.01, ***p < 0.001) between wild type and Ames dwarf. In the 3- and 12-month age groups n = 18 for wild type and n = 12 for Ames dwarf mice. In 24-month age group n = 12 for both wild type and Ames dwarf mice.
As in the acquisition phase, more dwarf mice appeared to use a spatial strategy compared to wild type siblings on day 5 as well as day 12 in all age groups but the difference was not statistically significant. Similarly, 3-, 12- and 24-month-old Ames dwarf mice tended to complete more trials as compared to wild type mice on day 5 and day 12 but the differences failed to reach statistical significance.
We also recorded the path length during the short-term and long-term retention trials and found that dwarf mice used a shorter total path length (cm) at 3 months of age on day 12 (p = 0.0586; wild type 463.60 ± 68.07 versus dwarf 335.0 ± 40.50) when compared to age-matched wild type mice. There were no genotype differences in path length between genotypes on day 5.
3.5. T-maze
3.5.1. Errors and latency during the acquisition phase on the T-maze
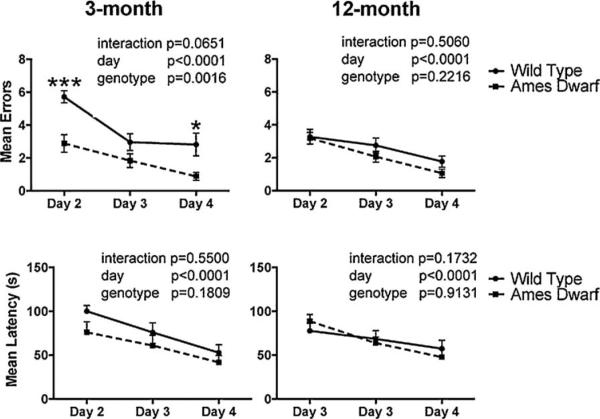
To substantiate the findings obtained in the Barnes maze, we employed a T-maze as an additional behavioral paradigm of spatial memory and tested 3- and 12-month-old mice (24-month-old mice were not available). During the acquisition period, Ames dwarf mice made fewer errors at 3 months of age [F(1,22) = 12.99; p = 0.0016] compared to age-matched wild type mice. No difference was detected between genotypes at 12 months of age [F(1,22) = 1.583; p = 0.2216]. Although, mean latency was not affected by genotype, a significant learning effect (p < 0.0001) was observed in both dwarf and wild type mice from day 2 to day 4 of the acquisition phase (Fig. 5). Similarly, 3-month-old Ames mice demonstrated a trend towards completion of more trials during the training phase of the T-maze with a statistically significant difference on day 1 (p = 0.0068), while at 12 months of age, the number of trials completed were equal. We also evaluated the type of strategy used on the T-maze during the probe trials day 5 and day 12 and found no genotype differences in either age group.
Fig. 5.
Mean errors and latency in the T-maze acquisition phase. Mean number of errors and latency(s) ± SEM are shown for each day of the trial. Ames dwarf mice are represented by dashed lines with closed squares and wild type mice are represented by solid lines with closed circles. Statistical comparison was performed by two-way repeated measures ANOVA. Asterisk (*) indicates a significant difference (*p < 0.05, ***p < 0.001) between wild type and Ames dwarf mice on the indicated day. In both age groups, n = 12 for wild type and n = 12 for Ames dwarf mice.
3.5.2. Short-term and long-term memory retention in T-maze
Ames dwarf mice made fewer errors during the probe trials on day 5 (short-term memory retention) and day 12 (long-term memory retention) compared to wild type mice (Fig. 6). On average, 3-month-old dwarf mice made less than one error on days 5 and 12 compared to wild type mice that made 5 or more errors on respective days (day 5, p = 0.0012; day 12, p = 0.0015). At the age of 12 months, dwarf mice made fewer errors than wild type mice on day 5 (p = 0.0299) only. Similarly, in 3-month-old Ames dwarf mice the latencies were shorter on day 5 (p = 0.0044) and showed the same trend on day 12 (p = 0.1185). Latencies were not different between genotypes at 12 months of age, in agreement with the Barnes maze results. Ames dwarf mice also tended to complete more T-maze trials during memory retention testing when compared to wild type mice. Regarding type of strategy, it was found that more 3-month-old Ames dwarf mice used a spatial strategy on day 12 compared to wild type mice (p = 0.0361; data not shown). At 12 months of age, there were no genotype differences in the type of strategy used during either the short-term or the long-term memory retention trials.
Fig. 6.
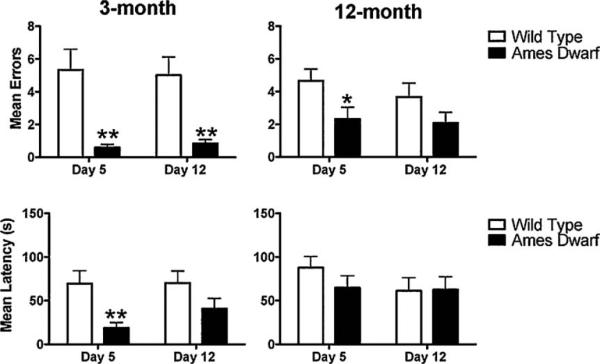
Mean number of errors and latency(s) on the probe trial on the T-maze. A probe trial was done on day 5 (short-term memory retention) and day 12 (long-term memory retention). During these trials the maze was rotated 1808, but all cues, including the experimenter, remained stationary. Data are presented as mean±SEM. Asterisk (*) indicates a significant difference (*p < 0.05, ** < 0.01) between wild type and Ames dwarf on the indicated day. In both age group n = 12 for wild type and n = 12 for Ames dwarf mice.
3.5.3. Kainic acid injection and seizure activity
To assess the effect of a hippocampal oxidative insult on spatial memory, KA was injected in a group of animals trained on the Barnes maze. This maze is considered a specific behavioral model for spatial memory and we had all 3 age groups of mice trained on this maze. Following KA injection, the mice were observed for two hours and were scored for seizure activity according to a modified Racine Scale. The preliminary dose response study showed that Ames dwarf mice were more sensitive to KA as indicated by pronounced seizures at lower doses (data not shown). Thus, we selected the 15 mg/kg dose of KA for both dwarf and wild type mice to avoid potential mortality. To match the seizure scores obtained in dwarf mice, an additional group of 3- and 12-month-old wild type mice were injected with 30 mg/kg KA. Seizure activity differed between genotypes in young mice (3 months old) given 15 mg/kg KA (mean seizure score p = 0.0355; Fig. 7). At 12 months of age, the seizure activity between wild type (15 mg/kg) and dwarf (15 mg/kg) mice also appeared different (p = 0.1032). In agreement, total seizure scores were also different (p = 0.0355) at 3 months of age between wild type (15 mg/kg) and dwarf (15 mg/ kg). However, the seizure activity was similar (p = 0.1032) in both genotypes at 15 mg/kg KA dose in old age group (24 months) indicating that sensitivity to KA increased in older wild type mice. Importantly, there was no difference between the seizure activity of Ames dwarf mice injected with 15 mg/kg KA and wild type mice with 30 mg/kg KA at 3 (p = 0.63990) and 12 months of age (p = 1.000; Fig. 7). Therefore, the effects of KA on various parameters were compared between the “equiseizure” dose of 15 mg/kg in dwarf and 30 mg/kg in wild type for 3 and 12 months of age but 15 mg/kg for both genotypes at 24 months of age. Within 24 h following KA, one 3-month-old dwarf, one 12-month-old dwarf, and one 3-month-old wild type (30 mg/kg) animal died and were therefore excluded from the post KA analysis. No seizure activity was observed in animals treated with an equal volume of normal saline.
Fig. 7.
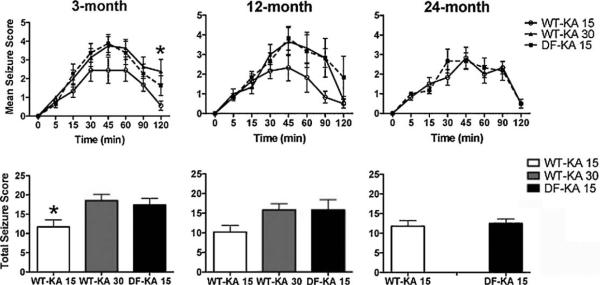
Mean and total seizure scores. Seizure score was assessed by the Racine scale for 2 h following KA injection. The treatment groups at different ages included—wild type mice receiving 15 mg/kg KA (WT-KA 15), wild type mice receiving 30 mg/kg KA (WT-KA 30), and Ames dwarf mice receiving 15 mg/kg KA (DF-KA 15). Data are presented as mean±SEM. The WT-KA 15 group is represented by solid lines with open circles, the WT-KA 30 group is represented by solid lines with closed triangles, and the DF-KA 15 group is represented by dashed lines with solid squares. Asterisk (*) indicates a significant difference (*p < 0.05) between WT-KA 15 and DF-KA 15. In all age groups and both genotypes n = 6 for each treatment group.
3.5.4. Kainic acid induced neurodegeneration in CA1 and CA3 subfields of hippocampus
We assessed neurodegeneration in the hippocampus seven days following KA injection using FJC as a marker for degenerating neurons in the CA1 and CA3 subfields of the hippocampus. As shown, there were FJC positive cells in the CA1 and CA3 areas of dwarf mice with 15 mg/kg and wild type mice with 30 mg/kg (Fig. 8A). There were no differences in the number of FJC positive cells between these two groups of mice (Fig. 8B) in CA1 (p = 0.9776) and CA3 (p = 0.4053) subfields. This finding further supports the use of the “equiseizure” dose in these mice; 15 mg/kg KA in Ames dwarf is comparable to 30 mg/kg KA in wild type mice. Saline injected animals did not show any FJC staining. Similarly, no neurodegeneration was observed with wild type animals injected with 15 mg/kg KA.
Fig. 8.
Hippocampal neurodegeneration as assessed by FluoroJade C (FJC) staining. Representative micrograph of FJC staining in the CA1 and CA3 subfields of hippocampi obtained from 3-month-old wild type and Ames dwarf mice seven days after KA administration. Ames dwarf mice at 15 mg/kg and wild type mice at 30 mg/kg showed neurodegeneration in CA1 and CA3 subfields as indicated by green fluorescence (A). Wild type mice with KA injection at the dose of 15 mg/kg and saline-injected wild type and dwarf mice did not show any neuronal loss. In both genotypes n = 3 for each treatment group. Scale bars represent 200 μm from A to E and 20 μm from F to O. The number of FJC positive cells was counted from 2 areas each in CA1 and CA3 subfields (3 sections/3 animals/genotype). Data are presented as mean±SEM (B).
3.5.5. Spatial memory following KA-induced hippocampal neurodegeneration
The mice were run on the maze seven days after the KA injection and the errors and latency were calculated. We found that at an equiseizure dose of KA, wild type mice showed a significant increase in the number of primary errors at 3 months (p < 0.01) and 12 months of age (p < 0.05) when compared to age-matched dwarf mice (Fig. 9). Similar to the increase in number of errors, the primary latencies also increased in these mice at 3 months (p < 0.05) and 12 months of age (p < 0.001) compared to dwarf mice (Fig. 9). Ames dwarf mice receiving an equiseizure dose of KA (15 mg/kg; that produced similar neurodegeneration in both CA1 and CA3 areas of the hippocampus when compared to wild type mice) did not show any increase in the number of errors or in escape latencies compared to saline-injected mice.
Fig. 9.
Post-KA primary and total errors on the Barnes maze. The mean primary and total errors were assessed on the Barnes maze in different age groups of wild type and Ames dwarf mice seven days following saline or KA injection. Errors and latency were evaluated as in acquisition phase of Barnes maze. Open bars represent the pre-KA data while closed bars represent post-KA data. Data are presented as mean±SEM. Asterisk (*) indicates a significant difference (*p < 0.05, ** < 0.01; ***p < 0.001) between pre- and post KA data. In all age groups for both genotypes n = 5–6 for each treatment group.
The use of spatial strategy as well as the number of trials completed decreased in both genotypes in each age group after KA injections. However, the differences were not significant because of the small number of animals. Similar to the results observed for errors and latency, the path length tended to increase in wild type mice at 3 and 12 months of age after KA treatment at the 30 mg/kg dose.
3.5.6. Gene expression and protein levels of IGF-1
The hippocampal protein levels for IGF-1 in the Ames dwarf were similar to the levels in wild type mice at 3 months of age (data not shown). There were no differences between Ames and wild type mice in basal gene expression of IGF-1 or the IGF-1 receptor in the hippocampus as assessed by real time RT-PCR. When IGF-1 mRNA expression levels were analyzed within the genotype, we found that there was a significant increase in IGF-1 mRNA in 24-month-old wild type as compared to 3 (p < 0.01) and 12 (p < 0.001) months old wild type mice. Similarly IGF-1 mRNA expression in 24-month-old dwarf mice was higher (p < 0.001) when compared to their young (3 and 12 months old) counterparts (Fig. 10).
Fig. 10.
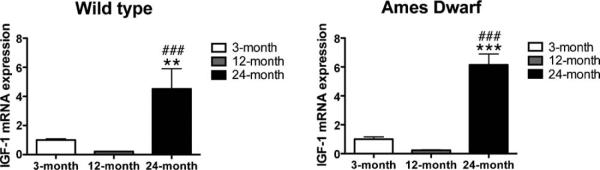
IGF-1 mRNA expression levels in the hippocampus of wild type and Ames dwarf mice. Baseline IGF-1 mRNA expression levels were assessed in different age groups of wild type and Ames dwarf mice. Data are presented as mean±SEM. Asterisk (*) indicates a significant difference (** < 0.01, ***p < 0.001) between 3- and 24-month-old mice; while # indicates a significant difference (p < 0.001) between 12- and 24-month-old mice. In all age groups for both genotypes n = 5–6.
4. Discussion
The hippocampus plays an important role in spatial learning and memory (O'Keefe and Dostrovsky, 1971) and forms the neuronal basis of the spatial cognitive maps (Sutherland and McDonald, 1990). The hippocampus is also known to be the brain area most vulnerable to aging and is associated with a decrease in IGF-1 levels and an increase in oxidative stress. There are no data in the literature on spatial memory of Ames dwarf mice despite their unique phenotype with peripheral IGF-1 deficiency, enhanced peripheral antioxidant status, and extended lifespan. A previous behavioral study showed that Ames dwarf mice do not experience an age related decline in locomotor activity when compared to their young counterparts (Kinney et al., 2001b). This interesting study also demonstrated that old dwarf mice did not differ from young dwarf mice in an inhibitory avoidance learning task, a test known to be sensitive to age-dependent changes in cognition. In addition, another study (Mattison et al., 2000) demonstrated that 18–21-month-old dwarf mice had increased retention in the inhibitory avoidance task at the 24-h and 7-day retention tests.
In this study we used a circular dry land Barnes maze and a T-maze to evaluate spatial memory. The Barnes maze is similar to a Morris water maze in terms of assessing hippocampal-based spatial memory, however the Barnes maze has been shown to be less stressful and physically less taxing than the Morris water maze (Harrison et al., 2009). The results indicate that in the Barnes maze, Ames dwarf mice made fewer errors and exhibited shorter escape latencies as compared to wild type mice. Younger Ames mice (3 and 12 months) also used more spatial strategy compared to their age-matched wild type siblings to solve the Barnes maze indicating better hippocampal-based learning and memory. Similarly, the data from the T-maze showed that Ames mice make fewer errors and have better memory at 3 months of age. Although, our 24-month-old dwarf mice were not found to be different from age-matched wild type mice, it was shown in another study that 18– 21-month-old dwarf mice exhibited significantly better memory retention than their age- and diet-matched normal counterparts (Mattison et al., 2000). This could be due to the fact that the behavioral paradigm used to study memory retention in the latter case was an inhibitory avoidance-learning task that uses shock as an aversive stimulus and the fact that our mice were 3–6 months older at the beginning of the experiments. In our study, we did not use any stimuli that might promote negative reinforcement.
Another possible explanation for a lack of behavioral differences between Ames dwarf and wild type mice at 24 months of age could be based on overall gene expression. The majority of genes expressed differentially in Ames dwarf mice change with age, initially their expression differs greatly between dwarf and wild type mice, but with time the expression of these genes in Ames dwarf mice tends to approach and overlap with those seen in wild type mice (Amador-Noguez et al., 2004).
We did not see an age-related decline in memory on the T-maze and the Barnes maze with wild type or dwarf mice as has been found in many other animal species (Barnes, 1979; Erickson and Barnes, 2003; Frick et al., 1995; Magnusson et al., 2003). It is possible that these two behavioral paradigms may not be the best test to assess age-associated cognitive impairment in the strain of mice used in our study. A memory paradigm that uses aversive stimuli might unmask the age associated memory impairment as compared to the T-maze and Barnes maze. However, we did not want to induce any stress on the animals and thus did not detect an age-associated decline in spatial memory. A study using the inhibitory avoidance learning task (that uses shock as an aversive stimulus) reported that aged wild type mice performed poorly while there was better memory retention in dwarf mice. Based on this study, in case of dwarf mice, it may indicate that there is a delay in cognitive aging (Kinney-Forshee et al., 2004; Mattison et al., 2000). It is also possible that in dwarfs, we may see the cognitive decline at ages greater than 24 months, but this has yet to be tested. As we were interested mainly in hippocampal-based spatial learning and memory, we used the present protocol to assess hippocampal function. We did not examine the reversal learning tasks as has been done in some studies as this task not only involves an intact hippocampus (Jarrard, 1993), but also prefrontal cortex (Kesner, 2000) and nucleus accumbens (Louilot et al., 1989).
One would expect to see a decrease in escape latency corresponding with fewer errors by dwarf mice. The reason that the Ames dwarf made significantly fewer errors but did not differ in latencies compared to wild type could be due to the smaller body size (1/2–1/3) of these animals thus, taking them longer to travel the same distance. In addition, it has been shown, based on stereotypy, that dwarf animals were more likely to move short distances compared to wild type mice (Meliska et al., 1997). Moreover, wild type mice were reported to exhibit more spontaneous locomotor activity than dwarfs but also showed that old dwarf mice were significantly more active than their younger counterparts (Kinney et al., 2001b).
When we tested for retention of memory, we found that on both the Barnes maze and T-maze, Ames dwarf mice performed better on the short-term and long-term memory tests in the 3-month-old group, and on short-term retention at 12 months of age. Studies have shown that memory retention was compromised in mice with altered growth hormone levels following a longer interval between test trials [GH transgenic (high plasma GH levels) versus wild type mice (Meliska et al., 1997); Ames dwarf versus wild type mice (Mattison et al., 2000)]. In contrast, middle-aged GH receptor knock out mice (no plasma IGF-1, high plasma GH) performed better than age matched normal animals in an inhibitory avoidance 28-day retention test (Kinney et al., 2001a). During memory retention assessment, it has been shown that the first trial plays the role of the reminding procedure, after which the task is performed correctly. This typical feature was observed in retrieval tests when there was an increase in latency of first trials during daily sessions (Arkhipov et al., 2008). In our study, the memory retention trial using the Barnes maze was conducted only once on the test day with no further testing. Therefore, no reminder was provided. In contrast, our T-maze protocol included 3 trials before the actual probe trial. Thus, multiple trials on the Barnes maze on day 5 and 12 may have allowed the actual differences in the retention of memory in these mice to be observed during both short- and long-term testing.
We found that Ames dwarf mice were more sensitive to KA in terms of neurodegeneration and seizure activity. A similar loss of hippocampal pyramidal cells in the CA1 and CA3 subfields with the equiseizure dose in Ames (15 mg/kg) and wild type (30 mg/kg) mice suggests that these two groups of mice were comparable. In the hippocampus, the highest density of KA receptors is found in the CA3 subfield, the area most severely damaged following KA administration. The neuronal injury with kainic acid in our study corresponded with that reported in other studies that used FluoroJade as a marker of neuronal loss (Bluthe et al., 2005; Mazarati et al., 2004). We used FJC as a marker of neuronal degeneration as it is sensitive and it specifically binds to degenerating neurons but not to healthy neurons, myelin, or vascular elements (Schmued et al., 1997, 2005; Schmued and Hopkins, 2000a,b). The problem with conventional techniques, such as hematoxylin and eosin (H and E) and Nissl's staining, is that even though they are technically simple procedures, there can be processing artifacts or significant alterations in cellular morphology. Such artifacts make it difficult to use neuronal shrinkage, vacoulation, and hypochromatism as parameters to infer neuro-degeneration. In this study, the degree of neuronal degeneration (as evident by FJC staining) produced by the lower dose (15 mg/kg) in dwarf mice was similar to wild type mice injected with 30 mg/kg KA at 3 months of age. Although this study did not directly assess neurodegeneration in adult (12 months) and aged (24 months) animals, it is appealing to suggest that a similar intensity of seizures will produce similar neurodegeneration in different age groups as reported in a previous study (Liang et al., 2007).
Behavioral symptoms caused by systemic injection of KA were comparable to those previously described as limbic seizures (Kraft et al., 2006; Liang et al., 2007; Sperk et al., 1983, 1985). The relationship between KA-induced behavioral seizures and neuro-degeneration is dose-and age-dependent with increased sensitivity to KA with increasing age (Benkovic et al., 2006; Hu et al., 1998; Schauwecker and Steward, 1997; Sperk et al., 1985). The genotype differences in susceptibility to KA observed in our study could be due to body fat differences. The percentage of body fat as well as fat distribution differs in adult dwarf mice when compared to the corresponding normal controls (Heiman et al., 2003). In addition, body weight has been shown to affect survival rate following KA administration (Chen et al., 2002). Differences in properties of the blood brain barrier may contribute to the difference in behavioral responses produced by systemic administration of KA, as KA was demonstrated to cause damage to the blood brain barrier (Benkovic et al., 2006).
In this study we found that at 24 months of age, low dose KA generated similar seizure activity in wild type and dwarf mice. This indicates that although dwarfs were more susceptible to KA compared to wild type mice at young ages, their sensitivity did not increase with age, as it did in wild type animals. This may result from a decreased antioxidant capacity in 24-month-old wild type animals as compared to age-matched dwarf mice (Brown-Borg and Rakoczy, 2005; Romanick et al., 2004). Kainic acid-induced seizure activity has been correlated with enhanced oxidative stress in various studies (Bruce and Baudry, 1995; Kim et al., 2000a,b, 2002; Shin et al., 2008).
We evaluated hippocampal-based spatial memory in the same setting seven days after KA injection. We chose this time interval following KA to assess spatial memory based on previous reports that showed that behavior of KA-treated animals was nearly normal in this period (Arkhipov et al., 2008; Zhang et al., 2010). Our results show that spatial memory was maintained in Ames dwarf mice even after KA-induced neuronal injury to the hippocampus. In contrast, KA-treated wild type mice showed deteriorations in memory. Primary errors and latencies were high in wild type mice (30 mg/kg KA) as compared to Ames dwarf mice with the equiseizure dose (15 mg/kg). Wild type mice with 15 mg/kg KA did not show deficiencies in memory like those with the higher dose possibly because lower “subconvulsive” doses of KA are not great enough for deterioration of spatial memory (rats; Arkhipov et al., 2008). A conflicting report in mice however, showed that systemic injection of KA at a dose that does not induce seizures or neuronal degeneration is able to impair spatial memory (Bluthe et al., 2005). One other possible reason for an increase in the number of errors after KA-induced neuronal loss could be due to a defect in selective attention. It has been demonstrated that rats with hippocampal dysfunction show excessive sensitivity to distractive stimulation resulting in long lasting exploratory activity (Oswald et al., 2002), thus this might increase the number of errors.
In our experiments, there were no differences in velocity between control and KA-treated wild type animals as revealed by comparing path length:latency ratios. Therefore, the increased latencies of the treated animals were most likely due to learning deficiencies rather than motor impairments. Previous work showing that KA produced impairment in spatial learning by affecting neither motor nor motivational capabilities also supports this conclusion (Connor et al., 1991; Morris et al., 1990). At 24 months of age, wild type mice showed a decrease in latency, which could be due to the fact that old animals are more sensitive to KA. Impairment in sensorial processes may also account for these learning deficits since retinal lesions have been observed with KA (Coyle et al., 1978), although in our study, we did not observe any abnormal visual-related behavior in our mice.
The mechanisms underlying cognitive deficits in learning and memory during aging are unclear. Neuronal injury may not be the only cause of cognitive deficit. Some studies have failed to correlate a loss of hippocampal neurons with age related cognitive deficit (Rapp and Gallagher, 1996; Wickelgren, 1996). In humans it has been demonstrated that hippocampal neuronal number remains stable during aging (West, 1993). Therefore, it is unclear why Ames dwarf mice retained their spatial memory despite neuronal injury. One of the possibilities is that in Ames dwarf mice, KA did not damage the hippocampal connections as effectively as it did in wild type mice. Another possibility is that neuronal reserves were greater in Ames dwarf mice to begin with as suggested by earlier work. Ames mice have been shown to exhibit higher levels of IGF-1 and more neurogenesis in the hippocampus as compared to wild type mice (Sun et al., 2005b). There is also evidence that systemic administration of KA increases the sprouting of mossy fibers in the hippocampal formation (Cronin and Dudek, 1988). Therefore, we could argue that Ames dwarf mice did lose spatial memory after KA injection but the recovery was faster due to enhanced neurogenesis. Ames dwarf mice may also consolidate memory sooner than the wild type mice and no longer need the hippocampus for retrieval as observed in other species (Ramos, 2009; Takashima et al., 2009). In our study, although we did not find elevated hippocampal IGF-1 levels in Ames dwarf mice as was previously reported, the levels were not different from wild type mice despite peripheral IGF-1 deficiency. In agreement, a recent study demonstrated that local brain levels of IGF-1 in two animal models with decreased plasma IGF-1 were similar to that in wild type counterparts (Adams et al., 2009). We believe that even though hippocampal IGF-1 levels were not higher in Ames dwarf mice, the locally produced IGF-1 might have different effects as compared to peripheral IGF-1 in wild type mice. IGF-1 has been shown to abrogate KA-induced memory impairment but the role of IGF-1 and effect of KA on neurogenesis needs further exploration by studying the time course of neurogenesis after KA injection in both wild type and Ames dwarf mice and the correlation with spatial memory (Bluthe et al., 2005). Increases in IGF-1 mRNA levels have been reported in non-brain tissue with age in Ames dwarf mice as opposed to decreases in wild type mice (Swindell, 2007). However, the increase in IGF mRNA levels in both wild type and dwarf mice in 24 months old as compared to their young counterparts (3 months old) suggest that IGF-1 levels may not correlate with spatial learning and memory.
The main mechanism by which KA induces neurodegeneration is by stimulation of kainate receptors in the CA3 area causing depolarization of CA3 pyramidal cells. This also leads to a secondary release of glutamate that acts on N-methyl d-aspartate (NMDA) receptors in the CA1 area causing more neuronal loss (Kim et al., 1999; Sperk, 1994). We cannot rule out the possibility that kainate and NMDA receptors in Ames dwarf mice are differentially expressed and exhibit different sensitivities to KA thus producing different effects. The stimulation of the kainate receptor and release of glutamate is responsible for excitotoxicity and free radical generation (Wang et al., 2005). This oxidative stress, in turn, is responsible for the neurodegeneration. Ames dwarf mice have been shown to exhibit enhanced antioxidative defense in the periphery in multiple studies as well as less vulnerability to induced oxidative stress (Bokov et al., 2009; Brown-Borg et al., 1999, 1996, 2004; Brown-Borg and Rakoczy, 2000, 2003, 2005). It has been shown that age-related loss of swim maze performance and locomotion was positively correlated with oxidative molecular damage in cerebral cortex and cerebellum in normal mice (Forster et al., 1996). Moreover, Ames mice were shown to resist beta-amyloid toxicity in hippocampal slice culture in comparison to wild type mice suggesting that they exhibit enhanced stress resistance in the CNS as well as the periphery (Schrag et al., 2008). Thus, Ames dwarf mice appear to exhibit an enhanced antioxidant capacity centrally, that is providing better protection against insult by KA and therefore maintaining spatial memory despite the neuronal damage. This study of KA-induced oxidative stress and the levels of antioxidant enzymes are currently ongoing in our lab and will be published in a companion paper.
Overall, KA-induced hippocampal damage and loss of spatial memory in wild type mice could be used as a unique mouse model simulating age-associated memory impairment in elderly populations. Ames dwarf mice resist both the age-related and oxidative stress-induced loss of spatial memory providing valuable insight into potential mechanisms responsible for maintaining spatial memory. It is tempting to speculate that the GH deficiency plays a role in maintenance of spatial memory based on the totality of information, but the prolactin and thyrotropin deficiencies have not been adequately studied at this point in time. Nevertheless, this is the first study in long-living Ames mice that evaluated hippocampal-based spatial memory across several ages and following an oxidative insult and serves as a beginning for further exploration of the mechanisms responsible.
Taken together, our results suggest that young Ames dwarf mice have better hippocampal-based spatial memory in terms of fewer errors and spatial strategy used when compared to age-matched wild type mice. Ames dwarf mice also have better short-term and long-term memory retention at young ages and maintain hippocampal-based spatial memory following KA-induced neuronal insult. We did not find a correlation between IGF-1 levels and spatial memory; however, the enhanced learning and memory in Ames dwarf mice could be due to enhanced antioxidative defenses, enhanced neurogenesis, and/or differential expression and sensitivity of kainate and NMDA receptors, all of which are under further exploration.
Acknowledgements
We wish to thank Drs. John Watt, Thad Rosenberger and Patrick Carr for providing assistance with immunohistochemistry. This work was supported by the Glenn Foundation for Medical Research, and by the Department of Pharmacology, Physiology and Therapeutics at the University of North Dakota School of Medicine and Health Sciences.
References
- Adams MM, Elizabeth Forbes M, Constance Linville M, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Stability of local brain levels of insulin-like growth factor-I in two well-characterized models of decreased plasma IGF-I. Growth Factors. 2009;27:181–188. doi: 10.1080/08977190902863639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sohal RS. Relationship between susceptibility to protein oxidation, aging, and maximum life span potential of different species. Exp. Gerontol. 1996;31:365–372. doi: 10.1016/0531-5565(95)02039-x. [DOI] [PubMed] [Google Scholar]
- Albert MS, Funkenstein HH. The effects of age: normal variation and its relation to disease. In: A.A.K., McKhann, G.M., M.W.I., editors. Diseases of the Nervous System: Clinical Neurobiology. Saunders; Philadelphia: 1992. pp. 598–611. [Google Scholar]
- Aleman A, Verhaar HJ, De Haan EH, De Vries WR, Samson MM, Drent ML, Van der Veen EA, Koppeschaar HP. Insulin-like growth factor-I and cognitive function in healthy older men. J. Clin. Endocrinol. Metab. 1999;84:471–475. doi: 10.1210/jcem.84.2.5455. [DOI] [PubMed] [Google Scholar]
- Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3:423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- Arkhipov V, Kulesskaja N, Lebedev D. Behavioral perseveration and impairment of long-term memory in rats after intrahippocampal injection of kainic acid in subconvulsive dose. Pharmacol. Biochem. Behav. 2008;88:299–305. doi: 10.1016/j.pbb.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Aging and the physiology of spatial memory. Neurobiol. Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C. Prolonged longevity of hypopituitary dwarf mice. Exp. Gerontol. 2001;36:21–28. doi: 10.1016/s0531-5565(00)00205-9. [DOI] [PubMed] [Google Scholar]
- Benkovic SA, O'Callaghan JP, Miller DB. Regional neuropathology following kainic acid intoxication in adult and aged C57BL/6J mice. Brain Res. 2006;1070:215–231. doi: 10.1016/j.brainres.2005.11.065. [DOI] [PubMed] [Google Scholar]
- Bizon J, Prescott S, Nicolle MM. Intact spatial learning in adult Tg2576 mice. Neurobiol. Aging. 2007;28:440–446. doi: 10.1016/j.neurobiolaging.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Frenois F, Kelley KW, Dantzer R. Pentoxifylline and insulin-like growth factor-I (IGF-I) abrogate kainic acid-induced cognitive impairment in mice. J. Neuroimmunol. 2005;169:50–58. doi: 10.1016/j.jneuroim.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived Ames dwarf mice are resistant to chemical stressors. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11:41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp. Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Growth hormone administration to long-living dwarf mice alters multiple components of the antioxidative defense system. Mech. Ageing Dev. 2003;124:1013–1024. doi: 10.1016/j.mad.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Glutathione metabolism in long-living Ames dwarf mice. Exp. Gerontol. 2005;40:115–120. doi: 10.1016/j.exger.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Sharma S, Bartke A. Long-living growth hormone receptor knockout mice: potential mechanisms of altered stress resistance. Exp. Gerontol. 2008 doi: 10.1016/j.exger.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters components of the glutathione metabolic pathway in Ames dwarf mice. Ann. N. Y. Acad. Sci. 2004;1019:317–320. doi: 10.1196/annals.1297.053. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech. Ageing Dev. 2005;126:389–398. doi: 10.1016/j.mad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Brown-Croyts LM, Caton PW, Radecki DT, McPherson SL. Phenobarbital pre-treatment prevents kainic acid-induced impairments in acquisition learning. Life Sci. 2000;67:643–650. doi: 10.1016/s0024-3205(00)00658-5. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Radic. Biol. Med. 1995;18:993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ljunggren HG, Bogdanovic N, Nennesmo I, Winblad B, Zhu J. Excitotoxic neurodegeneration induced by intranasal administration of kainic acid in C57BL/6 mice. Brain Res. 2002;931:135–145. doi: 10.1016/s0006-8993(02)02268-0. [DOI] [PubMed] [Google Scholar]
- Cherry KE, Park DC. Individual difference and contextual variables influence spatial memory in younger and older adults. Psychol. Aging. 1993;8:517–526. doi: 10.1037//0882-7974.8.4.517. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connor DJ, Langlais PJ, Thal LJ. Behavioral impairments after lesions of the nucleus basalis by ibotenic acid and quisqualic acid. Brain Res. 1991;555:84–90. doi: 10.1016/0006-8993(91)90863-q. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Biziere K, Schwarcz R. Neurotoxicity of excitatory amino acids in the neural retina. In: McGeer EG, Olney JW, McGeer PL, editors. Kainic Acid as a Tool in Neurobiology. Raven; New York, NY: 1978. pp. 177–188. [Google Scholar]
- Cronin J, Dudek FE. Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res. 1988;474:181–184. doi: 10.1016/0006-8993(88)90681-6. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav. Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Dik MG, Pluijm SM, Jonker C, Deeg DJ, Lomecky MZ, Lips P. Insulin-like growth factor I (IGF-I) and cognitive decline in older persons. Neurobiol. Aging. 2003;24:573–581. doi: 10.1016/s0197-4580(02)00136-7. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch. Biochem. Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp. Gerontol. 2003;38:61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Evans GW, Brennan PL, Skorpanich MA, Held D. Cognitive mapping and elderly adults: verbal and location memory for urban landmarks. J. Gerontol. 1984;39:452–457. doi: 10.1093/geronj/39.4.452. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol. Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav. Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- Gayoso MJ, Primo C, al-Majdalawi A, Fernandez JM, Garrosa M, Iniguez C. Brain lesions and water-maze learning deficits after systemic administration of kainic acid to adult rats. Brain Res. 1994;653:92–100. doi: 10.1016/0006-8993(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc. Natl. Acad. Sci. U.S.A. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn. Mem. 2006;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Watanabe K, Nishimura T, Iyo M, Shirayama Y, Minabe Y. Behavioral changes and expression of heat shock protein hsp-70 mRNA, brain-derived neurotrophic factor mRNA, and cyclooxygenase-2 mRNA in rat brain following seizures induced by systemic administration of kainic acid. Brain Res. 1998;804:212–223. doi: 10.1016/s0006-8993(98)00708-2. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Heiman ML, Tinsley FC, Mattison JA, Hauck S, Bartke A. Body composition of prolactin-, growth hormone, and thyrotropin-deficient Ames dwarf mice. Endocrine. 2003;20:149–154. doi: 10.1385/ENDO:20:1-2:149. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Thompson JL, Marchi T, Feldman DS. Behavioral effects of kainic acid administration on the immature brain. Epilepsia. 1988;29:721–730. doi: 10.1111/j.1528-1157.1988.tb04226.x. [DOI] [PubMed] [Google Scholar]
- Hu RQ, Koh S, Torgerson T, Cole AJ. Neuronal stress and injury in C57/BL mice after systemic kainic acid administration. Brain Res. 1998;810:229–240. doi: 10.1016/s0006-8993(98)00863-4. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav. Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology. 2000;28:219–228. [Google Scholar]
- Kim H, Bing G, Jhoo W, Ko KH, Kim WK, Suh JH, Kim SJ, Kato K, Hong JS. Changes of hippocampal Cu/Zn-superoxide dismutase after kainate treatment in the rat. Brain Res. 2000a;853:215–226. doi: 10.1016/s0006-8993(99)02254-4. [DOI] [PubMed] [Google Scholar]
- Kim HC, Bing G, Jhoo WK, Kim WK, Shin EJ, Park ES, Choi YS, Lee DW, Shin CY, Ryu JR, Ko KH. Oxidative damage causes formation of lipofuscin-like substances in the hippocampus of the senescence-accelerated mouse after kainate treatment. Behav. Brain Res. 2002;131:211–220. doi: 10.1016/s0166-4328(01)00382-5. [DOI] [PubMed] [Google Scholar]
- Kim HC, Bing G, Jhoo WK, Ko KH, Kim WK, Lee DC, Shin EJ, Hong JS. Dextromethorphan modulates the AP-1 DNA-binding activity induced by kainic acid. Brain Res. 1999;824:125–132. doi: 10.1016/s0006-8993(99)01155-5. [DOI] [PubMed] [Google Scholar]
- Kim HC, Jhoo WK, Kim WK, Suh JH, Shin EJ, Kato KKHK. An immunocytochemical study of mitochondrial manganese-superoxide dismutase in the rat hippocampus after kainate administration. Neurosci. Lett. 2000b;281:65–68. doi: 10.1016/s0304-3940(99)00969-6. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol. Behav. 2001a;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm. Behav. 2001b;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol. Behav. 2004;80:589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Kirasic KC, Bernicki MR. Acquisition of spatial knowledge under conditions of temporospatial discontinuity in young and elderly adults. Psychol. Res. 1990;52:76–79. doi: 10.1007/BF00867215. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Lee JM, Johnson DA, Kan YW, Johnson JA. Neuronal sensitivity to kainic acid is dependent on the Nrf2-mediated actions of the antioxidant response element. J. Neurochem. 2006;98:1852–1865. doi: 10.1111/j.1471-4159.2006.04019.x. [DOI] [PubMed] [Google Scholar]
- Liang LP, Beaudoin ME, Fritz MJ, Fulton R, Patel M. Kainate-induced seizures, oxidative stress and neuronal loss in aging rats. Neuroscience. 2007;147:1114–1118. doi: 10.1016/j.neuroscience.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. TTC, FluoroJade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J. Neurosci. Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louilot A, Taghzouti K, Simon H, Le Moal M. Limbic system, basal ganglia, and dopaminergic neurons. Executive and regulatory neurons and their role in the organization of behavior. Brain Behav. Evol. 1989;33:157–161. doi: 10.1159/000115920. [DOI] [PubMed] [Google Scholar]
- Lupberger J, Kreuzer KA, Baskaynak G, Peters UR, le Coutre P, Schmidt CA. Quantitative analysis of beta-actin, beta-2-microglobulin and porphobilinogen deaminase mRNA and their comparison as control transcripts for RTPCR. Mol. Cell Probes. 2002;16:25–30. doi: 10.1006/mcpr.2001.0392. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Aniya J, Wright KC, Ontl T, Xing Y, Bai L. Age-related deficits in mice performing working memory tasks in a water maze. Behav. Neurosci. 2003;117:485–495. doi: 10.1037/0735-7044.117.3.485. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Wright C, Bronson RT, Roth GS, Ingram DT, Bartke A. Studies of aging in Ames dwarf mice: effects of caloric restriction. J. Am. Aging Assoc. 2000;23:9–16. doi: 10.1007/s11357-000-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Lu X, Shinmei S, Badie-Mahdavi H, Bartfai T. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience. 2004;128:431–441. doi: 10.1016/j.neuroscience.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Kainic acid: the neurotoxic breakthrough. Crit. Rev. Toxicol. 1982;10:1–26. doi: 10.3109/10408448209033629. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Burke PA, Bartke A, Jensen RA. Inhibitory avoidance and appetitive learning in aged normal mice: comparison with transgenic mice having elevated plasma growth hormone levels. Neurobiol. Learn. Mem. 1997;68:1–12. doi: 10.1006/nlme.1997.3772. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Aging, stress and the hippocampus. Ageing Res. Rev. 2005;4:123–140. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Mitruskova B, Orendacova J, Racekova E. FluoroJade-B detection of dying cells in the SVZ and RMS of adult rats after bilateral olfactory bulbectomy. Cell. Mol. Neurobiol. 2005;25:1255–1264. doi: 10.1007/s10571-005-8502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiol. Aging. 2001;22:787–796. doi: 10.1016/s0197-4580(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Moore TE, Richards B, Hood J. Aging and the coding of spatial information. J. Gerontol. 1984;39:210–212. doi: 10.1093/geronj/39.2.210. [DOI] [PubMed] [Google Scholar]
- Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur. J. Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Oswald CJ, Yee BK, Rawlins JN, Bannerman DB, Good M, Honey RC. The influence of selective lesions to components of the hippocampal system on the orienting [correction of orientating] response, habituation and latent inhibition. Eur. J. Neurosci. 2002;15:1983–1990. doi: 10.1046/j.1460-9568.2002.02028.x. [DOI] [PubMed] [Google Scholar]
- Pompl PN, Mullan MJ, Bjugstad K, Arendash GW. Adaptation of the circular platform spatial memory task for mice: use in detecting cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. J. Neurosci. Methods. 1999;87:87–95. doi: 10.1016/s0165-0270(98)00169-1. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ramos JM. Is spatial memory transformed during the consolidation process: effect of reminding. Acta Neurobiol. Exp. (Wars) 2009;69:545–551. doi: 10.55782/ane-2009-1763. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech. Ageing Dev. 2004;125:269–281. doi: 10.1016/j.mad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000a;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol. Pathol. 2000b;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Schrag M, Sharma S, Brown-Borg H, Ghribi O. Hippocampus of Ames dwarf mice is resistant to beta-amyloid-induced tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus. 2008;18:239–244. doi: 10.1002/hipo.20387. [DOI] [PubMed] [Google Scholar]
- Sharps MJ, Gollin ES. Memory for object locations in young and elderly adults. J. Gerontol. 1987;42:336–341. doi: 10.1093/geronj/42.3.336. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Jeong JH, Bing G, Park ES, Chae JS, Yen TP, Kim WK, Wie MB, Jung BD, Kim HJ, Lee SY, Kim HC. Kainate-induced mitochondrial oxidative stress contributes to hippocampal degeneration in senescence-accelerated mice. Cell Signal. 2008;20:645–658. doi: 10.1016/j.cellsig.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech. Ageing Dev. 1994;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Sperk G. Kainic acid seizures in the rat. Prog. Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Sperk G, Lassmann H, Baran H, Kish SJ, Seitelberger F, Hornykiewicz O. Kainic acid induced seizures: neurochemical and histopathological changes. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- Sperk G, Lassmann H, Baran H, Seitelberger F, Hornykiewicz O. Kainic acid-induced seizures: dose-relationship of behavioural, neurochemical and histopathological changes. Brain Res. 1985;338:289–295. doi: 10.1016/0006-8993(85)90159-3. [DOI] [PubMed] [Google Scholar]
- Sun AY, Cheng Y, Bu Q, Oldfield F. The biochemical mechanisms of the excitotoxicity of kainic acid. Free radical formation. Mol. Chem. Neuropathol. 1992;17:51–63. doi: 10.1007/BF03159981. [DOI] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol. Aging. 2005a;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sun LY, Bartke A. Adult neurogenesis in the hippocampus of long-lived mice during aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:117–125. doi: 10.1093/gerona/62.2.117. [DOI] [PubMed] [Google Scholar]
- Sun LY, Evans MS, Hsieh J, Panici J, Bartke A. Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice. Endocrinology. 2005b;146:1138–1144. doi: 10.1210/en.2004-1115. [DOI] [PubMed] [Google Scholar]
- Sunyer B, Patil S, Hoger HGL. Barnes maze, a useful task to assess spatial reference memory in mice. Nat. Protocol. 2007;198:58–68. [Google Scholar]
- Sutherland RJ, McDonald RJ. Hippocampus, amygdala, and memory deficits in rats. Behav. Brain Res. 1990;37:57–79. doi: 10.1016/0166-4328(90)90072-m. [DOI] [PubMed] [Google Scholar]
- Swindell WR. Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics. 2007;8:353. doi: 10.1186/1471-2164-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis IL, Jensen O, Talamini LM, Rijpkema M, Fernandez G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J. Neurosci. 2009;29:10087–10093. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Lopez-Lopez C, Torres-Aleman I. Role of serum insulin-like growth factor I in mammalian brain aging. Growth Horm. IGF Res. 2004;14(Suppl A):S39–43. doi: 10.1016/j.ghir.2004.03.010. [DOI] [PubMed] [Google Scholar]
- van Dam PS, Aleman A. Insulin-like growth factor-I, cognition and brain aging. Eur. J. Pharmacol. 2004;490:87–95. doi: 10.1016/j.ejphar.2004.02.047. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005;31:3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr., Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- Wickelgren I. Is hippocampal cell death a myth? Science. 1996;271:1229–1230. doi: 10.1126/science.271.5253.1229. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Nadolski GJ, Samarut J, Chassande O, Redei EE. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor alpha. Behav. Brain Res. 2007;177:109–116. doi: 10.1016/j.bbr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, McNair KM, Noble J, Brownstein D, Hibberd C, Morton N, Mullins JJ, Morris RG, Cobb S, Seckl JR. Enhanced hippocampal long-term potentiation and spatial learning in aged 11beta-hydroxysteroid dehydrogenase type 1 knock-out mice. J. Neurosci. 2007;27:10487–10496. doi: 10.1523/JNEUROSCI.2190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Jin T, Quezada HC, Mix E, Winblad B, Zhu J. Kainic acid-induced microglial activation is attenuated in aged interleukin-18 deficient mice. J. Neuroinflamm. 2010;7:26. doi: 10.1186/1742-2094-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]