Abstract
The pyrethroid insecticide bifenthrin is frequently detected at ng/L concentrations in tributaries of the San Francisco Bay Delta. The estuary is also experiencing increasing salinity through climate change and water redirection. To evaluate the impacts of hypersaline conditions on bifenthrin toxicity in anadromous salmonids of the San Francisco Bay Delta (CA, USA), a 14-d laboratory exposure was performed using 2 strains of Oncorhynchus mykiss (rainbow trout and steelhead) acclimated to freshwater and to 8 g/L and 17 g/L salinity. The fish were then exposed to nominal concentrations of 0 μg/L, 0.1 μg/L, and 1.5 μg/L bifenthrin. Rainbow trout exhibited significant mortality following exposure to 1.5 μg/L (1.07 ± 0.35 μg/L measured) bifenthrin in freshwater. Elevated levels of Na+/K+ adenosine triphosphatase α1A mRNA subunit expression was observed in the gill of rainbow trout acclimated to hypersaline conditions relative to freshwater animals. No significant difference was noted in Na+/K+ adenosine triphosphatase subunit levels in brains of either strain in freshwater or hypersaline conditions. Likewise, significant differences were not observed in plasma vitellogenin or steroid hormone concentrations in either strain whether maintained in freshwater or saltwater. Saltwater acclimation significantly reduced nicotinamide adenine dinucleotide phosphate–catalyzed biotransformation of bifenthrin in liver microsomes of rainbow trout but not of steelhead. The present study showed that, relative to steelhead, rainbow trout have different responses to bifenthrin acute toxicity as well as different rates of hepatic bifenthrin biotransformation and regulation of Na+/K+ adenosine triphosphatase subunits in gills. These data indicate that significant differences exist between the strains and that animal life history may have important effects on the susceptibility of each strain to environmental contaminants.
Keywords: Bifenthrin, Pyrethroid, Salinity, Oncorhynchus mykiss, Biotransformation
INTRODUCTION
Pyrethroids are derivatives of naturally occurring pyrethrin insecticides used in urban pest control and agriculture [1–3]. By binding the voltage-gated sodium channels in neurons, pyrethroids prolong neuronal depolarization, causing paralysis and death [4]. In addition, pyrethroids inhibit Na+, K+, and Mg+ adenosine triphosphatase (ATPase) activity, and may therefore pose increased risk to fish that require Na+/K+ ATPase for cellular osmoregulation as well as neuronal function [5,6].
Of the pyrethroids, bifenthrin has consistently been measured in the ng/L range in northern California (USA) waterways and tributaries of the San Francisco Bay Delta [3,7–9]. Sources of bifenthrin to surface water include urban runoff from storm events and wastewater discharge through treatment facilities [7]. Given the continual output of low concentrations of bifenthrin into waterways, sublethal toxicity may occur. These types of exposures have the potential to affect developmental and reproductive endpoints that are more difficult to quantify and categorize than acute toxicity.
The occurrence of bifenthrin has also coincided with measurements of increased estrogenic activity in water extracts throughout the San Francisco Bay Delta region [9,10]. Recent studies have shown that pyrethroids cause estrogenic activity in fish [8,10–13] and cell lines [14]. Hydroxylated metabolites of permethrin produced stereoselectively from liver microsomes of rainbow trout had more estrogenic activity than the parent compound, as measured by vitellogenin (VTG) mRNA [12]. Because VTG is the precursor protein to egg yolk normally found in females, VTG measurements in juvenile and male fish can act as a biomarker for estrogenic exposure [15].
In addition to sublethal anthropogenic inputs, the San Francisco Bay Delta is also facing threats due to global climate change. Increasing temperatures result in a loss of freshwater input into the delta in the form of snowmelt [16]. Over time, this loss of freshwater input as well as additional freshwater removal for agriculture and transport to southern California have resulted in saltwater intrusion leading to increased salinity in the San Francisco Bay Delta [17]. Given saltwater intrusion of the delta, euryhaline aquatic species may be concomitantly exposed to higher saline water and pesticides.
Saline acclimation is a normal life history strategy for salmonids in the San Francisco Bay Delta. The catalytic activity of Na+/K+ATPase in the gill increases with increasing salinity [18,19]. Not only does Na+/K+ATPase increase the elimination of Na+, reducing cellular osmotic pressure in gill tissue, but it also regulates neuronal depolarization. In addition to inactivating voltage-gated Na channels of neurons, pyrethroids have also been shown to inhibit neuronal Na+/K+ATPase [6].
Plasma concentrations of cortisol also increase with increasing salinity [18,19]. Cortisol induces CYP3A27 [20], which catalyzes the hydroxylation of steroid hormones and biotransformation of many xenobiotics, including pesticides [21]. Previous studies have shown that CYP450 enzymes that activate pyrethroids to estrogenic metabolites are induced after hypersaline acclimation (particularly in salmonids) [22]. Therefore, coexposure to pyrethroids and hypersaline conditions could diminish acute neuronal toxicity but exacerbate estrogenic activities in areas where the combined exposure occurs.
The object of the present study was to evaluate the toxicity of bifenthrin in 2 strains of Oncorhynchus mykiss (rainbow trout and steelhead) following saltwater acclimation. Rainbow trout were obtained from a predominantly freshwater culture for lake and reservoir stocking. Steelhead trout were obtained from a hatchery that releases the fish in freshwater for eventual oceanic migration. Whereas steelhead are difficult to obtain because of their threatened status in California, rainbow trout are readily available at commercial hatcheries and serve as model salmonids for aquatic toxicology studies. In addition to acute toxicity, sublethal effects including steroid hormone and VTG concentrations were evaluated in plasma. Biotransformation of bifenthrin using liver microsomes was measured to determine the effect of saltwater acclimation on the relative conversion of bifenthrin to putatively less acutely toxic, but more estrogenic metabolites in each strain. Finally, the relative mRNA transcript levels of both Na+/K+ATPase α1a and α1b isoforms in the gills and brains of control fish were compared to assess differences in Na+/K+ATPase activity with increasing salinity.
MATERIALS AND METHODS
Chemicals
Bifenthrin (99.1% purity, Z-cis-bifenthrin isomer mixture) was purchased from ChemService. R-methyl(p)tolyl sulfoxide (MTSO) was obtained from Sigma Aldrich. Ethanol, acetonitrile, and n-hexane were all analytical grade (Fisher).
Fish acclimation and exposures
Juvenile rainbow trout (mean standard length 9.3 ± 1.0 cm and mean body weight 10.6 ± 3.4 g) were purchased from Jess Ranch Hatchery. Juvenile steelhead trout (mean standard length 9.6 ± 1.5 cm and mean body weight 10.6 ± 3.4 g) were obtained from the Nimbus Hatchery. On acquisition, they were kept in a 530-L living stream tank (from Fridge Units) with carbon-filtered municipal water at 11 °C to 12 °C. The fish were fed Silver Cup commercial feed every 48 h and were kept on a 14:10-h light:dark cycle. Fish were acclimated for approximately 2 wk prior to use.
For the exposure experiment, a total of 270 juvenile fish (135 rainbow trout and 135 steelhead) were first acclimated to freshwater and to 8 g/L and 17 g/L salinity according to the procedures described in Lavado et al. [22]. Briefly, fish were transferred to 8-L tanks starting at 4 g/L using a commercial salt mixture and acclimated for 48 h (CrystalSea Marine Mix; Marine Enterprises International). They were then transferred every 48 h to 8-g/L, 12-g/L, and 17-g/L tanks until the desired salinity was achieved. They were kept at the final salinity for 1 wk prior to bifenthrin exposure. The total salinity acclimation period lasted 14 d. There were no deaths during this period.
Bifenthrin exposures were carried out by exposing 3 replicate tanks (n = 5 fish/replicate tank) acclimated to each salinity to the solvent control (ethanol 0.01%) or to 0.1 μg/L or 1.5 μg/L bifenthrin (n = 3). Water changes and feedings were performed every 48 h for 14 d.
Sample collection and analysis
Throughout the exposure period, mortality was recorded per tank every 24 h. At the end of the exposure period, blood was collected from fish using heparinized needles and syringes. The blood was centrifuged at 10 000 g to obtain plasma, which was then stored at −80 °C until analysis. Plasma VTG protein levels were determined using an O. mykiss VTG sandwich enzyme-linked immunosorbent assay (ELISA) kit (Biosense Laboratories), and proteins were measured using the Coomassie Blue method (Pierce) with bovine serum albumin as the standard [23]. Fish were dissected, and livers were collected for microsomal incubations.
Testosterone (T), 17β-estradiol (E2), and 11-ketotestosterone (11-KT) steroid levels were determined using steroid-specific commercial competitive ELISA kits from Cayman Chemical. Prior to the steroid kit usage, plasma samples were extracted twice with approximately 3× diethyl ether, and the organic layer was collected and dried under a gentle stream of nitrogen gas before being reconstituted in the steroid ELISA kit dilution buffer solution.
The concentrations of bifenthrin in water samples from the exposure tanks were determined using solid-phase extraction and gas chromatography coupled with electron capture detection [24,25]. Water samples from random tanks were taken before and after exposures on days when water was changed in each tank to determine the mean measured bifenthrin concentrations for each treatment. The mean values from each replicate were pooled for the reported mean value for the treatment. One liter of water was collected from each tank and left undisturbed for several hours to allow solids to settle. Then, 500 mL of this water was transferred to an amber 2-L bottle, where methanol (MeOH) was added to a final 20% MeOH concentration. The mixture was percolated through solid-phase extraction cartridges (Waters) containing 360 mg of C18 silica (particle size 55–105 μm) at a flow rate of approximately 10 mL/min through a vacuum manifold system. The cartridges were preconditioned with 5 mL of MeOH, 5 mL of n-hexane, and 5 mL of Epure water. After the sample was passed through the cartridge, 200 mL of Epure water was passed through to eliminate salts from the cartridge. The cartridge was then dried for 30 min under vacuum before the retained analytes were eluted with 7 mL of hexane [25]. The elution was dried down under a gentle stream of nitrogen gas and resuspended in 1 mL n-hexane before analysis by gas chromatography (GC).
Quantification of the bifenthrin in the water extractions was carried out via gas chromatography using an Agilent 6890 GC equipped with a microelectron capture detector. Samples were introduced into the inlet at 250 °C in a pulsed splitless mode, and the separation was achieved on a VF-5MS capillary column (30 m Å ~ 0.25 mm Å ~ 0.25 μm film thickness, with a 10 m EZ-Guard precolumn [Varian]). The carrier gas was helium, and the flow rate was 1.5 mL/min. The oven program was initially set at 80 °C for 1 min, then increased to 300 °C at a rate of 15 °C/min, and then held at 300 °C for 10 min. The detector temperature was set at 310 °C [24]. The mean retention time of bifenthrin was 15.59 min. The quantitation of bifenthrin was achieved through external calibration using standards of known concentrations. The detection limit was 0.005 μg/L. Recovery rates of the extraction were determined by performing extractions on water samples of known bifenthrin concentrations. A recovery rate of 65.8% was observed for the 0.1-μg/L samples, and a rate of 96.7% was observed for the 1.5-μg/L nominal concentrations.
Subcellular fractionation
Microsomes were isolated from livers of fish from freshwater and saltwater acclimations according to Lavado et al. [22]. Livers of fish from each tank were pooled and homogenized in 1:5 w/v cold 100 mM KH2PO4/K2HPO4 buffer at pH 7.4. The solution contained 100 mM KCl and 1 mM ethylenediaminetetraacetic acid. Homogenates were centrifuged at 12 000 g for 30 min. The supernatant was collected and then centrifuged at 100 000 g for 60 min to obtain microsomal pellets. These pellets were resuspended in a small volume of the homogenization buffer with 20% (w/v) glycerol. Protein concentrations were determined by the Coomassie Blue method previously described [23].
Bifenthrin biotransformation
Liver microsomes of rainbow trout and steelhead from the 0 g/L and 17 g/L salinity acclimated negative control treatments were incubated with bifenthrin to determine conversion of the parent compound to polar metabolites. Briefly, in 1 mL total volume, 500 μg of protein were incubated with 500 μM bifenthrin, 2.5 mM nicotinamide adenine dinucleotide phosphate (NADPH), and 100 mM Tris-HCl, pH 7.4. Concentrations and conditions were determined from previous studies with permethrin [12]. Incubations were also carried out without NADPH and with boiled protein as negative controls. Samples (n = 3) were incubated for a total of 90 min with NADPH added every 30 min. The reactions were stopped by adding an equal volume of acetonitrile. An internal standard of R-methyl(p)tolyl sulfoxide (1 mg/mL) was added to each sample after the incubation. The samples were then centrifuged for 5 min at 13 000 g, and the supernatant was collected and injected (40 μL) into the high-performance liquid chromatography (HPLC) system.
The HPLC analysis was carried out on an SCL-10AVP Shimadzu system equipped with a 250 mm × 4.6 mm Hypersil ODS C18 (5 μm) reverse phase column (Thermo Scientific). Separation of bifenthrin metabolites employed a gradient system elution at a flow rate of 1 mL/min with a mobile phase composed of 90% acetonitrile and 10% water (solution A) and water brought to pH 1.7 with phosphoric acid (solution B). The run consisted of a 45-min linear gradient from 100% solution A to 70% solution B. Peaks were monitored with an ultraviolet (UV) detector (SPD-10AVP, Shimadzu) at 230 nm. Bifenthrin was quantified by coelution with authentic standard. The elution time of bifenthrin was 23.7 min.
Positive controls included phenobarbital-induced rat liver supersomes from XenoTech and human liver microsomes that were a gift from A. Rettie (University of Washington, Seattle, WA, USA). Incubations with fish microsomes were carried out at 25 °C, and mammalian microsomal incubations were carried out at 37 °C. The integrated peak area of bifenthrin in the boiled protein controls was used as a baseline to determine the reduction percentage of bifenthrin in incubations both with and without NADPH in humans, rats, and fish.
Na+/K+ ATPase mRNA measurements
The relative mRNA transcript levels of both Na/K α1a and Na/K α1b in the control fish were compared to determine whether the 2 isoforms do in fact increase and decrease with increasing salinity. Total mRNA was extracted from tissue (gill and brain) using an SV Total RNA Isolation Kit (Promega) following the manufacturer’s instructions. Primers for Na+/K+ ATPase α1a, Na+/K+ ATPase α1b, and elongation factor 1 alpha (ELF1α) have been described previously [26]. The sense primer for Na+/K+ ATPase α1a was 5′-GGCCGGCGAGTC-CAAT-3′, and the antisense primer was 5′-GAGCAGCTGTC-CAGGATCCT-3′. The sense primer for Na+/K+ ATPase α1b was 5′-CTGCTACATCTCAACCAACAACATT-3′, and the antisense primer was 5′-CACCATCACAGTGTTCATTGGAT-3′. We used ELF1α as housekeeping gene. The sense primer was 5′-GAGACCCATTGAAAAGTTCGAGAAG-3′, and the anti-sense primer was 5′-GCACCCAGGCATACTTGAAAG-3′. The RNA was quantified by quantitative polymerase chain reaction (qPCR) by using the iScript One-Step Real Time (RT)-PCR kit with SYBR Green (Bio-Rad). Each primer (100 nM, Na+/K+ ATPase α1a, Na+/K+ ATPase α1b, or ELF1α) was added to 25-μL PCR reactions containing SYBR Green RT-PCR Reaction Mix (Bio-Rad), 100 ng mRNA sample, and iScript Reverse Transcriptase for One-Step RT-PCR (Bio-Rad). The RT-PCR was performed on the iCycler-MyIQ (Bio-Rad) with the following protocol: 10 min at 50 °C (cDNA synthesis); 5 min at 95 °C (iScript reverse transcriptase inactivation); and 40 cycles of 10 s at 95 °C and 30 s at 62 °C. Fluorescence data were collected at the end of each cycle. Following the amplification reaction, a melting curve analysis was carried out between 60 °C and 95 °C, and fluorescence data were collected at intervals of 0.5 °C. A reaction excluding reverse transcriptase was included as a negative control. Relative qPCR expression was determined using RT-PCR Miner [27] and normalized to the transcript levels for ELF1α, because it did not vary across treatments. Data are presented as relative mRNA expression relative to the control.
Statistical analysis
All data from the experiment were tested for normality using a Kolmogorov–Smirnov test, and homogeneity of variance using Levene’s test. Comparisons between bifenthrin and salinity treatments in each strain/population were made using a two-way analysis of variance. Differences were considered significant at p <0.05. Statistical analyses were conducted using either GraphPad Prism Ver 5.0a for Windows or Software PASW Ver 18 (SPSS).
RESULTS
Bifenthrin water concentrations
The measured concentrations for the rainbow trout exposures were 0.1 μg/L was 0.025 ± 0.041 μg/L at the nominal concentration of 0.1 μg/L and 1.072 ± 0.348 μg/L at the 1.5-μg/L concentration. The control treatment measured concentrations were below the detection limit of 0.005 μg/L. The measured concentration for the steelhead exposures at the nominal concentration of 0.1 μg/L was 0.030 μg/L ± 0.016 μg/L; at the 1.5-μg/L concentration, it was 0.608 μg/L ± 0.182 μg/L. The control treatment measured concentrations were again below the detection limit of 0.005 μg/L.
Mortality
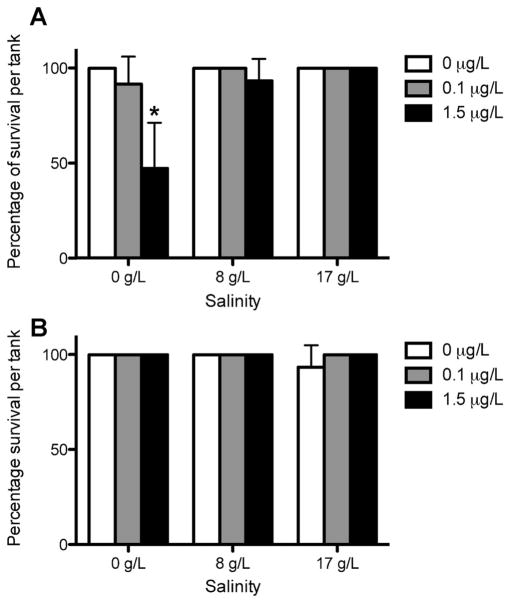
Rainbow trout showed no significant difference in survival at 8 g/L and 17 g/L in all bifenthrin treatment groups. However, a significant decrease (p = 0.0336) was observed at the 1.5-μg/L bifenthrin concentration within the freshwater (0 g/L) treatment. Significant mortality was not observed in steelhead (Figure 1).
Figure 1.
Survival of rainbow trout (A) and steelhead (B) Oncorhynchus mykiss in freshwater and in 8 g/L and 17 g/L salinity exposed to 0 μg/L, 0.1 μg/L, and 1.5 μg/L bifenthrin concentrations for 14 d. Data are expressed as mean survival percentage ± standard deviation of fish per tank (n = 5 fish per tank, 3 replicates). *p <0.05.
Vitellogenin and steroid hormones
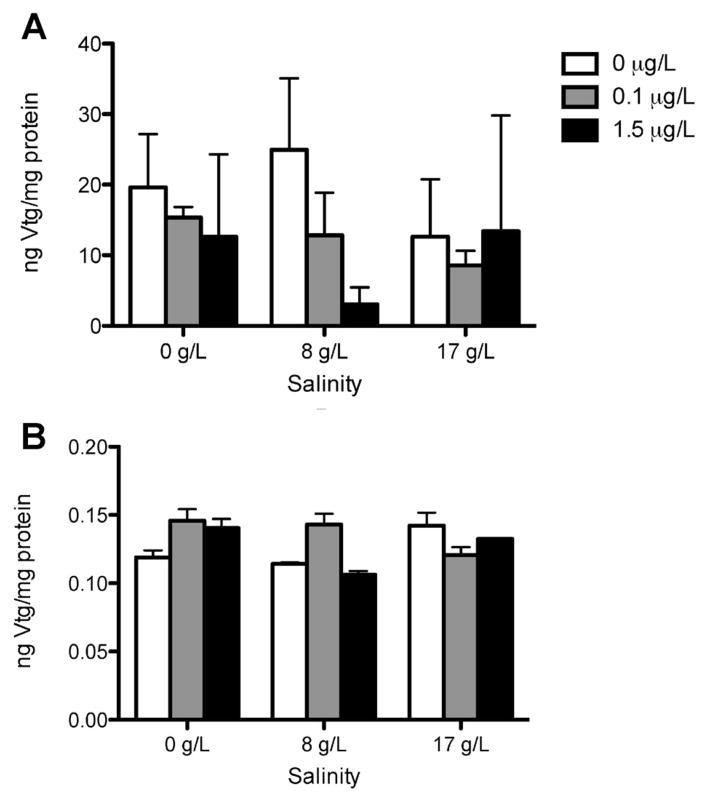
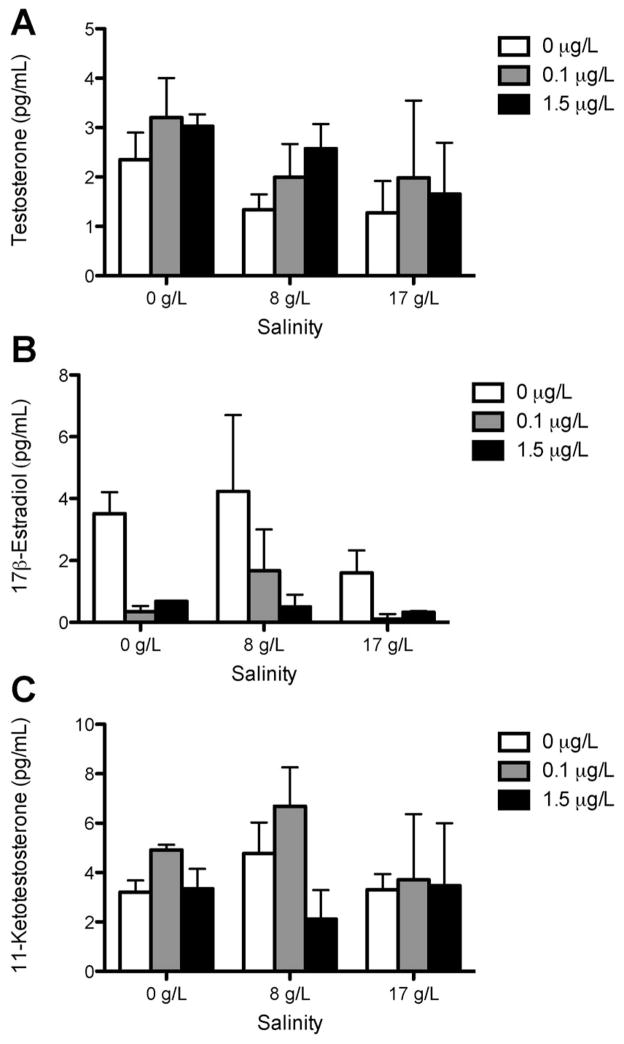
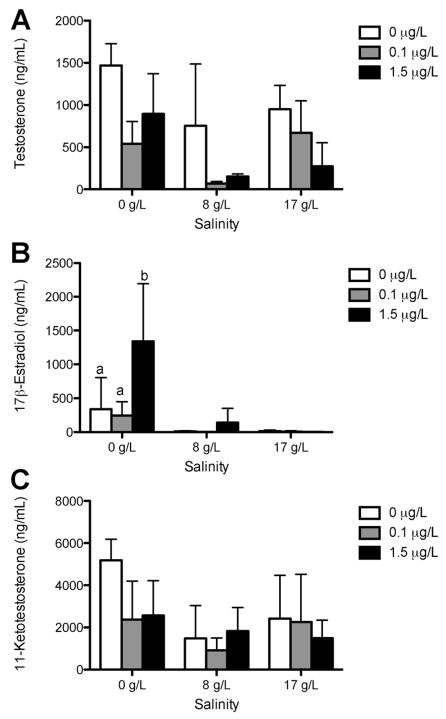
There was no significant difference in plasma VTG protein levels among rainbow trout and steelhead treatments (Figure 2). The VTG protein values for steelhead trout were often below the limit of detection (0.39 ng/mL) and were estimated using 50% of the detection limit. A nonsignificant trend toward reduced E2 concentrations was observed in steelhead after acclimation to saltwater and in rainbow trout in both freshwater and saltwater (Figures 3 and 4). The only significant change observed in sex steroid concentrations was in steelhead, in which treatment with 1.5 μg/L bifenthrin in freshwater significantly increased E2 concentrations (Figure 4). No significant alterations in 11-KT or T were observed in any other strain or treatment.
Figure 2.
Vitellogenin (VTG) plasma concentrations in rainbow trout (A) and steelhead (B) Oncorhynchus mykiss in freshwater and in 8 g/L and 17 g/L salinity exposed to 0 μg/L, 0.1 μg/L, and 1.5 μg/L bifenthrin concentrations for 14 d (n = 5 fish per tank, 3 replicates). Data are expressed as mean ± standard deviation.
Figure 3.
Sex steroid levels of testosterone (A), 17β-estradiol (B), and 11-ketotestosterone (C) in rainbow trout in freshwater and in 8 g/L and 17 g/L salinity exposed to 0 μg/L, 0.1 μg/L, and 1.5 μg/L bifenthrin concentrations for 14 d (n = 5 fish per tank, 3 replicates). Data are expressed as mean ± standard deviation.
Figure 4.
Sex steroid levels of testosterone (A), 17β-estradiol (B), and 11-ketotestosterone (C) in steelhead in freshwater and in 8 g/L and 17 g/L salinity exposed to 0 μg/L, 0.1 μg/L, and 1.5 μg/L bifenthrin concentrations for 14 d (n = 5 fish per tank, 3 replicates). Data are expressed as mean ± standard deviation. Bars with different letters represent significant differences at p = 0.0009.
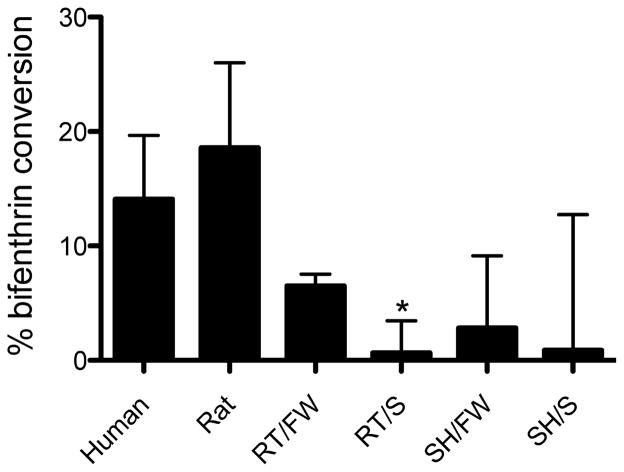
Bifenthrin biotransformation
The NADPH-dependent biotransformation of bifenthrin was highest in rat supersomes, followed by human microsomes and then O. mykiss. Bifenthrin biotransformation was significantly lower in salinity-acclimated rainbow trout (p = 0.0271, Student’s t test). Bifenthrin biotransformation in liver microsomes from freshwater- and saltwater-acclimated steelhead were detected, but no significant differences were observed between freshwater and hypersaline conditions (Figure 5).
Figure 5.
Percentage of conversion of bifenthrin in microsomal incubations containing nicotinaminde adenine dinucleotide phosphate compared with boiled protein controls (n = 3 replicate incubations per animal treatment). Data are expressed as mean ± standard deviation. Asterisk (*) indicates difference between freshwater (FW) and hypersaline (S) conditions; p = 0.0271. SH = steelhead; RT = rainbow trout.
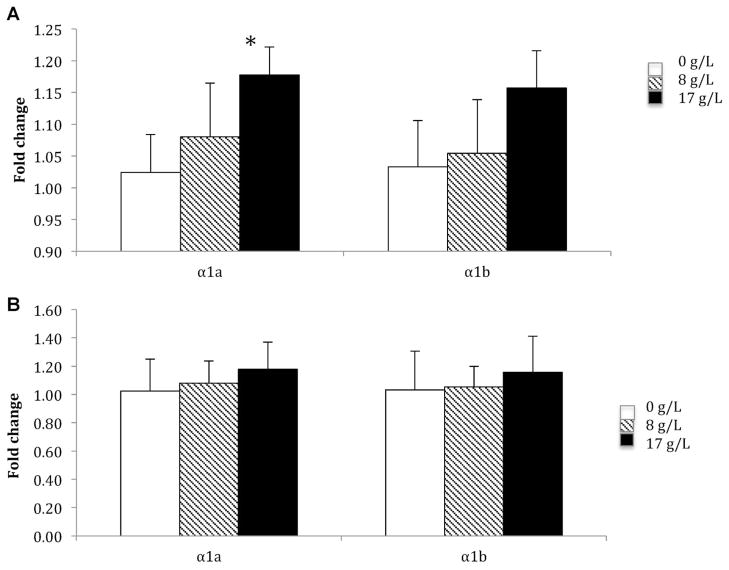
Na+/K+ ATPase mRNA expression
In gill, a salinity-dependent increase of Na+/K+ ATPase α1a (p = 0.024) and a trend toward an increase of α1b transcripts of Na+/K+ ATPase (p = 0.14) were observed in steelhead after salinity acclimation (Figure 6A). In rainbow trout, whereas statistically significant changes were not observed in the α1a transcript following salinity acclimation, a trend toward an increase in the α1b transcript of Na+/K+ ATPase was observed (Figure 6B). In contrast, significant differences (or trends) in isoform transcripts were not observed in brains from either strain or population following salinity acclimation (data not shown).
Figure 6.
Expression of Na+/K+ ATPase mRNA subunit isoforms α1a and α1b in gill of rainbow trout (A) and steelhead (B) (n = 5 fish per tank, 3 replicates). Data are expressed as average relative mRNA transcript expression ± standard deviation. * p = 0.002.
DISCUSSION
The aim of the present study was to evaluate the lethal and sublethal effects of environmentally relevant bifenthrin exposure on juvenile O. mykiss (rainbow trout and steelhead) acclimated to hypersaline conditions. Rainbow trout exhibited significant mortality following exposure to 1.5 μg/L bifenthrin in freshwater. Mortality was not observed in rainbow trout acclimated to higher salinities or steelhead across all treatments. Most worst-case detections of bifenthrin are in the low-ng/L concentration range in surface waters of California, including tributaries of the San Francisco Bay Delta [7,10]. Given saltwater intrusion of the San Francisco Bay Delta, we examined how acclimation of each strain or population to saltwater led to reductions of mortality in rainbow trout.
The initial hypothesis tested was that salinity acclimation detoxifies bifenthrin through enhanced biotransformation to hydroxylated metabolites that are not acutely toxic. Saltwater acclimation at 17 g/L has been shown to increase CYP3A27 in salmonids [22]. Therefore, increased levels of CYP3A in the saltwater-acclimated fish could potentially enhance the metabolism of bifenthrin to metabolites that do not cause acute neurotoxicity. Hydroxylated metabolites of permethrin (4-hydroxy-permethrin) and ester cleavage products (3-phenoxyl benzyl alcohol as well as 3-[4-hydroxy-phenoxy] benzyl alcohol) were found to increase VTG mRNA expression in primary rainbow trout hepatocytes [12]. Other in vitro studies have shown that these metabolites have demonstrated estrogen receptor binding and activation [14]. Recent studies in Menidia berylina demonstrated antiestrogenic activity of bifenthrin in vitro, but estrogenic activity at ng/L concentrations in vivo [8], indicating that biotransformation may have contributed to the estrogenic response [8]. Liver microsomes from humans, rats, steelhead, and rainbow trout catalyzed NADPH-dependent turnover of bifenthrin. However, in contrast to our hypothesis, bifenthrin metabolism was not induced by saltwater acclimation but rather was diminished in rainbow trout and unchanged by acclimation in steelhead. In vitro studies in microsomes from humans and rats reported that bifenthrin had the slowest internal clearance value of 5 pyrethroids evaluated, including permethrin [28]. Although specific metabolite profiles were not determined in the present study (or the human and rat study), the significantly lower turnover of bifenthrin in the fish suggested that metabolite formation was not great enough to elicit an estrogenic response.
Consistent with limited estrogenic metabolite formation, VTG was unaltered by either concentrations of bifenthrin or of hypersaline conditions following 14-d exposures. Significant increases in VTG mRNA expression were previously observed in Japanese medaka and zebrafish treated with bifenthrin [13,29]. However, the concentrations of bifenthrin were in the μg/L range and the duration of exposures ranged from 4 to 10 d. Whereas some studies measure VTG protein in either tissue homogenates or plasma, many other groups have used hepatic VTG mRNA to measure estrogenic activity because of its rapid response following exposure to estrogenic compounds [30–32]. Previous studies with rainbow trout in our laboratory have indicated that 14-d exposures to other pesticides as well as E2 positive controls are more than adequate to induce VTG protein [10,33]. However, additional time may be necessary to elicit estrogenic activity with compounds such as bifenthrin or its metabolites that likely have lower estrogenic efficacy or affect targets other than, or in addition to, the estrogen receptor.
Other possible targets may include enzymes or regulatory pathways that enhance biosynthesis or reduce clearance of E2. Whereas the present study showed trends toward reduction of steroid hormones in rainbow trout, E2 concentrations were statistically higher in steelhead following treatment with 1.5 μg/L bifenthrin in freshwater. Acclimation to saltwater did not affect concentrations in either strain. Other studies that separated male and female animals showed that bifenthrin reduced E2 in females (and oocyte development) after acclimation [34]. These results are consistent with studies in catfish (Heteropneustes fossilis), which reported a significant decrease in E2 and 11-KT levels after 45 d of exposure to 20 μg/L cypermethrin [35]. In contrast, the elevation of E2 in juvenile steelhead in the present study is consistent with estrogenic activity (e.g., VTG induction) observed in other fish (Menidia, Oryzias, Danio [8,11–13]), but VTG protein in plasma was unchanged in steelhead and no relationship was observed in linear regression comparisons of E2 with VTG within individuals (data not shown). It is unclear whether the failure to observe VTG protein following bifenthrin treatment may be due to kinetic issues among estrogen receptor binding, VTG mRNA transcription, or VTG translation, as discussed above, or the possibility that a longer duration of exposure is necessary to enhance body burdens of E2 to elicit VTG protein formation.
The differences in toxicity between steelhead and rainbow trout suggest that life history or strain differences (or both) may also have contributed to the response differences. Although genetically capable, the rainbow trout rarely undergo natural saltwater acclimation. In contrast, steelhead are released in freshwater, and are allowed to undergo natural migration to saltwater. One possible strain difference may be the imprinting of steelhead resulting in more efficient osmoregulatory function in animals of more advanced stages of development (i.e., older). In gill, Na+/K+ ATPase is a critical enzyme in salmonids undergoing saltwater acclimation, and catalytic activity is significantly induced during the smoltification process [36]. Previous work by Richards et al. [26] identified 2 isoforms of the Na+/K+ ATPase α subunit, 1a and 1b, that have been shown to decrease (Na+/K+ ATPase α1a) and increase (Na+/K+ ATPase α1b) expression with increasing salinity. In the present study, the lack of change in Na+/K+ ATPase α1b mRNA in either strain or population before or after acclimation to 17 ppt (~50%) is consistent with previous work suggesting that a higher concentration of salinity, 80% seawater (27 ppt), is required for induction [26]. Because pyrethroids inhibit Na+/K+ ATPase [6], animals with higher endogenous levels of the enzyme in the brain or peripheral nervous system may be more susceptible to the acute toxicity of pyrethroids because of additional targets for the pyrethroids. However, significant differences were not observed in Na+/K+ ATPase transcripts of brain in either rainbow trout or steelhead. Whether Na+/K+ ATPase α1a plays a more significant role in the peripheral nervous system or neuromuscular junction requires additional study.
In summary, the observation of diminished acute toxicity of bifenthrin after salinity acclimation in rainbow trout but not in steelhead was novel. Hepatic biotransformation does not appear to contribute to the acute detoxification of bifenthrin in saltwater-acclimated fish. The lack of VTG induction in either species under freshwater or saltwater conditions also does not support bifenthrin biotransformation in vivo and the formation of metabolites that have estrogenic activity. Further studies exploring the impacts of strain differences on enzymatic pathways involved in smoltification in salmonids is necessary to better understand the lethal and sublethal impacts of bifenthrin in salmonids in estuarine environments such as the San Francisco Bay Delta.
Acknowledgments
This material is based on work supported by the Delta Science Program under grant 2046. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Delta Science Program. We also thank the California Department of Fish and Game and the Nimbus Hatchery for providing some of the fish used in the present study. Water sample analyses were performed by W. Jiang and J. Gan of the University of California, Riverside. A special thank you also to R. Lavado and K. Forsgren for their technical and laboratory expertise.
References
- 1.Kuivila KM, Hladik ML. Understanding the occurrence and transport of current-use pesticides in the San Francisco Estuary Watershed. San Francisco Estuary and Watershed Science. 2008;6:1–21. [Google Scholar]
- 2.Spurlock F, Lee M. Synthetic pyrethroids use patterns, properties and environmental effects. In: Gan J, Spurlock F, Hendley P, Weston D, editors. Synthetic Pyrethroids: Occurrence and Behavior in Aquatic Environments, Vol 991—ACS Symposium Series. American Chemical Society; Washington, DC, USA: 2008. pp. 3–25. [Google Scholar]
- 3.Oros DR, Inge W. SFEI Contribution 415. White Paper. San Francisco Estuary Institute; Oakland, CA, USA: 2005. Pyrethroid insecticides: An analysis of use patterns, distributions, potential toxicity and fate in the Sacramento-San Joaquin Delta and Central Valley. [Google Scholar]
- 4.Vijverberg HPM, van den Bercken J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit Rev Toxicol. 1990;21:105–126. doi: 10.3109/10408449009089875. [DOI] [PubMed] [Google Scholar]
- 5.Roberts TR, Hutson DH. Insecticides and fungicides: Pyrethroids. In: Roberts TR, editor. Metabolic Pathways of Agrochemicals, Part 2. Royal Society of Chemistry; Cambridge, UK: 1999. pp. 579–725. [Google Scholar]
- 6.El-Toukhy MA, Giris RS. In vivo and in vitro studies on the effect of larvin and cypermehtrin on adenosine triphosphatase activity in male rats. J Environ Sci Health B. 1993;28:599–619. doi: 10.1080/03601239309372843. [DOI] [PubMed] [Google Scholar]
- 7.Weston DP, Holmes RW, Lydy MJ. Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ Pollut. 2009;157:287–294. doi: 10.1016/j.envpol.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Brander SM, Gouchun H, Smalling KL, Denison MS, Cherr GN. The in vivo estrogenic and in vitro anti-estrogenic activity of permethrin and bifenthrin. Environ Toxicol Chem. 2012;31:2848–2855. doi: 10.1002/etc.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlenk D, Lavado R, Loyo-Rosales J, Jones W, Maryoung L, Riar N, Werner I, Sedlak D. Reconstitution studies of pesticides and surfactants exploring the cause of estrogenic activity observed in surface waters of the San Francisco Bay Delta. Environ Sci Technol. 2012;46:9106–9111. doi: 10.1021/es3016759. [DOI] [PubMed] [Google Scholar]
- 10.Lavado R, Loyo-Rosales JE, Floyd E, Kolodziej EP, Snyder SA, Sedlak D, Schlenk D. Site-specific profiles of estrogenic activity in agricultural areas of California’s inland waters. Environ Sci Technol. 2009;43:9110–9116. doi: 10.1021/es902583q. [DOI] [PubMed] [Google Scholar]
- 11.Beggel S, Connon R, Werner I, Geist J. Changes in gene transcription and whole organism responses in larval fathead minnow (Pimephales promelas) following short-term exposure to the synthetic pyrethroid bifenthrin. Aquat Toxicol. 2009;105:180–188. doi: 10.1016/j.aquatox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Nillos MG, Chajkowski S, Gan J, Lavado R, Rimoldi JM, Schlenk D. Stereoselective biotransformation of permethrin to estrogenic metabolites in fish. Chem Res Toxicol. 2010;23:1568–1575. doi: 10.1021/tx100167x. [DOI] [PubMed] [Google Scholar]
- 13.Wang LM, Liu W, Yang C, Pan Z, Gan J, Xu C, Zhao M, Schlenk D. Enantioselectivity in estrogenic potential and uptake of bifenthrin. Environ Sci Technol. 2007;41:6124–6128. doi: 10.1021/es070220d. [DOI] [PubMed] [Google Scholar]
- 14.Tyler CR, Beresford N, van der Woning M, Sumpter JP, Thorpe K. Metabolism and environmental degradation of pyrethroid insecticides produce compounds with endocrine activities. Environ Toxicol Chem. 2000;19:801–809. [Google Scholar]
- 15.Denslow ND. Vitellogenin as a biomarker of exposure for estrogen or estrogen mimics. Ecotoxicology. 1999;8:385–398. [Google Scholar]
- 16.Knowles N, Cayan DR. Potential effects of global warming on the Sacramento/San Joaquin watershed and the San Francisco Estuary. Geophys Res Lett. 2002;29:32–42. [Google Scholar]
- 17.Peterson DH, Cayan DR, Dettinger MD, Noble M, Riddle LG, Schemel LE, Smith RE, Uncles RJ, Walters R. San Francisco Bay salinity: Observations, numerical simulation, and statistical models. In: Hollibaugh JT, editor. San Francisco Bay: The Ecosystem. American Association for the Advancement of Science; San Francisco, CA, USA: 1996. pp. 9–34. [Google Scholar]
- 18.Schreck CB. Stress and compensation in telostean fishes: Response to social and physical factors. In: Pickering AD, editor. Stress and Fish. Academic; New York, NY, USA: 1981. pp. 295–321. [Google Scholar]
- 19.Arjona FJ, Vargas-Chacoff L, Ruiz-Jarabo I, del Rio MPM, Mancera JM. Osmoregulatory response of Senegalese sole (Solea senegalensis) to changes in environmental salinity. Comp Biochem Physiol A. 2007;148:413–421. doi: 10.1016/j.cbpa.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Celander M. Impact of stress on animal toxicology. In: Balm PHM, editor. Stress Physiology in Animals. Sheffield Academic; Sheffield, UK: 1999. pp. 246–279. [Google Scholar]
- 21.Parkinson A, Ogilvie BW. Biotransformation of xenobiotics. In: Klaassen CD, editor. Cassarette and Doull’s Toxicology: The Basic Science of Poisons. McGraw Hill; New York, NY, USA: 2008. pp. 161–304. [Google Scholar]
- 22.Lavado R, Rimoldi JM, Schlenk D. Mechanisms of fenthion activation in rainbow trout (Oncorhynchus mykiss) acclimated to hypersaline environments. Toxicol Appl Pharmacol. 2009;235:143–152. doi: 10.1016/j.taap.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradford M. A rapid and sensitive method for the quantification of microgram protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Jiang W, Gan J, Haver D. Sorption and desorption of pyrethroid insecticide permethrin on concrete. Environ Sci Technol. 2011;45:602–607. doi: 10.1021/es1030323. [DOI] [PubMed] [Google Scholar]
- 25.Gil-Garcia MD, Barranco-Martinez D, Martinez-Galera M, Parrilla-Vazquez P. Simple, rapid solid-phase extraction procedure for the determination of ultra-trace levels of pyrethroids in ground and sea water by liquid chromatography/electrospray ionization mass spectroscopy. Rapid Commun Mass Spectrom. 2006;20:2395–2403. doi: 10.1002/rcm.2600. [DOI] [PubMed] [Google Scholar]
- 26.Richards JG, Semple JW, Bystriansky JS, Schulte PM. Na+/K+-ATPase α-isoform switching in gills of rainbow trout (Onchorhyncus mykiss) during salinity transfer. J Exp Biol. 2003;206:4475–4486. doi: 10.1242/jeb.00701. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1045–1062. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scollon EJ, DeVito MJ, Godin SJ, Hughes MF, Starr JM. In vitro metabolism of pyrethroid pesticides by rat and human hepatic microsomes and cytochrome p450 isoforms. Drug Metab Dispos. 2008;37:221–228. doi: 10.1124/dmd.108.022343. [DOI] [PubMed] [Google Scholar]
- 29.Jin M, Zhang X, Wang L, Huang C, Zhang Y, Zhao M. Developmental toxicity of bifenthrin in embryo-larval stages of zebrafish. Aquat Toxicol. 2009;95:347–354. doi: 10.1016/j.aquatox.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Hemmer MJ, Hemmer BL, Bowman CJ, Kroll KJ, Folmar LC, Marcovich D, Hoglund MD, Denslow ND. Effects of p-nonylphenol, methoxychlor, and edosulfan on vitellogenin induction and expression in sheepshead minnow (Cyprinodon variegatus) Environ Toxicol Chem. 2001;20:336–343. doi: 10.1897/1551-5028(2001)020<0336:eopnma>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci U S A. 2007;104:8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lattier DL, Reddy TV, Gordon DA, Lazorchak JM, Smith ME, Williams DE, Wiechman B, Flick RW, Miracle AL, Toth GP. 17α-Ethynylestradiol induced vitellogenin gene transcription quantified in livers of adult males, larvae, and gills of fathead minnows (Pimephales promelas) Environ Toxicol Chem. 2002;21:2385–2393. [PubMed] [Google Scholar]
- 33.Xie L, Thrippleton K, Irwin MA, Siemering GS, Mekebri A, Crane D, Berry K, Schlenk D. Evaluation of estrogenic activities of aquatic herbicides and surfactants using a rainbow trout vitellogenin assay. Toxicol Sci. 2005;87:391–398. doi: 10.1093/toxsci/kfi249. [DOI] [PubMed] [Google Scholar]
- 34.Forsgren KL, Riar N, Schlenk D. The effects of the pyrethroid insecticide, bifenthrin on steroid hormone levels and gonadal development of steelhead (Oncorhynchus mykiss) under hypersaline conditions. Gen Comp Endocrinol. 2013;186:101–107. doi: 10.1016/j.ygcen.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 35.Singh PB, Singh V. Cypermethrin induced histological changes in gonadotrophic cells, liver, gonads, plasma levels of estradiol-17β and 11-ketotestosterone, and sperm motility in Heteropneustes fossilis (Bloch) Chemosphere. 2008;72:422–431. doi: 10.1016/j.chemosphere.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Björnsson BT, Stefansson SO, McCormick SD. Environmental endocrinology of salmon smoltification. Gen Comp Endocrinol. 2011;170:290–298. doi: 10.1016/j.ygcen.2010.07.003. [DOI] [PubMed] [Google Scholar]