Abstract
Human herpesvirus-8 (HHV8)-positive effusion-based lymphomas have been termed primary effusion lymphoma (PEL) in the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Kaposi sarcoma herpesvirus (KSHV)/ HHV8-negative effusion-based lymphomas (KSHV/HHV8-negative EBLs) resembling PELs have been reported in the literature and in many cases have been (mis)classified as PEL-like lymphomas. Herein, we present a series of cases and a review of KSHV/HHV8-negative EBLs. This lymphoma, although cytomorphologically resembling PEL, is a distinct entity with characteristic clinical and pathologic features. Patients are older, generally human immunodeficiency virus negative and not immunosuppressed, frequently hepatitis C positive compared with the population baseline, and often have an underlying medical condition leading to fluid overload. The lymphoma cells express pan-B-cell antigens in 86.7%, and CD20 is expressed in 71.1% of the cases. The lymphoma is often of germinal center B or mixed germinal center B/activated B-cell signature with the Hans classifier, and Epstein-Barr virus is positive in nearly 30% of cases. Rare T-cell lymphomas were also reported. Clinical outcomes and response to therapy, including isolated aspiration, are relatively favorable compared with cases of PEL. We suggest that HHV8-negative effusion-based lymphoma is a distinct entity associated with fluid overload states.
Keywords: KSHV/HHV8-associated lymphoma, PEL-like lymphoma, primary effusion lymphoma, body cavity lymphoma, HHV8/KSHV-unrelated lymphoma, effusion lymphoma
Primary effusion lymphoma (PEL) is an uncommon, high-grade, large cell non-Hodgkin lymphoma typically of B-cell lineage that most often presents in immunocompromised patients as serous effusions without a primary tumor mass. Within the World Health Organization (WHO) classification schema, PEL remains unique, as its definition universally mandates the presence of Kaposi sarcoma herpesvirus (KSHV), also named human herpesvirus-8 (HHV8), in neoplastic cells.1 Morphologically, PEL is characterized by large pleomorphic cells displaying myriad appearances from immunoblastic to anaplastic. The characteristic immunophenotypic profile lacks pan-B-cell markers such as CD19, CD20, and CD79a, and both surface and cytoplasmic immunoglobulin with preservation of immunoglobulin heavy-chain hypermutation and lack of MYC gene rearrangements.1,2
Numerous reports have emerged in the last 15 years on KSHV/HHV8-negative effusion-based lymphomas (KSHV/HHV8-negative EBL), which demonstrate remarkably similar cytomorphologic characteristics to PEL but differ in immunophenotype, demographics, response to treatment, and clinical outcome. However, without standardized nomenclature to categorize these entities, terms such as PEL-like lymphoma, HHV8-unrelated PEL, body cavity–based high-grade lymphoma, and others have been used in the literature to describe these lymphomas. The resulting confusion and nonuniform diagnoses have thwarted accurate assessment of the pathogenesis, natural history, treatment, and prognosis of such lesions; this situation is made no less frustrating by the abysmal prognosis of classic PEL compared with reportedly better prognosis in PEL-like lymphomas.
Herein we discuss a series of 5 patients seen at our institution, as well as the largest literature review to date of the aforementioned entity, designated as KSHV/ HHV8-negative EBL. We believe that this represents a clinically and pathologically distinct entity compared with PEL; as such, its diagnostic criteria and nomenclature should be standardized in the interest of maintaining international uniformity.
MATERIALS AND METHODS
Cases in the literature were collected through a retrospective analysis of all reports indexed on Pubmed using a combination of the following search terms in both MeSH and non-MeSH queries: Primary Effusion Lymphoma; PEL; KSHV/HHV8 negative effusion lymphoma; Human Herpes Virus 8 lymphoma; and body cavity lymphoma. Cases were included in our study if they met the following inclusion criteria: lack of associated primary solid lymphoid malignancies; cytomorphologic features resembling PEL; and lack of KSHV/HHV8 expression.3–36 Six cases were excluded because their cytomorphology resembled that of Burkitt lymphoma and/or because of the presence of t(8:22)(q24;q11). We identified 40 cases from the published data and added a series of 5 new cases collected from our institution, bringing the total number of cases to 45.
Individual features of each case were studied, including patient demographics, clinical presentation and site of effusion, lymphoma morphology, immunophenotype, cytogenetic/molecular characteristics, method of therapeutic intervention, and eventual outcome (Tables 1–3). Cases are listed in order of their appearance in the literature and/or our institution.
TABLE 1.
Patient Demographics
| Case | References | Age | Sex | Clinical History | Effusion | HCV | HIV |
|---|---|---|---|---|---|---|---|
| 1 | Hermine et al3 | 52 | F | NA | PL, PC | NA | − |
| 2 | Carbone et al4 | 58 | M | NA | PL, PT | NA | + |
| 3 | Carbone et al4 | 90 | M | NA | PL | NA | − |
| 4 | Ichinohasama et al5 | 63 | M | HCV-C; HCC | PT | + | − |
| 5 | Rodriguez et al6 | 65 | M | EtOH-C; peritonitis, longstanding ascites | PT | − | − |
| 6 | Ashihara et al7 | 60 | F | Cholesteatoma 30 y earlier with VPS tube | PT | − | − |
| 7 | Hara et al8 | 65 | M | HCV-C | PT | + | − |
| 8 | Yamamoto et al9 | 72 | F | NA | PL, PT | NA | − |
| 9 | Ohori et al10 | 70 | M | HBV-C, s/p OLT 12 y earlier; recurrent HBV-C | PL | NA | NA |
| 10 | Saiki et al11 | 58 | F | DM; hypothyroidism | PT | + | − |
| 11 | Ohshima et al12 | 75 | M | NA | PL | NA | − |
| 12 | Ohshima et al12 | 32 | F | PLE | PT | NA | − |
| 13 | Ohshima et al12 | 81 | M | NA | PL | NA | − |
| 14 | Paner et al13 | 58 | M | HCV-C | PT | + | − |
| 15 | Hisamoto et al14 | 58 | F | CVID | PL, PC | − | − |
| 16 | Shimazaki et al15 | 90 | F | AF; orthopnea | PL | − | − |
| 17 | Nakamura et al16 | 51 | M | NA | Scrotal | − | − |
| 18 | Chiba et al17 | 55 | M | NA | PT | − | − |
| 19 | Inoue et al18 | 70 | F | DOE | PL, PC | − | − |
| 20 | Takao et al19 | 74 | F | HCV-C; allergic granulomatous angitis | PL, PC | + | − |
| 21 | Nonami et al20 | 32 | F | PLE; lymphangiomas; repeated systemic edema and years of chylous ascites | PL, PT | + | − |
| 22 | Fujiwara et al21 | 75 | F | NA | PC | − | − |
| 23 | Jenkins et al22 | 61 | M | EtOH-C | PT | − | − |
| 24 | Matsumoto et al23 | 90 | M | Pulmonary tuberculosis without chronic pyothorax or pneumothorax | PL | − | − |
| 25 | Matsumoto et al23 | 87 | F | WNL | PL | − | − |
| 26 | Venizelos et al24 | 27 | F | Renal transplant 5 y earlier | PT | NA | − |
| 27 | Youngster et al25 | 88 | M | Ischemic heart disease | PL | − | − |
| 28 | Terasaki et al26 | 68 | M | WNL | PL | − | − |
| 29 | Niino et al27 | 78 | M | WNL | PL, PC | − | − |
| 30 | Adiguzel et al28 | 89 | M | CAD; diabetes; HTN | PL | − | − |
| 31 | Tsagarakis et al29 | 77 | M | MI; prostate cancer s/p radiation | PL | − | − |
| 32 | De Filippi et al30 | 69 | M | HCV-C; renal cancer | PL, PT | + | − |
| 33 | Taira et la31 | 68 | F | NA | PL, PC | − | − |
| 34 | Takahashi et al32 | 82 | M | NA | PL, PC | − | − |
| 35 | Takahashi et al32 | 73 | M | NA | PL, PC, PT | − | − |
| 36 | Cooper et al33 | 44 | F | OA; DM; asthma; cholecystitis; EtOH abuse | PL | + | + |
| 37 | Kagoya et al34 | 74 | M | DOE | PC | − | − |
| 38 | Wang et al35 | 79 | M | HTN; OA; AD; DOE | PL | − | − |
| 39 | Terasaki et al36 | 99 | F | WNL | PL, PC | − | − |
| 40 | Terasaki et al36 | 85 | M | HTN; AF | PL, PC | − | − |
| 41 | This study | 85 | F | CAD; MI s/p CABG | PL | NA | NA |
| 42 | This study | 29 | M | Complex congenital heart disease s/p multiple surgeries; CHF; cirrhosis | PT | − | − |
| 43 | This study | 45 | M | EtOH-C; s/p OLT & redo OLT with subsequent HCV | PT | + | NA |
| 44 | This study | 72 | M | CAD; MI s/p angioplasty | PL | NA | NA |
| 45 | This study | 51 | M | EtOH-C; anasarca, diastolic dysfunction | PL | − | − |
AD indicates aortic dissection; AF, atrial fibrillation; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; CVID, common variable immunodeficiency; DM, diabetes mellitus; DOE, dyspnea on exertion; EtOH-C, alcohol-related cirrhosis; HBV-C; HBV-related cirrhosis; HCC, hepatocellular carcinoma; HCV-C, HCV-related cirrhosis; HTN, hypertension; MI, myocardial infarction; NA, not applicable; OA, osteoarthritis; OLT, orthotopic liver transplant; PC, pericardium; PL, pleura; PLE, protein-losing enteropathy; PT, peritoneum; s/p, status post; WNL, within normal limits.
TABLE 3.
Treatment/Clinical Outcome
| Case | Therapy | Effect | Overall Survival | Outcome |
|---|---|---|---|---|
| 1 | NA | NA | NA | NA |
| 2 | Aspiration | NA | 5 (mo) | Died (AIDS dementia) |
| 3 | Unspecified chemo | Aborted due to toxicity | 1 | Died |
| 4 | Aspiration | Spontaneous CR | 2 | Died (complications of HCC) |
| 5 | CHOP | CR; ascites remained | 12 | Died (traumatic subdural) |
| 6 | Aspiration | Spontaneous CR | 24 | Alive |
| 7 | Prednisolone, etoposide | CR | 8 | Alive |
| 8 | NA | NA | NA | Died |
| 9 | NA | NA | 8 | Alive |
| 10 | Aspiration | Spontaneous CR | 7 | Died (unrelated cerebral bleeding) |
| 11 | CHOP | CR | 15 | Died |
| 12 | CHOP, SCT | CR | 13 | Alive |
| 13 | Aspiration | NR | 2 | Alive |
| 14 | COP | PR, multiple recurrences | 5 | Died |
| 15 | Prednisolone | NR | 0.6 | Died |
| 16 | Aspiration | NR | 5 | Died |
| 17 | Orchiectomy, MEP-carboplatin, local radiation | CR | 8 | Alive |
| 18 | CHOP | CR | 5 | Died (pneumonia/pancreatitis) |
| 19 | CHOP, THP-COP, sobuzoxane | CR | 18 | Alive |
| 20 | R+THP-COP | CR | 26 | Alive |
| 21 | THP-COP, SCT | CR | 18 | Died (extensive bleeding from hemangioma) |
| 22 | CHOP | CR | 36 | Alive |
| 23 | Vincristine & cyclophosphamide | CR | NA | Alive |
| 24 | R+THP-COP, etoposide | PR | 25 | Alive |
| 25 | Rituximab | CR | 28 | Alive |
| 26 | Endoxan, farmorubicin, oncovin, prezolon | NA | 0.7 | Died (operative complications) |
| 27 | R+CHOP | CR | 9 | Alive |
| 28 | R+CHOP | Spontaneous CR after aspiration maintained after chemo | 22 | Alive |
| 29 | R+THP-COP | CR | 30 | Alive |
| 30 | Aspiration | Spontaneous CR; ascites returned without lymphoma | 40 | Alive |
| 31 | R+COP | NR | 3 | Alive |
| 32 | VelCD | Initial response, then recurrence | 2.1 | Died |
| 33 | CHOP | NR | 7 | Died |
| 34 | R+CHOP | CR; ascites remained | 12 | Alive |
| 35 | R+CHOP | CR; ascites remained | NA | Alive |
| 36 | CHOP | PR | 2 | Died (complications of Mallory Weiss tear) |
| 37 | R+CHOP | CR; ascites remained | NA | Died |
| 38 | Pleurodesis | Spontaneous CR | 55 | Alive |
| 39 | Aspiration | Spontaneous CR | 16 | Died |
| 40 | Aspiration | Spontaneous CR | 11 | Alive |
| 41 | Aspiration | NA | NA | NA |
| 42 | ICE, BCNU-ECM, SCT | Initial response, then recurrence | 4.5 | Died |
| 43 | CHOP | Aborted due to toxicity | 1.5 | Died |
| 44 | Aspiration | NA | NA | NA |
| 45 | Aspiration | NR | 0.03 | Died |
AIDS indicates acquired immunodeficiency syndrome; BCNU-ECM, BCNU, etoposide, cytarabine, melphalan; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; COP, cyclophosphamide, vincristine, prednisolone; ICE, ifosphamide, carboplatin, etoposide; MEP, mitoxantrone, etoposide, and prednisone; NA, not applicable; NR, no response; R, rituximab; SCT, stem cell transplant; THP-COP, pirarubicin, vincristine, cyclophosphamide, prednisolone; VelCD, bortezomib cyclo-phosphamide dexamethasone.
Our study was reviewed and approved by the UCLA Institutional Review Board.
Report of Internal Cases
We have summarized the patient demographics, lymphoma characteristics, and clinical outcomes for the internal cases (cases 41 to 45) alongside the data from our literature review (Tables 1–3). The presenting scenario for our patients has been briefly described below.
Case 41
An 85-year-old woman with a history of coronary artery disease status post myocardial infarction and 2-vessel coronary artery bypass graft presented with chest pain and shortness of breath. No lymphadenopathy, or-ganomegaly, or history of lymphoma was noted. Imaging revealed a large left-sided pleural effusion without associated mass lesion. Laboratory studies revealed a white blood cell (WBC) count of 12,960/μL, hematocrit of 33.7%, a platelet count of 469,000/μL, and lactate dehydrogenase (LD) of 586 U/L. Human immunodeficiency virus (HIV) and hepatitis status was unknown. Thoracocentesis extracted 1.2 L of dark tea-colored fluid with an LD of 2161 U/L. Microbiological studies were all negative. Cytomorphologic examination revealed large atypical lymphoid cells with irregular nuclei, multiple prominent nucleoli, coarse chromatin, and moderate cytoplasm. Many cells demonstrated plasmacytoid morphology. The patient declined treatment and opted to be transferred to hospice care, at which point she was lost to follow-up (Tables 1–3).
Case 42
A 29-year-old Egyptian man with a history of complex congenital heart disease status post multiple surgical corrections presented with congestive heart failure. Imaging showed hepatic cirrhosis with splenomegaly and ascites with no lymphadenopathy or other mass lesions. Laboratory studies revealed a WBC count of 7910/μL, hematocrit of 42.9%, a platelet count of 180,000/μL, and an LD of 1104 U/L. HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) studies were all negative. Paracentesis yielded 1.9 L of brown fluid with an LD of 6522 U/L. Microbiological studies were all negative. Cytomorphologic examination revealed neoplastic cells similar in morphology to those described in case 41.
The patient was treated with ifosphamide, carboplatin, and etoposide, followed by an additional round of BCNU, etoposide, cytarabine, melphalan, and an autologous stem cell transplant. However, he developed massive ascites and expired approximately 4½ months after the original lymphoma diagnosis (Tables 1–3).
Case 43
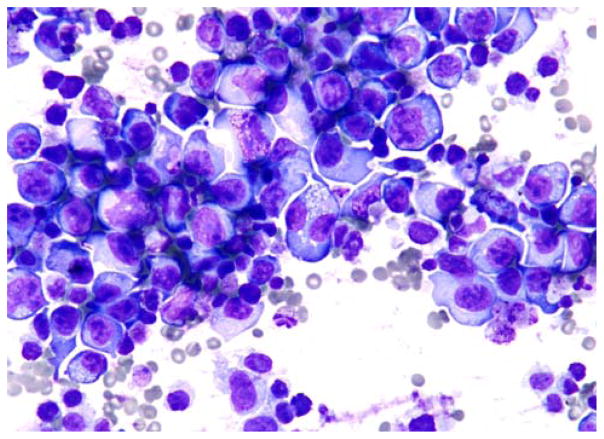
A 45-year-old man with a history of alcoholic cirrhosis status post orthotopic liver transplantation complicated by contraction of HCV presented with altered mental status and worsening abdominal distention. The imaging studies demonstrated an atrophic liver and diffuse abdominal and pelvic ascites without mass lesion or lymphadenopathy. Laboratory studies demonstrated a WBC count of 8300/μL, hematocrit of 33%, a platelet count of 107,000/μL, and an LD of 269 U/L. HBV and HCV studies were positive. Paracentesis extracted 1.9 L of clear fluid. Microbiological studies were all negative. Cytomorphologic features were similar to those described in previous cases (Fig. 1).
FIGURE 1.
Large, pleomorphic, atypical lymphoid cells with prominent nucleoli, basophilic cytoplasm, and clear vacuoles (Wright-Giemsa).
The patient received 1 cycle of cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy with resulting neutropenia and sepsis requiring readmission. Rapid decompensation ensued, and the patient expired approximately 1½ months after the initial lymphoma diagnosis (Tables 1–3).
Case 44
A 72-year-old man with a history of coronary artery disease and myocardial infarction status post angioplasty presented to our institution with dyspnea and cough. Imaging demonstrated a pleural effusion. Laboratory studies revealed a WBC count of 7300/μL, hematocrit of 40.8%, and a platelet count of 147,000/μL. Hepatitis and HIV status was unknown. Cytologic examination of the pleural fluid demonstrated features similar to those described in the previous cases. The patient was subsequently lost to follow-up (Tables 1–3).
Case 45
A 51-year-old man with a history of alcoholic cirrhosis presented with several days of worsening lower extremity edema and paroxysmal nocturnal dyspnea. Imaging revealed mild left-ventricular diastolic dysfunction, a cirrhotic liver, bilateral pleural effusions, borderline splenomegaly, and mild ascites without any lymphadenopathy or mass lesions. Laboratory studies revealed a WBC count of 3600/μL, hemoglobin of 8.3 g/ dL, a platelet count of 48,000/μL, and an LD of 1090 U/ L. HIV, hepatitis A virus, HBV, and HCV studies were all negative. Cytomorphologic examination of the pleural fluid demonstrated numerous large atypical cells similar in morphology to those previously described. The patient’s multiple confounding conditions and poor overall constitution led to rapid decompensation, and he expired on the day the lymphoma diagnosis was rendered (Tables 1–3).
RESULTS
Patient Demographics and Clinical Presentation of KSHV/HHV8-negative EBLs
The demographics and clinical characteristics are described in detail in Table 1. The patients were generally elderly (median 70 y) with a slight male-to-female predilection (62.2%). A striking proportion of patients were Japanese (60.0%), and more than half had a documented history of an underlying medical condition leading to fluid overload, including cirrhosis (1 due to HBV, 5 due to HCV, and 4 due to alcohol abuse), protein-losing enteropathy (2 patients), cardiac problems (10 patients), and a ventriculoperitoneal shunt placed 30 years before (1 patient). Seropositivity was observed in 4.9% and 26.5% of patients for HIV and HCV, respectively. Effusions most commonly arose within the pleura (66.7%), followed by the peritoneum (37.8%) and pericardium (26.7%). Multiple sites of effusion were demonstrated in 31.1% of cases, whereas in 1 case the effusion was entirely confined to the scrotum.16
Lymphoma Cytology, Immunophenotype, and Molecular Characteristics
The characteristics of lymphomatous cells are described in detail in Table 2. The neoplastic cells were morphologically medium to large with pleomorphic, immunoblastic, or plasmacytoid/plasmablastic morphology. Pan-B-cell antigens, defined as CD19, CD20, CD22, CD79a, or cytoplasmic/surface immunoglobulin, were present in 39 of 45 cases (86.7%). Thirty-two of 45 cases (71.1%) expressed CD20. One case lacked pan-B-cell antigens but demonstrated B-cell gene rearrangement. Two cases showed a T-cell immunophenotype, whereas 3 cases were indeterminate by either immunophenotype or the gene rearrangement studies. Twenty-four of 25 cases (96.0%) in which IgH gene rearrangement was performed demonstrated clonal rearrangement, whereas 1 case demonstrated T-cell gene rearrangement. Eleven of 17 (64.7%) cases carried a complex karyotype. Eleven of 38 cases (28.9%) demonstrated the presence of Epstein-Barr virus (EBV) sequences in malignant cells. The MYC gene demonstrated a germline configuration in 19 of 23 cases (82.6%).
TABLE 2.
Lymphoma Characteristics
| Case | Morphology | Immunophenotype | EBV | Ph/Ge | IgH | Cytogenetics | MYC |
|---|---|---|---|---|---|---|---|
| 1 | Large | CD45, CD19, CD20, CD22, HLA-DR | − | B | R | NA | NA |
| 2 | Large, pleomorphic | CD45, CD30, LLC | + | B | NA | NA | G |
| 3 | Large, pleomorphic | KLC | − | B | NA | NA | G |
| 4 | Large | CD45, CD19, CD20, CD22, LLC | − | B | R | t(9:14) & complex karyotype | G |
| 5 | Immunoblastic | CD19, LLC | − | B | R | NA | NA |
| 6 | Large, pleomorphic | CD45, CD7, CD19, CD20, CD22, HLA-DR | + | B | NA | Complex | G |
| 7 | Large, pleomorphic | CD19, CD20, CD22 | − | B | R | NA | G |
| 8 | Large | CD3, CD7, TCR α-β | NA | T | NA | del(1) (p11p22), +i(7)(ql0), and t(11:14)(q23;q11) | NA |
| 9 | Medium to large | CD19, CD20, LLC | + | B | R | NA | NA |
| 10 | Pleomorphic | CD4, CD5, CD19, CD20 | − | B | R | Hyperdiploid | R |
| 11 | Large | CD19, CD20, HLA-DR, KLC, IgM | − | B | R | Complex | G-A |
| 12 | Large | CD10, CD19, CD20, HLA-DR | − | B | R | Complex | G-A |
| 13 | Large | CD5, CD10, CD19, CD20, HLA-DR | − | B | R | Complex | G-A |
| 14 | Large, pleomorphic | CD45, CD10, CD19, CD20, CD22, FMC7, HLA-DR, KLC | − | B | R | NA | NA |
| 15 | Large, pleomorphic | CD19, CD20, CD22, HLA-DR | + | B | NA | No anomalies | G |
| 16 | Large, pleomorphic | CD20, CD79a, BCL2 | − | B | R | NA | R |
| 17 | Medium to large | CD19, CD20, CD45, CD79a | − | B | R | 46XY | NA |
| 18 | Large, plasmacytoid | CD20, CD38, CD45, CD79a, IgM, KLC | + | B | R | Complex | G |
| 19 | Large, pleomorphic | CD45, CD8, CD10, CD19, CD20, CD22, CD24, CD71, HLA-DR | − | B | R | Complex | G |
| 20 | NA | CD45, CD19, CD20, CD25, HLA-DR, KLC | − | B | R | NA | G-A |
| 21 | Large, pleomorphic | CD10, CD19, CD20, HLA-DR | − | B | NA | Complex | G-A |
| 22 | Large | CD20, CD79a | − | B | R | t(1;22)(q21;q11),t(14;17)(q32;q23) | G |
| 23 | Medium to large, plasmacytoid | CD38, CD138 | NA | ID | NA | NA | NA |
| 24 | Large, pleomorphic | CD19, CD20, CD30 | NA | B | R | Complex | R |
| 25 | Large | CD19, CD20, CD30, KLC | NA | B | NA | NA | NA |
| 26 | Immunoblastic to anaplastic | CD45, CD3, CD8, CD30 | − | T | TCR | NA | NA |
| 27 | Large, pleomorphic | CD45, CD20, CD30, CD79a | NA | B | NA | NA | NA |
| 28 | Large, pleomorphic | CD20, CD79a | NA | B | R | NA | G |
| 29 | Large, pleomorphic | CD19, CD20, CD22, HLA-DR, LLC, IgM, IgD | + | B | NA | Complex | G |
| 30 | Large, immunoblastic | CD45, CD30, CD38, CD71, HLA-DR | − | ID | NA | NA | NA |
| 31 | Large, pleomorphic | CD45, CD19, CD20, CD30, CD38, CD66, CD71, CD79a, EMA, LLC | + | B | NA | NA | G |
| 32 | Large, plasmacytoid | CD45, CD30, CD38, CD138, LLC | + | B | NA | NA | NA |
| 33 | NA | CD10, CD19 | NA | B | R | NA | NA |
| 34 | Medium to large, plasmacytoid | CD20, CD79a, LLC | + | B | NA | NA | NA |
| 35 | Large, pleomorphic | CD20 | − | B | NA | NA | NA |
| 36 | Large, pleomorphic | CD45, CD10, CD38 | + | B | R | NA | R |
| 37 | Medium to large | CD20, high Ki67 | − | B | NA | NA | NA |
| 38 | Large, pleomorphic to centroblastic | CD45, CD20, CD79a, BCL2, BCL6, MUM1 | − | B | R | NA | NA |
| 39 | Medium to large | CD5, CD19, CD20, CD25, LLC, IgM, IgD | − | B | R | NA | G-A |
| 40 | Medium to large | CD20 | − | B | R | Complex | G |
| 41 | Large, pleomorphic | CD45, CD20, CD79a, CD138, BOB1, OCT2, BCL6, MUM1 | − | B | NA | NA | NA |
| 42 | Large, pleomorphic | CD45, CD38, CD79a, CD138, PAX5, BOB1, OCT2, KLC | − | B | R | Constitutional paracentric inversion 10q | NA |
| 43 | Large, pleomorphic | CD45, CD38, CD56, CD138, KLC, Ki67 > 90% | + | B | G | NA | NA |
| 44 | Large, pleomorphic | CD45, CD20, CD30, CD43, BCL2 | − | B | NA | NA | NA |
| 45 | Large, plasmablastic | CD45, CD25, CD30, CD38, BLIMP1, granzyme, MUM1, TIA1 | − | B | NA | NA | NA |
G indicates germline; G-A, germline but amplified; ID, indeterminate; KLC, κ light chain restricted; LLC, λ light chain restricted; NA, not applicable; Ph/Ge, phenotype/genotype; R, rearranged.
Treatment and Clinical Outcome
Details of the treatment strategy and clinical outcome are outlined in Table 3. Patients were treated with either aspiration/pleurodesis alone (31.0%) or aspiration followed by chemotherapy regimens (69.0%). Three patients received postchemotherapy stem cell transplants, whereas 1 patient underwent an orchiectomy. Three patients had an unknown treatment/intervention course.
Data on the effects of the intervention strategy were available in the majority of cases; of these, complete remission (CR) or partial remission (PR) was achieved in 7 of 10 (70%) of postaspiration-only patients versus 23 of 28 (82.1%) of postchemotherapy patients. Survival and outcome data were available in 38 cases; of these, a subset of patients died of causes not attributable to the underlying lymphoma.4,5,6,11,17,20,24,33 Of the remaining 30 patients, the mean and the median survival were estimated to be 14.5 and 10 months, respectively, with 46.7% of patients surviving for at least 1 year after diagnosis.
DISCUSSION
In this study, we highlight our experience and published literature on KSHV/HHV8-negative EBLs, the majority of which is seen in individuals with an underlying medical condition such as cirrhosis and heart failure leading to fluid overload states. These cases often present with diagnostic/semantic dilemma for the pathologist, partly because they have not been specifically described in the current WHO classification.
On comparing our data of the 45 cases of KSHV/ HHV8-negative EBLs with a recent literature review of 142 KSHV/HHV8-positive PEL cases,37 the patterns that emerge strongly suggest that KSHV/HHV8-negative EBL is a distinct entity demonstrating unique demographic, immunophenotypic, and treatment response/survival characteristics (Table 4).
TABLE 4.
Comparison of KSHV/HHV8-negative EBL and PEL
| KSHV/HHV8- negative EBL | Traditional PEL* | |
|---|---|---|
| No. cases | 45 | 142 |
| Demographics | ||
| Age, median (range) | 70 (27–99) | 44 (26–101) |
| Sex (M/F) [n (%)] | 28/45 (62.2) | 129/136 (94.9) |
| Japanese origin [n (%)] | 27/45 (60.0) | NA |
| PMH leading to fluid overload [n (%)] | 23/45 (51) | NA |
| HIV+ [n (%)] | 2/41 (4.9) | 110/142 (77.5) |
| Hepatitis C [n (%)] | 9/34 (26.5) | 8/31 (25.8) |
| Effusion site | ||
| Pericardium [n (%)] | 12/45 (26.7) | 33/140 (23.6) |
| Peritoneum [n (%)] | 17/45 (37.8) | 53/140 (37.9) |
| Pleura [n (%)] | 30/45 (66.7) | 106/140 (75.7) |
| Multiple [n (%)] | 14/45 (31.1) | 46/140 (32.9) |
| Phenotype | ||
| HHV8+ [n (%)] | 0/45 (0) | 142/142 (100) |
| Pan-B-cell markers† [n (%)] | 39/45 (86.7) | 43/108 (39.8) |
| CD20+ [n (%)] | 32/45 (71.1) | 15/99 (15.1) |
| EBV+ [n (%)] | 11/38 (28.9) | 80/122 (65.6) |
| IgH rearrangement [n (%)] | 24/25 (96.0) | 71/87 (81.6) |
| Achieved CR/PR | ||
| Aspiration only [n (%)] | 7/10 (70.0) | 6/33 (18.2) |
| Chemotherapy [n (%)] | 23/28 (82.1) | 19/48 (39.6) |
| Survival—all | ||
| Mean (mo) | 12.8 | NA |
| Median (mo) | 8 | 4 |
| Survival >1 y [n (%)] | 16/38 (42.1) | 22/127 (17.3) |
| Survival—select‡ | ||
| Mean (mo) | 14.5 | NA |
| Median (mo) | 10 | NA |
| Survival >1 y [n (%)] | 14/30 (46.7) | NA |
Compared against previously published review from Kobayashi et al.37
Pan-B-cell markers defined as CD19, CD20, CD22, CD79a, and cytoplasmic or surface immunoglobulin.
Survival statistics exclude deaths unrelated to lymphoma (traumatic and complications of other underlying medical problems).
PMH indicates past medical history.
Compared with PELs, patients afficted with KSHV/HHV8-negative EBLs are older with a median age of 70 years versus 44 years. Patients are less frequently male (62.2% vs. 94.9%). HIV positivity in KSHV/HHV8-negative EBL is uncommon compared with PEL (4.9% vs. 77.5%).37 Patients of Japanese origin account for 60.0% of reported KSHV/HHV8-negative EBL cases, and more than half of the overall cases occur in patients with a documented history of a medical condition leading to fluid overload, although such a clinical history was not available for PEL cases.
The frequent null phenotype with lack of pan-B-cell markers has historically rendered PEL difficult to diagnose without ancillary studies.1,2 Although the reported overall expression of pan-B-cell markers in PEL has increased in recent years, its incidence was recently estimated at 39.8%.37 In the current series of KSHV/HHV8-negative EBL cases (Table 2), 86.7% of cases demonstrated pan-B-cell markers, with most cases expressing multiple such antigens. It is particularly noteworthy that given its therapeutic importance, CD20, the primary target of rituximab, demonstrates striking differential expression; 71.1% in our series versus 15.1% in reported PEL cases.37 A majority of cases in our series (7 of 9) were demonstrated to have either germinal center B or mixed germinal center B/activated B-cell immunosignatures unlike PEL in which lesional cells are typically of activated B-cell type.1 EBV sequences were found in 28.9% of KSHV/HHV8-negative EBL when compared with the 65.6% of PEL.37 IgH rearrangements are slightly more prevalent in KSHV/HHV8-negative EBL compared with PEL, with a rate of 96.0% versus 81.6%.37
In terms of prognosis, whereas PEL confers a uniformly abysmal clinical course on patients, KSHV/ HHV8-negative EBL appears less aggressive overall and may be associated with a more favorable prognosis. Treatments are often more efficacious with aspiration-only or chemotherapy interventions resulting in CR/PR in 70% and 82.1% of KSHV/HHV8-negative EBL patients versus 18.2% and 39.6% of PEL patients, respectively.37 The response to aspiration-only measures is particularly noteworthy for oncologists given that the older cohort of patients with KSHV/HHV8-negative EBL may be unable to tolerate chemotherapy and will seek alternative treatment modalities with acceptable outcomes; this sort of spontaneous regression has generated much speculation as regards the pathogenesis of KSHV/ HHV8-negative EBL, discussed below. In addition, overall survival encompassing all treatment modalities is considerably improved with a median and >1-year survival rate of 8 months and 42.1% in KSHV/HHV8-negative EBL versus 4 months and 17.3% in PEL, respectively.37 As previously described, survival statistics further improve when deaths from nonlymphoma causes are excluded, although no comparison data are available.
Numerous reports have in the past tried to address KSHV/HHV8-negative effusion lymphomas to better understand this entity. Ichinohasama et al5 proposed a potential 3-tiered classification system for effusion lymphomas on the basis of KSHV/HHV8 and MYC status: type I PEL (KSHV/HHV8 positive, germline MYC), type II PEL (KSHV/HHV8 negative, rearranged MYC), and type III PEL (KSHV/HHV8 negative, germline MYC), with the first 2 groups preferentially affecting HIV-sero-positive patients. Although this scheme has been referenced in numerous publications, we do not favor its usage for several reasons. The WHO has clearly stated that the term PEL be restricted to the cases that are KSHV/HHV8 positive. Furthermore, in the proposed system, the authors have labeled as type II PEL cases that were KSHV/ HHV8 negative with MYC gene rearrangement, which may be categorized as Burkitt lymphomas as further pointed out by Nador and colleagues.2,5
In a more recent review, Carbone and Gloghini38 classified effusion lymphomas using a constellation of features including cytomorphology, effusion location, presence of mass lesions, and EBV, KSHV/HHV8, and MYC status; lymphomas were subsequently categorized as primary lymphomas including PEL (KSHV/HHV8 positive, EBV negative, and MYC negative), Burkitt lymphoma (KSHV/HHV8 negative, EBV positive, MYC positive), and possibly KSHV/HHV8-unrelated EBL (KSHV/HHV8 negative, EBV positive or negative, and MYC negative) versus effusion lymphomas arising secondarily from lymphoid malignancies or body cavity–based masses that were KSHV/HHV8 negative. Although intuitive, this model raises the question of whether or not KSHV/HHV8-negative EBL is truly a “primary” lymphoma that arises in effusions secondary to other medical conditions leading to fluid overload. Unlike the classic “secondary” lymphoma, diffuse large B-cell lymphoma associated with chronic inflammation, and its prototype pyothorax association lymphoma, KSHV/HHV8-negative EBL maintains a distinct clinical picture; there is no radiologically evident thickening of serosal membranes, striking male predominance, or strong association with EBV.39
Theories regarding the pathogenesis of KSHV/ HHV8-negative effusions have failed to demonstrate a definite cause. Ichinohasama et al5 suggested that aberrations in PAX5, a B-cell-specific antigen, may play a role. Ohshima et al12 subsequently proposed that multistep genomic abnormalities including trisomy 8 and alterations of MYC were involved in lymphomagenesis. Previous epidemiologic studies have shown an association between HCV and B-cell non-Hodgkin lymphoma, which may be partially explained by the lymphotropic properties of HCV triggering clonal B-cell expansion.40–43 Co-infection with HCV was demonstrated in 26.5% of our cases of KSHV/HHV8-negative EBL, over an order of magnitude higher than the baseline prevalence rate of 2% for hepatitis C in the general population of the United States.44
A final suspect in the mechanism of lymphomagenesis that cannot be excluded is the effusion itself. A direct comparison may be drawn with diffuse large B-cell lymphoma associated with chronic inflammation, in which longstanding chronic inflammation of a site hosting EBV-transformed B-cells leads to escape from immune surveillance and subsequent malignant transformation.1 Rodriguez et al6 proposed that dysregulation of cytokines in the setting of chronic inflammation may predispose to KSHV/HHV8-negative EBL, a concept furthered by Ashihara et al7 who argued that localized serositis, in their case due to inflow of cerebrospinal fluid, may lead to transformation of an effusion into a malignant lymphoma. Several features in our case series support this possibility: more than half of the patients in our series had a documented medical condition either predisposing to or immediately causing a fluid overload state; 3 patients had a documented longstanding history of a benign effusion that demonstrated malignancy only after multiple aspirations; 7 of 10 patients achieved spontaneous remission after complete aspiration of their effusion without any additional treatment; 5 patients treated with chemotherapy experienced CR of the malignancy, whereas a benign effusion remained. Although none of these observations rises to the level of a causative association, they all support the concept that the lymphoma may in fact be secondary to a preexisting effusion.
Our study is not, however, without its inherent limitations. Although we have made every effort to meticulously collect data from all reported cases of KSHV/ HHV8-negative EBLs, our study is not a meta-analysis. Individual patients did not possess a standardized work up for their lymphoma in terms of immunophenotypic or cytogenetic/molecular diagnostic panels. Furthermore, the true length of time during which a patient had an effusion before a potential malignant transformation remains unknown in most cases. Finally, whereas the aggregate data demonstrate a favorable overall survival compared with PEL, 3 of our 5 internal cases succumbed to their illness within 4 months of diagnosis. However, given our status as a tertiary-care referral center, our patients tend to present with more advanced disease and liver dysfunction, exacerbating intolerance to treatment and potentially accounting for their poor outcomes.
Although the data above delineate a clear set of unique features in KSHV/HHV8-negative EBL, characteristics similar to PEL remain and have been the impetus for vague nomenclature over the years. The distinct cytomorphology, location of effusions, lack of solid malignancy, and lack of MYC rearrangements all parallel to that of PEL, and it is precisely this mimicry that has spawned confusing labels such as PEL-like lymphoma and HHV8-unrelated PEL, which is of particular concern given this entity’s reported frequency at 20% of overall primary lymphomatous effusions.38 However, such labels do no service to pathologists, who may be unable to render accurate diagnoses in the absence of agreed-upon criteria, clinicians, who struggle with ideal treatment regimens and inconsistent response data, and patients, who no longer know what survival statistics to believe. So then the question remains—given their uniqueness, where and how should these lymphomas be classified in the pantheon of B-cell lymphomas?
In summary, we have presented data from the largest overall series of KSHV/HHV8-negative EBL cases, including the largest single-institution collection. Our analysis demonstrates that KSHV/HHV8-negative EBLs, although sharing cytomorphologic characteristics with PEL, is a distinct entity: lymphoma cells are KSHV/ HHV8 negative and express pan-B-cell antigens; patients are older with less male predominance, are generally HIV negative, often hepatitis C positive, and often have an underlying medical condition leading to fluid overload; and clinical outcomes and response to therapy are much improved. Furthermore, the striking association with underlying fluid overload states predating the malignant effusion raises the possibility that these lymphomas are in fact secondary to chronic serosal stimulation. Although the WHO description of PEL alludes to reports of KSHV/HHV8-negative EBL arising in the peritoneal cavities of patients with HCV cirrhosis, we believe such patients represent a small subset of a more global entity related to fluid overload at different sites with lymphomagenesis not inextricably tied to HCV.1 Given the distinct clinicopathologic features of KSHV/HHV8-negative EBL, a provisional subtype of effusion-based KSHV-negative lymphomas may be a consideration.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Said J, Cesarman E. Primary effusion lymphoma. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Lyon, France: IARC WHO Classification of Tumours; 2008. pp. 260–261. [Google Scholar]
- 2.Nador RG, Cesarman E, Chadburn A, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 3.Hermine O, Michel M, Buzyn-Veil A, et al. Body-cavity-based lymphoma in an HIV-seronegative patient without Kaposi’s sarcoma-associated herpesvirus-like DNA sequences. N Engl J Med. 1996;334:272–273. doi: 10.1056/NEJM199601253340417. [DOI] [PubMed] [Google Scholar]
- 4.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma-associated herpesvirus DNA sequences in AIDS-related and AIDS-unrelated lymphomatous effusions. Br J Haematol. 1996;94:533–543. doi: 10.1046/j.1365-2141.1996.d01-1826.x. [DOI] [PubMed] [Google Scholar]
- 5.Ichinohasama R, Miura I, Kobayashi N, et al. Herpes virus type 8-negative primary effusion lymphoma associated with PAX-5 gene rearrangement and hepatitis C virus: a case report and review of the literature. Am J Surg Pathol. 1998;22:1528–1537. doi: 10.1097/00000478-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez J, Romaguera JE, Katz RL, et al. Primary effusion lymphoma in an HIV-negative patient with no serologic evidence of Kaposi’s sarcoma virus. Leuk Lymphoma. 2001;85:185–189. doi: 10.3109/10428190109057969. [DOI] [PubMed] [Google Scholar]
- 7.Ashihara E, Shimazaki C, Hirai H, et al. Human herpes virus 8-negative primary effusion lymphoma in a patient with a ventriculoperitoneal shunt tube. Int J Hematol. 2001;74:327–332. doi: 10.1007/BF02982069. [DOI] [PubMed] [Google Scholar]
- 8.Hara T, Nishi S, Horimoto A, et al. Primary effusion lymphoma in a patient with hepatitis C virus-related liver cirrhosis. J Gastroenterol Hepatol. 2001;16:948–949. [PubMed] [Google Scholar]
- 9.Yamamoto Y, Kitajima H, Sakihana H, et al. CD3+CD4-CD8-TCR-alphabeta+ T-cell lymphoma with clinical features of primary effusion lymphoma: an autopsy case. Int J Hematol. 2001;74:442–446. doi: 10.1007/BF02982089. [DOI] [PubMed] [Google Scholar]
- 10.Ohori NP, Whisnant RE, Nalesnik MA, et al. Primary pleural effusion posttransplant lymphoproliferative disorder: distinction from secondary involvement and effusion lymphoma. Diagn Cytopathol. 2001;25:50–53. doi: 10.1002/dc.2001. [DOI] [PubMed] [Google Scholar]
- 11.Saiki M, Saitoh T, Inoue M, et al. Human herpesvirus-8 negative primary effusion lymphoma with complete clinical remission after removal of ascites. Rinsho Ketsueki. 2002;43:548–553. [PubMed] [Google Scholar]
- 12.Ohshima K, Ishiguro M, Yamasaki S, et al. Chromosomal and comparative genomic analyses of HHV-8-negative primary effusion lymphoma in five HIV-negative Japanese patients. Leuk Lymphoma. 2002;43:595–601. doi: 10.1080/10428190290012100. [DOI] [PubMed] [Google Scholar]
- 13.Paner GP, Jensen J, Foreman K, et al. HIV and HHV-8 negative primary effusion lymphoma in a patient with hepatitis C virus-related liver cirrhosis. Leuk Lymphoma. 2003;44:1811–1814. doi: 10.1080/1042819031000104015. [DOI] [PubMed] [Google Scholar]
- 14.Hisamoto A, Yamane H, Hiraki A, et al. Human herpes virus-8-negative primary effusion lymphoma in a patient with common variable immunodeficiency. Leuk Lymphoma. 2003;44:2019–2022. doi: 10.1080/1042819031000110955. [DOI] [PubMed] [Google Scholar]
- 15.Shimazaki M, Fujita M, Tsukamoto K, et al. An unusual case of primary effusion lymphoma in a HIV-negative patient not pathogenetically associated with HHV8. Eur J Haematol. 2003;71:62–67. doi: 10.1034/j.1600-0609.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y, Tajima F, Omura H, et al. Primary effusion lymphoma of the left scrotum. Intern Med. 2003;42:351–353. doi: 10.2169/internalmedicine.42.351. [DOI] [PubMed] [Google Scholar]
- 17.Chiba H, Matsunaga T, Kuribayashi K, et al. Autoimmune hemolytic anemia as a first manifestation of primary effusion lymphoma. Ann Hematol. 2003;82:773–776. doi: 10.1007/s00277-003-0734-x. [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y, Tsukasaki K, Nagai K, et al. Durable remission by sobuzoxane in an HIV-seronegative patient with human herpesvirus 8–negative primary effusion lymphoma. Int J Hematol. 2004;79:271–275. doi: 10.1532/ijh97.03107. [DOI] [PubMed] [Google Scholar]
- 19.Takao T, Kobayashi Y, Kuroda J, et al. Rituximab is effective for human herpesvirus-8-negative primary effusion lymphoma with CD20 phenotype associated hepatitis C virus-related liver cirrhosis. Am J Hematol. 2004;77:419–420. doi: 10.1002/ajh.20227. [DOI] [PubMed] [Google Scholar]
- 20.Nonami A, Yokoyama T, Takeshita M, et al. Human herpes virus 8-negative primary effusion lymphoma (PEL) in a patient after repeated chylous ascites and chylothorax. Intern Med. 2004;43:236–242. doi: 10.2169/internalmedicine.43.236. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara T, Ichinohasama R, Miura I, et al. Primary effusion lymphoma of the pericardial cavity carrying t(1;22)(q21;q11) and t(14;17)(q32;q23) Cancer Genet Cytogenet. 2005;156:49–53. doi: 10.1016/j.cancergencyto.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins C, Sorour Y, Blake E, et al. Human-immunodeficiency-virus-negative, human-herpes-virus-8-negative abdominal cavity primary effusion lymphoma. Clin Oncol (R Coll Radiol) 2005;17:636–638. doi: 10.1016/j.clon.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto Y, Nomura K, Ueda K, et al. Human herpesvirus 8-negative malignant effusion lymphoma: a distinct clinical entity and successful treatment with rituximab. Leuk Lymphoma. 2005;46:415–419. doi: 10.1080/10428190400018364. [DOI] [PubMed] [Google Scholar]
- 24.Venizelos I, Tamiolakis D, Lambropoulou M, et al. An unusual case of posttransplant peritoneal primary effusion lymphoma with T-cell phenotype in a HIV-negative female, not associated with HHV-8. Pathol Oncol Res. 2005;11:178–181. doi: 10.1007/BF02893396. [DOI] [PubMed] [Google Scholar]
- 25.Youngster I, Vaisben E, Cohen H, et al. An unusual cause of pleural effusion. Age Ageing. 2006;35:94–96. doi: 10.1093/ageing/afj009. [DOI] [PubMed] [Google Scholar]
- 26.Terasaki Y, Okumura H, Saito K, et al. HHV-8/KSHV-negative and CD20-positive primary effusion lymphoma successfully treated by pleural drainage followed by chemotherapy containing rituximab. Intern Med. 2008;47:2175–2178. doi: 10.2169/internalmedicine.47.1565. [DOI] [PubMed] [Google Scholar]
- 27.Niino D, Tsukasaki K, Torii K, et al. Human herpes virus 8-negative primary effusion lymphoma with BCL6 rearrangement in a patient with idiopathic CD4 positive T-lymphocytopenia. Haematologica. 2008;93:21–23. doi: 10.3324/haematol.12085. [DOI] [PubMed] [Google Scholar]
- 28.Adiguzel C, Bozkurt SU, Kaygusuz I, et al. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: report of a rare case and review of the literature. APMIS. 2009;117:222–229. doi: 10.1111/j.1600-0463.2008.00005.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsagarakis NJ, Argyrou A, Gortzolidis G, et al. Report of an HIV and HHV-8 negative case of primary effusion lymphoma with idiopathic T4 lymphocytopenia. Int J Hematol. 2009;90:94–98. doi: 10.1007/s12185-009-0343-0. [DOI] [PubMed] [Google Scholar]
- 30.De Filippi R, Iaccarino G, Frigeri F, et al. Elevation of clonal serum free light chains in patients with HIV-negative primary effusion lymphoma (PEL) and PEL-like lymphoma. Br J Haematol. 2009;147:405–408. doi: 10.1111/j.1365-2141.2009.07846.x. [DOI] [PubMed] [Google Scholar]
- 31.Taira T, Nagasaki A, Okudaira T, et al. HIV- and HHV-8-negative primary effusion lymphoma-like lymphoma presenting with lymphomatous effusions complicated by cardiac tamponade-a case report. Gan To Kagaku Ryoho. 2009;36:1195–1198. [PubMed] [Google Scholar]
- 32.Takahashi T, Hangaishi A, Yamamoto G, et al. HIV-negative, HHV-8-unrelated primary effusion lymphoma-like lymphoma: report of two cases. Am J Hematol. 2010;85:85–87. doi: 10.1002/ajh.21568. [DOI] [PubMed] [Google Scholar]
- 33.Cooper AR, Burack WR, Allerton JP. A case of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8-unrelated but Epstein-Barr virus-positive primary effusion lymphoma-like lymphoma in the setting of human immunodeficiency virus and hepatitis C virus infection. Leuk Lymphoma. 2010;51:2303–2305. doi: 10.3109/10428194.2010.520775. [DOI] [PubMed] [Google Scholar]
- 34.Kagoya Y, Takahashi T, Yoshimoto T, et al. Recurrent pericardial effusion after treatment for primary effusion lymphoma-like lymphoma: an autopsied case. Ann Hematol. 2011;90:219–220. doi: 10.1007/s00277-010-0975-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Nava VE, Schechter GP, et al. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: a patient successfully treated with pleurodesis. J Clin Oncol. 2011;29:e747–e750. doi: 10.1200/JCO.2011.35.7509. [DOI] [PubMed] [Google Scholar]
- 36.Terasaki Y, Yamamoto H, Kiyokawa H, et al. Disappearance of malignant cells by effusion drainage alone in two patients with HHV-8-unrelated HIV-negative primary effusion lymphoma-like lymphoma. Int J Hematol. 2011;94:279–284. doi: 10.1007/s12185-011-0906-8. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi Y, Kamitsuji Y, Kuroda J, et al. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: report of 2 cases and review of 212 cases in the literature. Acta Haematol. 2007;117:132–144. doi: 10.1159/000097460. [DOI] [PubMed] [Google Scholar]
- 38.Carbone A, Gloghini A. PEL and HHV8-unrelated effusion lymphoma. Classification and diagnosis. Cancer. 2008;114:225–227. doi: 10.1002/cncr.23597. [DOI] [PubMed] [Google Scholar]
- 39.Chan JKC, Aozasa K, Gaulard P. DLBCL associated with chronic inflammation. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Lyon, France: IARC WHO Classification of Tumours; 2008. pp. 245–246. [Google Scholar]
- 40.Imai Y, Oshawa M, Tanaka H, et al. High prevalence of HCV infection in patients with B-cell non-Hodgkin’s lymphoma: comparison with birth cohort- and sex-matched blood donors in a Japanese population. Hepatology. 2002;35:974–976. doi: 10.1053/jhep.2002.32149. [DOI] [PubMed] [Google Scholar]
- 41.Vallisa D, Berte R, Rocca A, et al. Association between hepatitis C virus and non-Hodgkin’s lymphoma, and effects of viral infection on histologic subtype and clinical course. Am J Med. 1999;106:556–560. doi: 10.1016/s0002-9343(99)00069-8. [DOI] [PubMed] [Google Scholar]
- 42.Franzin F, Efremov DG, Pozzato G, et al. Clonal B-cell expansion in peripheral blood of HCV-infected patients. Br J Haematol. 1995;90:548–552. doi: 10.1111/j.1365-2141.1995.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 43.Lerat H, Rumin S, Habersetzer F. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood. 1998;91:3841–3849. [PubMed] [Google Scholar]
- 44.Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]