Abstract
Objective
to assess the phospholipase activity of endothelial (EL) and hepatic lipase (HL) in post-heparin plasma of subjects with Metabolic Syndrome (MS)/obesity and their relationship with atherogenic and antiatherogenic lipoproteins. Additionally, to evaluate Lipoprotein lipase (LPL) and HL activity as TG-hydrolyses to complete the analyses of SN1 lipolytic enzymes in the same patient.
Approach and results
plasma EL, HL and LPL activities were evaluated in 59 patients with MS and 36 controls. A trend towards higher EL activity was observed in MS. EL activity was increased in obese compared with normal weight group (p=0.009) and was negatively associated with HDL-cholesterol (p=0.014 and p=0.005) and apoAI (p=0.045 and p=0.001) in Control and MS group, respectively. HL activity, as triglyceride (TG) hydrolase, was increased in MS (p=0.025); as well as in obese (p=0.017); directly correlated with LDL-cholesterol (p=0.005) and apoB (p=0.003) and negatively with HDL-C (p=0.021) in Control group. LPL was decreased in MS (p<0.001); as well as in overweight and obese compared with normal weight group (p=0.015 and p=0.004 respectively); inversely correlated %TG-VLDL (p=0.04) and TG/apoB index (p=0.013) in Control group. These associations were not found in MS.
Conclusions
we describe for the first time EL and HL activity as phospholipases in MS/Obesity, being both responsible of HDL catabolism. Our results elucidate part of the remaining controversies about SN-1 lipases activity in MS and different grades of obesity. The impact of insulin-resistance on the activity of the three enzymes determines the lipoprotein alterations observed in these states.
Keywords: Endothelial Lipase, Hepatic Lipase, Lipoprotein Lipase, phospholipase activity, obesity, metabolic syndrome
INTRODUCTION
Lipoprotein lipase (LPL), hepatic lipase (HL) and endothelial lipase (EL) constitute a family of lipases involved in lipoprotein metabolism. These enzymes share a similar sequence structure at the genetic and protein level thus indicating a common ancestral origin. However, they are expressed in different tissues and act on different lipoprotein substrates, indicating that they may have evolved to specific roles.1 The three proteins are heparin-binding lipases anchored to the endothelial surface and mediate the hydrolysis of triglycerides (TG) and phospholipids (PL) at the SN1 position within circulating lipoproteins. Lipid hydrolysis results in structural changes within the lipoprotein species which affects their removal from the plasma and releases fatty acids to be taken up by tissues. Even though the three lipases have both TG and PL lipase activity, LPL is predominantly a TG lipase, EL principally hydrolyses PL and HL has an intermediate TG and PL lipase activity.2 LPL is responsible for the hydrolysis of chylomicrons and very low-density lipoproteins (VLDLs). Lower LPL activity has been associated with severe hypertriglyceridemia accompanied by low levels of high density lipoprotein cholesterol (HDL-C). 3,4 HL is involved in HDL metabolism as well as being responsible for the metabolism of apolipoprotein (apo) B containing lipoproteins (especially intermediate (IDL) and large low density lipoproteins (LDL)).5 Higher concentrations and activity of HL, as TG hydrolase, have been associated with increased levels of small dense LDL6–8 and lower levels of HDL. 7,8 EL is primarily involved in HDL metabolism and higher concentrations have been correlated with lower HDL levels. 9,10 Moreover, recently it has been shown that EL activity is responsible for the low HDL-C levels in hemodialysis patients.11
Alterations in the levels of plasma lipoproteins (high LDL-C and low HDL-C) are hallmarks of cardiovascular disease (CVD). Several co-morbidities including diabetes, obesity and metabolic syndrome (MS) have been shown to increase the risk of CVD12, in part due to the changes in lipoprotein profile, including increased levels of TG rich lipoproteins, low levels of HDL-C and increased levels of small dense LDL. This profile, characteristic of insulin resistance (IR) states, is mainly attributed to abdominal obesity; however it must be considered that the increase in body mass index does not always reflect IR. In addition there is evidence that weight change and IR individually can affect the atherogenic plasma lipid profile.13,14 The behavior of LPL and HL (as TG hydrolases) have been widely studied in IR states and obesity,15–18 however to our knowledge, EL and HL activity, as phospholipases, have never been evaluated in these situations. The altered lipoprotein profile observed in IR patients and during obesity could be in part a consequence of differing lipolysis of lipoproteins in these states. Furthermore, there are still controversies about the SN-1 lipases activity in different grades of obesity.19–22
Our aim was to assess the phospholipase activity of EL and HL in post-heparin plasma of subjects with MS/obesity and their relationship with atherogenic and antiatherogenic lipoprotein levels. Additionally, we evaluated LPL and HL activity as TG-hydrolyses to ascertain the individual roles of the three plasma lipolytic enzymes in the same patient and the consequent lipoprotein profile.
MATERIALS AND METHODS
Materials and Methods are available in the online- Data Supplement.
RESULTS
Characteristics of the Study Population
The clinical and biochemical characteristics of MS and Control group are shown in Table 1. In MS group, 43 patients were women and 16 men, whereas in the Control group, 20 were women and 16 were men. Patients with MS were older (p<0.001) and presented higher BMI (p<0.001) and waist circumference (p<0.001) than Controls.
Table 1.
Clinical and Biochemical Characteristics of Control and MS Groups
| Control (n=36) |
MS (n=59) |
p= | |
|---|---|---|---|
| Age (years) | 35±14 | 48±11 | 0.001 |
| Gender (W/M) | 20/16 | 43/16 | 0.083 |
| BMI (Kg/m2) | 23.5 ± 2.7 | 34.2 ± 5.9 | 0.001 |
| Waist circumference(cm) | 81.5 ± 11.6 | 105.3 ± 10.1 | 0.001 |
| TG (mmol/l) | 1.1 (0.4–2.9) | 2.1 (1.0–5.6) | 0.001 |
| Total-C (mmol/l) | 4.6 (3.3–7.6) | 5.3(3.6–8.1) | 0.042 |
| LDL-C (mmol/l) | 3.0 ± 1.1 | 3.7 ± 0.9 | 0.001 |
| HDL-C (mmol/l) | 1.5 ± 0.4 | 1.1 ± 0.2 | 0.001 |
| apoA-I (g/l) | 1.7 ± 0.4 | 1.4 ± 0.3 | 0.001 |
| apoB-100 (g/l) | 0.9 ± 0.3 | 1.1 ± 0.3 | 0.002 |
Data are expressed as mean±SD or median (range) for skewed distributed data. MS indicates Metabolic Syndrome; W, women; M, men; BMI, body mass index; Total-C, total cholesterol; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; apoA-I, apolipoprotein A-I; apoB, apolipoprotein B.
Regarding lipid and lipoprotein profile, the MS group presented higher TG (p<0.001), total cholesterol (p=0.042), LDL-C (p<0.001) and apo-B100 (p=0.002) and lower HDL-C and apo-AI levels (p<0.001).
In reference to IR and inflammatory state, as expected, values of glucose, insulin, HOMA-IR, TG/HDL-C, FFA and hs-CRP were higher (p<0.001), and adiponectin lower in MS patients compared to Controls (p<0.001) (Table 2).
Table 2.
Insulin Resistance and Inflammatory Markers in Control and MS Groups
| Control (n=36) |
MS (n=59) |
p= | |
|---|---|---|---|
| Glucose (mmol/l) | 4.8 (3.9–5.6) | 5.4 (4.5–7.0) | 0.001 |
| Insulin (pmol/l) | 36.1 (13.9–118.0) | 66.0 (13.9–479.2) | 0.001 |
| HOMA-IR | 1.2 (0.3–3.6) | 2.5 (0.5–20.6) | 0.001 |
| TG/ HDL-chol | 1.7 (0.5–7.9) | 4.4 (2.1–16.2) | 0.001 |
| FFA(mmol/l) | 0.5 (0.1–0.8) | 0.6 (0.3–1.1) | 0.011 |
| Adiponectin (µg/ml) | 12.3 (4.8–28.3) | 5.6 (1.9–20.6) | 0.001 |
| hs-CRP (mg/l) | 1.3 (0.1–11.7) | 3.1(0.3–29.7) | 0.001 |
Data are expressed as median (range) for skewed distributed data. HOMA-IR indicates homeostasis model assessment for insulin resistance index; hs-CRP, high-sensitivity C-reactive protein; HDL-chol, high-density lipoprotein-cholesterol; TG, triglycerides; MS, Metabolic Syndrome.
Furthermore, in both groups VLDL, IDL and sdLDL were isolated and characterized. As shown in table 3, in patients with MS a prevalence of larger VLDL was observed, enriched in TG, as well as an increase of remnants and sdLDL.
Table 3.
VLDL, IDL and sdLDL in Control and MS patients.
| Control (n=36) |
MS (n=59) |
p= | |
|---|---|---|---|
| Large VLDL (%) | 7.8 (1.0–21.9) | 33.5 (1.2–72.9) | 0.010 |
| VLDL-TG (%) | 49±12 | 56±6 | 0.018 |
| VLDL-C(%) | 13±3 | 14±3 | ns |
| VLDL-pt (%) | 14±4 | 16±4 | ns |
| VLDL-pl (%) | 18±4 | 14±3 | 0.002 |
| TG/apoB | 6.2 ± 2.8 | 11.7 ± 4.7 | 0.001 |
| IDL-C (mg/dl) | 0.1 ± 0.06 | 0.2 ± 0.08 | 0.036 |
| sd LDL (%) | 11.3±6.3 | 28.7±5.4 | 0.001 |
Data are expressed as mean±SD. MS indicates Metabolic Syndrome; TG, triglycerides; C, cholesterol; pt, protein; pl, phospholipids; sdLDL, small and dense low density lipoprotein.
The baseline characteristics of the subjects divided according to obesity degree are shown in Table 4.
Table 4.
Baseline characteristics of the subjects according to obesity degree
| NW (n=27) | OW (n=20) | OB (n=48) | |
|---|---|---|---|
| BMI (Kg/m2) | 23.0 (16.7–24.9) | 26.7 (25.2–29.1)α | 34.5 (30.0–54.7)αγ |
| Age (years) | 36±15 | 45±14* | 46±11α |
| Gender (W/M) | 18/9 | 11/9 | 34/14 |
| Waist circumf (cm) | 78±11 | 95±7α | 107±10αγ |
| TG (mmol/l) | 1.0 (0.4–2.9) | 1.8 (0.5–4.6)α | 2.1 (1.0–5.6)α |
| Total-C (mmol/l) | 4.7 (3.3–7.6) | 6.2 (3.4–7.9)α | 5.2 (3.6–8.1) |
| LDL-C (mmol/l) | 2.8±1.1 | 4.1±1.2α | 3.5±0.8α |
| HDL-C (mmol/l) | 1.6±0.4 | 1.2±0.4α | 1.0±0.2α |
| apoA-I (g/l) | 1.8±0.4 | 1.6±0.3 | 1.4±0.2α |
| apoB-100 (g/l) | 0.9±0.3 | 1.1±0.3* | 1.0±0.3* |
| Glucose (mmol/l) | 4.78 (3.9–5.4) | 5.3 (4.4–6.6)α | 5.5 (4.4–7.0)α |
| Insulin (pmol/l) | 32.5 (13.9–66.7) | 43.0 (13.9–147.2)α | 76.4 (27.1–479.2)γ |
| HOMA-IR | 0.9 (0.3–2.0) | 1.4 (0.5–15.6)* | 2.7 (0.9–20.6)αγ |
| TG/ HDL-C | 1.5 (0.7–7.9) | 3.6 (0.5–15.7) | 4.4 (2.1–16.2)α |
| FFA(mmol/l) | 0.5 (0.1–0.8) | 0.6 (0.1–1.1) | 0.6 (0.3–1.1) |
| Adiponectin (µg/ml) | 12.6 (7.0–28.2) | 7.3 (1.9–20.6)α | 5.6 (1.9–16.4)α |
| hs-CRP (mg/l) | 1.3(0.1–11.7) | 2.3 (0.2–6.2) | 3.4 (0.3–29.7)* |
Data are expressed as mean±SD or median (range) for skewed distributed data. W indicates women; M, men; BMI, body mass index; Total-C, total cholesterol; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; apoA-I, apolipoprotein A-I; apoB, apolipoprotein B; HOMA-IR, homeostasis model assessment for insulin resistance index; FFA, free fatty acids; hs-CRP, high-sensitivity C-reactive protein. vs NW
p<0.05
p<0.01; vs OW
p<0.05
p<0.01.
Phospholipase Activities
-EL activity
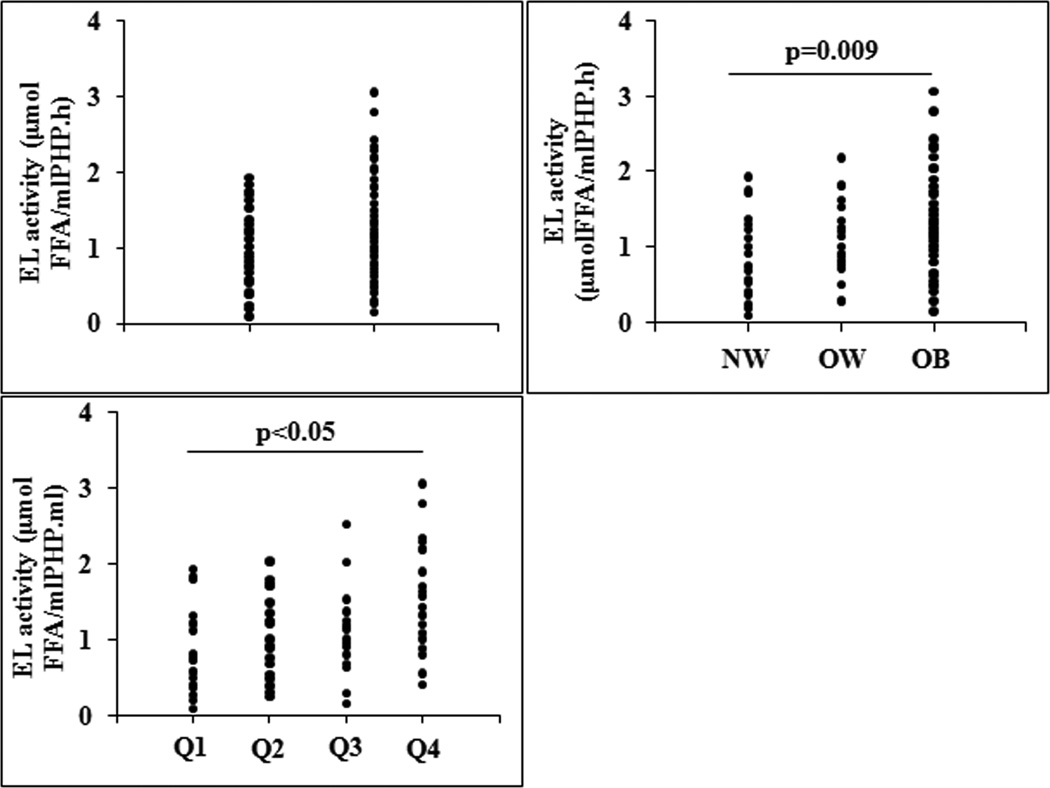
EL activity was evaluated in Control and MS group. A trend towards higher EL activity was observed in MS however it did not reach significance: 0.92 (0.09–1.93) vs 1.11 (0.15–3.06) μmol FFA/ml PHP.h, p=0.097 (Figure 1A). There was no difference in EL activity between men and women: 1.25 (0.29–3.06) vs 1.0 (0.09–2.53) μmol FFA/ml PHP.h, p=0.330.
Figure 1.
Endothelial lipase activity (EL) in: A) Control and Metabolic Syndrome (MS) group; B) different obesity grade: Normal weigth (NW), Overweigth (OW) and Obese (OB); and C) different obesity grade according to HOMA-IR quartile (Q): Q1, HOMA-IR≤ 1.02; Q2, 1.03<HOMA-IR≤ 1.76; Q3, 1.77 <HOMA-IR≤ 3.30 and Q4, HOMA-IR≥ 3.31. FFA indicates free fatty acids.
EL activity was not associated with age (r=−0.167; p=0.147) nor with waist circumference (r=0.183; p=0.126). Given the direct association between EL activity and BMI in the whole population (r=0.291; p=0.01), we analyzed the behavior of the enzyme according to the obesity degree of the subjects. EL activity was significantly increased in OB group compared with NW group: 1.25 (0.15–3.06) vs 0.71 (0.09–1.93) μmol FFA/ml PHP.h, p=0.009 (Figure 1B). Even though no correlations with age and gender were observed, we performed an ANCOVA analysis including both variables. Difference between OB and NW group persisted significant (F= 6.9, p=0.004 and F= 4.8, p=0.01, respectively).
In addition, in Control and MS group, EL activity was negatively associated with HDL-C (r= −0.369, p=0.014 and r=−0.480, p=0.005 respectively) and apoAI (r=−0.311, p=0.045 and r=−0.559, p=0.001 respectively) highlighting the role of EL on HDL catabolism. Similarly, in both groups EL activity was positively correlated with insulin (r=0.301, p=0.05 and r=0.390, p=0.027 respectively) and HOMA-IR (r=0.310, p=0.047 and r=0.413, p=0.019 respectively). In contrast, EL activity negatively correlated with adiponectin (r=−0.515; p=0.006) only in Control group.
Given that there was no difference in EL activity between MS and Control group, but a positive association between EL activity and HOMA-IR was observed, individuals were divided according to HOMA-IR quartile. The quartiles were defined according to the following range: quartile 1: HOMA-IR≤ 1.02; quartile 2: 1.03<HOMA-IR≤ 1.76; quartile 3: 1.77 <HOMA-IR≤ 3.30 and quartile 4: HOMA-IR≥ 3.31.It was observed that individuals with the highest grade of IR (quartile 4) showed a significant increase in EL activity respect to individuals with the lowest grade of IR (quartile 1) (p <0.05) (Figure 1C)
-HL activity
When HL was evaluated as phospholipase, there was no difference between Control and MS group: 5.85 (1.99–14.0) vs 5.62 (0.66–16.58) μmol FFA/ml PHP.h, p=0.750, neither between NW, OW and OB group: 5.33 (1.99–12.89) vs 5.20 (1.54–14.0) vs 5.87 (0.66–16.58) μmol FFA/ml PHP.h, p=0.912. In turn, in the whole population, HL as phospholipase was increased in men compared to women: 7.31 (1.61- 16.58) vs 4.38 (0.66–16.17) μmol FFA/ml PHP.h, p<0.001.
Although no difference in HL activity was found between groups, regarding lipoprotein profile, in Control group, HL activity was negatively correlated with HDL-C (r=−0.639; p=0.001) and apoA-I levels (r=−0.623; p=0.001) while in MS group only a tendency with HDL-C was observed (r=−0.281; p=0.062). An inverse association with adiponectin was observed only in Control group (r=−0.441; p=0.021).
Effect of EL and HL as phospholipase on HDL
Given that EL and HL as phospholipase were associated with HDL-C, the impact of both enzymes activities on HDL-C was analyzed through a multivariate regression analyses to distinguish the contribution of each one. In MS group HDL-C decrease remained mainly associated with EL activity (β=−0.35; p= 0.01).
TG-lipase Activities
-HL activity
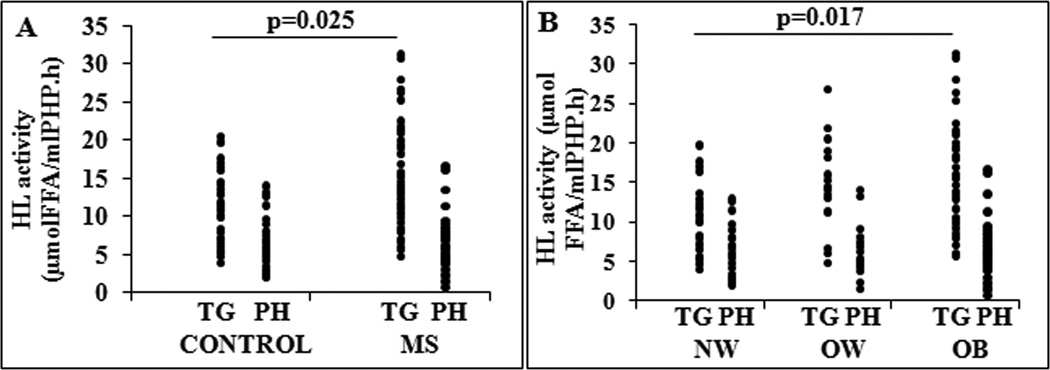
As expected, HL activity, as TG hydrolase, was increased in MS compared to control group: 14.53±6.33 vs 11.26±4.92 μmol FFA/ml PHP.h, p=0.025 (Figure 2A). Similarly to its activity as phospholipase men presented higher values of HL than women (16.7±5.8 μmol FFA/ml PHP.hvs 11.8±5.5 μmol FFA/ml PHP.h, p<0.001). In reference to obesity degree, HL activity as TG-hydrolase was significantly increased in OB group compared with NW group: 15.0±6.3 vs 10.8±4.8μmol FFA/ml PHP.h, p=0.017 (Figure 2B). HL activity was not associated with age (r=0.031; p=0.802). In reference to lipids and lipoproteins profile, in Control group HL activity was directly correlated with LDL-C (r=0.526; p=0.005), apoB (r=0.560; p=0.003) and negatively correlated with HDL-C (r=−0.442; p=0.021). These correlations were not found in MS group in whom HL activity showed a weak inverse correlation with IDL-C (r=−0.365; p=0.040). With respect to IR markers, HL activity was positively associated with insulin (r=0.378; p=0.011) and HOMA-IR (r=0.323; p=0.032) in MS group.
Figure 2.
Hepatic lipase (HL) activity in: A) Control and Metabolic Syndrome (MS) group; and B) HL activity in different obesity grade: Normal weigth (NW), Overweigth (OW) and Obese (OB). FFA indicates free fatty acids.
Given that HL activity was higher in men than women, we performed an ANCOVA analysis including gender as independent variable. Differences in HL activity remained significant between MS and Controls (F= 8.9, p=0.004) and among obesity degree groups (F= 6.4, p=0.003). Even though HL activity was not associated with age we also included this variable in the ANCOVA analysis. Differences in HL activity persisted significant between MS and Controls (F= 5.2, p=0.02) and among obesity degree groups (F= 4.1, p=0.02).
It is important to point out that HL as TG hydrolase directly correlated with HL as phospholipase: r= 0.79, p<0.001.
-LPL activity
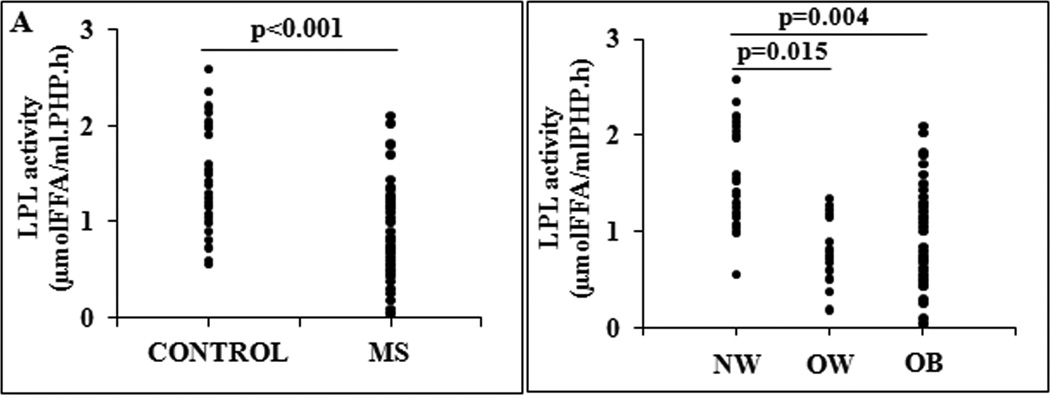
Patients with MS presented lower LPL activity than Controls: 0.75 (0.04–2.10) vs 1.38 (0.56–2.58) μmol FFA/ml PHP.h, p<0.001. In the whole population, there was no difference in LPL activity between men and women: 0.99 (0.26–1.55) vs 1.15 (0.04–2.58) μmol FFA/ml PHP.h, p=0.160 and it was not associated with age (r=−0.171; p=0.173).
When obesity degree was considered, LPL activity was significantly decreased in OW group: 0.81 (0.19–1.34) μmol FFA/ml PHP.h and OB group: 0.75 (0.04–2.10) μmol FFA/ml PHP.h compared with NW group: 1.54 (0.56–2.58) μmol FFA/ml PHP.h, p=0.015 and p=0.004 respectively (Figure 3).
Figure 3.
Lipoprotein lipase (LPL) activity in: A) Control and Metabolic Syndrome (MS) group; and B) different obesity grade: Normal weigth (NW), Overweigth (OW) and Obese (OB). FFA indicates free fatty acids.
Although no correlations with age and gender were observed, we performed an ANCOVA analysis including age and gender as independent variables. Differences in LPL activity remained significant between MS and Control group (F= 10.7, p=0.002 and F= 12.1, p=0.001, respectively) and among obesity degree groups (F= 5.9, p=0.004 and F= 6.6, p=0.002, respectively)
The expected relationship between LPL activity and %TG-VLDL (r=−0.636, p=0.04) and TG/apoB index (r=−0.783, p=0.013) were indeed found in Control group. However, in this group only weak associations between LPL activity and insulin (r=−0.462, p=0.04) and HOMA-IR index (r=−0.468, p=0.037) were found.
DISCUSSION
In the present study, we evaluated the role of EL, HL and LPL activity in the lipoprotein abnormalities associated with MS and obesity. It is important to highlight that this is the first time that EL and HL as phospholipases were evaluated in MS and obesity. Moreover, to our knowledge, this study is the first that report all three lipoprotein lipases activity in the same population.
It is well known that obesity and associated IR are main contributors to cardiovascular disease.23 These relationships are directly linked with lipids and lipoproteins alterations which include elevated TG and lower HDL-C.
In this study, we showed for the first time that EL activity is increased in individuals with higher obesity degree and is associated with lower HDL-C and apoA-I levels. Previous studies have shown that overexpression of EL in mice results in a dramatic decrease in HDL-C and apoA-I levels, leading to the production of smaller HDL particles.10 Furthermore, several studies in human plasma demonstrated higher expression of EL in MS24 and obesity25 associated with a significant decrease of HDL-C levels. In this study, we evaluated EL activity and no significant difference between Controls and MS patients was found. However, there was a trend towards increase EL activity in MS and the lack of significance may reflect the inherent high assay variability. In this respect a direct EL activity assay where HL is removed by immunoprecipitation would be useful. Further studies including larger number of patients would possibly allow us to obtain significant differences between groups. When the subjects were analyzed according to their obesity degree, those with the highest obesity grade presented the highest EL activity. Our results support the previous findings of Badellino et al26 who showed that plasma EL concentration is positively correlated with markers of adiposity, such as BMI and waist circumference in healthy individuals. Part of the association observed between EL and obesity could be attributed to the impact of the IR state, corroborated by the correlation between EL activity and the HOMA index in individuals with MS and Controls. This finding is held up by the increased EL activity observed at the highest HOMA quartile. In addition, it is important to highlight that, in contrast to LPL and HL, EL activity was associated with lower HDL-C and apoA-I levels in individuals with MS and Controls. Although IR would be the best predictor of EL behavior, this study is the first to report an inverse association between EL activity and adiponectin in individuals without MS. It should be noted that adiponectin was also associated with HL phospholipase activity, suggesting a possible role of this cytokine in phospholipase activity regulation. Until now, it has only been described an inverse association between EL concentration and adiponectin levels in healthy individuals.26 However, in vitro studies showed that TNF-α may stimulate EL secretion thus, adiponectin would affect EL indirectly by inhibiting inhibiting TNF-α.26 Further studies are necessary to elucidate this finding.
Regarding HL, as expected, TG-activity was increased in MS group. HL activity appears to be regulated by several factors including age,27 gender. 27,28 It has been reported that men have twice as high HL activity than women,29 and in accordance, we have observed that men presented higher HL activity than women; however, the higher HL activity in MS group was independent of gender and age. Even more, HL activity was increased in OB group; these results suggest that the severe states of obesity would be implicated in the regulation of the enzyme, as described in other studies.21 The specific mechanism that links HL activity to hyperinsulinemia and IR remains unclear. It is known that type 1 diabetic patients present low HL activity17 and that chronic hyperinsulinemic states show increased activity of the enzyme.18 It was suggested that secondary factors might contribute to the regulation of HL in obesity and IR states30; in fact in our study, only in MS group a weak association between HL activity and IR markers was found. HL activity directly correlated with atherogenic lipoprotein profile only in Control group. When analyzing HL as phospholipase, no differences between groups, MS or obesity degree, were observed. HL phospholipase activity inversely correlated with HDL and apoA-I levels in Control group. HL enzyme activities, as TG lipase and phospholipase, were directly correlated within individuals. The differences in importance within a disease state may reflect their different in vivo substrates: VLDL and LDL for TG-lipase activity and HDL for phospholipase activity.
According to our results, HDL-C levels would be influenced by phospholipase activities of EL as well as HL, with EL mainly responsible of HDL catabolism. Both HL and EL variants have been shown to affect HDL-C.31,32 Since often they are similarly regulated it is important to which is the predominant determinant of HDL-C. The present data along with our previous analysis of individuals undergoing hemodialysis11 suggest that HDL-C decrease is mainly associated with EL activity. Our findings extend previous reports about factors that can modulate HDL-C levels, such as lecithin cholesterol acyl transferase, and ATP-binding cassette sub-family A member 1 expression, among others.33
In reference to LPL, this study shows a significant decrease in plasma activity in individuals with MS. Previous studies reported lower LPL mass in individuals with MS,14 as well as negative correlations with BMI. 34 Our results of LPL activity in PHP are in agreement with the reported findings; we found a significant decrease from OW situations with a clear decrease in OB. Recent studies have shown that the expression and activity of LPL in PHP is lower in obese diabetic patients with respect to obese individuals without diabetes and controls.20 Moreover, it has been reported a decrease in LPL gene expression in the visceral adipose tissue from morbidly obese individuals compared to obese and lean individuals. 35 In our study we observed that LPL activity was inversely associated with surrogate markers of IR only in individuals without MS. In reference to the role of LPL in VLDL catabolism, our data revealed the expected inverse correlation with VLDL-TG content and size in Control group; however these association were not found in MS patients. These results are in accordance with previous studies which suggest that other factors have more important regulatory roles in the removal of postprandial lipoproteins in IR states36,37
With respect to hs-CRP, in this study no significant association between this chronic inflammation marker and lipolytic enzymes activity was found. Different studies have shown controversial results according to the effect of CRP on the expression and activity of the enzymes.30,26,38,39 In this study, the lack of association between hs-CRP and the lipolytic enzymes should not exclude more complex inflammatory mechanisms in the regulation of enzyme activity.
Finally, in this study we describe for the first time the activity of the three main lipolytic enzymes, evaluated in the same population, highlighting the specific role of each one on the different lipoproteins metabolism. EL and HL as phospholipase are both responsible of the HDL catabolism. Our results elucidate part of the remaining controversies about the SN-1 lipases activity in different grades of obesity. The impact of IR and obesity on the three enzymes behavior (table 5) determines the lipoproteins alterations in these pathologic situations. Overall, lipolytic enzymes would be an interesting potential therapeutic target as a strategy to improve lipoprotein profile and reduce cardiovascular risk in IR and obese patients.
Table 5.
LPL, HL and EL behavior in Metabolic Syndrome and obesity
| LPL | HL-TG | HL-PL | EL | |
|---|---|---|---|---|
| MS | ↓ | ↑ | = | =/↑ |
| Obesity | ↓ | ↑ | = | ↑ |
Supplementary Material
SIGNIFICANCE.
The novelty of this study is that this is the first time that EL and HL activity as phospholipases have been evaluated in Metabolic Syndrome and Obesity. In addition, this is the first study that all three SN1 lipases were evaluated in the same population. The altered lipoprotein profile observed in IR patients and during obesity could be in part a consequence of differing lipolysis of lipoproteins in these states.
ACKNOWLEDGEMENTS
None
SOURCES OF FUNDING
This work was supported by University of Buenos Aires Grants (20020110100041) and NIH Grant HL-055323 from the National Heart, Lung, and Blood Institute.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Hasham S, Pillarisetti S. Vascular lipases, inflammation and atherosclerosis. Clin Chim Acta. 2006;372:179–183. doi: 10.1016/j.cca.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 2.McCoy MG, Sun GS, Marchadier D, Maugeais C, Glick JM, Rader DJ. Characterization of the lipolytic activity of endothelial lipase. J Lipid Res. 2002;43:921–929. [PubMed] [Google Scholar]
- 3.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 4.Krentz AJ. Lipoprotein abnormalities and their consequences for patients with type 2 diabetes. Diabetes Obes Metab. 2003;5(Suppl 1):S19–S27. doi: 10.1046/j.1462-8902.2003.0310.x. [DOI] [PubMed] [Google Scholar]
- 5.Zambon A, Deeb SS, Bensadoun A, Foster KE, Brunzell JD. In vivo evidence of a role for hepatic lipase in human apoB-containing lipoprotein metabolism, independent of its lipolytic activity. J Lipid Res. 2000;41:2094–2099. [PubMed] [Google Scholar]
- 6.Berg G, Siseles N, González A, Ortiz O, Tempone A, Wikinski R. Higher values of hepatic lipase activity in postmenopause: relationship with atherogenic intermediate density and low density lipoproteins. Menopause. 2001;8:51–57. doi: 10.1097/00042192-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Brunzell J, Zambon A, Deeb S. The effect of hepatic lipase on coronary artery disease in humans is influenced by the underlying lipoprotein phenotype. Biochim Biophys Acta. 2012;1821:365–372. doi: 10.1016/j.bbalip.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miksztowicz V, Lucero D, Zago V, Cacciagiú L, Lopez G, Gonzalez Ballerga E, Sordá J, Fassio E, Schreier L, Berg G. Hepatic lipase activity is increased in nonalcoholic fatty liver disease beyond insulin resistance. Diabetes Metab Res Rev. 2012;28:535–541. doi: 10.1002/dmrr.2312. [DOI] [PubMed] [Google Scholar]
- 9.Jin W, Marchadier D, Rader DJ. Lipases and HDL-C metabolism. Trends Endocrinol Metab. 2002;13:174–178. doi: 10.1016/s1043-2760(02)00589-1. [DOI] [PubMed] [Google Scholar]
- 10.Maugeais C, Tietge UJ, Broedl UC, Marchadier D, Cain W, McCoy MG, Lund-Katz S, Glick JM, Rader DJ. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation. 2003;108:2121–2126. doi: 10.1161/01.CIR.0000092889.24713.DC. [DOI] [PubMed] [Google Scholar]
- 11.Miksztowicz V, McCoy MG, Schreier L, Cacciagiú L, Elbert A, Gonzalez AI, Billheimer J, Eacho P, Rader DJ, Berg G. Endothelial lipase activity predicts highdensity lipoprotein catabolism in hemodialysis: novel phospholipase assay in postheparin human plasma. Arterioscler Thromb Vasc Biol. 2012;32:3033–3040. doi: 10.1161/ATVBAHA.112.300110. [DOI] [PubMed] [Google Scholar]
- 12.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005;47:201–210. [PubMed] [Google Scholar]
- 13.Mäntyselkä P, Kautiainen H, Saltevo J, Würtz P, Soininen P, Kangas AJ, Ala-Korpela M, Vanhala M. Weight change and lipoprotein particle concentration and particle size: a cohort study with 6.5-year follow-up. Atherosclerosis. 2012;223:239–243. doi: 10.1016/j.atherosclerosis.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Liu EY, Freudenreich O, Goff D, Henderson DC, Fan X. Phenotypic characteristics in metabolically obese but normal weight non-diabetic patients with schizophrenia. Schizophr Res. 2010;124:49–53. doi: 10.1016/j.schres.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Hanyu O, Miida T, Obayashi K, Ikarashi T, Soda S, Kaneko S, Hirayama S, Suzuki K, Nakamura Y, Yamatani K, Aizawa Y. Lipoprotein lipase (LPL) mass in preheparin serum reflects insulin sensitivity. Atherosclerosis. 2004;174:385–390. doi: 10.1016/j.atherosclerosis.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Saiki A, Oyama T, Endo K, Ebisuno M, Ohira M, Koide N, Murano T, Miyashita Y, Shirai K. Preheparin serum lipoprotein lipase mass might be a biomarker of metabolic syndrome. Diabetes Res Clin Pract. 2007;76:93–101. doi: 10.1016/j.diabres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Caixàs A, Pérez A, Payés A, Otal C, Carreras G, Ordóñez-Llanos J, Reviriego J, Anderson JH, de Leiva A. Effects of a short-acting insulin analog (Insulin Lispro) versus regular insulin on lipid metabolism in insulin-dependent diabetes mellitus. Metabolism. 1998;47:371–376. doi: 10.1016/s0026-0495(98)90045-2. [DOI] [PubMed] [Google Scholar]
- 18.Syvänne M, Ahola M, Lahdenperä S, Kahri J, Kuusi T, Virtanen KS, Taskinen MR. High density lipoprotein subfractions in non-insulin-dependent diabetes mellitus and coronary artery disease. J Lipid Res. 1995;36:573–582. [PubMed] [Google Scholar]
- 19.Eckel R, Yost T. Weight reduction increases adipose tissue lipoprotein lipase responsiveness in obese women. J Clin Invest. 1987;80:992–997. doi: 10.1172/JCI113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costabile G, Annuzzi G, Di Marino L, De Natale C, Giacco R, Bozzetto L, Cipriano P, Santangelo C, Masella R, Rivellese A. Fasting and post-prandial adipose tissue lipoprotein lipase and hormone-sensitive lipase in obesity and type 2 diabetes. J Endocrinol Invest. 2011;34:110–114. doi: 10.1007/BF03347469. [DOI] [PubMed] [Google Scholar]
- 21.Pardina E, Baena-Fustegueras J, Catalán R, Galard R, Lecube A, Fort J, Allende H, Vargas V, Peinado-Onsurbe J. Increased expression and activity of hepatic lipase in the liver of morbidly obese adult patients in relation to lipid content. Obes Surg. 2009;19:894–904. doi: 10.1007/s11695-008-9739-9. [DOI] [PubMed] [Google Scholar]
- 22.Després JP, Ferland M, Moorjani S, Nadeau A, Tremblay A, Lupien PJ, Thériault G, Bouchard C. Role of hepatic-triglyceride lipase activity in the association between intraabdominal fat and plasma HDL cholesterol in obese women. Arteriosclerosis. 1989;9:485–492. doi: 10.1161/01.atv.9.4.485. [DOI] [PubMed] [Google Scholar]
- 23.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Badellino K, Wolfe M, Reilly M, Rader D. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 2006:e22. doi: 10.1371/journal.pmed.0030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paradis M, Badellino K, Rader D, Tchernof A, Richard C, Luu-The V, Deshaies Y, Bergeron J, Archer W, Couture P, Bergeron N, Lamarche B. Visceral adiposity and endothelial lipase. J Clin Endocrinol Metab. 2006;91:3538–3543. doi: 10.1210/jc.2006-0766. [DOI] [PubMed] [Google Scholar]
- 26.Badellino K, Wolfe M, Reilly M, Rader D. Endothelial lipase is increased in vivo by inflammation in humans. Circulation. 2008;117:678–685. doi: 10.1161/CIRCULATIONAHA.107.707349. [DOI] [PubMed] [Google Scholar]
- 27.Huttunen JK, Ehnholm C, Kekki M, Nikkilä EA. Post-heparin plasma lipoprotein lipase and hepatic lipase in normal subjects and in patients with hypertriglyceridaemia: correlations to sex, age and various parameters of triglyceride metabolism. Clin Sci Mol Med. 1976;50:249–260. doi: 10.1042/cs0500249. [DOI] [PubMed] [Google Scholar]
- 28.Krauss RM, Levy RI, Fredrickson DS. Selective measurement of two lipase activities in postheparin plasma from normal subjects and patients with hyperlipoproteinemia. J Clin Invest. 1974;54:1107–1124. doi: 10.1172/JCI107855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr MC, Hokanson JE, Zambon A, Deeb SS, Barrett PH, Purnell JQ, Brunzell JD. The contribution of intraabdominal fat to gender differences in hepatic lipase activity and low/high density lipoprotein heterogeneity. J Clin Endocrinol Metab. 2001;86:2831–2837. doi: 10.1210/jcem.86.6.7586. [DOI] [PubMed] [Google Scholar]
- 30.Schneider J, von Eynatten M, Schiekofer S, Nawroth P, Dugi K. Low plasma adiponectin levels are associated with increased hepatic lipase activity in vivo. Diabetes Care. 2005;28:2181–2186. doi: 10.2337/diacare.28.9.2181. [DOI] [PubMed] [Google Scholar]
- 31.Zambon A, Deeb SS, Hokanson JE, Brown BG, Brunzell JD. Common variants in the promoter of the hepatic lipase gene are associated with lower levels of hepatic lipase activity, buoyant LDL, and higher HDL2 cholesterol. Arterioscler Thromb Vasc Biol. 1998;18:1723–1729. doi: 10.1161/01.atv.18.11.1723. [DOI] [PubMed] [Google Scholar]
- 32.Brown RJ, Edmondson AC, Griffon N, Hill TB, Fuki IV, Badellino KO, Li M, Wolfe ML, Reilly MP, Rader DJ. A naturally occurring variant of endothelial lipase associated with elevated HDL exhibits impaired synthesis. J Lipid Res. 2009;50:1910–1916. doi: 10.1194/jlr.P900020-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaziri N. Causes of dysregulation of lipid metabolism in chronic renal failure. Semin Dial. 2009;22:644–651. doi: 10.1111/j.1525-139X.2009.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi J, Saito K, Fukamachi I, Taira K, Takahashi K, Bujo H, Saito Y. Preheparin plasma lipoprotein lipase mass: correlation with intra-abdominal visceral fat accumulation. Horm Metab Res. 2001;33:412–416. doi: 10.1055/s-2001-16230. [DOI] [PubMed] [Google Scholar]
- 35.Clemente-Postigo M, Queipo-Ortuño M, Fernandez-Garcia D, Gomez-Huelgas R, Tinahones F, Cardona F. Adipose tissue gene expression of factors related to lipid processing in obesity. PLoS One. 2011;6:247–283. doi: 10.1371/journal.pone.0024783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg IJ, Vanni-Reyes T, Ramakrishnan S, Holleran S, Ginsberg HN. Circulating lipoprotein profiles are modulated differently by lipoprotein lipase in obese humans. J Cardiovasc Risk. 2000;7:41–47. doi: 10.1177/204748730000700108. [DOI] [PubMed] [Google Scholar]
- 37.Olivecrona T, Bengtsson-Olivecrona G. Lipoprotein lipase and hepatic lipase. Curr Opin Lipidol. 1993;4:187–196. [Google Scholar]
- 38.Maingrette F, Li L, Renier G. C-reactive protein enhances macrophage lipoprotein lipase expression. J Lipid Res. 2008;49:1926–1935. doi: 10.1194/jlr.M800024-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Ma G, Zhang X, Wang J. Lipoprotein lipase and premature coronary artery disease. Acta Cardiol. 2009;64:379–383. doi: 10.2143/AC.64.3.2038025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.