Abstract
The ureABC genes of Mycobacterium tuberculosis were cloned. By using a set of degenerate primers corresponding to a conserved region of the urease enzyme (EC 3.5.1.5), a fragment of the expected size was amplified by PCR and was used to screen a M. tuberculosis cosmid library. Three open reading frames with extensive similarity to the urease genes from other organisms were found. The locus was mapped on the chromosome, using an ordered M. tuberculosis cosmid library. A suicide vector containing a ureC gene disrupted by a kanamycin marker (aph) was used to construct a urease-negative Mycobacterium bovis bacillus Calmette-Guérin mutant by allelic exchange involving replacement of the ureC gene with the aph::ureC construct. To our knowledge, allelic exchange has not been reported previously in the slow-growing mycobacteria. Homologous recombination will be an invaluable genetic tool for deciphering the mechanisms of tuberculosis pathogenesis, a disease that causes 3 x 10(6) deaths a year worldwide.
Full text
PDF


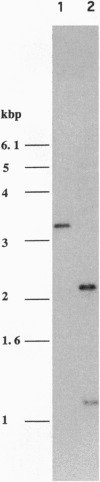
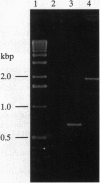
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Husson R. N., Young R. A. The uraA locus and homologous recombination in Mycobacterium bovis BCG. J Bacteriol. 1993 Nov;175(22):7282–7289. doi: 10.1128/jb.175.22.7282-7289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Collins C. M., D'Orazio S. E. Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol Microbiol. 1993 Sep;9(5):907–913. doi: 10.1111/j.1365-2958.1993.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Davis E. O., Jenner P. J., Brooks P. C., Colston M. J., Sedgwick S. G. Protein splicing in the maturation of M. tuberculosis recA protein: a mechanism for tolerating a novel class of intervening sequence. Cell. 1992 Oct 16;71(2):201–210. doi: 10.1016/0092-8674(92)90349-h. [DOI] [PubMed] [Google Scholar]
- Davis E. O., Sedgwick S. G., Colston M. J. Novel structure of the recA locus of Mycobacterium tuberculosis implies processing of the gene product. J Bacteriol. 1991 Sep;173(18):5653–5662. doi: 10.1128/jb.173.18.5653-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. O., Thangaraj H. S., Brooks P. C., Colston M. J. Evidence of selection for protein introns in the recAs of pathogenic mycobacteria. EMBO J. 1994 Feb 1;13(3):699–703. doi: 10.1002/j.1460-2075.1994.tb06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin P. J., Raviglione M. C., Kochi A. Global tuberculosis incidence and mortality during 1990-2000. Bull World Health Organ. 1994;72(2):213–220. [PMC free article] [PubMed] [Google Scholar]
- Eaton K. A., Brooks C. L., Morgan D. R., Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991 Jul;59(7):2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero R. L., Labigne A. Cloning, expression and sequencing of Helicobacter felis urease genes. Mol Microbiol. 1993 Jul;9(2):323–333. doi: 10.1111/j.1365-2958.1993.tb01693.x. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Tuckman M., Bloom B. R. Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature. 1987 Jun 11;327(6122):532–535. doi: 10.1038/327532a0. [DOI] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol. 1988 Aug;170(8):3342–3349. doi: 10.1128/jb.170.8.3342-3349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: nucleotide sequence determination and comparison with jack bean urease. J Bacteriol. 1989 Dec;171(12):6414–6422. doi: 10.1128/jb.171.12.6414-6422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpana G. V., Bloom B. R., Jacobs W. R., Jr Insertional mutagenesis and illegitimate recombination in mycobacteria. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5433–5437. doi: 10.1073/pnas.88.12.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Timm J., Rauzier J., Gomez-Lus R., Davies J., Gicquel B. Transposition of an antibiotic resistance element in mycobacteria. Nature. 1990 Jun 21;345(6277):739–743. doi: 10.1038/345739a0. [DOI] [PubMed] [Google Scholar]
- McAdam R. A., Weisbrod T. R., Martin J., Scuderi J. D., Brown A. M., Cirillo J. D., Bloom B. R., Jacobs W. R., Jr In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect Immun. 1995 Mar;63(3):1004–1012. doi: 10.1128/iai.63.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L., David H. L. Evaluation de l'activité uréase et de l'activité beta-glucosidase pour l'identification pratique des mycobactéries. Ann Microbiol (Paris) 1979 Oct;130B(3):323–332. [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney S. B., Lynch M. J., Mobley H. L., Hausinger R. P. Purification, characterization, and genetic organization of recombinant Providencia stuartii urease expressed by Escherichia coli. J Bacteriol. 1988 May;170(5):2202–2207. doi: 10.1128/jb.170.5.2202-2207.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. J., Styblo K., Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990 Mar;65(1):6–24. [PubMed] [Google Scholar]
- Otal I., Martín C., Vincent-Lévy-Frebault V., Thierry D., Gicquel B. Restriction fragment length polymorphism analysis using IS6110 as an epidemiological marker in tuberculosis. J Clin Microbiol. 1991 Jun;29(6):1252–1254. doi: 10.1128/jcm.29.6.1252-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I. S., Carr M. B., Hausinger R. P. In vitro activation of urease apoprotein and role of UreD as a chaperone required for nickel metallocenter assembly. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3233–3237. doi: 10.1073/pnas.91.8.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddles P. W., Whan V., Blakeley R. L., Zerner B. Cloning and sequencing of a jack bean urease-encoding cDNA. Gene. 1991 Dec 15;108(2):265–267. doi: 10.1016/0378-1119(91)90443-f. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Lugosi L., Jekkel A., Melton R. E., Kieser T., Bloom B. R., Jacobs W. R., Jr Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6987–6991. doi: 10.1073/pnas.85.18.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Melton R. E., Mustafa S., Kieser T., Jacobs W. R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990 Nov;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Sriwanthana B., Island M. D., Maneval D., Mobley H. L. Single-step purification of Proteus mirabilis urease accessory protein UreE, a protein with a naturally occurring histidine tail, by nickel chelate affinity chromatography. J Bacteriol. 1994 Nov;176(22):6836–6841. doi: 10.1128/jb.176.22.6836-6841.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L. A., Rose R. E. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 1988 Jan 11;16(1):358–358. doi: 10.1093/nar/16.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Brisson-Noël A., Vincent-Lévy-Frébault V., Nguyen S., Guesdon J. L., Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990 Dec;28(12):2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm J., Lim E. M., Gicquel B. Escherichia coli-mycobacteria shuttle vectors for operon and gene fusions to lacZ: the pJEM series. J Bacteriol. 1994 Nov;176(21):6749–6753. doi: 10.1128/jb.176.21.6749-6753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]