Abstract
Rationale
While accumulating data support the efficacy of intramyocardial cell-based therapy to improve LV function in patients with chronic ischemic cardiomyopathy undergoing CABG, the underlying mechanism and impact of cell injection site remain controversial.Mesenchymal stem cells (MSCs) improve LV structure and function through several effects including: reducing fibrosis, neoangiogenesis and neomyogenesis.
Objective
To test the hypothesis that the impact on cardiac structure and function following intramyocardial injections of autologous MSCs results from a concordance of pro-recovery phenotypic effects.
Methods and Results
Six patients were injected with autologous MSCs into akinetic/hypokinetic myocardial territories not receiving bypass graft for clinical reasons. MRI was used to measure scar, perfusion, wall thickness and contractility at baseline, 3, 6 and 18 months and to compare structural and functional recovery in regions that received MSC injections alone, revascularization alone, or neither. A composite score of MRI variables was used to assess concordance of antifibrotic effects, perfusion, and contraction at different regions. After 18 months, subjects receiving MSCs exhibited increased LVEF (+9.4±1.7%, p=0.0002) and decreased scar mass (-47.5±8.1%; p<0.0001) compared to baseline. MSC-injected segments had concordant reduction in scar size, perfusion and contractile improvement (concordant score: 2.93±0.07), whereas revascularized (0.5±0.21) and non-treated segments (-0.07±0.34) demonstrated non-concordant changes (p<0.0001 vs. injected segments).
Conclusions
Intramyocardial injection of autologous MSCs into akinetic yet non-revascularized segments produces comprehensive regional functional restitution, which in turn drives improvement in global LV function. These findings, although inconclusive due to lack of placebo group, have important therapeutic and mechanistic hypothesis-generating implications.
Keywords: Stem Cell, Mesenchymal stem cells, bypass surgery
Introduction
Cell-based therapy is emerging as a potential new therapeutic strategy that as an adjunct to revascularization could offer additional clinical benefits1 by virtue of producing both structural and functional cardiac improvements. There is a particular interest in testing the impact of delivering bone marrow-derived stem cells during CABG, and accumulating studies2-5 suggest that the improvement in several functional indices is greater than that achieved with CABG alone.
Preclinical studies provide evidence that bone marrow stem cells contribute to cardiac function and reverse remodeling after ischemic damage6 acting both locally7, 8 and remotely (possibly through paracrine mechanisms)9-11. There appear to be at least three dominant mechanisms that underlie the cardiac reparative response: reduction in tissue fibrosis, neovascularization, and neomyogenesis. One critical question that emerges from the deployment of MSCs during CABG relates to the mechanisms responsible for the MSC-induced effects, an issue with major clinical implications in terms of where and how the cells should be administered during cardiac surgical revascularization.
In studies to date, investigators have either infused12 or injected2, 3 bone marrow-derived cells in areas that were undergoing revascularization. This strategy, although rational, has not allowed the detection of cell mechanisms of action that are independent of revascularization. While the reported benefits can thus be attributed to a potential synergy13 between the favorable environment created by the revascularization of the region and the MSCs, the precise delineation of each contribution, however, remains unknown.
Here, we test the hypothesis that intramyocardial injections of autologous MSCs delivered to segments of myocardium not receiving surgical revascularization improve regional cardiac structure and function. Our study design stipulated that cells would be injected in areas deemed unfit for revascularization to test the hypothesis that MSCs act both independently of revascularization and as an adjunct to CABG. Moreover this trial employed cardiac MRI imaging that permitted direct measurement of the phenotype of functional and anatomical restitution observed after MSCs delivery. This trial, which originally was designed to enroll 45 patients, was suspended after 9 patients were enrolled due to slow accrual. Here we present the comprehensive cardiac phenotypic data obtained from the six enrolled subjects that received MSCs during CABG surgery.
Methods
Eligible patients had a diagnosis of chronic ischemic left ventricular dysfunction secondary to MI and were scheduled to undergo CABG. The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial was approved by the institutional review boards of the University of Miami Miller School of Medicine, The Johns Hopkins University School of Medicine, and the Miami Veterans Affairs Hospital; all patients gave written informed consent. Patients underwent bone marrow aspiration from the iliac crest, isolation and expansion of MSCs14-17. MSC characterization is summarized in online table I. After randomization, MSCs were transepicardially delivered, under direct visualization, using a syringe and 25-gauge needle following the completion of the CABG procedure in areas of the myocardium not deemed fit for surgical revascularization.
Cardiac MRI for structure and function was conducted at baseline, 3, 6 months, and 18 months after the product delivery. Global and regional measurements18 were made for all time points and the results were grouped based on whether left ventricular areas that contained scar received injections of MSCs alone in the absence of revascularization (injected only), were only revascularized but not injected with MSCs (revascularized only) or received neither revascularization nor MSCs (untreated).
Detailed regional MRI studies included: 1) delayed contrast-enhancement, 2) first pass perfusion, 3) end-diastolic wall thickness, 4) end systolic wall thickening, 5) peak systolic circumferential strain, 6) peak diastolic strain rate19 and 7) topographic analyses20 of the injected infarcted areas.
A concordant improvement score (CIS) was devised to investigate the phenotype of recovery in response to stem cell therapy. Five MRI variables were chosen in order to reflect the biology of the three proposed17 mechanisms with which MSCs exert their effects: 1) antifibrotic21 effects were measured by using delayed contrast-enhancement images, 2) proangiogenic22 effects were quantified by first pass perfusion, and 3) regenerative effects9, 23 that lead to restoration of contractile function were measured by using end diastolic wall thickness, end systolic thickening and tagged images (circumferential strain). Improvement compared to baseline was graded with one point and deterioration with -1, while no change (±5%) was graded with zero points. Accordingly, in each segment a cumulative score was calculated by adding the points graded for changes in: 1) infarct size, 2) perfusion at rest and 3) the average of the points for changes in end diastolic wall thickness, systolic thickening and circumferential strain. A concordant improvement in all 5 variables yielded a cumulative CIS of 3 points whereas a deterioration in all variables, a score of -3 points.
An expanded Methods section is available in the Online Supplement.
Results
Demographic and perioperative data
Preoperative baseline characteristics of the patients are summarized in table 1 and their medication at entry as well as at 18 months are shown in online table II. Six patients that were enrolled and randomized to the MSC group (2 injected with the low dose and 4 with the higher dose).All patients were male with average age of 54.9 years, and had hypertension and hyperlipidemia, 83.3% were smokers, two had a history of congestive heart failure and all were in NYHA functional class I/II.The average time between last MI and the time of study enrollment was 675 days (range 70-1565 days). An average of 3±0.3 coronary arteries were grafted (range 2 to 4). All patients received a left internal thoracic artery graft anastomosed to the left anterior descending coronary artery. One patient received bilateral internal mammary artery grafts. Ten venous grafts were used to graft right coronary arteries, left circumflex arteries and diagonal branches. The median number of stem cell injections was 10 (range 8 to 20), for a total volume of 5ml.
Table 1. Patient Baseline Characteristics.
| Baseline Characteristics | MSCs plus CABG (N=6) |
|---|---|
| Age, mean(SEM) years | 54.9 (4.2) |
| Male sex | 6 (100.0%) |
| White Race | 5 (83.3%) |
| Hispanic Ethnicity | 4 (66.7%) |
| History of prior coronary revascularization | 2 (33.3%) |
| History of atrial or ventricular arrhythmia | 1 (16.7%) |
| History of hypertension | 6 (100.0%) |
| History of congestive heart failure | 2 (33.3%) |
| I | 3 (50.0%) |
| II | 3 (50.0%) |
| III | 0 (0.0%) |
| Anterior, inferior, lateral, septal | 2 |
| Anterior | 0 |
| Anterior, septal | 3 |
| Inferior | 2 |
| Inferior, lateral | 2 |
| Revascularized Vessels | |
| LAD | 6 |
| LCx | 4 |
| RCA | 2 |
| History of valvular heart disease | 0 (0.0%) |
| History of smoking | 5 (83.3%) |
| Current smoker | 2 (33.3%) |
| History of diabetes | 4 (66.7%) |
| History of hyperlipidemia | 6 (100.0%) |
| Peak VO2, mean (SEM), mL/kg/min | 19.1 (0.9) |
| 6-minute walk test, mean (SEM) , m | 432.8 (60.3) |
| % Predicted FEV1, mean(SEM) | 82.5 (7.5) |
| MLHFQ, mean (SEM) | 30.17 (9.11) |
Abbreviations: AICD, automatic implantable cardioverter-defibrillator; CT, computed tomography; FEV1, forced expiratory volume in first second of expiration; LV, left ventricular; MI, myocardial infarction; LAD, Left Anterior Descending artery; LCx, Left Circumflex artery; RCA, Right Coronary Artery; MLHFQ, Minnesota Living with Heart Failure Questionnaire; V. O2, oxygen consumption per unit time. Data presented as No. (%) unless otherwise specified. New York Heart Association class I indicates no limitation; class II, slight limitation of physical activity; and class III, marked limitation of physical activity. Current smoker indicates patient actively smoking at time of enrollment.
All injections were well tolerated and no safety concerns were observed as summarized in online table III.
Autologous MSCs injections reduce scar size and improve the LVEF in patients undergoing CABG
Global MRI analyses in MSC treated patients demonstrated an increased end-diastolic volume (EDV) and decreased end-systolic volume (ESV) at 18 months compared to baseline (Table 2) as described previously for post CABG patients24, 25.There was a significant increase of left ventricular stroke volume (77.92±6.21ml to 105.92±5.03ml at 18 months, p=0.001) that resulted in a significant improvement of the LVEF (from 41.2±4.9% at baseline to 51.3%±5.4 at 18 months, p=0.0002; an increase of 26.26±7.1% at 18 months, p=0.02). Importantly and in agreement with previous studies15, 26, 27 scar tissue mass was significantly decreased in the MSC treated group from 23.92±8.85g to 15.20±6.76g at 18 months (-47.5±8.1%; p<0.0001) accompanied with a simultaneous increase of viable tissue (from 93.95±9.98g to 126.86±18.04g at 18 months, p=0.02; an increase of 30.6±13.31% p=0.03; Figure 1). Increase in viable tissue correlated with the increase in LVEF (r=0.88, p=0.04) (Figure 2A). Scar size as a percent of the total LV mass decreased in all patients (from 19.36±5.93% to 9.37±3.57% at 18 months, p=0.004), corresponding to a 53.64±5.4% decrease (P<0.0001).
Table 2. Cardiac MRI parameters preoperatively and at 18 months.
| Cardiac MRI parameters | MSCs plus CABG (N=6) | ||
|---|---|---|---|
| Baseline | 18 months | P | |
| LV ejection fraction, mean (SEM), % | 41.16 (4.91) | 51.34 (5.38) | 0.0002 |
| End-diastolic volume, mean (SEM), mL | 191.42 (32.89) | 221.26 (38.73) | 0.005 |
| End-systolic volume, mean (SEM), mL | 119.78 (31.66) | 115.33 (34.84) | 0.03 |
| Stroke volume, mean (SEM), mL | 77.97 (6.21) | 105.92 (5.03) | 0.001 |
| Viable mass, mean (SEM), (g) | 94.03 (9.96) | 126.69 (18.10) | 0.02 |
| Scar mass, mean (SEM), g | 23.84 (8.87) | 15.37 (6.75) | <0.0001 |
| Scar as % of LV mass, mean (SEM), % | 19.27 (5.94) | 9.9 (3.56) | 0.004 |
Figure 1. Scar size reduction and increase of viable tissue due to intramyocardial injection of autologous bone marrow MSCs in conjunction with CABG.
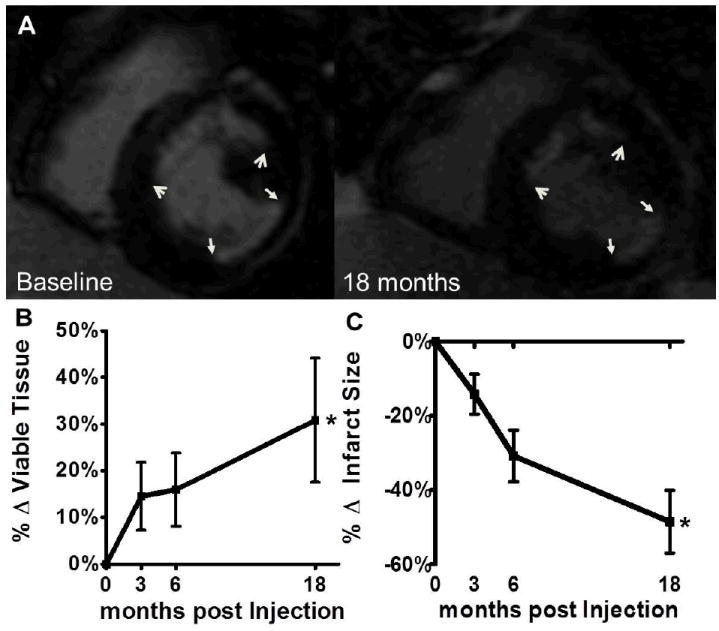
(Panel A) Delayed enhancement MRI scar size images show durable reduction in scar size in patients who underwent both CABG and autologous MSCs injection, 18 months post CABG. Arrows correspond to edges of anterior wall (open arrowheads) and inferolateral wall (closed arrowheads) infarct scars, both at baseline and at 18 months post MSCs injection during CABG (Panel B) There was a significant increase of viable tissue in CABG patients who received adjunct autologous MSCs injections compared to baseline. (Panel C) Infarct size was reduced with MSC therapy during CABG compared to baseline. *p<0.05, two way ANOVA repeated measures. MSCs indicate mesenchymal stem cells; CABG, coronary artery bypass grafting. Shown are mean±SEM at each time point.
Figure 2. Increase of viable tissue determines degree of left ventricular functional restitution with autologous MSC therapy and the number of injections and of cells injected in each segment the degree segmental scar size reduction.
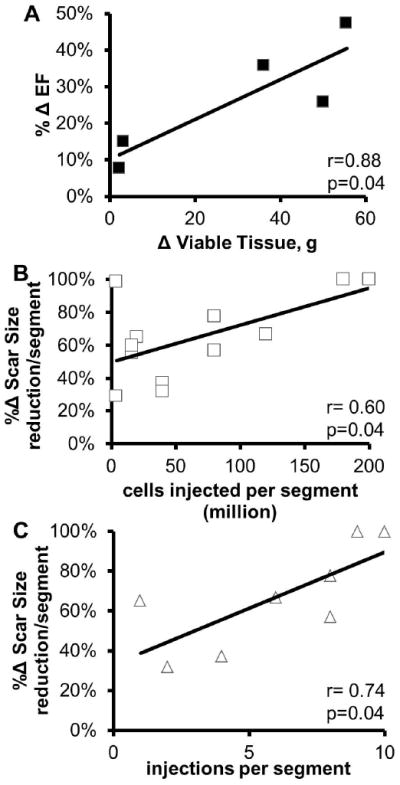
(Panel A) Correlation between the change in viable tissue from baseline (preinjection) to 18 months post-surgery with the percentage change in EF suggests that increase of viable tissue with MSC therapy drives functional improvement in ischemic cardiomyopathy.(Panel B) The number of MSC cells injected per segment correlated with the reduction in scar tissue (r=0.6, p=0.04). (Panel C) A similar correlation was observed between the number of injections each segment received and the reduction of scar size in the respective segment in the patients that received the higher dose of MSCs (r=0.74, p=0.04). MSC indicates mesenchymal stem cells; EF ejection fraction.
MSCs produce significant regional improvements in perfusion and contractility
Next, we examined the impact of MSCs injection on regional scar mass, tissue perfusion and regional contractility. We analyzed 384 segments from six patients at baseline, 3, 6 and 18 months. Then we identified segments that contained scar at baseline that received either only MSCs (by study design these segments were not revascularized; n=12), segments that represented territories that were only revascularized and not injected (n=63) and segments that were untreated (n=8). From the second group (revascularized only), we distinguished segments that were adjacent to the MSC-injected segments (n=16) and those that were remote (n=47; Table 3).
Table 3. Segmental analysis of cardiac MRI parameters at baseline in patients receiving MSCs injections during CABG.
| Cardiac MRI parameter | Injected (n=12) | All Revascularized (n=63) | Adjacent Revascularized (n=16) | Remote Revascularized (n=47) | p value |
|---|---|---|---|---|---|
| Scar tissue per segment, mean (SEM), g | 1.83 (0.55) | 1.74 (0.58) | 2.05 (0.75) | 1.71 (0.53) | 0.79 |
| Diastolic wall thickness, mean (SEM), mm | 6.44 (0.35) | 6.96 (0.72) | 6.42 (0.56) | 7.11 (0.86) | 0.42 |
| Systolic Wall Thickening, mean (SEM), % | 26.06 (5.01) | 32.9 (6.48) | 33.27 (7.93) | 32.77 (6.57) | 0.28 |
| Eulerian circumferential Strain at midwall, mean (SEM) | -17.26 (1.97) | -16.42 (1.38) | -17.45 (1.81) | -16.06 (1.46) | 0.32 |
| Average Upslope/LVBP, mean (SEM), % | 12.29 (0.44) | 13.55 (0.6) | 13.2 (0.74) | 13.8 (0.64) | 0.27 |
Scar tissue decreased at 18 months compared to baseline in both MSC only-treated segments (from 1.83±0.55g/segment to 0.98±0.36g/segment, p=0.003; a decrease of 57.55±7.24%, p=0.005) and revascularized only (from 1.74±0.58g/segment to 1.06±0.5g/segment, p=0.01; a decrease of 48.93±10.15%, p=0.01) but not in untreated segments (n=8, from 0.69±0.31g/segment to 0.64±0.29g/segment, p=0.21). Additionally, in MSC only-treated segments, tissue perfusion at rest rose (from 12.29±0.44% to 15.86±0.77%, p=0.02), end diastolic thickness improved (from 6.44±0.35mm to 7.50±0.53mm, p=0.04), end systolic wall thickening increased (from 26.06±5.01% to 53.22±9.4%, p=0.02), tissue tagging (Eulerian circumferential strain; Ecc) improved (from -17.26±1.97 to -22.01±2.42, p=0.04) and peak diastolic strain rate improved (from 0.28±0.06s-1 to 0.40±0.04s-1, p=0.03). Importantly, in MSC injected segments, contractility (tissue tagging) and perfusion increased to a level similar to non-scarred segments at 18 months after treatment (Online Figure II). There was a borderline improvement of systolic wall thickening in the revascularized only segments but no significant change in any of the rest of the metrics (perfusion, wall thickness, Ecc and peak diastolic strain rate). In the untreated segments no change was noted.
When the revascularized only segments were distinguished between those adjacent and those remote to the MSC injection sites there was a uniform decrease of scar tissue both adjacently and remotely to the injection sites at 18 months (-55.01±15.16%, p=0.03 and -48.14±12.68%, p=0.0007 vs. baseline respectively).The improvement in systolic wall thickening (p=0.048, vs. baseline) was noted preferentially in revascularized segments adjacent to MSC injection sites (from 33.27±7.93% to 57.78±9.71%, p=0.04).The rest of the metrics remained unchanged in both subgroups.
Concordance Index Score reveals MSCs’ extent of action around the injection sites
We indexed the concordance of regional improvement in function, scar reduction, and tissue perfusion with a CIS. At 18 months, the injected only segments had a significantly greater CIS compared to revascularized only and non-treated segments (2.93±0.07 vs. 0.50±0.21 vs. -0.07±0.34 respectively, p<0.0001). When segments were compared according to their proximity to the injection sites we found that at 18 months, the injected non-revascularized segments had a greater CIS than both the adjacent segments and remote segments (p<0.0001), and the segments adjacent to the site of MSC injection had a greater CIS compared to remote and non-treated segments (1.00±0.29 vs. 0.42±0.30 vs. -0.07±0.34 respectively, p=0.03; Figure 4).
Figure 4. Concordance Index Score is an indicator of simultaneous and comprehensive improvement.
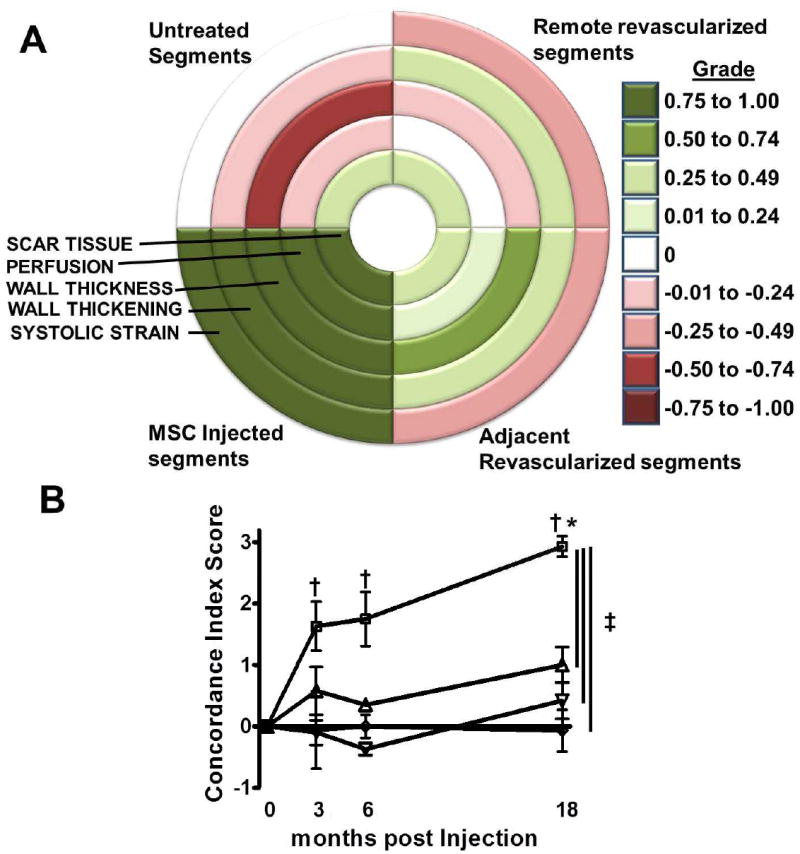
(Panel A) Bulls Eye map depicting the concordance of change for each variable used for the Concordance Index Score based on the average grade for each group at 18 months post treatment. A grade closer to 1 signifies a concordant improvement in the variable and a grade closer to -1 represents deterioration. The concordance index score is then derived by adding the grades for changes in scar tissue size, perfusion and the average of the grades for changes in wall thickness, wall thickening and systolic strain.The highest value (3) signifies simultaneous improvement in all 5 CMR indices and the lowest (-3) a simultaneous deterioration respectively. (Panel B) In the MSCs plus CABG group, the injected non-revascularized segments improved comprehensively and thus had a higher concordant improvement score compared to all other groups. The effect of the MSCs dissipated by a function of distance from the actual injection site (p=0.03 adjacent vs. remote revascularized and non-treated segments).□ =MSC Injected, ▲=Adjacent Revascularized, ▼=Remote Revascularized, ◇=untreated. *p<0.05 one way ANOVA repeated measures, †p<0.05 vs. baseline, Bonferroni posttests, ‡p<0.05 two way ANOVA
The number of cells injected correlates with the decrease of scar size
Each patient received an average of 11±1.3 injections (range: 8-20 injections) and each injected segment received an average of 5.6±0.9 injections (range 1-9 injections). There was a correlation between the number of cells injected in each segment and the observed decrease in segmental scar mass (r=0.6, p=0.04; Figure 2). A similar correlation was observed between the number of injections per segment and the reduction of scar size in that segment in the patients that received the highest dose of MSCs (r=0.74, p=0.04; Figure 2). The improvement in circumferential strain or perfusion did not correlate with the cell dose.
Impact of infarct transmurality
Out of twelve infarct areas that were injected with MSCs, 4 were characterized by transmural scar (>50%) and 8 were non-transmural. The reduction in infarct size at 18 months was not different (between groups p=0.35) in transmural (-52±11.4%, p=0.02) compared with non-transmural infarcts (-67±9.2%, p=0.001) at 18 months. The same was true for all other metrics where the transmural infarcts exhibited a similar behavior to their non-transmural counterparts (online Table IV).
Topographic Analysis of the Injected Infarcted Segments
Injection of MSCs produced reductions in both thickness and circumferential extent of infarct scar, at 18 months. Both endocardial length (distance of the endocardium occupied by scar; from 32.3±3.5mm at baseline to 18.18±2.25mm at 18 months, p=0.002) and scar thickness (from 4.6±0.42mm at baseline to 2.72±0.53mm at 18 months, p=0.005) decreased significantly at 18 months post therapy. There was also an increase in the epicardial cap thickness (radial length of viable tissue that occupied space between scar and epicardial border; from 2.7±0.3mm at baseline to 5.26±0.38mm at 18 months, p<0.001; Figure 5)
Figure 5. Topographic Analyses of MSCs Injected Scarred Segments.
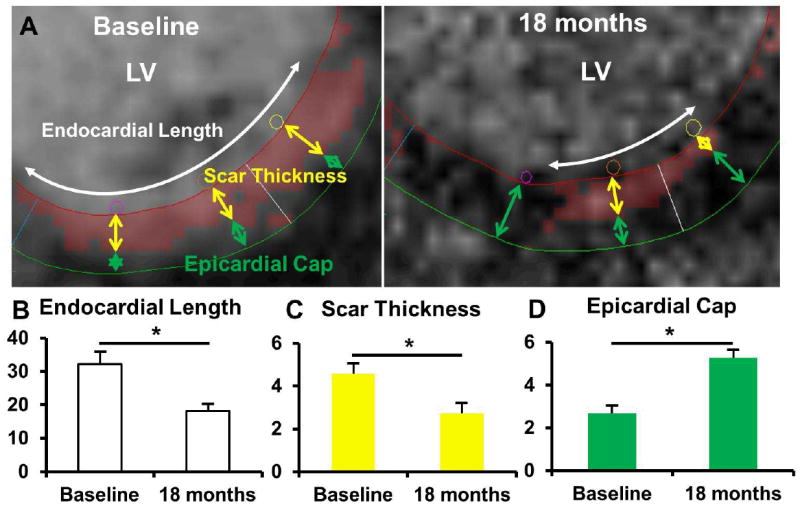
(Panel A) Topographic analysis of a late contrast enhanced image that depicts scar (red color). The total endocardial length (white double headed arrow) of the scar was measured at baseline. The center of the scar on the endocardial border was then noted and scar thickness was measured (distance from the endocardial contour to the distal border of the scar; yellow double headed arrows) at that point. The distance from the epicardial contour to the distal border of the scarred area was also measured at this point (Epicardial Cap; green double headed arrows). Then the same measurements were repeated at half the length from the lateral edges of the scar. The final values for each scar were derived by averaging the respective measurements. The same points were then identified at a slice in the same area at 18 months and the calculations were repeated. (Panel B). At 18 months the endocardial length decreased. (Panel C) Scar thickness also decreased at 18 months. (Panel D) There was also an increase in the epicardial cap thickness at 18 months. *p<0.05, paired t-test.
Role of collaterals in perfusion
From data acquired during the patients’ pre-surgery catheterizations and coronary angiograms collateral vessels were visualized in four patients. To eliminate a major role28, 29 of collateral circulation on the increase in tissue perfusion, we analyzed perfusions excluding 10 segments that contained scar at baseline (2 that were injected only and 8 that were only revascularized). Perfusion increase was significantly different in the remaining 10 injected only segments that were not perfused by collaterals, compared to the remaining 55 revascularized only segments (36.6±13.6% vs 6.3±4.8% respectively, p=0.04).
Discussion
Our study addresses the effects of autologous MSC injections in non-revascularized infarcted areas of the left ventricle in patients with LV dysfunction undergoing CABG. We show that MSCs orchestrate a comprehensive improvement in scar reduction, tissue perfusion and regional function that occurs predominantly at the site of MSC injection. These findings suggest that MSCs exert their benefits predominantly at a regional level, providing important guidance for the use of this strategy clinically. Additional phenotypic improvement, albeit to a lesser degree, occurs remotely, supporting the possibility of paracrine effects17, 30. Importantly in this study the regional effects were of sufficient magnitude to drive a major increase in ejection fraction.
Our findings are in agreement with earlier studies of cell-based therapy in the setting of cardiac surgery, in which increases in LVEF2 and perfusion3, decrease of scar tissue12 and increased wall motion and contractility31 compared to patients undergoing only CABG are reported. Other studies, however, such as that of Hendrikx at al5, confirmed the safety of autologous bone marrow transplantation during CABG, but did not demonstrate additional benefits beyond surgical revascularization. Taken together in totality, the aforementioned studies, although having different designs and employing different cell populations, dosages and delivery techniques, tended to show that bone marrow cell derivatives offer a benefit over that of CABG alone. In our study we have shown that there was significant decrease of scar tissue, a corresponding increase in viable tissue and an increase in regional contractile performance (as measured by both systolic wall thickening and tissue tagging), which together yielded a significant increase of global LVEF. The seemingly paradoxical simultaneous increase in EDV and LVEF, which is well described in other trials post-CABG24, 25, 32, is attributable to the interactions of an intact pericardium (or the lack thereof post-surgery) and the ventricular hemodynamics33 without directly affecting the contractile performance32, 34.
Our findings also agree and offer perspective on our previously published POSEIDON trial15 in which we also found a marked reduction in scar tissue in response to catheter delivery of autologous or allogeneic MSCs. In a subanalysis of POSEIDON35, scar tissue reduced in both the injected and the non-injected segments but the segmental EF was improved only in the injected segments. Interestingly in POSEIDON, we observed an increase in LVEF only in the group of patients receiving a low dose (20 M) of MSCs. This effect is likely attributable to cell concentration rather than cell number, per se. The findings here of a dramatic increase in LVEF support this contention as the cell concentration was lower, and the delivery technique used here obviated the passage of a highly concentrated MSC suspension through the bore of the catheter. A significant decrease of scar size and an improvement in contractility (peak Ecc) at the site of injection was also observed in the Transendocardial Autologous Cells (hMSC or hBMC) in Ischemic Heart Failure Trial (TAC-HFT)16. Importantly, both the POSEIDON and TAC-HFT trials document improvement in clinical outcomes (6-minute walk test and Minnesota Living with Heart Failure Questionnaire) in patients receiving intramyocardial cell therapy.
From preclinical and translational studies in an acute or subacute ischemic environment, it is known that MSCs act in a multifactorial manner36-39. MSCs release anti-fibrotic matrix metaloproteases, modulate cardiac stem cell proliferation and differentiation9, stimulate neovascularization17, 22,40, establish intercellular coupling with cardiomyocytes41, mediate Akt-dependant change in calcium and upregulate C×4342. All these mechanisms can explain scar reduction, improved perfusion and contractility. In a recently published preclinical study26 from our group, where allogeneic MSCs were employed in a swine model of chronic ischemic left ventricular dysfunction, infarct size reduction was the driving force of the reversal of remodeling that also led to an increase in LVEF.
The mechanism(s) of action of transplanted MSCs during CABG remain poorly delineated. In such a setting43, 44, the inflammation associated with an acute MI has subsided, fibrous tissue and remodeling have ensued and the extent of hibernating myocardial tissue varies. All previous study designs targeted scarred areas that were revascularized, either by using the actual revascularized vessel or by injecting in the proximity of the revascularized region. The design of our study specifically targeted regions of scar that were not eligible for revascularization, thus enabling the study of differences in the behavior of scar that was either injected and not revascularized vs. areas that were revascularized and did not receive MSCs. We acknowledge that there is an inherent limitation in the segmental analysis approach given that the segments of the heart are not insulated from one another. The employment of MRI for the assessment of diastolic function still remains a controversial issue45 and criteria for MRI-derived diastolic function indices still need to be determined. Finally, the lack of a control group constitutes an important limitation to this study reducing the conclusiveness of our results. Nevertheless these findings are hypothesis-generating and can guide the design of future trials.
Despite these limitations, our data support that injected segments respond substantially better compared with the rest of the heart, despite the lack of regional revascularization. We also show a progressive drop-off in regional phenotypic improvement as distance from injection site increases. Together these findings are of important clinical value for designing and refining the strategy of incorporating cell based therapy into surgical revascularization.
Conclusion
Together, these findings are consistent with the finding that MSCs act preferentially at the site of injection. In addition, there is a radius of action of the injected MSCs with a drop-off of activity as a function of distance from injection site. These findings coupled with the correlation of the number of injections and of the cells injected per segment with phenotypic outcome have mechanistic and therapeutic implications. Whereas cell dosing15, 27 and timing46 of stem cell therapy have been previously addressed, the present study illustrates the importance of the location and delivery method in the algorithm of achieving an optimal response to cell therapy in chronically scarred myocardium. Furthermore, employing sophisticated imaging (MRI or CT) for identifying scarred areas coupled with a strategy to treat as much area of the scarred myocardium as possible is likely to contribute to the success of cell-based therapy.
Supplementary Material
Figure 3. MSCs injections lead to regional improvement.
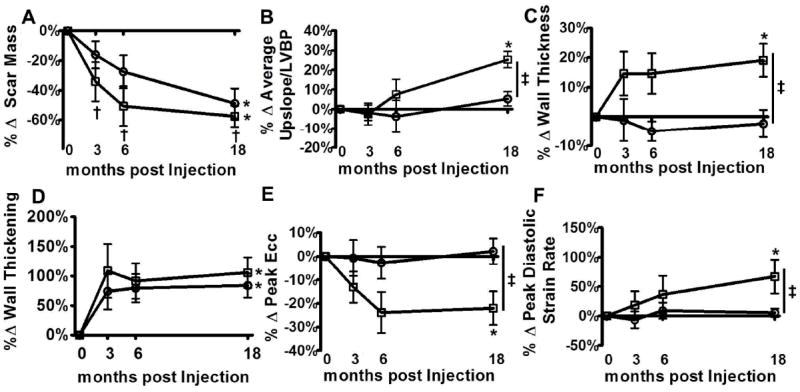
(Panel A) Infarct size was decreased significantly at 3 months that was sustained at 6 and at 18 months in the injected only segments. In the revascularized only segments, the decrease of the infarct size became significant at 18 months. (Panel B) The average upslope corrected for the left ventricular blood pool intensity is a measure of regional perfusion. After MSCs injection there was a significant increase of perfusion in the injected segments that differed significantly from the revascularized only segments.(Panel C) MSCs injections led to a significant regional increase of wall thickness compared to the revascularized only segments. (Panel D) Systolic wall thickening improved in the injected segments and in the revascularized only segments.(Panel E) Peak Ecc is a measure of contractility with lower values signifying better contractility. There was significant improvement of peak Ecc at the injected segments over time and compared to revascularized only segments. (Panel F) Peak diastolic strain rate is an index of diastolic function and a higher value signifies improved diastolic function. There was a significant improvement in diastolic function at the injected segments compared to revascularized only. □ = MSC injected only segments, ○ = revascularized only segments *p<0.05 one way ANOVA repeated measures, †p<0.05 vs. baseline, Bonferroni posttests, ‡p<0.05 two way ANOVA. Shown are mean±SEM at each time point.
What Is Known?
Bone marrow stem cells (MSCs) reduce infarct size, improve regional function, and contribute to reverse remodeling in ischemic cardiomyopathy.
There are at least three dominant mechanisms underlying the improved cardiac phenotype following MSC administration: anti-fibrotic effects, neovascularization, and neomyogenesis.
Accumulating clinical evidence support that MSC can accentuate cardiac structural and functional responses when delivered during CABG.
What New Information Does This Article Contribute?
MSCs orchestrate a concordant improvement in cardiac structure by producing scar reduction, enhanced regional function, and improved tissue perfusion with effects occurring predominantly at sites of cell injection.
There is a progressive drop-off in regional phenotypic improvement as a function of distance from injection sites.
The employment of sophisticated MRI imaging allows for the detailed identification of scarred areas facilitating the targeting of treatment sites as well as for non-invasive assessment of the mechanisms of action of MSCs in humans.
This study employed MRI imaging to independently assess regional changes in several important phenotypic parameters occurring in the ventricle in response to MSC injection. The study was designed to distinguish the effects of MSC injections from revascularization, per se, by selecting sites of injection as hypokinetic/akinetic segments that were not amenable for revascularization for clinical reasons. Our data show that injected segments respond substantially better compared to the rest of the heart, despite the lack of regional revascularization. We also show a progressive drop-off in regional phenotypic improvement as a function of distance from injection site. This study offers important insights into the mechanism of action of MSC therapy and provides novel guidance for cell injection strategies as future trials are planned for cell therapy for patients with ischemic heart disease.
Acknowledgments
Sources of Funding
This study was funded by the Specialized Center for Cell Therapeutics award (HL081028). Dr. Hare is also supported by NIH grants: RO1 HL110737-01, R01 HL107110, R01 HL094849, CCTRN UM1 HL113460, and R01HL084275. Dr. Karantalis is supported by an award from the American Heart Association.
Non-standard Abbreviations and Acronyms
- MI
Myocardial Infarction
- CABG
Coronary Artery Bypass Graft
- MSCs
Mesenchymal Stem Cells
- LVEF
Left Ventricular Ejection Fraction
- DE
Delayed Enhancement
- NYHA
New York Heart Association
- ANOVA
Analysis Of Variance
- CIS
Concordant Improvement Score
Footnotes
Disclosures
Drs. Hare and Heldman disclose a relationship with Vestion that includes equity, board membership, and consulting. Vestion did not contribute funding to this study. Dr. Hare discloses equity in Kardia and a grant from Biocardia.
References
- 1.Zhao Q, Ye X. Additive value of adult bone-marrow-derived cell transplantation to conventional revascularization in chronic ischemic heart disease: A systemic review and meta-analysis. Expert Opin Biol Ther. 2011;11:1569–1579. doi: 10.1517/14712598.2011.616491. [DOI] [PubMed] [Google Scholar]
- 2.Patel AN, Geffner L, Vina RF, Saslavsky J, Urschel HC, Jr, Kormos R, Benetti F. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: A prospective randomized study. J Thorac Cardiovasc Surg. 2005;130:1631–1638. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 3.Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski D, Freund M, Steinhoff G. Intramyocardial delivery of cd133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 4.Chachques JC, Acar C, Herreros J, Trainini JC, Prosper F, D’Attellis N, Fabiani JN, Carpentier AF. Cellular cardiomyoplasty: Clinical application. Ann Thorac Surg. 2004;77:1121–1130. doi: 10.1016/j.athoracsur.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 5.Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: Results from a randomized controlled clinical trial. Circulation. 2006;114:I101–107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 6.Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Li T, Wei X, Bianchi G, Hu J, Sanchez PG, Xu K, Zhang P, Pittenger MF, Wu ZJ, Griffith BP. Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction. Stem Cells Transl Med. 2012;1:685–695. doi: 10.5966/sctm.2012-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki G, Iyer V, Lee TC, Canty JM., Jr Autologous mesenchymal stem cells mobilize ckit+ and cd133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 11.Wendel JS, Ye L, Zhang P, Tranquillo RT, Zhang J. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A. 2013 doi: 10.1089/ten.tea.2013.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu S, Liu S, Zheng Z, et al. Isolated coronary artery bypass graft combined with bone marrow mononuclear cells delivered through a graft vessel for patients with previous myocardial infarction and chronic heart failure: A single-center, randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol. 2011;57:2409–2415. doi: 10.1016/j.jacc.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 13.Gokhale AG, Chelluri LK, Kumaresan K, Subramanyam G, Sudhakar K, Vemuri S, Debnath T, Ratnakar KS. Evaluation of the autologous bone marrow mononuclear therapy and functional restoration in the scarred myocardium by imaging analysis. J Cardiovasc Dis Res. 2011;2:133–136. doi: 10.4103/0975-3583.83037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva JS, Hare JM. Cell-based therapies for myocardial repair: Emerging role for bone marrow-derived mesenchymal stem cells (MSCs) in the treatment of the chronically injured heart. Methods Mol Biol. 2013;1037:145–163. doi: 10.1007/978-1-62703-505-7_8. [DOI] [PubMed] [Google Scholar]
- 15.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldman AW, Difede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azevedo CF, Amado LC, Kraitchman DL, Gerber BL, Osman NF, Rochitte CE, Edvardsen T, Lima JA. Persistent diastolic dysfunction despite complete systolic functional recovery after reperfused acute myocardial infarction demonstrated by tagged magnetic resonance imaging. Eur Heart J. 2004;25:1419–1427. doi: 10.1016/j.ehj.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Becker LC, Schuster EH, Jugdutt BI, Hutchins GM, Bulkley BH. Relationship between myocardial infarct size and occluded bed size in the dog: Difference between left anterior descending and circumflex coronary artery occlusions. Circulation. 1983;67:549–557. doi: 10.1161/01.cir.67.3.549. [DOI] [PubMed] [Google Scholar]
- 21.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 22.Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 23.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samady H, Elefteriades JA, Abbott BG, Mattera JA, McPherson CA, Wackers FJ. Failure to improve left ventricular function after coronary revascularization for ischemic cardiomyopathy is not associated with worse outcome. Circulation. 1999;100:1298–1304. doi: 10.1161/01.cir.100.12.1298. [DOI] [PubMed] [Google Scholar]
- 25.Mintz LJ, Ingels NB, Jr, Daughters GT, 2nd, Stinson EB, Alderman EL. Sequential studies of left ventricular function and wall motion after coronary arterial bypass surgery. Am J Cardiol. 1980;45:210–216. doi: 10.1016/0002-9149(80)90637-2. [DOI] [PubMed] [Google Scholar]
- 26.Williams AR, Suncion VY, McCall F, Guerra D, Mather J, Zambrano JP, Heldman AW, Hare JM. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J Am Heart Assoc. 2013;2:e000140. doi: 10.1161/JAHA.113.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 29.Werner GS, Emig U, Mutschke O, Schwarz G, Bahrmann P, Figulla HR. Regression of collateral function after recanalization of chronic total coronary occlusions: A serial assessment by intracoronary pressure and doppler recordings. Circulation. 2003;108:2877–2882. doi: 10.1161/01.CIR.0000100724.44398.01. [DOI] [PubMed] [Google Scholar]
- 30.Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2013 doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Liu S, Zheng Z, Yin G, Song L, Chen H, Chen X, Chen Q, Jiang S, Tian L, He Z, Hu S, Zhao S. A pilot trial of autologous bone marrow mononuclear cell transplantation through grafting artery: A sub-study focused on segmental left ventricular function recovery and scar reduction. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2013.01.217. [DOI] [PubMed] [Google Scholar]
- 32.Tischler MD, Rowan M, LeWinter MM. Increased left ventricular mass after thoracotomy and pericardiotomy. A role for relief of pericardial constraint? Circulation. 1993;87:1921–1927. doi: 10.1161/01.cir.87.6.1921. [DOI] [PubMed] [Google Scholar]
- 33.Smiseth OA, Kingma I, Refsum H, Smith ER, Tyberg JV. The pericardial hypothesis: A mechanism of acute shifts of the left ventricular diastolic pressure-volume relation. Clin Physiol. 1985;5:403–415. doi: 10.1111/j.1475-097x.1985.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Q, Du F, Zhu X, Zhang P, Suntharalingam P, Ippolito J, Kamdar FD, Chen W, Zhang J. Atp production rate via creatine kinase or atp synthase in vivo: A novel superfast magnetization saturation transfer method. Circ Res. 2011;108:653–663. doi: 10.1161/CIRCRESAHA.110.231456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suncion VY, Ghersin E, Fishman J, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally? An analysis from the POSEIDON randomized trial. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naftali-Shani N, Itzhaki-Alfia A, Landa-Rouben N, et al. The origin of human mesenchymal stromal cells dictates their reparative properties. J Am Heart Assoc. 2013;2:e000253. doi: 10.1161/JAHA.113.000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM. Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol. 2012;303:H256–270. doi: 10.1152/ajpheart.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong Q, Ye L, Zhang P, Lepley M, Swingen C, Zhang L, Kaufman DS, Zhang J. Bioenergetic and functional consequences of cellular therapy: Activation of endogenous cardiovascular progenitor cells. Circ Res. 2012;111:455–468. doi: 10.1161/CIRCRESAHA.112.269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishii M, Shibata R, Numaguchi Y, Kito T, Suzuki H, Shimizu K, Ito A, Honda H, Murohara T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler Thromb Vasc Biol. 2011;31:2210–2215. doi: 10.1161/ATVBAHA.111.231100. [DOI] [PubMed] [Google Scholar]
- 41.Mureli S, Gans CP, Bare DJ, Geenen DL, Kumar NM, Banach K. Mesenchymal stem cells improve cardiac conduction by upregulation of connexin 43 through paracrine signaling. Am J Physiol Heart Circ Physiol. 2013;304:H600–609. doi: 10.1152/ajpheart.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeSantiago J, Bare DJ, Semenov I, Minshall RD, Geenen DL, Wolska BM, Banach K. Excitation-contraction coupling in ventricular myocytes is enhanced by paracrine signaling from mesenchymal stem cells. J Mol Cell Cardiol. 2012;52:1249–1256. doi: 10.1016/j.yjmcc.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canty JM, Jr, Suzuki G. Myocardial perfusion and contraction in acute ischemia and chronic ischemic heart disease. J Mol Cell Cardiol. 2012;52:822–831. doi: 10.1016/j.yjmcc.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizzello V, Poldermans D, Bax JJ. Assessment of myocardial viability in chronic ischemic heart disease: Current status. Q J Nucl Med Mol Imaging. 2005;49:81–96. [PubMed] [Google Scholar]
- 45.Westenberg JJ. Cmr for assessment of diastolic function. Curr Cardiovasc Imaging Rep. 2011;4:149–158. doi: 10.1007/s12410-011-9070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


