Abstract
Objective
ANGPTL3 and ANGPTL4 are secreted proteins that inhibit lipoprotein lipase (LPL) in vitro. Genetic variants at the ANGPTL3 and ANGPTL4 gene loci are significantly associated with plasma lipid traits. The aim of this study was to evaluate the association of plasma angiopoietin-like protein 3 (ANGPTL3) and 4 (ANGPTL4) concentrations with lipid and metabolic traits in a large community-based sample.
Approach and Results
Plasma ANGPTL3 and ANGPTL4 levels were measured in 1770 subjects using a validated ELISA assay. A Pearson unadjusted correlation analysis and a linear regression analysis adjusting for age, gender and race were performed. ANGPTL3 levels were significantly positively associated with low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels (both P < 2 ×10−5) but not triglycerides. In contrast, ANGPTL4 levels were significantly negatively associated with LDL-C and HDL-C (both P < 2 × 10−5) and positively associated with triglycerides (P=0.003). In addition, ANGPTL4, but not ANGPTL3, levels were significantly positively associated with fasting blood glucose and metabolic syndrome.
Conclusions
Despite having similar biochemical effects in vitro, plasma ANGPTL3 and ANGPTL4 concentrations have nearly opposite relationships with plasma lipids. ANGPTL4 is strongly negatively associated with LDL-C and HDL-C and positively with multiple features of the metabolic syndrome including triglycerides, whereas ANGPTL3 is positively associated with LDL-C and HDL-C and not with metabolic syndrome traits including triglycerides. While ANGPTL3 and ANGPTL4 both inhibit LPL in vitro and influence lipoprotein metabolism in vivo, the physiology of these related proteins and their effects on lipoproteins is clearly divergent and complex.
Keywords: Angiopoietin-Like Protein 3, Angiopoietin-Like Protein 4, lipid metabolism, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, epidemiology, lipids, lipoproteins, metabolic syndrome, diabetes mellitus
Introduction
The family of angiopoietin-like proteins (ANGPTLs) are secreted proteins characterized by key structural motifs including an N-terminal signal peptide directing secretion, a N-terminal coiled-coiled domain, a linker region, and a C-terminal fibrinogen-like domain.1 Two of the members that have been extensively studied are ANGPTL3 and ANGPTL4. Functional studies in vitro have shown that ANGPTL3 reversibly inhibits and ANGPTL4 irreversibly inhibits lipoprotein lipase (LPL), suggesting a role in lipoprotein metabolism.1 Transgenic mouse models have shown that ANGPTL3 and ANGPTL4 overexpression leads to increased triglyceride (TG) levels. 1–4 ANGPTL3 knockout mice have at least a 50% reduction in both plasma TG and high-density lipoprotein cholesterol (HDL-C). 5 ANGPTL4 knockout mice have a 65–90% decrease in TG levels, lower circulating very low-density lipoprotein cholesterol (VLDL-C) levels and increased LPL activity. 6, 7
Consistent with these data, genome wide-association studies identified that common variants at the ANGPTL3 locus are associated with TG and low-density lipoprotein cholesterol (LDL-C) and at the ANGPTL4 locus are associated with HDL-C levels.8, 9 Population-based resequencing in the Dallas Heart Study found that nonsynonymous loss-of-function variants in both proteins were associated with lower TG levels.10, 11 Exome sequencing in a family with familial combined hypolipidemia identified loss-of-function mutations in ANGPTL3 that were causally associated with decreased TG, LDL-C, and HDL-C.12
Despite the biological interest in these proteins, the fact that they are secreted proteins detectable in the plasma, and their potential for therapeutic targeting, there are very limited data on plasma levels of ANGPTL3 and ANGPTL4 and their relationship with plasma lipids and metabolic parameters. 3, 5, 13–18 We therefore undertook the first large-scale study to assay plasma ANGPTL3 and ANGPTL4 levels and address their association with lipids and metabolic traits.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Distribution and demographic associations with plasma ANGPTL3 and ANGPTL4 levels
The characteristics of the study population (n=1770) are described in Table 1. The sample was predominantly white, majority male, and baseline median age was 53 years. Approximately half of the sample had metabolic syndrome or type 2 diabetes.
Table 1.
Characteristics of Study Participants
| n = 1770 | |
|---|---|
| Age, years | 53 (46–61) |
| Female, % | 35 |
| Black, % | 6 |
| Metabolic Syndrome, % | 49 |
| Type 2 Diabetes, % | 44 |
| Blood Pressure, mmHg | |
| Systolic | 128 (118–139) |
| Diastolic | 76 (70–83) |
| Current tobacco abuse, % | 11.2 |
| Medications, % | |
| Aspirin | 26 |
| Statins | 35 |
| Fibrates | 5 |
| Niacin Derivatives | 3 |
| Metformin | 26 |
| Sulfonylureas | 15 |
| Thiazolidinediones | 11 |
| Insulin | 7 |
| Total Cholesterol, mg/dl | 190 (162–218) |
| Triglycerides, mg/dl | 120 (88–176) |
| HDL-C, mg/dl | 47 (39–57) |
| LDL-C, mg/dl | 111 (88–135) |
| VLDL-C, mg/dl | 27 (18–39) |
| Apolipoprotein A-I (mg/dl) | 127 (113–141) |
| Apolipoprotein A-II (mg/dl) * | 33 (30–36) |
| Apolipoprotein B (mg/dl) | 90 (76–105) |
| Apolipoprotein C-III (mg/dl) † | 14.3 (11.3–18.3) |
| Body mass index, kg/m2 | 30.1 (26.5–34.3) |
| Waist circumference, inches | 40 (36–44) |
| Fasting blood glucose, mg/dl | 95 (79–118) |
| Hemoglobin A1C, % * | 6.2 (5.6–7.0) |
| Adiponectin (µg/ml) * | 12.3 (7.7–19.2) |
| Leptin (ng/ml) * | 10.3 (5.8–19.9) |
| Insulin (pmol/l) * | 10.4 (6.2–16.7) |
Data are expressed as median and interquartile range (IQR) or as percentages, unless otherwise noted. LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol.
Measured for subset of population (n=1154).
Measured for subset of population (n=711).
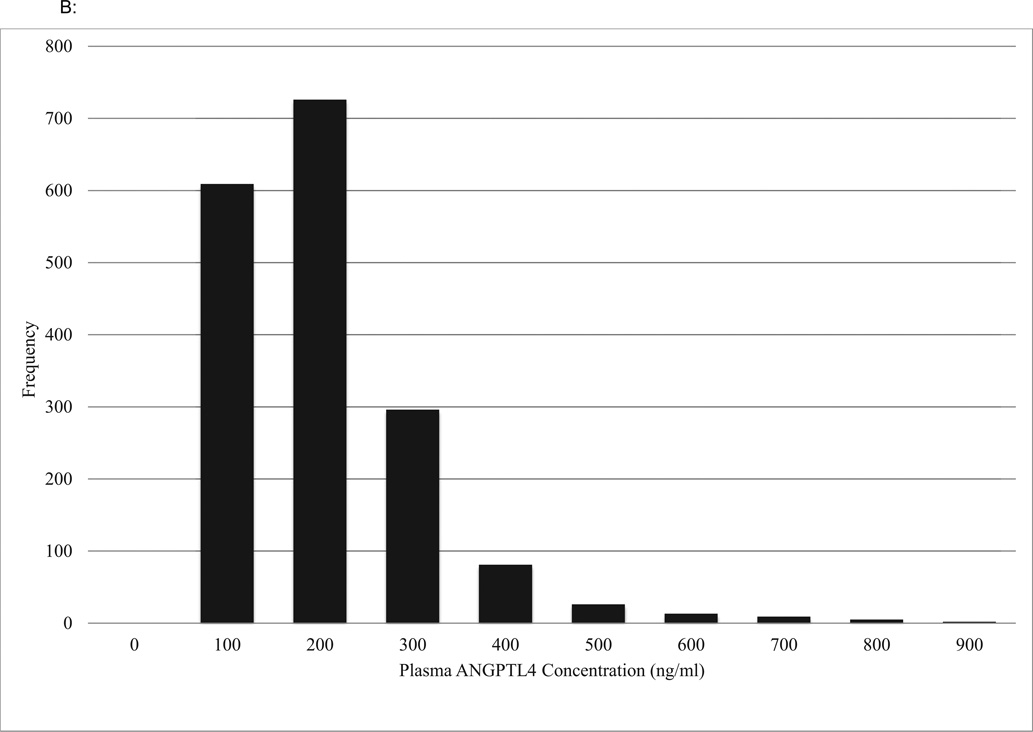
The distribution of plasma ANGPTL3 levels is shown in Figure 1A (mean 236 ± 87 ng/ml and median 224 ng/ml) and of plasma ANGPTL4 levels in Figure 1B (median 128 [IQR: 85 – 198] ng/ml).
Figure 1.
A: Distribution of ANGPTL3 Plasma Concentrations
ANGPTL3, Angiopoietin-like protein 3
B: Distribution of ANGPTL4 Plasma Concentrations
ANGPTL4, Angiopoietin-like protein 4
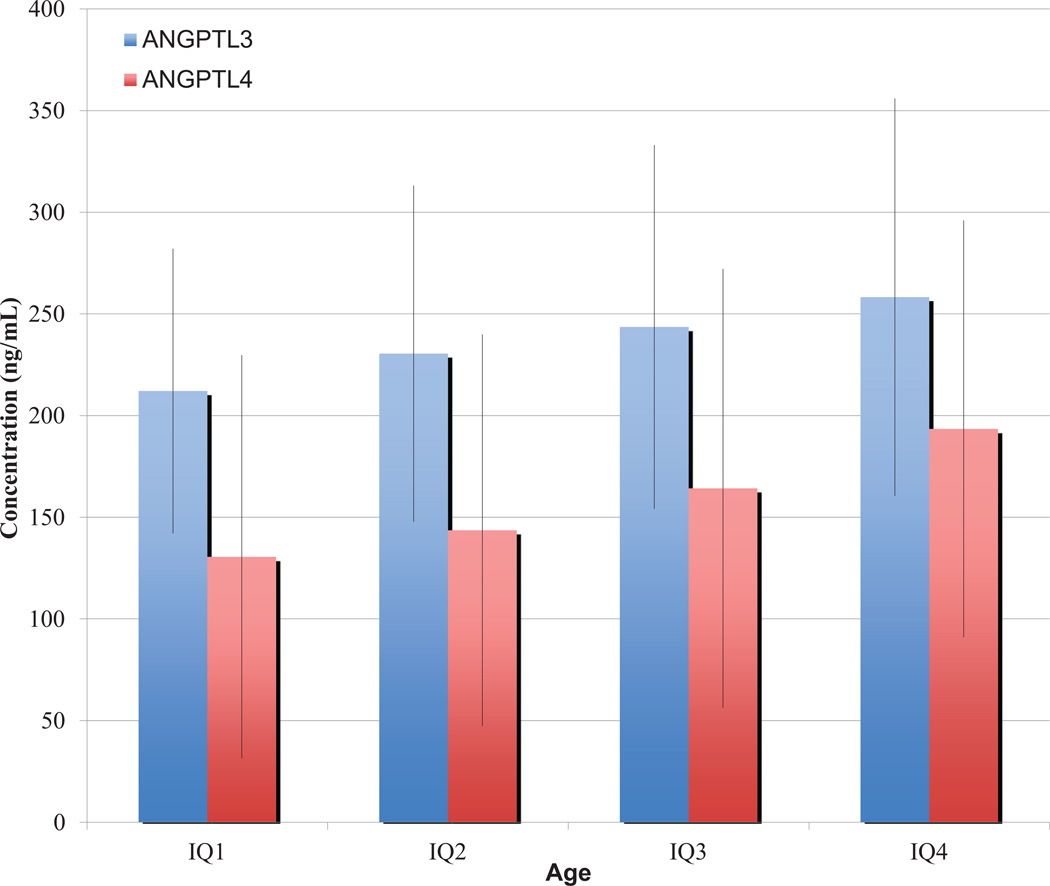
Higher levels of ANGPTL3 and ANGPTL4 levels were observed with increasing age quartiles as shown in Figure 2 (r = 0.17 and r = 0.31, respectively; P < 1 × 10−13 for both) based on Pearson correlation.
Figure 2.
Association of ANGPTL3 and ANGPTL4 with Age
Mean concentrations are plotted with 1 standard deviation error bars for each interquartile range (IQ). ANGPTL3, Angiopoietin-like protein 3; ANGPTL4, Angiopoietin-like protein 4.
ANGPTL3 levels were significantly lower in males (mean 227 ng/ml ± 82 ng/ml) than females (mean 253 ng/ml ± 94 ng/ml) (P = 2.38 × 10−9) in a logistic regression analysis. There was no significant difference in ANGPTL4 levels between males (mean 160 ng/ml ± 108 ng/ml and median 130 ng/ml) and females (153 ng/ml ± 96 ng/ml and median 126 ng/ml). Although the African American population was small (6%), there were significant racial differences in ANGPTL3 and ANGPTL4, with African American subjects having lower plasma levels for both proteins (P < 0.01 for both) in a logistic regression analysis.
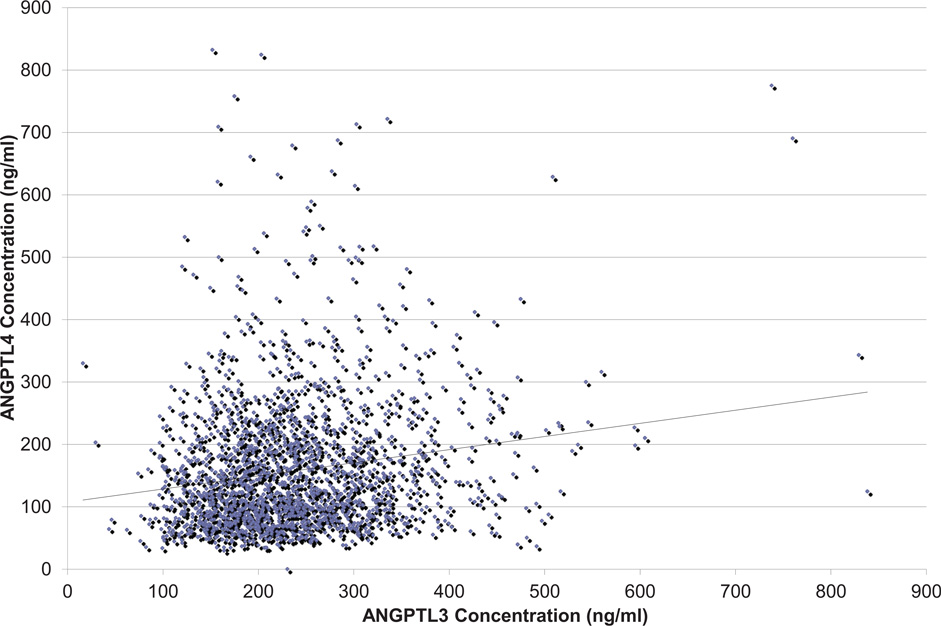
Plasma levels of ANGPTL3 and ANGPTL4 were weakly but significantly correlated with each other. Figure 3 shows a scatter plot of the measured ANGPTL3 and ANGPTL4 concentrations. Based on Pearson’s correlation, ANGPTL3 and ANGPTL4 were correlated (r = 0.18; P < 0.0001). When adjusted for age, gender, and race, the same findings held true (P < 0.0001).
Figure 3.
Association of ANGPTL3 with ANGPTL4 Plasma Levels
ANGPTL3, Angiopoietin-like protein 3; ANGPTL4, Angiopoietin-like protein 4.
ANGPTL3 and ANGPTL4 are associated with LDL-C and HDL-C in opposing directions
Plasma ANGPTL3 levels were significantly positively associated with LDL-C (P <0.002), HDL-C (P < 10−8), and total cholesterol (P < 10−7), but were not associated with triglycerides or VLDL-C (Table 2). The age, sex, and race-adjusted linear regression analysis supported these findings (Supplemental material, Table I). In a second model, a linear regression analysis adjusting for demographics and statin use showed no significant change in the associations observed (Supplemental material, Table II). Lastly, the same associations were observed between ANGPTL3 and lipid phenotypes among diabetics and non-diabetics. Apolipoprotein A-I was positively associated with plasma ANGPTL3 levels based on Pearson correlation and linear regression analysis. Apolipoproteins A-II, B, and C-III were not associated with ANGPTL3 levels in a demographic-adjusted linear regression model (Supplemental material, Table I).
Table 2.
ANGPTL3 and ANGPTL4 Association with Lipid Parameters
| ANGPTL3 | ANGPTL4 | |||
|---|---|---|---|---|
| r | P | r | P | |
| Triglycerides, mg/dl | 0.024 | 0.27 | 0.081 | 0.0008 |
| VLDL-C, mg/dl | −0.002 | 0.95 | 0.07 | 0.02 |
| LDL-C, mg/dl | 0.072 | 0.002 | −0.097 | 5.82 × 10−5 |
| HDL-C, mg/dl | 0.138 | 4.61 × 10−9 | −0.107 | 7.84 × 10−6 |
| Total cholesterol, mg/dl | 0.129 | 4.50 × 10−8 | −0.084 | 0.0005 |
| Apolipoprotein A-I, mg/dl | 0.099 | 4.22 × 10−5 | −0.067 | 0.01 |
| Apolipoprotein A-II, mg/dl | −0.013 | 0.65 | −0.097 | 0.003 |
| Apolipoprotein B, mg/dl | −0.004 | 0.86 | −0.134 | 2.11 × 10−7 |
| Apolipoprotein C-III, mg/dl | 0.034 | 0.47 | 0.114 | 0.02 |
ANGPTL3, Angiopoietin-like protein 3; ANGPTL4, Angiopoietin-like protein 4; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol
In contrast, plasma ANGPTL4 levels were significantly negatively associated with LDL-C, HDL-C, and total cholesterol (all P < 10−4), and positively associated with triglycerides and VLDL-C (P < 0.001) (Table 2). The age, sex, and race-adjusted linear regression analysis supported these findings (Supplemental material, Table I). A second model adjusting for age, gender, race, and statin use showed no significant change from the association observed for the demographic-adjusted models (Supplemental material, Table II).
An interaction analysis was performed between ANGPTL4 and diabetes status to determine if diabetes was affecting the association of ANGPTL4 with lipid parameters. For HDL-C, there was a significant interaction with ANGPTL4 and diabetes status (P =0.02). Interaction analysis of ANGPTL4 and diabetes was not significant when performing analysis for TG and LDL-C. Therefore, a stratified analysis was performed which showed that among non-diabetics, there was no significant association between ANGPTL4 and HDL-C, LDL-C, total cholesterol, and TG. Among diabetics, there was a significant negative association between ANGPTL4 and HDL-C (P < 10−3) and a positive association with triglycerides (P = 0.02).
In addition, a sub-stratified analysis was performed with metabolic syndrome in diabetics and non-diabetics. First, there was no association observed between lipid parameters and ANGPTL4 in non-diabetics with or without metabolic syndrome. All the diabetic patients had metabolic syndrome; therefore a sub-stratified analysis could not be performed.
In support of the lipid findings, apolipoproteins A-I and B were also negatively associated with ANGPTL4 levels (P < 0.01), whereas apolipoprotein C-III, a marker of triglyceride-rich lipoproteins, was positively associated with ANGPTL4 levels (P < 0.02) (Table 2). In a demographic-adjusted linear regression analysis, apolipoproteins A-I, B, and C-III showed similar findings as the Pearson’s correlation analysis (Supplemental material, Table I).
ANGPTL4 has a much stronger relationship with metabolic parameters than ANGPTL3
In an unadjusted model, ANGPTL4 had a more significant positive association with BMI and waist circumference (P < 2 ×10−16 for both) compared to ANGPTL3 (P < 10−4; Table 3). In addition, ANGPTL4, but not ANGPTL3, was significantly and positively associated with fasting plasma glucose (P < 2 × 10−16). ANGPTL4 levels in diabetics were two times higher than non-diabetics (181 [IQR: 136–237] ng/ml vs. 93 [IQR: 69–134] ng/ml; P < 2.0 × 10−16). ANGPTL4 was also significantly and positively associated with insulin, free fatty acids and leptin whereas plasma adiponectin levels were significantly and negatively associated with ANGPTL4 levels. In an age, sex, and race-adjusted model, linear regression analysis supported the above findings (Supplemental material, Table III).
Table 3.
ANGPTL3 and ANGPTL4 Association with Metabolic Parameters
| ANGPTL3 | ANGPTL4 | |||
|---|---|---|---|---|
| r | P | r | P | |
| Body mass index, kg/m2 | 0.12 | 1.85 × 10−7 | 0.218 | 2.20 × 10−16 |
| Waist Circumference, inches | 0.098 | 4.03 × 10−5 | 0.272 | 2.20 × 10−16 |
| Blood Pressure, mmHg | ||||
| Systolic | 0.009 | 0.70 | 0.121 | 1.63 × 10−11 |
| Diastolic | −0.08 | 0.001 | −0.08 | 0.002 |
| Fasting Blood Glucose, mg/dl | 0.014 | 0.56 | 0.316 | 2.20 × 10−16 |
| Hemoglobin A1C, % | 0.096 | 0.0008 | 0.329 | 2.20 × 10−16 |
| Insulin, IU/ml | 0.099 | 0.0008 | 0.348 | 2.20 × 10−16 |
| Free Fatty Acids, mEq/ml | 0.099 | 0.001 | 0.287 | 2.20 × 10−16 |
| Leptin, ng/ml | 0.166 | 1.72 × 10−7 | 0.137 | 2.34 × 10−5 |
| Adiponectin, mg/ml | 0.121 | 0.0001 | −0.177 | 2.98 × 10−8 |
ANGPTL3, Angiopoietin-like protein 3; ANGPTL4, Angiopoietin-like protein 4; IU, international units
In contrast, while modest positive associations were observed with ANGPTL3 and waist circumference, BMI, hemoglobin A1C, insulin, free fatty acids, and leptin on unadjusted analyses, after adjustment for demographic parameters, ANGPTL3 was only modestly significantly associated with BMI, waist circumference, adiponectin and leptin (Supplemental material, Table III).
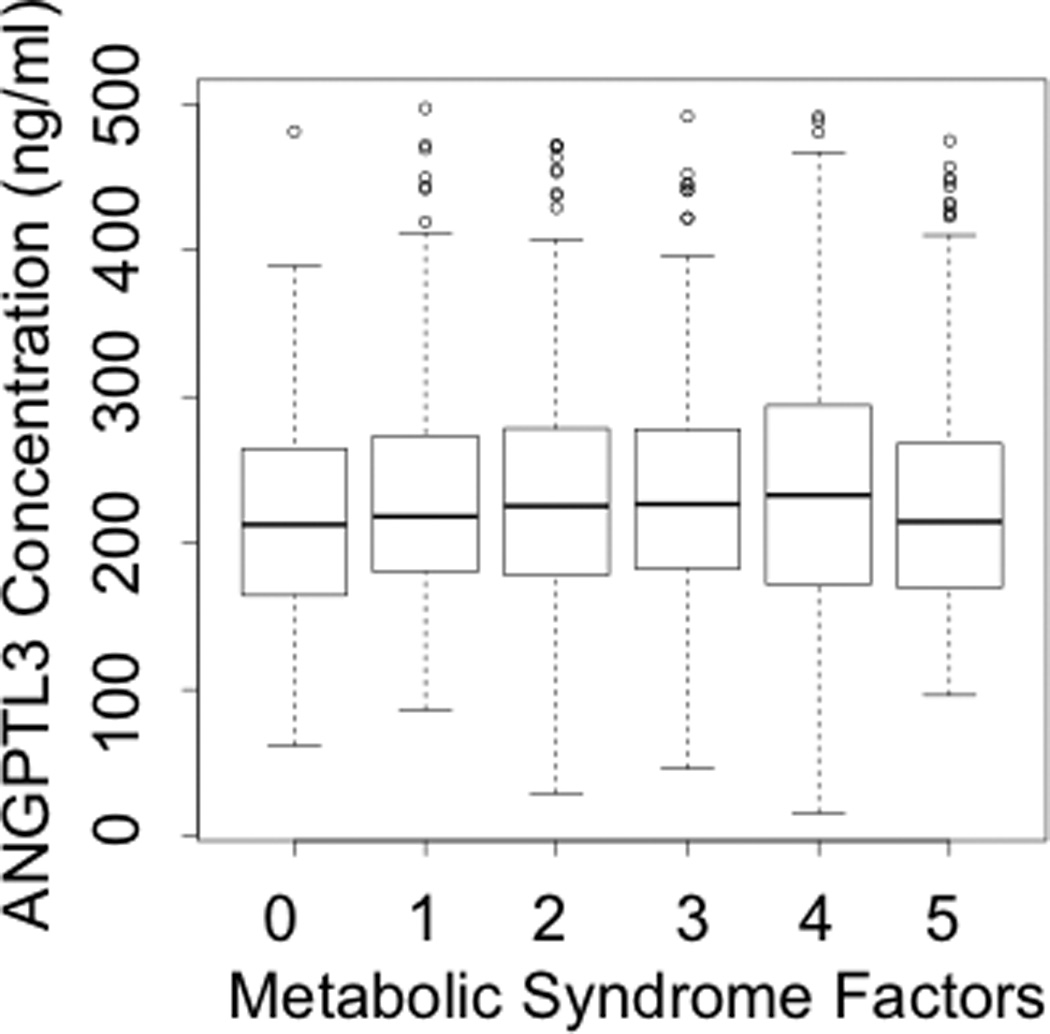
Given the significant associations observed with all factors of metabolic syndrome: waist circumference, systolic blood pressure, blood glucose, TG, and HDL-C, there was a clear relationship between plasma ANGPTL4 levels and the number of metabolic syndrome parameters, as observed in Figure 4B (P < 2 × 10−16). In contrast, no association was observed between plasma ANGPTL3 levels and metabolic syndrome (Figure 4A).
Figure 4.
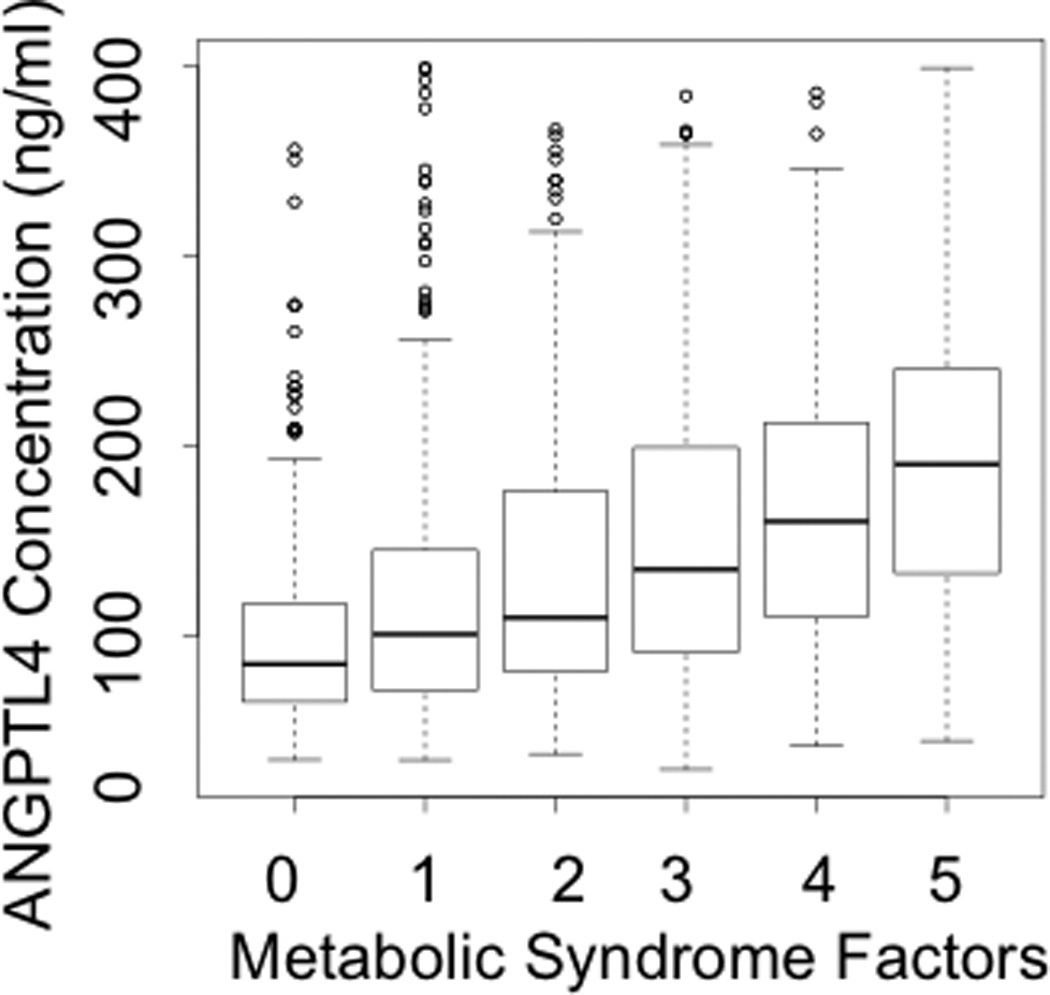
A: Association of ANGPTL3 with Metabolic Syndrome
ANGPTL3, Angiopoietin-like protein 3. Box plots show median, interquartile range, maximum value, minimum value and upper range outliers (open circles).
B: Association of ANGPTL4 with Metabolic Syndrome
ANGPTL4, Angiopoietin-like protein 4. Box plots show median, interquartile range, maximum value, minimum value and upper range outliers (open circles).
Discussion
In the largest study of plasma concentrations of ANGPTL3 and ANGPTL4 to date, we identified highly significant correlations of each protein with lipid and metabolic traits. Remarkably, despite their known similar biochemical properties (specifically inhibition of LPL), plasma concentrations of these two proteins have relatively weak associations with plasma TG levels and opposite relationships with LDL-C and HDL-C levels. ANGPTL3 concentrations are significantly positively correlated with LDL-C and HDL-C levels; conversely ANGPTL4 concentrations are significantly negatively correlated with LDL-C and HDL-C levels. While ANGPTL4 has an expected (albeit modest) positive correlation with TG levels, ANGPTL3 has no correlation with TGs. Strikingly, ANGPTL4 is strongly associated with multiple features of the metabolic syndrome, while ANGPTL3 has much less association. Previous studies had small sample sizes or less accurate assays, which resulted in inconsistent associations between these proteins and phenotypic parameters. 3, 5, 13–18 Thus, these results provide new information about these two related proteins that are clearly important in lipoprotein biology in humans.
In humans, ANGPTL3 expression occurs predominantly in the liver. ANGPTL3 expression is markedly induced by activation of Liver X receptor (LXR), which binds to its response element in the ANGPTL3 promoter. ANGPTL3 is proteolytically cleaved by proprotein convertases to yield the biologically active N-terminal fragment and an inactive C-terminal fragment. The N-terminal fragments forms multi-subunit complexes to protect against degradation. N-terminal ANGPTL3 modestly suppresses LPL catalytic activity in vitro.1 Studies of gain of function of ANGPTL3 in mouse models have shown elevation of fasting TG levels due to suppression of VLDL clearance via inhibition of LPL, which is more pronounced in the fed state.19 Loss of function in mice results in reduction of both TG levels and HDL-C levels.5
Common variants at the ANGPTL3 gene locus are significantly associated with LDL-C and TGs. Sequencing studies of the coding region of ANGPTL3 revealed that the variant M259T among African Americans (mean allele frequency = 5%) was significantly associated with lower TG levels. 10 The M259T variant was not associated with other metabolic parameters such as glucose and BMI. Most compellingly, exome sequencing of a large family with pan-hypolipidemia revealed two rare ANGPTL3 nonsense mutations that co-segregated with reduced plasma lipid levels.12 While increased LPL activity due to lack of ANGPTL3 might explain the reduced TG levels in this family, it does not explain the reduced LDL-C or HDL-C levels. ANGPTL3 also inhibits endothelial lipase (EL), which hydrolyzes HDL-C phospholipids, and the effect on HDL-C levels could be related to ANGPTL3 inhibition of EL activity.5 However, the effect on LDL-C levels remains unexplained.
Studies of plasma ANGPTL3 are sparse and provide conflicting results. One study with 250 Finnish subjects showed that ANGPTL3 was positively associated with HDL-C and negatively associated with TG.14 In a second population of predominantly end-stage kidney disease and healthy subjects (n = 394), ANGPTL3 was positively associated with HDL-C and LDL-C on univariate analysis.15 Our finding in a significantly larger community-based sample that plasma levels of ANGPTL3 are strongly and positively associated with LDL-C and HDL-C levels is novel and largely consistent with the human genetic data. However, we did not observe the expected positive association with triglycerides. This suggests that there are other factors involved in modulating the relationship between ANGPTL3 levels and triglyceride metabolism.
In humans, ANGPTL4 expression occurs in the liver, adipose tissue, small intestine and heart. ANGPTL4 expression is stimulated by peroxisome-proliferator activated receptor α (PPARα), which binds to response elements in the ANGPTL4 promoter. Oligomerization occurs with intermolecular disulfide bonds followed by secretion of the protein. ANGPTL4 is proteolytically cleaved via proprotein convertases into an active N-terminal fragment and inactive C-terminal fragment containing the fibrinogen-like domain. N-terminal ANGPTL4 interacts with the extra-cellular matrix through heparin-sulfate proteoglycans and has been shown to irreversibly inhibit LPL.1 Studies of gain and loss of function of ANGPTL4 in mouse models have shown both elevation and reduction of TG, respectively. 21
Common variants at the ANGPTL4 gene locus are significantly associated with HDL-C. Population-based resequencing of the coding regions in 3551 subjects from the Dallas Heart Study found one non-synonymous sequence variant, E40K, in European Americans (MAF 1.3%) associated with lower plasma TG and LDL-C levels and higher HDL-C levels (P = 0.004).11 These findings were reproduced in two larger patient populations.11 This variant was not genotyped in our study population. ANGPTL4 also inhibits hepatic lipase, which is involved in HDL-C and LDL-C metabolism.20 Unlike ANGPTL3, no patients with genetic deficiency of ANGPTL4 have been reported.
Thus far, the largest scale study of plasma ANGPTL4 levels was in 666 healthy men, and showed that plasma ANGPTL4 levels were inversely associated with HDL-C, but not associated with LDL-C or TG.17 Smaller scale studies in a Finnish population showed no correlation of plasma ANGPTL4 levels with TG, LDL-C, or HDL-C. Metabolic and inflammatory parameters such as BMI, free fatty acids, and CRP were positively associated with the protein. 14, 17 Our current study showed that plasma ANGPTL4 concentrations are significantly inversely associated with LDL-C and HDL-C and positively associated with TG. While the directional associations with TG and HDL-C are consistent with predictions from the human genetics, the inverse association with LDL-C is not. This suggests that there are other factors involved in modulating the relationship between ANGPTL4 levels and LDL metabolism
The biological explanation for the difference between the associations of ANGPTL3 and ANGPTL4 levels with lipid traits is not obvious. Their mechanisms of inhibition of LPL are different. 1 ANGPTL3 inhibits endothelial lipase and ANGPTL4 inhibits hepatic lipase, and while it is not clear that these are distinct qualitative differences between the two proteins, this could provide some explanation. Of note, there is remarkable variation in their amino acid sequence, and only 30% similarity between the two proteins exists. 21 They differ in tissue expression, with ANGPTL4 being more broadly expressed including in intestine and adipose whereas ANGPTL3 is more specific to liver. Finally, regulation of expression differs, with ANGPTL3 more responsive to LXR and ANGPTL4 to PPARα.
Furthermore, we found that ANGPTL4 levels were highly significantly associated with all aspects of the metabolic syndrome (increased BMI and waist circumference, increased systolic blood pressure, elevated TG, low HDL-C, elevated glucose and hemoglobin A1C). There is limited data on the effects of ANGPTL4 and glucose metabolism. ANGPTL4 is directly stimulated by free fatty acids via activation of PPARα. ANGPTL4 inhibits extracellular LPL-mediated lipolysis to prevent excess free fatty acid uptake into the cell. ANGPTL4 knockout mice studies show that ANGPTL4 deficiency has a significant inhibitory effect on macrophage foam cell production.7 However, in transgenic mice, ANGPTL4 overexpression decreased macrophage content by 41% (P < 0.05). Human recombinant ANGPTL4 significantly decreased macrophage uptake of oxidized LDL-C. 22
By an unknown mechanism, ANGPTL4 stimulates intracellular lipolysis in adipocytes and myocytes, particularly in the fasting state. 21 At hyperinsulinemic-euglycemic clamp in 24-hour fasting mice, transgenic ANGPTL4 mice showed reduced glucose utilization compared to controls (125% versus 59%; P < 0.05). This data suggests that there is peripheral insulin resistance seen with ANGPTL4 overexpression. 20 Further mechanistic studies are needed to better elucidate the metabolic functions of ANGPTL4.
Some of the limitations of this study were the predominantly Caucasian population; therefore, the observed associations may not be generalizable across races. The study populations were enriched for diabetes and metabolic syndrome. In addition, the assays that were used for measuring plasma ANGPTL3 and ANGPTL4 concentrations may have included truncated portions of the protein, which are not involved in lipid metabolism. Since this is as a cross-sectional analysis, causal relationships of ANGPTL3 or ANGPTL4 cannot be inferred with lipid and metabolic parameters; only associations can be drawn.
The goal of this study was to determine relationships between ANGPTL3 and ANGPTL4 with various lipid and metabolic parameters. These circulating proteins clearly influence human physiology and may be potential targets for lipid lowering therapies.
Supplementary Material
Significance.
Angiopoietin-like protein 3 (ANGPTL3) and 4 (ANGPTL4) are secreted proteins that inhibit lipoprotein lipase in vitro. Genetic variants at the ANGPTL3 and ANGPTL4 gene loci are significantly associated with plasma lipid traits. However, few data exist regarding the relationship of plasma levels of ANGPTL3 and ANGPTL4 with lipid and metabolic traits. The current study is the largest-scale study that highlights the associations between these two biomarkers and lipid and energy metabolism. Despite have similar biochemical effects in vitro, plasma ANGPTL3 and ANGPTL4 concentrations have nearly opposite relationships with plasma lipids. ANGPTL4, but not ANGPTL3, is strongly negatively associated with LDL-C and positively with multiple features of the metabolic syndrome, whereas ANGPTL3 is positively associated with LDL-C and HDL-C. These novel biomarkers may be potential targets for lipid lowering therapies.
Acknowledgements
Sources of Funding:
This work was supported by grants R37 HL055323 and RC2 HL101834 from the National Heart, Lung, and Blood Institute.
Abbreviations
- ANGPTL3
Angiopoietin-Like Protein 3
- ANGPTL4
Angiopoietin-Like Protein 4
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- TG
Triglycerides
- CAD
Coronary artery disease
- VLDL-C
Very low-density lipoprotein cholesterol
- LPL
Lipoprotein lipase
- EL
Endothelial lipase
- LXR
Liver X receptor
- PPARα
Peroxisome-proliferator activated receptorα
Footnotes
Disclosures: none
References
- 1.Mattijssen F, Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochimica et biophysica acta. 2012;1821:782–789. doi: 10.1016/j.bbalip.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, Horikoshi H, Furukawa H. Angptl3 regulates lipid metabolism in mice. Nature genetics. 2002;30:151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 3.Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Muller M, Kersten S. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. The Journal of biological chemistry. 2006;281:934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 4.Koster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin-like (angptl) 4 overexpression and targeted disruption of angptl4 and angptl3: Regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 5.Shimamura M, Matsuda M, Yasumo H, et al. Angiopoietin-like protein3 regulates plasma hdl cholesterol through suppression of endothelial lipase. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 6.Yin W, Romeo S, Chang S, Grishin NV, Hobbs HH, Cohen JC. Genetic variation in angptl4 provides insights into protein processing and function. The Journal of biological chemistry. 2009;284:13213–13222. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi H, Fujiwara Y, Kondo T, et al. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochemical and biophysical research communications. 2009;379:806–811. doi: 10.1016/j.bbrc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Willer CJ, Sanna S, Jackson AU, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nature genetics. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nature genetics. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, Cohen JC. Rare loss-of-function mutations in angptl family members contribute to plasma triglyceride levels in humans. The Journal of clinical investigation. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. Population-based resequencing of angptl4 uncovers variations that reduce triglycerides and increase hdl. Nature genetics. 2007;39:513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, angptl3 mutations, and familial combined hypolipidemia. The New England journal of medicine. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HF, Hesselink MK, Schrauwen P, Muller M. Caloric restriction and exercise increase plasma angptl4 levels in humans via elevated free fatty acids. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 14.Robciuc MR, Tahvanainen E, Jauhiainen M, Ehnholm C. Quantitation of serum angiopoietin-like proteins 3 and 4 in a finnish population sample. Journal of lipid research. 2010;51:824–831. doi: 10.1194/jlr.M002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoji T, Hatsuda S, Tsuchikura S, Kimoto E, Kakiya R, Tahara H, Koyama H, Emoto M, Tabata T, Nishizawa Y. Plasma angiopoietin-like protein 3 concentration is associated with uremic dyslipidemia. Atherosclerosis. 2009;207:579–584. doi: 10.1016/j.atherosclerosis.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Hatsuda S, Shoji T, Shinohara K, Kimoto E, Mori K, Fukumoto S, Koyama H, Emoto M, Nishizawa Y. Association between plasma angiopoietin-like protein 3 and arterial wall thickness in healthy subjects. Journal of vascular research. 2007;44:61–66. doi: 10.1159/000098153. [DOI] [PubMed] [Google Scholar]
- 17.Smart-Halajko MC, Robciuc MR, Cooper JA, et al. The relationship between plasma angiopoietin-like protein 4 levels, angiopoietin-like protein 4 genotype, and coronary heart disease risk. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2277–2282. doi: 10.1161/ATVBAHA.110.212209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stejskal D, Karpisek M, Humenanska V, Solichova P, Stejskal P. Angiopoietin-like protein 3: Development, analytical characterization, and clinical testing of a new elisa. General physiology and biophysics. 2007;26:230–233. [PubMed] [Google Scholar]
- 19.Kersten S. Regulation of lipid metabolism via angiopoietin-like proteins. Biochemical Society transactions. 2005;33:1059–1062. doi: 10.1042/BST20051059. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein L, Berbee JF, van Dijk SJ, van Dijk KW, Bensadoun A, Kema IP, Voshol PJ, Muller M, Rensen PC, Kersten S. Angptl4 upregulates cholesterol synthesis in liver via inhibition of lpl- and hl-dependent hepatic cholesterol uptake. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2420–2427. doi: 10.1161/ATVBAHA.107.151894. [DOI] [PubMed] [Google Scholar]
- 21.Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin-like 4: A decade of research. Bioscience reports. 2012;32:211–219. doi: 10.1042/BSR20110102. [DOI] [PubMed] [Google Scholar]
- 22.Georgiadi A, Wang Y, Stienstra R, Tjeerdema N, Janssen A, Stalenhoef A, van der Vliet JA, de Roos A, Tamsma JT, Smit JWA, Tan NS, Muller M, Rensen PCN, Kersten S. Overexpression of angiopoietin-like protein 4 protects against atherosclerosis development. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1529–1537. doi: 10.1161/ATVBAHA.113.301698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.