Abstract
The human eye is subjected constantly to oxidative stress due to daily exposure to sunlight, high metabolic activities, and oxygen tension. Reactive oxygen species generated from environmental insults and pathological conditions render the human eye particularly vulnerable to oxidative damage. The ocular surface composed of the tear film, the cornea, and the aqueous humor forms the first physical and biochemical barrier of the eye and plays a pivotal role in combating free radicals. These ocular compartments are enriched in certain antioxidants in the form of metabolic enzymes or small molecules. Such an antioxidant defense system in the ocular surface is essential for the maintenance of redox homeostasis in the eye and protection against oxidative damage. Herein, we review the properties and functions of key constituent antioxidants of the ocular surface.
Keywords: age-related eye disease, antioxidants, free radicals, ocular defense system, reactive oxygen species, vitamin A, vitamin E
I. INTRODUCTION
Daily exposure to sunlight and atmospheric oxygen challenges the eye with an intense burden of oxygen free radicals, making it highly vulnerable to oxidative damage. Generation of reactive oxygen species (ROS) and resultant oxidative stress have been implicated in the pathogenesis of numerous forms of eye disease, including photokeratoconjunctivities,1 photokeratitis,2 pingueculae and pterygia,3 cataract,4–6 glaucoma, and macular degeneration.7,8 The cornea covers the outermost layer of the eye, serving two fundamental functions: 1) It is the initial barrier of the eye that protects the inner ocular tissues (such as lens and retina) against external insults; and 2) together with the lens, it constitutes the “refraction unit” of the eye to permit light to enter and focus on the retina.9
The cornea consists of three cellular layers, including a stratified squamous epithelium; a thick stroma containing collagen fibers, proteoglycans, glycosaminoglycans and keratocytes; and a posterior single layer of endothelium. Being a unique avascular tissue, the cornea depends on the tear film and the aqueous humor to provide its anterior and posterior surfaces, respectively, with nutrients and protective molecules. The ocular surface, including the tear film, the cornea, and the aqueous humor, is the first line to encounter environmental insults and play a pivotal role in protecting the inner ocular tissues against oxidative damage. In doing so, the ocular surface has developed a range of defense mechanisms in the form of enzymatic and non-enzymatic antioxidant molecules. Some of these molecules act to scavenge or neutralize ROS, while others function to repair the damage caused by these free radicals.
II. FORMATION OF REACTIVE OXYGEN SPECIES IN THE EYE
ROS derive from diatomic oxygen (O2 [Figure 1]) and are produced as byproducts during cellular metabolism. As such, the generation of ROS normally correlates with cellular metabolic rate.10 The addition of one electron to dioxygen forms the superoxide anion radical (O2−•), which occurs mostly during the process of mitochondrial respiration.11 The dismutation of this molecule by the enzymes superoxide dismutases forms hydrogen peroxide (H2O2).12 H2O2 is mostly scavenged efficiently by the enzymes glutathione peroxidase in the mitochondria13 or by the enzyme catalase in peroxisomes.14 Although hydrogen peroxide is less reactive than superoxide, the breakdown of this molecule by various transition metals (Fe2+, Cu+, and others) via Fenton reaction can generate the highly reactive hydroxyl radical (OH•).15 The reaction of superoxide or hydroxyl radical with polyunsaturated fatty acids generates the peroxyl radical (LOO•).16 In addition to the mitochondrial origin, ROS in the form of superoxide anion and hydrogen peroxide can be generated through the activities of cytoplasmic oxidases, such as xanthine oxidase, histamine oxidase, and monoamine oxidase.17
Figure 1.
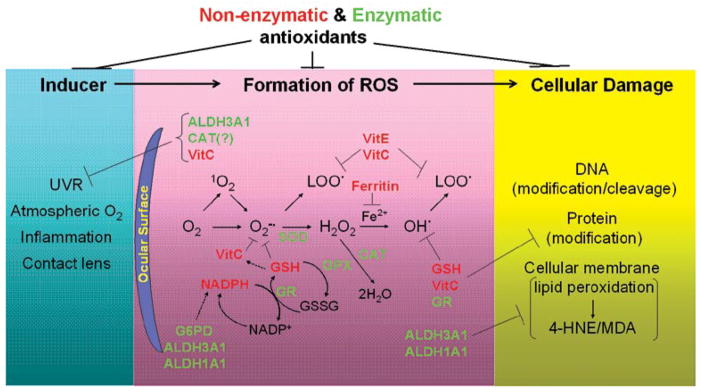
Formation of reactive oxygen species and antioxidant defenses in the eye. Environmental exposures (such as solar ultraviolet radiation and high atmospheric oxygen) and certain pathological conditions (such as inflammation and prolonged contact lens wearing) induce the generation of reactive oxygen species (ROS) in ocular tissues. ROS are derived from diatomic oxygen (O2), including superoxide anion radical (O2−•), hydrogen peroxide (H2O2), hydroxyl (OH•), and peroxyl radicals (LOO•). ROS have high potential to react with DNA, proteins and cellular membranes, resulting in modifications of these macromolecules and consequent cellular damage. The antioxidant defense systems in the ocular surface function to combat ROS and protect ocular tissues from oxidative damage. Superoxide dismutases (SODs), catalase (CAT), glutathione peroxidases (GPXs), glutathione reductase (GR) and aldehyde dehydrogenases (ALDH3A1 and ALDH1A1) represent enzymatic antioxidants. Glutathione (GSH), ascorbic acid (VitC), α-tocopherol (VitE), NADPH and ferritin represent nonenzymatic anti-oxidants. The properties and functions of each antioxidant are discussed in detail in the text.
Harboring unpaired electron(s), ROS provide a great degree of reactivity to induce reduction-oxidation (redox) reactions, thereby having the potential to attack important biomolecules. Under physiological conditions, cellular redox homeostasis is maintained by a delicate balance between ROS generation and antioxidant systems (Figure 1). Oxidative stress occurs in biological systems when such balance is disrupted so that ROS is overproduced. Excessive ROS can cause damage to DNA, cellular lipids, and proteins, thereby inhibiting their biological functions.18 As the most reactive molecule, hydroxyl radical has the potential to react with each component of the DNA molecule, damaging the purine, pyrimidine bases and the deoxyribose backbone19; such permanent modification of DNA initiates the process of mutagenesis and carcinogenesis. The amino acid residues of proteins, especially cysteine and methionine residues, are prone to oxidation by ROS; oxidations of structurally or functionally important sites result in protein inactivation or misfolding.20 The cellular membrane component is another target of ROS; lipid peroxidation occurs after the formation of peroxyl radicals and generates cytotoxic products, such as 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA).16
Given its intense exposure to light, robust metabolic activity, and high oxygen tension, the human eye is constantly subjected to oxidative stress. Solar ultraviolet radiation (UVR) is the major environmental inducer of ROS formation in the eye. UVR consists of UVA (315–400 nm), UVB (280–315 nm), and UVC (100–280 nm). All UVC and most UVB are absorbed by the cornea, whereas UVA is primarily absorbed by the lens (Figure 2). No UVC or UVB and very little UVA (<1%) reach the retina.21 Absorption of UVR by these ocular tissues, particularly associated with the shorter and higher energy wavelengths of UVC and UVB, lead to photochemically generated ROS, including singlet oxygen (1O2), superoxide anion, hydroxyl and peroxyl radicals.21
Figure 2.
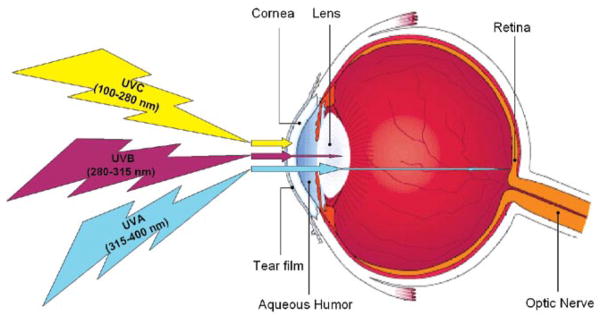
The absorption of solar ultraviolet radiation by the eye. Solar ultraviolet radiation (UVR) consists of UVA at 315–400 nm, UVB at 280–315 nm, and UVC at 100–280 nm. The cornea absorbs all UVC and most UVB, whereas UVA is primarily absorbed by the lens. No UVC or UVB and very little UVA (<1%) reach the retina.
All three spectrums of UVR have been shown to cause strand breakage, pyrimidine and thymine dimer formation, and protein cross-linking.22,23 These UVR-induced molecular modifications in the eye have been associated with eye pathologies, including cataract formation and corneal and retinal degeneration.24 In addition, oxidative stress is a common threat to ocular tissues in certain pathological conditions of the eye, such as inflammation and diabetes, where a substantial amount of ROS can be produced.25,26 Xanthine oxidase-mediated ROS formation in the tear film has been suggested to contribute to the hypoxia-reoxygenation injury of the cornea associated with prolonged wearing of hydrophilic contact lenses.27 Facing such challenge, the ocular surface, with its robust antioxidant defense systems, functions crucially in maintaining the redox homeostasis of the eye.
III. ANTIOXIDANTS IN THE CORNEA
A. Enzymatic Antioxidants
To date, the enzymatic antioxidants that have been documented to be present in the cornea include superoxide dismutases (SODs), catalase (CAT), glutathione peroxidases (GPXs) and reductase (GR), and glucose-6-phospate dehydrogenase (G6PD [Table 1]). It is reported that, in normal corneal epithelium, the total level of SOD activity is much higher than CAT activity, which is higher than that of GPX.28 In addition, certain members of aldehyde dehydrogenase (ALDH) superfamily that are expressed abundantly in the cornea are recognized to act as antioxidants.
Table 1.
Antioxidants present in the ocular surface
| Compartment | Antioxidants | |
|---|---|---|
| Enzymatic | Nonenzymatic | |
| Tear film | Superoxide dismutases | Uric acid Ascorbic Acid Glutathione L-tyrosine L-cysteine |
| Cornea | Superoxide dismutases Glutathtione peroxidases Glutathione reductase Catalase Glucose-6-Phosphodehydrogenase Aldehyde dehydrogenase 3A1 Aldehyde dehydrogenase 1A1 |
Ascorbic Acid Glutathione NADPH α-tocopherol Retinol Ferritin Albumin |
| Aqueous humor | Superoxide dismutases | Ascorbic Acid L-tyrosine Uric Acid L-cysteine Glutathione |
The properties and functions of each antioxidant are discussed in detail in the text.
1. Superoxide Dismutases
SODs (EC 1.15.1.1) catalyze the reaction for the formation of less reactive hydrogen peroxide from superoxide radical (Figure 1).29 Three different SOD isozymes have been found to be constitutively expressed in the cornea of various species, including human: the extracellular SOD containing copper-zinc (EC-SOD, homotetrameric with a 26kDa subunit), the cytosolic SOD containing copper-zinc (CuZn-SOD, homodimeric with a 16kDa subunit), and the mitochondrial SOD containing manganese (Mn-SOD, homotetrameric with a 21–25kDa subunit).30,31 More importantly, these enzymes are biologically functional in the corneal epithelial and endothelial layers.32
The activity of SOD in the cornea seems to vary depending on species, ranging from high in rabbits and guinea pigs to low in pigs and almost absent in cattle.33 Behndig et al have reported a comparable level of activity (~85 U/mg protein) for EC-SOD and CuZn-SOD in human corneas, whereas Mn-SOD has the lowest level among the three (~5.7 U/mg protein).31 Three individual mouse lines harboring the deficiency in Sod1, Sod2 or Sod3, the gene encoding the cytosolic CuZn-SOD, mitochondrial Mn-SOD or extracellular EC-SOD, respectively, have been generated.34–37 Homozygous mutants of these mouse lines reveal distinct eye phenotypes (Table 2), indicating a differential role for the SOD isozymes in eye physiology. In particular, the protective role of extracellular SOD in the cornea is evidenced in EC-SOD knockout animals, which show increased spontaneous age-related loss of endothelial cells and increased susceptibility to oxidative stress and inflammatory damage.34
Table 2.
Antioxidant-deficient rodent models displaying eye phenotypes
| Gene | Antioxidant deficiency | Eye Phenotypes |
|---|---|---|
| Superoxide dismutase 1 (Sod1) | Cytosolic CuZn-SOD | Homozygous mutants are sensitive to diabetes-induced cataracts formation.37 |
| Superoxide dismutase 2 (Sod2) | Mitochondrial Mn-SOD | Homozygous mutants show retinal pathologies before they die by 2.5 weeks.35 |
| Superoxide dismutase 3 (Sod3) | Extracellular CuZn-SOD | Homozygous mutants show age-related loss of corneal endothelial cells and increased susceptibility to LPS-induced inflammatory endothelial damage.34 |
| Glutathione peroxidase 1 (Gpx1) | Cellular GPX | Homozygous mutants show progressive lens pathologies with age and develop mature cataracts after 15 months.42 |
| Aldehyde dehydrogenase 3A1 (Aldh3a1) | ALDH3A1 | Homozygous mutants develop cataracts and punctate opacities in lens cortex by 1 month.56 |
| Aldehyde dehydrogenase 1A1 (Aldh1a1) | ALDH1A1 | Homozygous mutants develop cataracts at 6–9 months of age.56 |
| Aldh3a1 and Aldh1a1 | ALDH3A1 and ALDH1A1 | Homozygous double mutants develop cataracts and punctate opacities in lens cortex by 1 month.56 |
| γ-glutamyl transpeptidase1 (Ggt1) | GSH | Homozygous mutants develop bilateral cataracts by 2–3 months of age.81 |
2. Glutathione Peroxidases and Glutathione Reductase
GPXs (EC 1.11.1.9) represent a family of enzymes that catalyze the reduction of H2O2 or organic hydroperoxides to water or corresponding alcohols using glutathione (GSH) as the electron donor.13,34 By catalyzing this reaction, GPXs bring about an end to the peroxide-dependent chain reaction of free radical generation and resultant membrane damage of ocular tissues.38 GR functions to recycle GSH from its oxidized form (GSSG), thereby maintaining the level of this important nonenzymatic antioxidant (see below) in the cornea. In addition, GR can reduce protein thiols to their native state. It has been reported that corneal GR activity is elevated significantly in inflamed corneas, suggesting a role of GR in combating inflammation-derived ROS.39 The activities of GPX and GR have been found in both corneal epithelium and endothelium.40,41 Deficiency of GPX1, the isoform identified in the cytosol, nucleus, and mitochondria, results in increased oxidative damage and associated pathology in lens and retina in a gene-knockout mouse model42; however, no corneal pathology has been reported in this animal model (Table 2).
3. Catalase
CAT (EC 1.11.1.6), a homotetramer with a 60kDa subunit, is an important scavenger of hydrogen peroxide present outside of mitochondria (Figure 1). It functions not only to protect ocular tissues against hydrogen peroxide, but also to protect superoxide dismutases from inactivation.43 This enzyme in its bioactive form has been detected in normal corneal epithelium and endothelium.39,44 In corneal cell cultures, overexpression or inhibition of CAT confers protection or susceptibility, respectively, to ROS-induced injury.45,46 Interestingly, Heck et al proposed a novel function of this enzyme in response to UVB exposure in skin keratinocytes.47 They hypothesized that CAT absorbs UVB light directly and, as a product of this absorption, it mediates the production of ROS that can be detoxified by other antioxidants. It is worth speculating that such a UVB-filtering property of CAT may contribute to its protective role in the cornea aside from the antioxidant function of this enzyme. Catalase null mice display increased sensitivity to ROS-mediated tissue injuries; however, no eye pathology has been reported in the mutant mice.48
4. Glucose-6-phosphate Dehydrogenase
G6PD (EC 1.1.1.49) contributes to the enzymatic defense mechanisms of the cornea against oxidation by maintaining the cellular reductive potential in the form of nicotinamide adenine dinucleotide phosphate (NADPH, see below) through the pentose phosphate pathway.49 This enzyme supplies NADPH as the electron donor for GR-mediated reduction of GSSG and consequently acts to balance the cellular pool of GSH. The important role of GR activity in corneal defense against UV-induced oxidative stress is implicated by enhanced GR activity in porcine corneas exposed to UVA or a small dose of UVC.50 Nevertheless, no eye pathologies have been reported in human subjects or rodent models with G6PD deficiencies.51,52
5. Aldehyde Dehydrogenases 3A1 and 1A1
ALDH3A1 (EC 1.2.1.5) and ALDH1A1 (EC 1.2.1.36) belong to a superfamily of NAD(P)+-dependent enzymes that catalyze the oxidation of a wide variety of endogenous and exogenous aldehydes to their corresponding acids.53 ALDH3A1 accumulates in high levels in the cornea of most mammals, including human, making up about 5–50% of the total water-soluble corneal proteins, depending on species.54 The rabbit cornea, on the other hand, exceptionally expresses ALDH1A1 instead of ALDH3A1.55 These enzymes are considered “corneal crystallins” and are believed to contribute to the transparent and refractory properties of the cornea.55
In addition to serving a structural role, ALDH3A1 and ALDH1A1 have been shown in rodent models to confer protection against lens oxidative damage and cataract formation (Table 2),56 which is associated with UVR exposure. Similarly, it has been found that decreased ALDH3A1 activity is associated with pathologic corneas.57 Given the abundant expression of these enzymes in the cornea, it is believed that ALDH3A1 and ALDH1A1 play a key role in protecting the eye from UV-induced damage by multifactorial mechanisms58: 1) acting as antioxidants by scavenging directly UV-induced free radicals or by producing the antioxidant NADPH, 2) direct absorption of UV light, and 3) metabolism of toxic aldehydes produced by UV-induced lipid peroxidation. Consistent with an antioxidant role for ALDH3A1 is the observation that overexpression of human ALDH3A1 prevents GSH depletion caused by oxidative agents in rabbit corneal fibroblast cells and protects human corneal epithelial cells from oxidant-induced DNA damage.59,60
B. Nonenzymatic Antioxidants
1. Ascorbic Acid
Among the nonenzymatic antioxidants present in the cornea, ascorbic acid (vitamin C) is believed to be the most important one.9,33,61,62 This water soluble molecule is a strong electron donor, readily reacting with a broad spectrum of free radicals, including superoxide anion and hydroxyl and peroxyl radicals. Dehydroascorbate, the oxidized product of ascorbate, is then reduced back to ascorbate, using GSH or NADPH as electron donors.63
Ascorbate is abundant in various ocular compartments in diurnal animals that do or do not possess de novo ascorbate synthesis,64 emphasizing a significant role for this antioxidant in the eye. The accumulation of ascorbate in the eye is achieved primarily by active transport through the iris-ciliary body into aqueous humor and subsequent transport into the cornea and lens.65 The distribution of ascorbate in the cornea has been investigated in the bovine eye.66 The highest ascorbate concentration (~1.56 mg/g) was found in the corneal epithelium, particularly in the central region covering the papillary area. A much lower level (~0.2 mg/g), although exceeding that of serum, was observed in the corneal stroma and endothelium. Based on this pattern of ascorbate distribution in the cornea and the contribution of corneal fractions to UV absorption, it is proposed that ascorbate may absorb the UVB spectrum of light between 280–310 nm, thereby protecting internal ocular structures against UVR-induced damage.66 The difference in the levels of ascorbate in corneas of diurnal and nocturnal animals also supports an important role of this antioxidant in protecting the eye from light-induced stress and tissue damage.67 It is suggested that ambient radiation plays a role in sustaining the high ascorbate concentration in the corneal epithelium.68 Despite all these facts, however, oral supplementation of ascorbate that increases ascorbate concentration of lens by 53% is not protective against UVB-induced cataract in guinea pigs.69 It should be noted that ascorbate serves as an essential cofactor for collagen synthesis by corneal fibroblasts, by which ascorbate promotes healing of damaged corneal tissues and reduces the incidence of corneal ulceration and perforation.70
2. Glutathione
GSH, a tripeptide composed of glutamate, cysteine, and glycine, is ubiquitously synthesized in all cell types.71 It is the most abundant cellular non-protein thiol, and it pairs with GSSG to function as the major cellular redox buffer. GSH scavenges hydroxyl radical and superoxide directly and serves as a cofactor for the enzyme GPXs in metabolizing hydrogen peroxide, as well as lipid peroxides (Figure 1).72 Through the action of the glutathione S-transferases (GSTs), GSH can be conjugated to a great variety of electrophilic endogenous compounds and foreign chemicals, resulting in efficient and safe elimination.73 Furthermore, GSH is able to regenerate other important antioxidants, Vitamins C and E, back to their active forms. The cellular redox status of GSH (GSH/GSSG ratio) has been suggested to be an important determinant of cell fate, including proliferation, apoptosis, and senescence.74,75
In addition to its role as a major antioxidant, GSH has been shown to participate in other physiological processes, including nucleotide metabolism, formation of lipid second messengers, regulation of nitric oxide homeostasis, and post-translational modification of proteins.76 Corneal GSH is in the mM range (4~7 mM), and it accumulates mostly in the epithelium, where GSH concentration is five-fold higher than that in the stroma.76,77 Unlike ascorbate, GSH in the corneal cellular layers can be synthesized via its de novo biosynthetic pathway, which is mediated by two cytosolic enzymes, glutamate-cysteine ligase (GCL) and glutathione synthetase (GSS).78 Under oxidative stress, fast turnover of corneal GSH via its synthetic pathway and recycling by GR are required to counteract free radicals. In the cornea, GSH plays a pivotal role in maintaining the normal hydration level,41 protecting the integrity of the cellular membrane and degrading xenobiotics.
Disrupted GSH homeostasis has been associated with various human diseases affecting cornea, such as viral infections and diabetes.79,80 In rodent models with pharmacologically or genetically induced systemic GSH deficiency, ocular pathologies have been observed. For instance, in the mouse model with disrupted γ-glutamyl transpeptidase (Ggt1) gene encoding the membrane-bound enzyme that cleaves GSH to recycle precursor amino acids for GSH re-synthesis, lens GSH levels decrease by 90% and cataracts develop at an earlier age ubiquitously compared to normal mice (Table 2).81 In a preliminary study on a mouse model with combined deficiency in GSH and ascorbate, pathologies in the cornea and lens have been noted (Chen and Vasiliou, unpublished observation). These studies provide convergent evidence to support a protective role of GSH against oxidative damage in the ocular tissues.
3. Reduced Nicotinamide-Adenine Dinucleotide Phosphate
NADPH and its oxidized product NADP form the other important cellular redox buffer. The cellular concentration of NADPH is in the μM range, but has been found to be higher in ocular tissues, probably because of the presence of abundant NADPH-dependent “enzyme-crystallins” in the cornea and lens.82 NADPH functions as an antioxidant in the cornea via multiple mechanisms: 1) It is a coenzyme of GR in the GPX/GR system for the regeneration of GSH from GSSG83; 2) It may act as a direct antioxidant by reducing glutathiyl, tyrosyl, and peroxynitrite radicals generated during oxidative stress84; 3) It is a UVR filter85; 4) It may protect other antioxidant enzymes from ROS-induced inactivation86; and, 5) It maintains a reducing potential for pyridine nucleotide-dependent redox-active enzymes, such as isocitrate dehydrogenase, malic dehydrogenase and 6-phosphate dehydrogenase, which are involved in protecting eye tissues.87
4. α-Tocopherol and Retinol
α-Tocopherol (the most active form of vitamin E) is a fat-soluble antioxidant, and it functions uniquely to break the chain reaction of lipid peroxidation by quenching peroxyl radicals.88,89 This action of α-tocopherol is crucial for the maintenance of cellular membrane integrity. In addition, α-tocopherol can regenerate other antioxidants, including GSH and ascorbate.90 A protective role of vitamin E in the eye is implicated by studies showing significant correlation between high plasma α-tocopherol levels and lesser prevalence of cataract.91 In agreement with this, vitamin E-enriched diet has been reported to protect UVR-induced damage in the cornea and lens.92
Retinol (vitamin A) not only plays a physiological role in normal vision, but also acts as a hydrophobic antioxidant by reducing oxidative stress and preventing apoptosis in corneal endothelial cells. It has been shown that vitamin A supplementation in the culture medium provides considerable protection in murine corneal endothelial cells against lipid peroxidation and oxidative damage induced by iron overload; this effect of vitamin A is greater than that of the combination of vitamin C and Vitamin E.93 The antioxidant role of vitamin A is believed to be partially due to the polyene hydrophobic unit, which can quench singlet oxygen species, neutralize radicals, and stabilize peroxyl radicals.94
5. Ferritin
In the presence of free iron, UVR generates the hydroxyl radical, which triggers lipid peroxidation and ocular tissue injury. Considering the amount and duration of UVR exposure of the cornea during a lifetime, it is highly necessary that the level of free iron is tightly regulated in this tissue and kept at a safe low level. This sequestering of iron in the cytoplasm is brought about by the iron-binding protein ferritin.22 Cai et al showed that ferritin is a developmentally regulated protein in avian corneal epithelial cells and is expressed in the nucleus.95 This nuclear ferritin protein is indistinguishable from the cytoplasmic form and can sequester iron, and, thus, acts as an antioxidant.22,95,96 This function of nuclear ferritin in corneal epithelial cells has been suggested to be protective against UVR-induced DNA damage.97
6. Albumin
Serum albumin diffuses from peripheral blood vessels present around the cornea toward its center. Levels of albumin at 12–25% of total water soluble proteins have been reported in mammalian corneas,98 suggesting an important role for this protein in the cornea. Albumin is distributed exclusively in the stromal layer of the cornea. Aside from being a binding and transporting protein, albumin has been shown to act as an antioxidant by scavenging hydrogen peroxide.98
IV. ANTIOXIDANTS IN THE TEAR FILM AND AQUEOUS HUMOR
The tear film and aqueous humor are important components of defense mechanisms in the ocular surface. The tear film covers the anterior surface of the cornea and is the first line of defense against external insults. It creates a smooth refractive surface on the cornea and protects the cornea from dehydration and environmental damage. It also functions in nourishing the anterior cornea and concentrating locally released biochemical metabolites. Consistent with a protective role against oxidative stress, the tear film contains both nonenzymatic and enzymatic antioxidants. In human tears, ascorbic acid (665 μM) and uric acid (328 μM) account for ~50% of the total antioxidant activity, with ascorbic acid being the most abundant and uric acid the second; other small molecules including GSH (107 μM), L-cysteine (48 μM) and L-tyrosine (45 μM) make up the rest.99 The only reported antioxidant enzyme in the tear film is SOD, which displays a activity at 1~32 U/mg protein.31,100
The aqueous humor secreted by ciliary bodies is a clear and slightly alkaline liquid that occupies the space between the cornea and lens. It plays a crucial role in nourishing and protecting the corneal endothelium and the anterior epithelial lining of the lens. It also removes the metabolic wastes and biochemical products generated by the cornea and lens. Thus, ROS can be continuously generated in the aqueous humor in the form of hydrogen peroxide, superoxide anion, singlet oxygen, and peroxyl radicals. The antioxidant profile of the aqueous humor resembles that of the tear film.101 In the order of abundance in human aqueous humor, non-enzymatic antioxidants include ascorbic acid (530 μM), L-tyrosine (78 μM), uric acid (43 μM), L-cysteine (14.3 μM) and glutathione (5.5 μM). Quite differently in nocturnal rat, the aqueous humor is concentrated in thiol antioxidant GSH (125 μM) and L-cysteine (63 μM). The SOD activity at 2.2 ± 0.3, 2.7 ± 1.2 and <0.2 U/ml for EC-SOD, CuZn-SOD and Mn-SOD, respectively, has been reported in the aqueous humor; it is believed that this trace amount of SOD activity does not contribute significantly to the antioxidant defense mechanisms of the aqueous humor.31
The high concentration of ascorbic acid in aqueous humors of diurnal species, likely resulting from more efficient sequestering of this molecule,102 strongly supports an important role of ascorbic acid in protecting against light (UVR)-mediated damage. Indeed, systemic administration of ascorbic acid that increases the ascorbic acid level in the aqueous humor by 30-fold has been shown to protect lens epithelium against UVR-induced DNA damage in rat.103 It is believed that three different mechanisms are involved in such a protective effect of ascorbic acid compartmentalized in the aqueous humor104: direct absorption of UVR; quenching the fluorescence of biomolecules; and controlling the fluorescence-mediated biotransformation. This quenching of fluorescence also facilitates the decrease of light scattering.104 Amino acid L-tyrosine is electrochemically active and scavenges hydroxyl radicals and singlet oxygen species.101,105 Uric acid is a purine metabolite and its antioxidant property has been documented in extracellular fluids, including the tear film and the aqueous humor.106 The concentration of uric acid in human ocular fluids is only a fraction of the plasma concentration.101,107 This water-soluble molecule has high reactivity toward singlet oxygen and hydroxyl radicals, serving as a potent scavenger for these ROS.107,109 In addition, it has been proposed that uric acid regulates the redox state of GSH-ascorbate system.107,108 Amino acid L-cysteine replenishes the tissue GSH pool by supplying the rate-limiting substrate for GSH biosynthesis71; it also acts as an antioxidant directly via the thiol group.
Taken together, the extracellular fluid in the ocular surface, namely the tear film and the aqueous humor, is replete with water-soluble and low-molecular-weight antioxidants, which enrich the defense mechanisms of the cornea against oxidative stress.
V. CONCLUDING REMARKS
The ocular surface is equipped with a battery of redundant and diverse antioxidants to combat oxidative stress arising from environmental exposure. While serving their functions, these antioxidants are consumed by ROS and/or damaged by radiations.33,64,102 As age progresses, the production of these antioxidants and their functioning tend to decrease in the eye and almost completely halt during the later stages of life.2,110,111 It is during and at this crucial stage that the pro-oxidant species begin to advance, resulting in certain pathological conditions of the eye, such as cataract and glaucoma.2,93,110 Based on these observations, the effect of pharmacological supplements, mainly GSH and vitamins, on aging-related eye diseases have been investigated in experimental animal models. Results derived from these studies are contradictory,69,80,112–116 suggesting the complexity in the disease etiologies.
The antioxidant defense network in the ocular surface, as well as in other ocular compartments, is characterized by the cross-talk between pathways or individual antioxidants, which is essential in maintaining the overall cellular redox balance in the eye. Classical examples include GPX-GSH-GR-NADPH and GSH-VitC-VitE systems. The fact that deficiency of one antioxidant is not always associated with eye pathologies may be explained by the redundancy of the antioxidant defense system in the ocular surface. On the other hand, the distinct role of certain antioxidants in ocular surface defense is reflected by ocular phenotypes resulting from their disruption. For instance, Aldh3a1 null mice develop cataracts and punctate opacities in lens cortex as early as in 1 month, whereas Aldh1a1 null mice develop cataracts much later in life (6–9 months).56 Given the abundant expression of ALDH3A1 in the cornea, the protective effect of ALDH3A1 is most likely due to its filtering property. On the other hand, ALDH1A1 may protect against cataract formation by detoxifying the products of lipid peroxidation in both the cornea and lens. Furthermore, the highest incidence and most severe lenticular phenotypes are observed in Aldh3a1/1a1 null mice, indicating an additive effect of ALDH3A1 and ALDH1A1. Future studies aimed at understanding the interplay between these antioxidants and the underlying mechanisms will provide valuable information on the pathogenesis and therapeutic interventions of ocular diseases.
Acknowledgments
We thank Dr. David Thompson for critical reading and discussion of this manuscript.
Supported in part by National Institutes of Health (NIH) grants EY11490 and EY17963.
Abbreviations
- ALDH
Aldehyde dehydrogenases
- CAT
catalase
- CuZn-SOD
Copper-zinc superoxide dismutases
- EC-SOD
Extracellular superoxide dismutases
- G6PD
Glucose-6-phosphate dehydrogenase
- GPXs
glutathione peroxidases
- GR
glutathione reductase
- GSH
Glutathione
- GSSG
Oxidized form of glutathione
- GST
Glutathione S-transferases
- H2O2
Hydrogen peroxide
- LOO
Peroxyl radical
- Mn-SOD
Manganese-containing superoxide dismutases
- NADPH
Nicotinamide adenine dinucleotide phosphate
- OH
Hydroxyl radical
- ROS
Reactive oxygen species
- SODs
superoxide dismutases
- UVR
ultraviolet radiation
Footnotes
The authors have no commercial or proprietary interest in any concept or product discussed in this article.
References
- 1.van Kuijk FJ. Effects of ultraviolet light on the eye: role of protective glasses. Environ Health Perspect. 1991;96:177–84. doi: 10.1289/ehp.9196177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts JE. Ocular phototoxicity. J Photochem Photobiol B. 64:136–43. doi: 10.1016/s1011-1344(01)00196-8. 200. [DOI] [PubMed] [Google Scholar]
- 3.Tenkate TD. Ultraviolet radiation: human exposure and health risks. J Environ Health. 2009;61:9–15. [Google Scholar]
- 4.Andley UP, Rhim JS, Chylack LT, Jr, Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest Ophthalmol Vis Sci. 1994;35:3094–102. [PubMed] [Google Scholar]
- 5.Zigman S. Lens UVA photobiology. J Ocul Pharmacol Ther. 2000;16:161–5. doi: 10.1089/jop.2000.16.161. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanian D. Ultraviolet radiation and cataract. J Ocul Pharmacol Ther. 2000;16:285–97. doi: 10.1089/jop.2000.16.285. [DOI] [PubMed] [Google Scholar]
- 7.Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor HR, Munoz B, West S, et al. Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc. 1990;88:163–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Piatigorsky J. Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the refracton hypothesis. Cornea. 2001;20:853–8. doi: 10.1097/00003226-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol. 2005;37:2478–503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Cadenas E, Sies H. The lag phase. Free Radic Res. 1998;28:601–9. doi: 10.3109/10715769809065816. [DOI] [PubMed] [Google Scholar]
- 12.Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–52. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387(10–11):1329–35. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 14.Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;1763:1755–66. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37:1921–42. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Aikens J, Dix TA. Perhydroxyl radical (HOO.) initiated lipid peroxidation. The role of fatty acid hydroperoxides. J Biol Chem. 1991;266:15091–8. [PubMed] [Google Scholar]
- 17.del Rio LA, Sandalio LM, Palma JM, et al. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radic Biol Med. 1992;13:557–80. doi: 10.1016/0891-5849(92)90150-f. [DOI] [PubMed] [Google Scholar]
- 18.Bergendi L, Benes L, Duracková Z, Ferencik M. Chemistry, physiology and pathology of free radicals. Life Sci. 1999;65:1865–74. doi: 10.1016/s0024-3205(99)00439-7. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B. Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Problems, resolutions and preliminary results from nutritional supplementation studies. Free Radic Res. 1998;29:469–86. doi: 10.1080/10715769800300531. [DOI] [PubMed] [Google Scholar]
- 20.Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324 (Pt 1):1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergmanson JP, Soderberg PG. The significance of ultraviolet radiation for eye diseases. A review with comments on the efficacy of UV-blocking contact lenses. Ophthalmic Physiol Opt. 1995;15:83–91. [PubMed] [Google Scholar]
- 22.Cai CX, Birk DE, Linsenmayer TF. Nuclear ferritin protects DNA from UV damage in corneal epithelial cells. Mol Biol Cell. 1998;9:1037–51. doi: 10.1091/mbc.9.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu ZG, Baskaran R, Lea-Chou ET, et al. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature. 1996;384(6606):273–6. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 24.Sacca SC, Bolognesi C, Battistella A, et al. Gene-environment interactions in ocular diseases. Mutat Res. 2009;667:98–117. doi: 10.1016/j.mrfmmm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Elner SG, Bian ZM, et al. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–72. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cejkova J, Labsky J, Vacik J. Reactive oxygen species (ROS) generated by xanthine oxidase in the corneal epithelium and their potential participation in the damage of the corneal epithelium after prolonged use of contact lenses in rabbits. Acta Histochem. 1998;100:171–84. doi: 10.1016/s0065-1281(98)80025-1. [DOI] [PubMed] [Google Scholar]
- 28.Cejková J, Stípek S, Crkovská J, et al. UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Res. 2004;53:1–10. [PubMed] [Google Scholar]
- 29.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 30.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 31.Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci. 2009;39:471–5. [PubMed] [Google Scholar]
- 32.Redmond TM, Duke EJ, Coles WH, et al. Localization of corneal superoxide dismutase by biochemical and histocytochemical techniques. Exp Eye Res. 1984;38:369–78. doi: 10.1016/0014-4835(84)90192-1. [DOI] [PubMed] [Google Scholar]
- 33.Kovaceva J, Pláteník J, Vejrazka M, et al. Differences in activities of antioxidant superoxide dismutase, glutathione peroxidase and prooxidant xanthine oxidoreductase/xanthine oxidase in the normal corneal epithelium of various mammals. Physiol Res. 2007;56:105–23. doi: 10.33549/physiolres.930889. [DOI] [PubMed] [Google Scholar]
- 34.Behndig A, Karlsson K, Brännström T, et al. Corneal endothelial integrity in mice lacking extracellular superoxide dismutase. Invest Ophthalmol Vis Sci. 2001;42:2784–8. [PubMed] [Google Scholar]
- 35.Sandbach JM, Coscun PE, Grossniklaus HE, et al. Ocular pathology in mitochondrial superoxide dismutase (Sod2)-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:2173–8. [PubMed] [Google Scholar]
- 36.Reddy VN, Kasahara E, Hiraoka M, et al. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res. 2004;79:859–68. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Olofsson EM, Marklund SL, Behndig A. Enhanced diabetes-induced cataract in copper-zinc superoxide dismutase-null mice. Invest Ophthalmol Vis Sci. 2009;50:2913–8. doi: 10.1167/iovs.09-3510. [DOI] [PubMed] [Google Scholar]
- 38.Atalla LR, Sevanian A, Rao NA. Immunohistochemical localization of glutathione peroxidase in ocular tissue. Curr Eye Res. 1988;7:1023–7. doi: 10.3109/02713688809015149. [DOI] [PubMed] [Google Scholar]
- 39.Kenney MC, Chwa M, Atilano SR, et al. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol Vis Sci. 2005;46:823–32. doi: 10.1167/iovs.04-0549. [DOI] [PubMed] [Google Scholar]
- 40.Bhuyan KC, Bhuyan DK. Regulation of hydrogen peroxide in eye humors. Effect of 3-amino-1H-1,2,4-triazole on catalase and glutathione peroxidase of rabbit eye. Biochim Biophys Acta. 1977;497:641–51. doi: 10.1016/0304-4165(77)90284-7. [DOI] [PubMed] [Google Scholar]
- 41.Riley MV. A role for glutathione and glutathione reductase in control of corneal hydration. Exp Eye Res. 1984;39:751–8. doi: 10.1016/0014-4835(84)90074-5. [DOI] [PubMed] [Google Scholar]
- 42.Reddy VN, Giblin FJ, Lin LR, et al. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Invest Ophthalmol Vis Sci. 2001;42:3247–55. [PubMed] [Google Scholar]
- 43.Bayliak MM, Semchyshyn HM, Lushchak VI. Possible accumulation of non-active molecules of catalase and superoxide dismutase in S. Cerevisiae cells under hydrogen peroxide induced stress. Central Eur J Biol. 2009;2:326–36. [Google Scholar]
- 44.Atalla L, Fernandez MA, Rao NA. Immunohistochemical localization of catalase in ocular tissue. Curr Eye Res. 1987;6:1181–7. doi: 10.3109/02713688709025227. [DOI] [PubMed] [Google Scholar]
- 45.Choi SI, Kim TI, Kim KS, et al. Decreased catalase expression and increased susceptibility to oxidative stress in primary cultured corneal fibroblasts from patients with granular corneal dystrophy type II. Am J Pathol. 2009;175:248–61. doi: 10.2353/ajpath.2009.081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hudde T, Comer RM, Kinsella MT, et al. Modulation of hydrogen peroxide induced injury to corneal endothelium by virus mediated catalase gene transfer. Br J Ophthalmol. 2002;86:1058–62. doi: 10.1136/bjo.86.9.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heck DE, Vetrano AM, Mariano TM, Laskin JD. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem. 2003;278:22432–6. doi: 10.1074/jbc.C300048200. [DOI] [PubMed] [Google Scholar]
- 48.Ho YS, Xiong Y, Ma W, et al. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279:32804–12. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 49.Ho HY, Cheng ML, Chiu DT. Glucose-6-phosphate dehydrogenase--from oxidative stress to cellular functions and degenerative diseases. Redox Rep. 2007;12:109–18. doi: 10.1179/135100007X200209. [DOI] [PubMed] [Google Scholar]
- 50.Tsubai T, Matsuo M. Ultraviolet light-induced changes in the glucose-6-phosphate dehydrogenase activity of porcine corneas. Cornea. 2002;21:495–500. doi: 10.1097/00003226-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Pandolfi PP, Sonati F, Rivi R, et al. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–15. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 53.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piatigorsky J. Multifunctional lens crystallins and corneal enzymes. More than meets the eye. Ann N Y Acad Sci. 1998;842:7–15. doi: 10.1111/j.1749-6632.1998.tb09626.x. [DOI] [PubMed] [Google Scholar]
- 55.Jester JV, Budge A, Fisher S, Huang J. Corneal keratocytes: phenotypic and species differences in abundant protein expression and in vitro light-scattering. Invest Ophthalmol Vis Sci. 2005;46:2369–78. doi: 10.1167/iovs.04-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lassen N, Bateman JB, Estey T, et al. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knockout mice. J Biol Chem. 2007;282:25668–76. doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gondhowiardjo TD, van Haeringen NJ, Völker-Dieben HJ, et al. Analysis of corneal aldehyde dehydrogenase patterns in pathologic corneas. Cornea. 1993;12:146–54. doi: 10.1097/00003226-199303000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Lassen N, Black WJ, Estey T, Vasiliou V. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin Cell Dev Biol. 2008;19:100–12. doi: 10.1016/j.semcdb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Pappa A, Brown D, Koutalos Y, et al. Human aldehyde dehydrogenase 3A1 inhibits proliferation and promotes survival of human corneal epithelial cells. J Biol Chem. 2005;280:27998–8006. doi: 10.1074/jbc.M503698200. [DOI] [PubMed] [Google Scholar]
- 60.Lassen N, Pappa A, Black WJ, et al. Antioxidant function of corneal ALDH3A1 in cultured stromal fibroblasts. Free Radic Biol Med. 2006;41:1459–69. doi: 10.1016/j.freeradbiomed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Pappa A, Estey T, Manzer R, et al. Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization in the cornea. Biochem J. 2003;376(Pt 3):615–23. doi: 10.1042/BJ20030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pappa A, Sophos NA, Vasiliou V. Corneal and stomach expression of aldehyde dehydrogenases: from fish to mammals. Chem Biol Interact. 2001;130–132(1–3):181–91. doi: 10.1016/s0009-2797(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 63.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 64.Ringvold A, Anderssen E, Kjonniksen I. Ascorbate in the corneal epithelium of diurnal and nocturnal species. Invest Ophthalmol Vis Sci. 1998;39:2774–7. [PubMed] [Google Scholar]
- 65.Rose RC, Bode AM. Ocular ascorbate transport and metabolism. Comp Biochem Physiol A Comp Physiol. 1991;100:273–85. doi: 10.1016/0300-9629(91)90470-w. [DOI] [PubMed] [Google Scholar]
- 66.Ringvold A, Anderssen E, Kjonniksen I. Distribution of ascorbate in the anterior bovine eye. Invest Ophthalmol Vis Sci. 2000;41:20–3. [PubMed] [Google Scholar]
- 67.Suh MH, Kwon JW, Wee Wr, et al. Protective effect of ascorbic Acid against corneal damage by ultraviolet B irradiation: a pilot study. Cornea. 2008;27:916–22. doi: 10.1097/ICO.0b013e31816f7068. [DOI] [PubMed] [Google Scholar]
- 68.Ringvold A, Anderssen E, Kjonniksen I. Impact of the environment on the mammalian corneal epithelium. Invest Ophthalmol Vis Sci. 2003;44:10–5. doi: 10.1167/iovs.02-0173. [DOI] [PubMed] [Google Scholar]
- 69.Mody VC, Jr, Kaka M, Elfving A, et al. Drinking water supplementation with ascorbate is not protective against UVR-B-induced cataract in the guinea pig. Acta Ophthalmol. 2008;86:188–95. doi: 10.1111/j.1600-0420.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 70.Pfister RR, Paterson CA, Hayes SA. Effects of topical 10% ascorbate solution on established corneal ulcers after severe alkali burns. Invest Ophthalmol Vis Sci. 1982;22:382–5. [PubMed] [Google Scholar]
- 71.Chen Y, Shertzer HG, Schneider SN, et al. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem. 2005;280:33766–74. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- 72.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57:1825–35. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rinaldi R, Eliasson E, Swedmark S, Morgenstern R. Reactive intermediates and the dynamics of glutathione transferases. Drug Metab Dispos. 2002;30:1053–8. doi: 10.1124/dmd.30.10.1053. [DOI] [PubMed] [Google Scholar]
- 74.Watson WH, Chen Y, Jones DP. Redox state of glutathione and thioredoxin in differentiation and apoptosis. Biofactors. 2003;17:307–14. doi: 10.1002/biof.5520170130. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, Johansson E, Fan Y, et al. Early onset senescence occurs when fibroblasts lack the glutamate-cysteine ligase modifier subunit. Free Radic Biol Med. 2009;47:410–8. doi: 10.1016/j.freeradbiomed.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalton TP, Chen Y, Schneider SN, et al. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–26. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 77.Kannan R, Tang D, Mackic JB, et al. A simple technique to determine glutathione (GSH) levels and synthesis in ocular tissues as GSH-bimane adduct: application to normal and galactosemic guinea-pigs. Exp Eye Res. 1993;56:45–50. doi: 10.1006/exer.1993.1007. [DOI] [PubMed] [Google Scholar]
- 78.Meister A. Glutathione metabolism. Methods Enzymol. 1995;251:3–7. doi: 10.1016/0076-6879(95)51106-7. [DOI] [PubMed] [Google Scholar]
- 79.Kannan R, Mackic JB, Zlokovic BV. Corneal transport of circulating glutathione in normal and galactosemic guinea pigs. Cornea. 1999;18:321–7. doi: 10.1097/00003226-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 80.Nucci C, Palamara AT, Ciriolo MR, et al. Imbalance in corneal redox state during herpes simplex virus 1-induced keratitis in rabbits. Effectiveness of exogenous glutathione supply. Exp Eye Res. 2000;70:215–20. doi: 10.1006/exer.1999.0782. [DOI] [PubMed] [Google Scholar]
- 81.Chévez-Barrios P, Wiseman AL, Rojas E, et al. Cataract development in gamma-glutamyl transpeptidase-deficient mice. Exp Eye Res. 2000;71:575–82. doi: 10.1006/exer.2000.0913. [DOI] [PubMed] [Google Scholar]
- 82.Rao PV, Zigler JS., Jr Extremely high levels of NADPH in guinea pig lens: correlation with zeta-crystallin concentration. Biochem Biophys Res Commun. 1990;167:1221–8. doi: 10.1016/0006-291x(90)90654-6. [DOI] [PubMed] [Google Scholar]
- 83.Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 84.Kirsch M, De Groot H. NAD(P)H, a directly operating anti-oxidant? FASEB J. 2001;15:1569–74. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- 85.Atherton SJ, Lambert C, Schultz J, et al. Fluorescence studies of lens epithelial cells and their constituents. Photochem Photobiol. 1999;70:823–8. [PubMed] [Google Scholar]
- 86.Kirkman HN, Rolfo M, Ferraris AM, Gaetani GF. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J Biol Chem. 1999;274:13908–14. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]
- 87.Pappa A, Chen C, Koutalos Y, et al. Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative damage. Free Radic Biol Med. 2003;34:1178–89. doi: 10.1016/s0891-5849(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 88.Liebler DC. The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol. 1993;23:147–69. doi: 10.3109/10408449309117115. [DOI] [PubMed] [Google Scholar]
- 89.Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278(5706):737–8. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- 90.Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- 91.Wu SY, Leske MC. Antioxidants and cataract formation: a summary review. Int Ophthalmol Clin. 2000;40 (Fall):71–81. doi: 10.1097/00004397-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Wegener A, Heinitz M, Dwinger M. Experimental evidence for interactive effects of chronic UV irradiation and nutritional deficiencies in the lens. Dev Ophthalmol. 2002;35:113–24. doi: 10.1159/000060815. [DOI] [PubMed] [Google Scholar]
- 93.Serbecic N, Beutelspacher SC. Vitamins inhibit oxidant-induced apoptosis of corneal endothelial cells. Jpn J Ophthalmol. 2005;49:355–62. doi: 10.1007/s10384-005-0209-9. [DOI] [PubMed] [Google Scholar]
- 94.Palace VP, Khaper N, Qin Q, Singal PK. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic Biol Med. 1999;26:746–61. doi: 10.1016/s0891-5849(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 95.Cai CX, Birk DE, Linsenmayer TF. Ferritin is a developmentally regulated nuclear protein of avian corneal epithelial cells. J Biol Chem. 1997;272:12831–9. doi: 10.1074/jbc.272.19.12831. [DOI] [PubMed] [Google Scholar]
- 96.Balla G, Jacob HS, Balla J, et al. Ferritin: a cytoprotective anti-oxidant strategem of endothelium. J Biol Chem. 1992;267:18148–53. [PubMed] [Google Scholar]
- 97.Cai C, Ching A, Lagace C, Linsenmayer T. Nuclear ferritin-mediated protection of corneal epithelial cells from oxidative damage to DNA. Dev Dyn. 2008;237:2676–83. doi: 10.1002/dvdy.21494. [DOI] [PubMed] [Google Scholar]
- 98.Nees DW, Fariss RN, Piatigorsky J. Serum albumin in mammalian cornea: implications for clinical application. Invest Ophthalmol Vis Sci. 2003;44:3339–45. doi: 10.1167/iovs.02-1161. [DOI] [PubMed] [Google Scholar]
- 99.Gogia R, Richer SP, Rose RC. Tear fluid content of electrochemically active components including water soluble antioxidants. Curr Eye Res. 1998;17:257–63. doi: 10.1076/ceyr.17.3.257.5213. [DOI] [PubMed] [Google Scholar]
- 100.Barsouk A, Fraiture B, Peele K, et al. Levels of manganese superoxide dismutase activity are increased in tears from patients with thyroid-associated ophthalmopathy. Orbit. 1996;15:205–11. [Google Scholar]
- 101.Richer SP, Rose RC. Water soluble antioxidants in mammalian aqueous humor: interaction with UV B and hydrogen peroxide. Vision Res. 1998;38:2881–8. doi: 10.1016/s0042-6989(98)00069-8. [DOI] [PubMed] [Google Scholar]
- 102.Reiss GR, Werness PG, Zollman PE, Brubaker RF. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch Ophthalmol. 1986;104:753–5. doi: 10.1001/archopht.1986.01050170143039. [DOI] [PubMed] [Google Scholar]
- 103.Reddy VN, Giblin FJ, Lin LR, Chakrapani B. The effect of aqueous humor ascorbate on ultraviolet-B-induced DNA damage in lens epithelium. Invest Ophthalmol Vis Sci. 1998;39:344–50. [PubMed] [Google Scholar]
- 104.Ringvold A. The significance of ascorbate in the aqueous humour protection against UV-A and UV-B. Exp Eye Res. 1996;62:261–4. doi: 10.1006/exer.1996.0031. [DOI] [PubMed] [Google Scholar]
- 105.Whiteman M, Halliwell B. Protection against peroxynitrite-dependent tyrosine nitration and alpha 1-antiproteinase inactivation by ascorbic acid. A comparison with other biological antioxidants. Free Radic Res. 1996;25:275–83. doi: 10.3109/10715769609149052. [DOI] [PubMed] [Google Scholar]
- 106.Horwath-Winter J, Kirchengast S, Meinitzer A, et al. Determination of uric acid concentrations in human tear fluid, aqueous humour and serum. Acta Ophthalmol. 2009;87:188–92. doi: 10.1111/j.1755-3768.2008.01215.x. [DOI] [PubMed] [Google Scholar]
- 107.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Becker BF, Reinholz N, Leipert B, et al. Role of uric acid as an endogenous radical scavenger and antioxidant. Chest. 1991;100(3 Suppl):176S–181S. doi: 10.1378/chest.100.3_supplement.176s. [DOI] [PubMed] [Google Scholar]
- 109.Sevanian A, Davies KJ, Hochstein P. Serum urate as an antioxidant for ascorbic acid. Am J Clin Nutr. 1991;54(6 Suppl):1129S–1134S. doi: 10.1093/ajcn/54.6.1129s. [DOI] [PubMed] [Google Scholar]
- 110.Cejková J, Vejrazka M, Pláteník J, Stípek S. Age-related changes in superoxide dismutase, glutathione peroxidase, catalase and xanthine oxidoreductase/xanthine oxidase activities in the rabbit cornea. Exp Gerontol. 2004;39:1537–43. doi: 10.1016/j.exger.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 111.Samiec PS, Drews-Botsch C, Flagg EW, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 112.Ayala MN, Soderberg PG. Vitamin E can protect against ultraviolet radiation-induced cataract in albino rats. Ophthalmic Res. 2004;36:264. doi: 10.1159/000081206. [DOI] [PubMed] [Google Scholar]
- 113.Fernandez MM, Afshari NA. Nutrition and the prevention of cataracts. Curr Opin Ophthalmol. 2008;19:66–70. doi: 10.1097/ICU.0b013e3282f2d7b6. [DOI] [PubMed] [Google Scholar]
- 114.Maraini G, Sperduto RD, et al. Clinical Trial of Nutritional Supplements and Age-Related Cataract Study Group. A randomized, double-masked, placebo-controlled clinical trial of multivitamin supplementation for age-related lens opacities. Clinical trial of nutritional supplements and age-related cataract report no. 3. Ophthalmology. 2008;115:599–607. doi: 10.1016/j.ophtha.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 115.Moriarty-Craige SE, Adkison J, Lynn M, et al. Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am J Ophthalmol. 2005;140:1020–6. doi: 10.1016/j.ajo.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 116.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119:1439–52. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]


