Abstract
Liposomes are becoming increasingly important as drug delivery systems, to target a drug to specific cells and tissues and thereby protecting the recipient from toxic effects of the contained drug. Liposome preparations have been described to activate complement. In this study, we have investigated complement activation triggered by neutral dimyristoyl-phosphocholine (DMPC) liposomes in human plasma and whole-blood systems. Incubation in plasma led to the generation of complement activation products (C3a and sC5b-9). Unexpectedly, investigations of surface-bound C3 revealed contact activated, conformationally changed C3 molecules on the liposomes. These changes were characterized by Western blotting with C3 monoclonal antibodies, and by incubating liposomes with purified native C3 and factors I and H. Quartz crystal microbalance analysis confirmed binding of C3 to planar DMPC surfaces. In addition, we demonstrated that DMPC liposomes bound to or were phagocytized by granulocytes in a complement-dependent manner, as evidenced by the use of complement inhibitors. In summary, we have shown that C3 is activated both by convertase-dependent cleavage, preferentially in the fluid phase, by mechanisms which are not well elucidated, and also by contact activation into C3(H2O) on the DMPC surface. In particular, this contact activation has implications for the therapeutic regulation of complement activation during liposome treatment.
Keywords: Liposome, Complement, Immune response, Contact activation
1. Introduction
Liposomes are widely used as tailor-made delivery vehicles, acting as carriers of peptides, proteins, and other active substances for pharmaceutical, cosmetic, and biochemical purposes [1,2]. In addition, liposomes are commonly studied as biomimetic models for complex biological systems, since amphiphilic phospholipid molecules are major components of biological membranes [3]. Although phospholipids are natural components of the human body, it is well known that they initiate immune responses in vitro [4] and in vivo [5-7]. A crucial factor in these detrimental reactions is the complement system.
The complement system is part of the innate immune system and one of the main effector mechanisms of antibody-mediated immunity. There are three different activation pathways for complement activation: the classical, alternative, and lectin pathways. All complement pathways converge at the major complement protein C3 [8]. The native C3 molecule is composed of an α-chain (110 kDa) and a β-chain (75 kDa), connected by a disulfide bond and non-covalent forces. C3 is activated by distinct C3 convertases of the classical/lectin pathway and the alternative pathway. These convertases cleave C3 between residues 726 and 727 (Arg–Ser) of the α-chain [9,10], generating two fragments: C3a (9 kDa), an anaphylatoxin [11]; and C3b (175 kDa), which binds covalently to the surface when a thioester is cleaved [12] and opsonize artificial and target (e.g., bacterial) surfaces for phagocytosis and cytotoxicity.
Formation of the alternative pathway convertases (C3bBb) depends on an initial deposition of C3b molecules. These molecules can be provided by either of the three activation pathways or by so-called “tick-over” [13]. Tick-over is suggested to be caused by a non-proteolytic activation of C3 as the result of a nucleophilic attack of the internal thioester bond hidden in the α-chain of the native C3 by water or by other nucleophilic substances (e.g., NH3 and methylamine) [14]. This mechanism generates a C3b-like molecule known as C3(H2O) [15,16], which is able to form fluid-phase convertases (C3(H2O)Bb) that deposit initial C3b molecules on the surface.
Unlike native C3, C3b and C3(H2O) can be cleaved by factor I, together with a co-factor, into the inactivated forms iC3b and iC3(H2O), respectively. It is also well established that like C3b, both C3(H2O) and iC3(H2O) interact with C3 receptors, including CR1 (CD35) [8], CR2 (CD21) [17], and a CR3 (CD11b/CD18)-like molecule from Candida albicans [18].
The interaction between liposomes and the complement system has previously been described [2,4,6,7,19], and interactions between liposomes and C3 have been demonstrated [20]. The capacity of liposomes to activate the complement system has been studied, and the degree of activation has been found in these reports to correlate with the lipid composition, i.e., the degree of lipid saturation [4,21], cholesterol content [4,22], and/or the presence of charged phospholipids [4]. The composition of the liposomes with regard to cholesterol content drastically influences complement activation, in a strong dose-dependent manner [4]. In addition to their net charge and cholesterol content, the size and curvature of the liposomes affect their ability to activate the complement system [23] with larger liposomes being more efficient activators than smaller liposomes [24].
Modification of liposomes with PEG, metals, artificial lipids, drugs, and homing ligands has resulted in distinctive surface chemistries that can provoke activation of both the complement and coagulation systems [25]. These blood-contact issues are well known clinically, and several in vivo studies have attempted to prevent liposome-driven complement activation by pre-treatment with complement inhibitors [26,27]. The effects on phagocytosis mediated by charged liposomes have also been investigated and shown to be dependent on complement activation [28].
In the present study, we investigated the activation of the complement system on artificial phospholipid bilayer membranes. Here we focused on the phosphocholine (PC) surface because PC is one of the main components of cellular membranes. Dimyristoyl-phosphocholine (DMPC) liposomes were used to mimic biological membranes, which are exposed to human blood. Generation of fluid-phase complement activation products (C3a and sC5b-9) by DMPC liposomes was observed in human blood plasma. Unexpectedly, the C3 bound to the liposome surface was not the result of convertase (proteolytic)-mediated complement activation. By using various monoclonal antibodies, we characterized the bound C3 molecules as C3(H2O), which has similar functional properties as C3b and that can be cleaved by factor I and co-factor H. Flow cytometry analysis showed complement-dependent binding or uptake of liposomes by polymorphonuclear monocytes (PMNs). The discovery of C3(H2O) generation on the surface of liposomes has implications for the regulation of complement-mediated binding or uptake and destruction of therapeutic liposomes.
2. Materials and methods
2.1. Purified proteins and antibodies
C3 and factor H were purified according to Hammer et al. from human plasma and serum, respectively [29]. The first step of the factor H purification involved a euglobulin precipitation, as described by Nilsson and Müller-Eberhard [30]. Factor I was prepared from human plasma according to Fearon [31]. C3b was generated by incubating C3 with trypsin, which produces C3a and C3b fragments. The C3a was removed by gel filtration. Incubating C3 with factor I and factor H as a co-factor leads to formation of iC3b. The anti-C3 monoclonal antibodies (mAbs) 4SD17.3, 7D.236.2, 7D.398.1, and 7D.9.2 were produced according to Nilsson et al. [32] and were affinity-purified on protein A-Sepharose. Culture supernatants containing mAbs were adjusted to 1.5 m glycine/3 M NaCl, pH 8.9, and applied to a 4-ml protein A-Sepharose column. After the column was washed, the antibodies were eluted with 0.1 m glycine, pH 2.8, and then dialyzed against PBS. Both ethylenediaminetetraacetic acid (EDTA) and ethylene glycol tetraacetic acid (EGTA) were purchased from Merck (Merck KGaA, Darmstadt, Germany). Compstatin analog (Ac-ICV(1MeW) QDWGAHRCT-NH2) and C5a receptor antagonist (C5aRA) PM×53 were provided by Prof. John D. Lambris. APC-conjugated monoclonal mouse anti-human CD11b (ICRF44), Pacific Blue-conjugated mouse anti-human CD16 (3G8), APC-conjugated mouse IgG1 isotype control (MOPC-21), and Pacific blue-conjugates IgG1 isotype control (MOPC-21) were all purchased from BD Pharmingen (BD Biosciences, San Jose, CA, USA).
2.2. Blood, plasma, and serum preparation
Blood samples were obtained from healthy volunteers who had not received any medication for at least 10 days prior to donation. Blood was drawn into 6 ml vacutainer tubes (BD Vacutainer Z, Plymouth, UK) containing the specific thrombin inhibitor lepirudin (70 μm; Refludan; Schering AG, Berlin, Germany). To prepare plasma, whole blood was centrifuged twice at 2000 g for 15 min at room temperature (RT); the plasma samples will be referred to as lepirudin plasma. In other experiments, blood was drawn into 5 ml vacutainer tubes. To prepare normal human serum, whole blood was allowed to clot for 1 h and then centrifuged at 2500 g for 15 min at RT.
2.3. Liposome preparation
1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) phospholipids (Lipoid, Ludwigshafen, Germany) dissolved in ethanol (99% purity, AppliChem GmbH, Darmstadt, Germany), either pure or containing 2 mol% Atto 590-1,2-dihexdecanoyl-sn-glycero-3-phosphatidyl-ethanolamin (DPPE-Atto 590; Atto-Tec, Siegen, Germany), were evaporated and resuspended in PBS buffer (pH 7.4) to a final lipid concentration of 20 mm. The suspension was extruded with a hand extruder through two 200 nm or 50 nm polycarbonate track etch membranes (Whatman, Maidstone, UK) at 40–50 °C to a mean diameter of 169 ± 4 nm or 75 ± 5 nm (polydispersity index <0.1), as measured by dynamic light scattering (DLS; Zetasizer ZS90, Malvern, Herrenberg, Germany). Zetapotential was determined as well (Zetasizer ZS90) in PBS to an average of −5.5 ± 2.7 mV. Liposomes were frozen at −24 °C. Before use, liposomes were ultrasonicated, and the size and monodispersity of several batches were checked after freezing. The size of liposome was unaffected by freezing (169 ± 4 nm before freezing and 167 ± 4 nm after freezing). An endotoxin assay (Pierce LAL Chromogenic Endotoxin Quantitation Kit, Thermo Scientific, Rockford, USA) showed an endotoxin content below the lowest point of the standard curve. In a liposome preparation with a concentration of 12 mm, an endotoxin concentration of 0.04 EU/ml in PBS was obtained.
2.4. Measuring complement activation – sample preparation
To study the DMPC liposomes-mediated complement activation, liposomes were incubated in lepirudin-anticoagulated plasma or normal human serum. Treatment with lepirudin, a specific thrombin inhibitor, ensures that no activation of the clotting system has occurred, but it does not influence the complement system and its convertases [33]. DMPC liposomes (1.5 mg/ml) were incubated in the lepirudin plasma or normal human serum for up to 60 min at 37 °C.
In other experiments, lepirudin plasma was preincubated with either 10 μM of the specific C3 inhibitor Compstatin [34], 10 mm EDTA, or 10 mM EGTA with 2.5 mm Mg2+ prior to the addition of 1.5 mg/ml liposomes. The reaction was stopped immediately or after 15, 30, or 60 min of incubation at 37 °C by the addition of 10 mm EDTA.
2.5. Enzyme-linked immunosorbent assays (ELISAs)
The liposome-mediated complement activation in the fluid phase was measured by ELISA. C3a and sC5b-9 levels in lepirudin plasma and normal human serum were analyzed. PBS with 0.05% Tween20 (Sigma–Aldrich Inc, St. Louis, MO, USA) was used as a washing buffer; the washing buffer also containing 1% (w/v) bovine serum albumin (BSA; Sigma–Aldrich), and 10 mm EDTA was used as a working buffer. Zymosan-activated serum was used as calibration standard. As a substrate, 3,3′,5,5′-tetramethylbenzidine (TMB; Invitrogen) was used for all assays.
C3a Anti-C3a monoclonal antibody 4SD17.3 [35] was used as the capture anti-body. Bound C3a was detected with a biotinylated anti-C3a antibody, followed by horseradish peroxidase (HRP)-conjugated streptavidin (Amersham, Little Chalfont, UK).
sC5b-9 Anti-human C5b-9 (Diatec, Oslo, Norway) was used as the capture antibody. Bound sC5b-9 complexes were detected with the Ig fraction of a polyclonal antibody recognizing complement C5 (Acris, Herford, Germany), followed by HRP-conjugated polyclonal anti-sheep immunoglobulin antibody (Dako, Glostrup, Denmark). DeltaSoft (BioMetallics Inc, Princeton NJ, USA) software was used to determine the concentrations of C3a and sC5b-9 in each sample.
2.6. SDS-PAGE and Western blot analysis
Western blot analysis was performed to study binding of complement proteins, to the liposomes. Liposomes (7.5 mg/ml) were incubated in lepirudin plasma in the absence or presence of 10 μm Compstatin for up to 30 min at 37 °C, which gave a liposome:C3 ratio of 5:1, assuming that normal human plasma has a C3 concentration of 1.5 mg/ml. Compstatin was added to investigate whether the binding of the complement proteins was mediated by complement activation or not. The reaction was stopped immediately or after 30 min by adding 10 mm EDTA. The samples were centrifuged at 20,000 g for 45 min at 4 °C, and the supernatants were carefully removed. Samples were then washed three times with 1 mm phenyl-methylsulfonylfluoride (PMSF; Sigma–Aldrich) in PBS, to inhibit putative proteases that could cleave C3.
In other experiments, liposomes at 1 mg/ml were incubated in 0.2 mg/ml purified C3 for 15 min at 37 °C. (In order to facilitate comparison of Western blots performed after incubation of liposomes in plasma and purified C3, respectively, the same liposome:C3 ratio (5:1) was chosen for both sets of experiments.) Either buffer or factor I (8 μg/ml) and factor H (32 μg/ml) were added and incubated for a further 10 min at 37 °C. The samples were boiled under reducing conditions and subjected to 7.5% or 10% SDS-PAGE. Proteins were transferred to a PVDF membrane (polyvinylidene fluoride; Bio-Rad, Hercules, CA, USA). After blocking with 1% BSA, the membranes were incubated in biotinylated anti-human C3c polyclonal antibody (Dako). To further investigate the proteolytic forms of the bound C3 molecules, individual biotinylated mouse anti-human C3 monoclonal antibodies (anti-C3 mAbs) were used to detect different C3 molecule epitopes: 4SD17.3 to detect C3a (α1), mAb 7D.398.1 to detect the 20 kDa polypeptide of C3c (α2), mAb 7D.9.2 to detect C3d (α4) and mAb 7D.236.2 to detect the 40 kDa polypeptide of C3c (α5), as designated in Fig. 4B [35,36]. After washing, the bound antibodies were detected with HRP-conjugated streptavidin followed by diaminobenzidine (DAB; Sigma–Aldrich) for color development. C3, C3b, and iC3b were used as controls at a concentration of 0.5 μg/ml each.
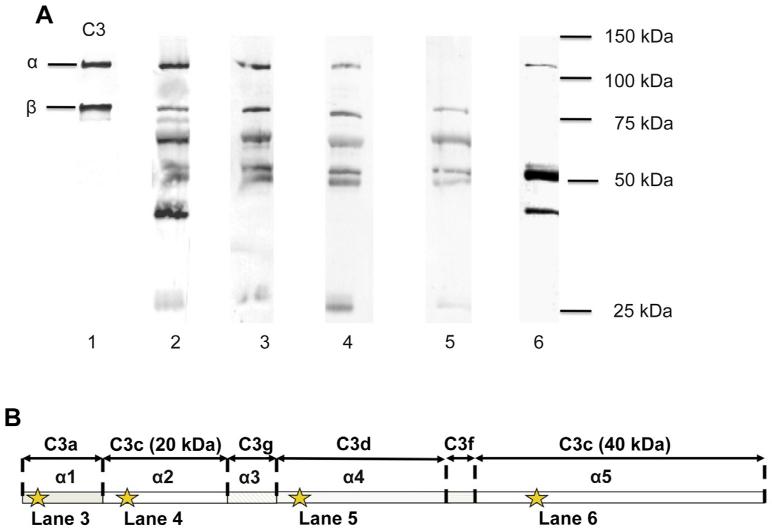
Fig. 4.
Characterization of liposome-bound C3 was further investigated by the use of different mAbs. (A): 4SD17.3 (lane 3) against the C3a epitope (α1), 7D.398.1 (lane 4) against the 20 kDa C3c epitope (α2), 7D.9.2 (lane 5) against C3d (α4), and 7D.236.2 (lane 6) against the 40 kDa C3c epitope (α5). The binding was compared to that of a polyclonal anti-C3c (lane 2) and a C3 control (lane 1). Liposomes were incubated in lepirudin plasma for 30 min. After boiling, the samples were subjected to SDS-PAGE under reducing conditions and blotted to a PVDF membrane. Epitope locations for the anti-C3 mAbs is shown in panel (B).
2.7. Flow cytometry
Flow cytometry was used to monitor the activation of PMNs and the binding or uptake of liposomes by PMNs. Atto 590 fluorescence-labeled liposomes (1.5 mg/ml) were incubated in whole blood for 30 min at 37 °C in the absence or presence of either 50 μM Compstatin or 10 μM of C5aRA [37]. The reaction was stopped with EDTA, followed by incubation with the following antibodies for 30 min at RT: Pacific Blue-labeled mouse anti-human CD16 (BD Pharmingen) was used to identify PMNs, APC-conjugated monoclonal mouse anti-human CD11b (BD Pharmingen) to measure the degree of activation of PMNs, APC-conjugated mouse IgG1, and Pacific blue-conjugates IgG1 were both used as isotype controls according to the instructions of the manufacturer (BD Pharmingen). The erythrocytes were then lysed in FACS lysis buffer (Becton Dickinson, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. The cells were washed twice with buffer containing PBS with 2% fetal calf serum (Invitrogen) and 0.1% sodium azide (NaN3; Sigma–Aldrich). After the last wash, the cells were resuspended in 1% paraformaldehyde (PFA; Apoteket, Gothenburg, Sweden) in PBS. The bound fluorochrome-labeled antibodies were monitored in a LSR II flow cytometer (Becton Dickinson, San Jose, CA, USA) using BD FACSDiva software. A total of 7500 CD16-positive counts were acquired, and the data are presented as double-positivity between Pacific Blue CD16-labeled PMNs and Atto 590-labeled liposomes. (In some experiments, the blood was incubated with FITC-labeled liposomes and the experiment was done as described above. However, those samples were analyzed by flow cytometry prior to and after incubation with 7.5% trypan blue (Sigma–Aldrich) for 5 min at RT.)
2.8. Confocal microscopy
To confirm uptake or binding of fluorescence-labeled liposomes by monocytes and PMNs, samples prepared for flow cytometry were fixed on a microscope slide, and the fluorochrome-labeled PMNs and liposomes were monitored using confocal microscopy (Zeiss LSM 510; Carl Zeiss, Jena, Germany).
2.9. Quartz crystal microbalance with dissipation (QCM-D)
Quartz crystal microbalance experiments with dissipation monitoring (QCM-D 300; Q-Sense, Gothenburg, Sweden) were carried out to observe the binding of C3 to DMPC surfaces in real-time. The underlying technique is based on an oscillating quartz crystal as a sensor chip. Frequency and dissipation are monitored and are proportional to the mass adsorption on the sensor chip due to the amount of coupled mass [38,39]. The relationship is described by the Sauerbrey relation with the overtone number n, the frequency shift Δf and the mass sensitivity constant C (= 17.7 ng Hz −1 cm −2 at f = 5 MHz sensor chip):
| (1) |
In these measurements, a SiO2 sensor chip (Q-Sense, Gothenburg, Sweden) was mounted, and exchange of the solution was performed perpendicular to the surface. All experiments were carried out at a temperature of 37 ± 0.05 °C. A solution containing 0.1 mg/ml of 75 ± 5 nm DMPC liposomes in PBS was added. This low concentration of lipids is necessary to guarantee, that a bilayer forms [40]. After a rinse with PBS, C3 (200 μg/ml) was added, and the surface was again washed with 1.2 ml PBS to demonstrate binding to the lipid surface. In order to study the conformation of the C3 molecules bound to the lipid surface, factor H (32 μg/ml) was added to the surface and after two washing cycles with PBS, factor I (8 μg/ml) was added in sequence followed by another washing cycle using PBS. Changes in the frequency Δf and the dissipation ΔD were measured at the fundamental frequency (n = 1, i.e., f ~ 5 MHz), third (n = 3, i.e., f ~ 15 MHz), fifth (n = 5, i.e., f ~ 5 MHz) and seventh (n = 7, i.e., f ~ 35 MHz) overtone. To analyze the data, Δf was normalized to the fundamental resonance:
| (2) |
for simplicity and consistency. QSoft 301 (Q-Sense) was used for analyzing the data.
2.10. Statistical analysis
Results are presented as means ± SEM. Origin 8.7 was used for statistical analysis. Here, n refers to n independent experiments using plasma from n different donors. In all our experiments, we used 6 different donors. Flow cytometry data were analyzed using Prism 6 for Macintosh software (GraphPad, San Diego, CA, USA). One-way ANOVA and Bonferroni’s multiple comparison test was applied for calculating differences in data containing more than two variables.
3. Results
3.1. Detection of complement activation
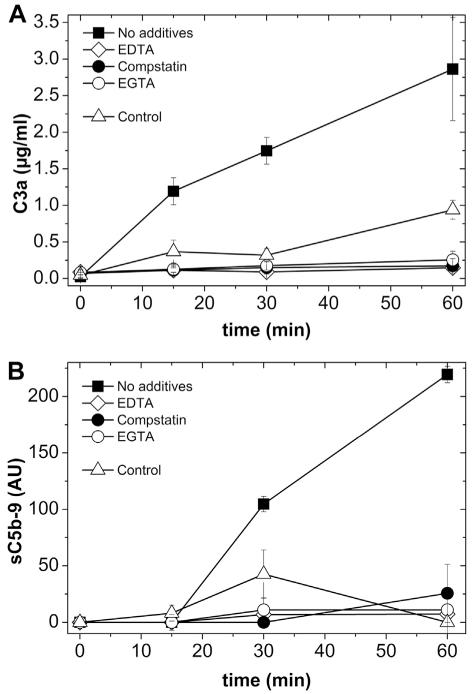
Complement activation initiated by DMPC liposomes of 170-nm diameter was studied by incubating liposomes in lepirudin plasma for up to 60 min, in the absence and presence of various complement inhibitors. The generation of complement activation products, C3a and sC5b-9, was assessed by ELISA. A significant increase in C3a (Fig 1A) and sC5b-9 (Fig 1B) levels was observed in lepirudin plasma that had been incubated with DMPC liposomes. Both C3a and sC5b-9 were inhibited to background levels by Compstatin, EDTA, and Mg2+/EGTA. Lepirudin plasma without the addition of liposomes was used as a control and showed only background level activation of C3a and sC5b-9.
Fig. 1.
Liposome-induced complement activation, measured as generation of C3a (A) and sC5b-9 (B). Lepirudin plasma incubated with DMPC liposomes for up to 60 min in the absence or presence of EDTA, EGTA, or 10 μm Compstatin. Lepirudin plasma without liposomes was used as a control.
3.2. Binding of C3 on the liposome surface
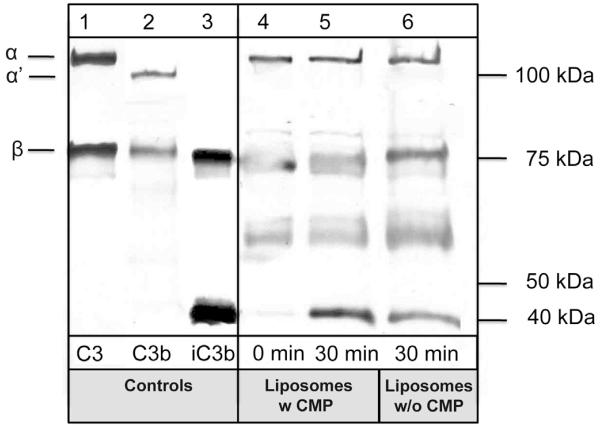
The binding of C3 to the liposome surface was investigated by SDS-PAGE and Western blotting. Liposomes were incubated in lepirudin plasma in the absence and presence of 10 μM Compstatin for up to 30 min at 37 °C (Fig. 2). Western blot analysis using a polyclonal antibody, which recognizes the C-terminal C3c-derived part of the α-chain (α) and the entire β-chain of C3 (b) and activation/degradation fragments (C3b, iC3b; lanes 1–3) as controls, revealed rapid binding of substantial amounts of C3 to the liposome surface immediately after incubation (lane 4). No α′ chain was observed in this sample, thereby indicating that the deposition has not been driven by convertase-mediated activation. The occurrence of a 40-kDa band after 30 min of incubation (lane 5) suggested a partial proteolytic processing, most likely by factor I-mediated degradation. The lack of significant effect of Compstatin supports the interpretation of an independence of convertase-mediated activation and advocated the hypothesis that C3 adheres to liposomes in a C3(H2O)-type conformation.
Fig. 2.
Binding of C3 to DMPC liposomes was investigated by SDS-PAGE and Western blotting with a polyclonal anti-C3c antibody. Liposomes were incubated in lepirudin plasma in the absence (lane 4–5) or presence of 10 μm Compstatin (CMP; lane 6). After incubation in lepirudin plasma for 0 min (lane 4) or 30 min (lane 5–6), the liposomes were washed and subjected to SDS-PAGE under reducing conditions and blotted to a PVDF membrane. C3, C3b, and iC3b (lane 1–3) were used as controls. Addition of Compstatin did not attenuate the binding of C3 to liposomes.
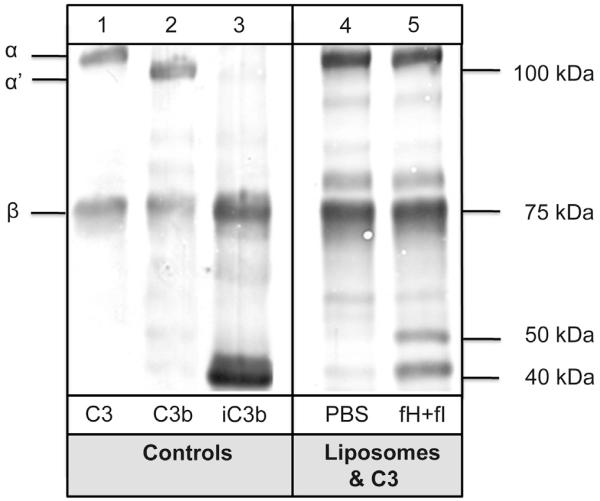
The binding of C3 to liposomes was confirmed using a system, where liposomes were incubated with purified C3 for 15 min (Fig. 3). After 15 min of incubation, either PBS (lane 4) or factor I together with co-factor H (lane 5) were added to the sample and incubated for another 10 min. Cleavage of the C3 molecule similar to the effects seen in lepirudin plasma was demonstrated when factor I and factor H were added to the sample.
Fig. 3.
Adsorption and cleavage of C3 on the liposome surface was further confirmed using DMPC liposomes and purified native C3 (200 μg/ml). Liposomes were incubated with native C3 in the absence (lane 4) or presence of factor H (32 μg/ml) and factor I (8 μg/ml) (lane 5) for 10 min. After washing, the liposomes were analyzed by SDS-PAGE and Western blotting with a polyclonal anti-C3c. C3, C3b, and iC3b (lane 1–3) were used as controls.
To further characterize the bound C3 on the liposome surfaces after incubation in lepirudin plasma (i.e., to ascertain that it was not C3b generated by proteolytic cleavage), we used a range of α-chain-specific mAbs (Fig. 4A). The binding specificity of the various antibodies is shown in Fig. 4B. As expected, the whole α-chain was intact at 0 min. After 30 min of incubation at 37 °C, a substantial amount of cleavage had occurred. Antibodies against a C3a epitope, a 20 kDa C3c epitope, and a C3d epitope all showed the same pattern, demonstrating that the 20 kDa polypeptide of C3c always was accompanied by the C3a fragment, confirming that the convertase site was not cleaved into C3a and C3b. The reactivity of the mAb against the 40 kDa epitope of C3c suggested that the initial cleavages occurred at or close to the first and second factor I cleavage sites.
3.3. Activation of cells and phagocytosis
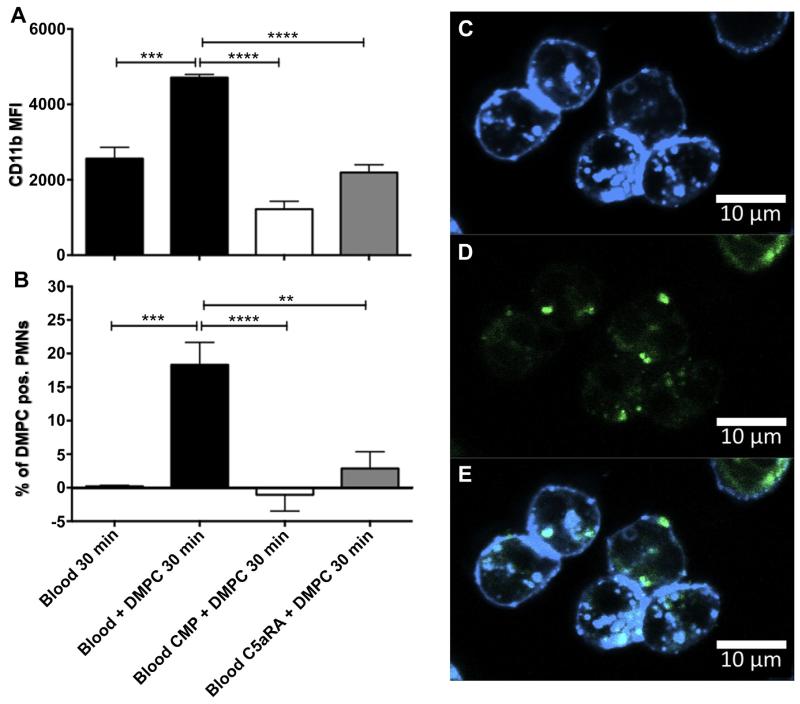
The physiological relevance of the liposome-driven complement activation was examined by flow cytometry analysis, measuring activation of PMNs as expression of CD11b, as well as binding or uptake of the complement protein-bearing liposomes by the PMNs. Incubation of DMPC liposomes in whole blood for 30 min led to an upregulation of CD11b on PMNs, which was inhibited by both Compstatin and C5aRA (Fig 5A). Compstatin or C5aRA added to blood, in the absence of liposomes did not affect the expression of CD11b on PMNs (not shown). In addition, flow cytometry analysis showed a significant double-positivity between PMNs and fluorescent-labeled liposomes, demonstrating a binding or phagocytosis of the liposomes by the PMNs. This phenomenon was also dependent on the fluid-phase liposome-driven complement activation and was therefore inhibited significantly by the addition of either Compstatin or C5aRA (Fig. 5B).
Fig. 5.
The physiological relevance of the liposome-driven complement activation was investigated by flow cytometry as expression of CD11b (A) and uptake of liposomes by the same type of cells (B). Liposomes where incubated in whole blood for 30 min in the absence (black) and presence of 50 μm Compstatin (CMP, white) or 10 μm C5aRA (gray). Incubation of Atto 590-labeled liposomes for 30 min in whole blood increased both activation of PMNs and led to uptake of liposomes by the activated PMNs. Binding of DMPC liposomes to PMNs was confirmed using confocal microscopy (C–E). In (C) PMNs labeled with Pacific blue anti-CD16 and in (D) Atto 590-labeled liposomes are shown. In (E) both markers are superimposed. Data are expressed mean ± SEM; n = 6 (**P < 0.01; ***P < 0.001; ****P < 0.0001). Binding of DMPC liposomes to PMNs was con firmed using confocal microscopy (C–E). (C) PMNs labeled with Pacific blue anti-CD16. (D) Atto 590-labeled liposomes and (E) shows an overlay of both markers. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The binding to or the uptake of liposomes by PMNs was confirmed using confocal microscopy. Samples prepared for flow cytometry were fixed on microscope slides and analyzed for double-positivity between CD16-labeled PMNs and Atto 590-labeled liposomes. The results demonstrate an equal distribution of the liposomes between those phagocytosed by the cells and those bound to the cell membrane (Fig. 5C-E). The distribution of the cell surface-bound liposomes and those which were phagocytosed was confirmed using trypan blue, which quenches FITC-signal from liposomes that are bound to cell surface. In some experiments, blood was incubated with FITC-conjugated DMPC liposomes. These samples were then analyzed by flow cytometry prior to and after incubation with trypan blue. The FITC-signal from CD16-positive PMNs was reduced by approximately 40–50% due to incubation with trypan blue, which confirmed the results from the confocal microscopy (data not shown).
3.4. Real-time monitoring of C3 adsorption to lipid surface
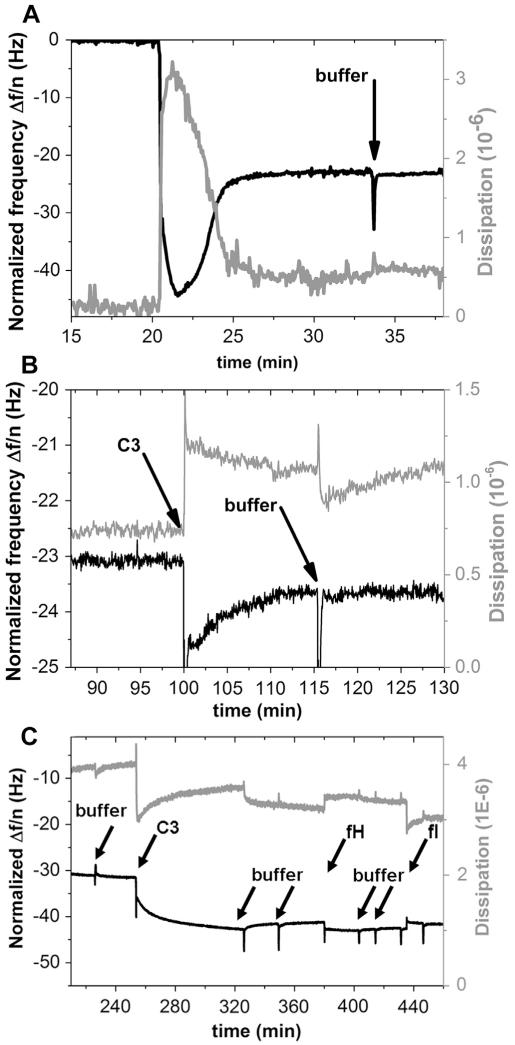
QCM-D measurements (Fig. 6) indicated the binding of C3 to the lipid surface and a more quantitative investigation was possible. By SDS-PAGE and Western Blot analysis, a binding and a conformational change was measurable but no quantitative analysis of the bound proteins neither using a system of liposomes and human plasma nor liposomes and pure C3. Assuming, that a liposome surface with a diameter of 170 nm resembles a planar surface for the protein due to its small dimensions given in Ref. [9], i.e., 117 × 156 × 271 Å, the QCM-D experiments should lead to equal binding behavior compared to the investigations done by Western Blot studies using liposome surfaces and pure C3. A stable DMPC lipid bilayer was formed. Subsequently, the adsorption of C3 was studied, by monitoring the frequency shift and the dissipation, which were both stable after rinsing with buffer and equilibration. C3 bound to the lipid surface at a density of 22 ng/cm2 which corresponds to a total number of 7.1 × 1010 molecules C3/cm2, assuming a molecular weight of 185 kDa. Using the dimensions of C3 given in Ref. [9], i.e., 117 × 156 × 271 Å, we estimated that each molecule covers a maximum area of 4.22 × 10-12 cm2, leading to a theoretical maximal binding of 23.7 × 1010 molecules C3/cm2. Consequently, under these experimental conditions, up to 30% of the area (7.1 × 1010/23.7 × 1010) was covered by C3 molecules.
Fig. 6.
Adsorption of C3 on a DMPC lipid bilayer as monitored by QCM-D. Bilayer formation took place after 15 min at 37 °C (A). (B) shows the binding of C3 (200 μg/ml) to the surface. C3 binding to the surface was confirmed after rinsing with buffer (black arrows). (C) In order to ascertain that the C3 on the DMPC lipid bilayer was in the form of C3(H2O), factor H (32 μg/ml) and factor I (8 μg/ml) were added in sequence. Binding of factor H to C3 was confirmed by a decrease in frequency and an increase in dissipation. After the addition of factor I, the frequency increased as a result of a loss of mass due to a lower affinity between factor H and the factor I-generated iC3(H2O).
In other experiments, we added factor H and factor I in sequence, in order to ascertain that the C3 was in the form of C3(H2O) on the surface. We found a decrease in frequency and an increase in dissipation, which confirmed that factor H bound to C3. After the addition of factor I, the frequency increased which is equivalent to a loss in mass. This loss is due to the fact that factor H has a lower affinity for the factor I-cleaved product (iC3(H2O)). The dissipation on the other hand decreased, which means that the more elastic surface after the factor H binding, was reduced by factor I.
4. Discussion
In this study, we have demonstrated that neutral DMPC liposomes activate complement in lepirudin-anticoagulated human plasma. Usually, poly(ethylene glycol) (PEG) modified liposomes made of dipalmitoylphosphatidylcholine (DPPC) and cholesterol are used for drug delivery. Cholesterol is added to lower the transition temperature from gel-to-liquid crystal phase. The transition temperature (Tc) of DPPC alone is 41 °C which leads to a low membrane fluidity for a lipid bilayer membrane and further on to poor biological properties at physiological 37 °C. However, the addition of cholesterol complicates the studies of complement activation since cholesterol also affects the complement system[4,22]. We therefore used pure DMPC liposomes as model liposomes and cellular membranes since DMPC has a lower Tc (24–25 °C) and shows a high membrane fluidity at 37 °C. With this compromise liposome, we get a biologically relevant membrane which gives us a deeper understanding into how complement activation is activated and bound to liposomes, drug delivery systems and biological membranes.
The generation of the complement activation products C3a and sC5b-9 in the fluid phase, showed a dependence on both time and dose (number of liposomes). The activation was inhibited by the C3 blocking peptide Compstatin [41,42], and by the chelators EDTA and EGTA. The mechanism of recognition and activation of the complement system by lipids used in different drug delivery systems are not fully understood and still needs to be elucidated. Previous studies have shown that negatively charged phospholipids activate the complement system via the classical pathway and through direct interactions of C1q with anionic phospholipids [43], but it has been suggested that the activation mechanism is different from direct immunoglobulin-dependent C1q activation and may involve CRP [46]. By contrast, the presence of positively charged phospholipids activates the alternative pathway [21].
Liposomes consisting of neutral phospholipids as in this study, are generally considered to be poor complement activators[4,7,21,44], but prolonged incubation of neutral liposomes in human serum or plasma leads to the binding of complement proteins to the liposomes [45]. In the present study, Mg2+/EGTA inhibited complement activation, demonstrating that the activation was dependent on Ca2+ and therefore both the classical and the lectin pathway activation may be involved. Preliminary studies showed this in that C1q was present on the liposomes (data not shown).
In our study and studies by others [47,48], SDS-PAGE of liposomes incubated in serum show a broad variety of bound proteins. Sabin et al. [49] showed the interaction between fibrinogen and IgG with DMPC liposomes. Deposition of C3 on the surface of liposomes has previously been reported [19,50]. In the present study, SDS-PAGE, followed by Western blotting with a polyclonal antibody directed against the 40 kDa α5 C-terminal fragment of the α-chain and the entire β-chain was used. Using the mentioned antibodies, we demonstrated that the bound C3 α-chain was not cleaved into the C3 α′-chain, as in the activated form of C3b. In addition, we saw the generation of polypeptides similar to those generated during factor I-mediated cleavage, corroborating the hypothesis that C3(H2O) was deposited on the liposomes. In order to demonstrate the binding of C3(H2O) to the liposomes, we performed further studies in which we used a set of mAbs directed against α1, α2, α4, and α5. This analysis confirmed that the C3 α-chain was initially cleaved at the first and second factor I cleavage sites and also in the C3d region and in α5. We cannot rule out the possibility that the cleavage in the C3d region results from a spontaneous cleavage at the thiol ester site in native C3, which has been described to occur during heating in preparation for SDS-PAGE [51]. However, all N-terminal polypeptides contained C3a (α1), clearly demonstrating that they were generated as a result of cleavages of C3(H2O) and not from C3b, which does not contain a1 (i.e., C3a). In addition, SDS-PAGE/Western blotting showed that C3 deposition was not inhibited by complement inhibitors, further supporting our view that C3 was not bound to the liposomes as a result of proteolytic (convertase) activation.
The question is how the transition from native C3 to C3(H2O) is brought about. In this study, the generation of C3(H2O) was detected by its conformational changes (conformational epitopes), the remaining C3a, and its susceptibility to factor I cleavages (in the presence of co-factors e.g. factor H). It has previously been demonstrated that by this definition, C3(H2O) occurs on polystyrene surfaces [52], on human platelets [53], and at blood-gas [54] and plasmae–gas interfaces [8]. The polystyrene surface and the platelet membrane are solid surfaces when compared to the variable surfaces formed by gas bubbles. In the latter case, C3(H2O) can be assessed by ELISA in the fluid phase, since the gas obviously disappears from the fluid after release. All these examples demonstrates that native C3 upon contact with a surface is prone to transform into C3(H2O).
We have recently shown that the phospholipid surfaces of activated platelets (and platelet-derived microparticles) in whole blood bind C3 in the form of C3(H2O) [53]. A similar interaction of C3 with the DMPC liposomes may explain the generation of C3(H2O) in the present study. In order to quantify the binding, we performed QCM-D experiments in which purified native C3 was allowed to deposit onto DMPC bilayers. These experiments confirmed that native C3 formed a stable coat on the phospholipid bilayer and it could be estimated that the deposited C3 covered up to 30% of the lipid surface, under the experimental conditions used (Fig. 6, calculations in 3.4). We have previously demonstrated that C3 adsorbed on artificial polymer surfaces is completely unfolded at such low density [55]. Thus, the low packing density per se corroborates that C3 is bound in the form of C3(H2O). Sequential addition of factors H and I, showed that the C3 was affected by the interaction with factor I and H which confirmed the transition of C3 to C3(H2O).
The conformational changes that occur during activation of C3 to surface-bound C3b, iC3b and C3dg are reflected in the exposure of neoepitopes on the surface of the C3 molecule. We have also shown that native C3 changes its conformation in a similar fashion after coming into contact with a surface [52], suggesting that it may possess biological properties similar to bound C3 fragments and activation products. In order to investigate whether the complement activation and the binding of C3(H2O) have any functional impact on liposome turnover in whole blood, we tested these liposome preparations in whole-blood phagocytosis. We found that the particles were both integrated with the membrane and found in the cytoplasm as a result of ingestion. Both Compstatin and the C5aR antagonist inhibited the binding to and the uptake of liposomes into PMNs, demonstrating that the fluid-phase complement activation which occurs in the fluid phase and generates C5a was crucial for the activation of PMNs and for the upregulation of CD11b on PMNs so that the C3(H2O)-bearing liposomes can be bound or ingested (Fig. 7).
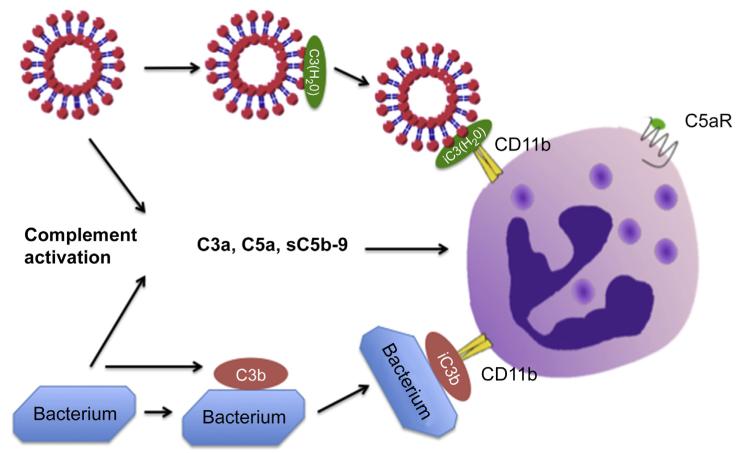
Fig. 7.
Model for how DMPC liposomes activate complement in the fluid phase leading to generation of C3a, C5a and sC5b-9. The formed C5a will activate nearby PMNs via the C5a receptor. In addition, C3 binds to the liposomes in the form of C3(H2O), which, when digested to iC3(H2O) will bind to CD11b on PMNs, promoting phagocytosis of the opsonized liposomes. For comparison, complement activation by a bacterium leading to deposition of C3b on the surface, as well as generation of C3a, C5a and sC5b-9, is shown. The bound C3b is digested to iC3b, promoting phagocytosis of the opsonized bacteria, via CD11b on the PMNs.
5. Conclusion
In the present study, we demonstrate that neutral DMPC liposomes activate complement in two ways: 1) in the fluid phase tentatively due to binding of C1q and 2) by binding C3 in the form of C3(H2O). The generation of C3(H2O) represents a new activation mechanism leading to a non-proteolytic activation of C3 into a functionally active C3b-like molecule. These actions are likely to have an impact on the turnover of liposomes in vivo. Complement activation in the fluid phase, with the generation of anaphylatoxins such as C3a and C5a, will activate phagocytosing cells and, since C3(H2O) acts as a ligand to C3 receptors, it will affect the fate of the liposomes (Fig. 7). Attempts to increase the half-life of blood-borne liposomes by using complement inhibitors may be partially thwarted because the binding of C3(H2O) is not dependent on proteolytic complement activation. In certain cases, additional measures such as receptor blockade will therefore be necessary in order to comprehensively prevent liposome-induced complement activation. In sum, these findings are consistent with binding of the C3b-like molecule, C3(H2O), to the liposomes with the implications for the fate of liposomes and the analysis and treatment of complement-mediated adverse effects during treatment with liposome formulations.
Acknowledgments
We thank Dr. Deborah McClellan for excellent editorial assistance.
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013/under REA grant agreement No 324275 and under RTD grant agreement No 602699; the Swedish Research Council (VR) 2009-4675, and 2012-2407; the Swedish Research Council and Stem Therapy; AFA insurances 1100156; the faculty of Health and Life Sciences, Lin-næus University; the Karlsruhe House of Young Scientists (KHYS), the Karlsruhe School of Optics and Photonics (KSOP), and the Deutsche Forschungsgemeinschaft (DFG) through the Center of Functional Nanostructures (CFN) and by U.S. National Institutes of Health grants AI030030 and AI068730.
References
- [1].Ulrich AS. Biophysical aspects of using liposomes as delivery vehicles. BiosciRep. 2002;22:129–50. doi: 10.1023/a:1020178304031. [DOI] [PubMed] [Google Scholar]
- [2].Szebeni J, Muggia F, Gabizon A, Barenholz Y. Activation of complement bytherapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv Drug Deliv Rev. 2011;63:1020–30. doi: 10.1016/j.addr.2011.06.017. [DOI] [PubMed] [Google Scholar]
- [3].Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Liposome Res. 1985;26:1015–35. [PubMed] [Google Scholar]
- [4].Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146:4234–41. [PubMed] [Google Scholar]
- [5].Baron LG, Meyer KB, Szoka JFC. Effects of complement depletion on the pharmacokinetics and gene delivery mediated by cationic lipid-DNA complexes. Hum Gene Ther. 1998;9:315–23. doi: 10.1089/hum.1998.9.3-315. [DOI] [PubMed] [Google Scholar]
- [6].Szebeni J, Moghimi S. Liposome triggering of innate immune response: a perspective on benefits and adverse reactions. J Liposome Res. 2009;19:85–90. doi: 10.1080/08982100902792855. [DOI] [PubMed] [Google Scholar]
- [7].Ishida T, Harashima H, Kiwada H. Liposome clearance. Biosci Rep. 2002;22:197–224. doi: 10.1023/a:1020134521778. [DOI] [PubMed] [Google Scholar]
- [8].Ekdahl KN, Nilsson B, Pekna M, Nilsson UR. Generation of iC3 at the interface between blood and gas. Scand J Immunol. 1992;35:85–91. doi: 10.1111/j.1365-3083.1992.tb02837.x. [DOI] [PubMed] [Google Scholar]
- [9].Janssen BJC, Huizinga EG, Raaijmakers HCA, Roos A, Daha MR, Ekdahl KN, et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437:505–11. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- [10].Ross GD, Newman SI, Lambris JD, Devery-Pocius JE, Cain JA, Lachmann PJ. Generation of three different fragments of bound C3 with purified factor 1 or serum. J Exp Med. 1983;158:334–52. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hugli TE. Human anaphylatoxin (C3a) from the third component of complement. J Biol Chem. 1975;250:8293–301. [PubMed] [Google Scholar]
- [12].Sim RB, Twose TM, Paterson DS, Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981;193:115–27. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pangburn MK, Müller-Eberhardt HJ. Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Ann N Y Acad Sci. 1983;421:291–8. doi: 10.1111/j.1749-6632.1983.tb18116.x. [DOI] [PubMed] [Google Scholar]
- [14].Pangburn MK, Müller-Eberhardt HJ. Relation of a putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980;152:1102–14. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Law S, Dodds AW. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 1997;6:263–74. doi: 10.1002/pro.5560060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pangburn MK, Schreiber RD, Müller-Eberhardt HJ. Formation of the initial C3 convertase of the alternative complement pathway. J Exp Med. 1981;154:856–67. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schwendinger MG, Spruth M, Schoch J, Dierich MP, Prodinger WM. A novel mechanism of alternative pathway complement activation accounts for the deposition of C3 fragments on CR2-expressing homologous cells. J Immunol. 1997;158:5455–63. [PubMed] [Google Scholar]
- [18].Alaei S, Larcher C, Ebenbichler C, Prodinger WM, Janatova J, Dierich MP. Isolation and biochemical characterization of the iC3b receptor of Candida albicans. Infect Immun. 1993;61:1395–9. doi: 10.1128/iai.61.4.1395-1399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moghimi S, Hamad I. Liposome-mediated triggering of complement cascade. J Liposome Res. 2008;18:195–209. doi: 10.1080/08982100802309552. [DOI] [PubMed] [Google Scholar]
- [20].Ishida T, Kojima H, Harashima H, Kiwada H. Biodistribution of liposomes and C3 fragments associated with liposomes evaluation of their relationship. Int J Pharm. 2000;205:183–93. doi: 10.1016/s0378-5173(00)00511-1. [DOI] [PubMed] [Google Scholar]
- [21].Devine DV, Wong K, Serrano K, Chonn A, Cullis PR. Liposome-complement interactions in rat serum: implications for liposome survival studies. Biochim Biophys Acta. 1994;1191:43–51. doi: 10.1016/0005-2736(94)90231-3. [DOI] [PubMed] [Google Scholar]
- [22].Alving CR, Richards RL, Guirguis AA. Cholesterol-dependent human complement activation resulting in damage to liposomal model membranes. J Immunol. 1977;118:342–7. [PubMed] [Google Scholar]
- [23].Liu D, Hu Q, Song YK. Liposome clearance from blood: different animal species have different mechanisms. Biochim Biophys Acta. 1995;1240:277–84. doi: 10.1016/0005-2736(95)00184-0. [DOI] [PubMed] [Google Scholar]
- [24].Harashima H, Sakata K, Funato K, Kiwada H. Enhanced hepatic uptake of liposomes through complement activation depending on the size of liposomes. Pharm Res. 1994;11:402–6. doi: 10.1023/a:1018965121222. [DOI] [PubMed] [Google Scholar]
- [25].Pham CTN, Mitchell LM, Huang JL, Lubniewskie CM, Schall OF, Killgore JK, et al. Variable antibody-dependent activation of complement by functionalized phospholipid nanoparticle surfaces. J Biol Chem. 2011;286:123–30. doi: 10.1074/jbc.M110.180760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Szebeni J, Alving CR. Complement-mediated acute effects of liposome-encapsulated hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 1999;27:23–41. doi: 10.3109/10731199909117481. [DOI] [PubMed] [Google Scholar]
- [27].Szebeni J. Complement activation-related pseudoallergy caused by amphiphilic drug carriers: the role of lipoproteins. Curr Drug Deliv. 2005;2:443–9. doi: 10.2174/156720105774370212. [DOI] [PubMed] [Google Scholar]
- [28].Scieszka JF, Maggiora LL, Wright SD, Cho J. Role of complements C3 and C5 in the phagocytosis of liposomes by human neutrophils. Pharm Res. 1997;8:65–9. doi: 10.1023/a:1015830306839. [DOI] [PubMed] [Google Scholar]
- [29].Hammer CH, Wirtz GH, Renfer L, Gresham HD, Tack BF. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981;256:3995–4006. [PubMed] [Google Scholar]
- [30].Nilsson UR, Müller-Eberhardt HJ. Isolation of beta If-globulin from human serum and its characterization as the fifth component of complement. J Exp Med. 1965;122:277–98. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fearon DT. Purification of C3b inactivator and demonstration of its two polypeptide chain structure. 1977;119:1248–52. [PubMed] [Google Scholar]
- [32].Nilsson B, Svensson KE, Borwell P, Nilsson UR. Production of mouse monoclonal antibodies that detect distinct neoantigenic epitopes on bound C3b and iC3b but not on the corresponding soluble fragments. Mol Immunol. 1987;24:487–94. doi: 10.1016/0161-5890(87)90023-x. [DOI] [PubMed] [Google Scholar]
- [33].Bexborn F, Engberg AE, Sandholm K, Mollnes TE, Hong J, Ekdahl KN. Hirudin versus heparin for use in whole blood in vitro biocompatibility models. J Biomed Mater Res A. 2008;89:951–9. doi: 10.1002/jbm.a.32034. [DOI] [PubMed] [Google Scholar]
- [34].Katragadda M, Magotti P, Sfyroera G, Lambris J. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, Compstatin. J Med Chem. 2006;49:4616–22. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- [35].Nilsson B, Svensson KE, Inganäs M, Nilsson UR. A simplified assay for the detection of C3a in human plasma employing a monoclonal antibody raised against denatured C3. J Immunol Methods. 1988;107:281–7. doi: 10.1016/0022-1759(88)90229-3. [DOI] [PubMed] [Google Scholar]
- [36].Alsenz J, Becherer JD, Nilsson B, Lambris JD. Structural and functional analysis of C3 using monoclonal antibodies. Curr Top Microbiol. 1990;153:235–48. doi: 10.1007/978-3-642-74977-3_13. [DOI] [PubMed] [Google Scholar]
- [37].Finch A, Wong A, Paczkowski N, Wadi S, Craik D, Fairlie D, et al. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. 1999;42:1965–74. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- [38].Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z Physiother. 1959;55:206–22. [Google Scholar]
- [39].Rodahl M, Höök F, Krozer A, Brzezinski P, Kasemo B. Quartz-crystal micro-balance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev Sci Instrum. 1995;66:3924–30. [Google Scholar]
- [40].Cho NJ, Curtis WF, Kasemo B, Höök F. Quartz-crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat Protoc. 2010;5:1096–106. doi: 10.1038/nprot.2010.65. [DOI] [PubMed] [Google Scholar]
- [41].Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–92. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190:3839–47. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Devine DV, Marjan JM. The role of immunoproteins in the survival of liposomes in the circulation. Crit Rev Ther Drug Carrier Syst. 1997;14:105–31. [PubMed] [Google Scholar]
- [44].Yan X, Scherphof G, Kamps J. Liposome opsonization. J Liposome Res. 2005;15:109–39. doi: 10.1081/lpr-64971. [DOI] [PubMed] [Google Scholar]
- [45].Szebeni J, Baranyi L, Savay S, Milosevits J, Bodo M, Bunger R, et al. The interaticion of liposomes with the complement system: in vitro and in vivo assays. Meth Enzymol. 2003;373:136–54. doi: 10.1016/S0076-6879(03)73010-9. [DOI] [PubMed] [Google Scholar]
- [46].Jiang H, Robey FA, Gewurz H. Localization of sites through which C-reactive protein binds and activates complement to residues 14–26 and 76–92 of thehuman C1q A chain. J Exp Med. 1992;175:1373–9. doi: 10.1084/jem.175.5.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Johnstone A, Masin D, Mayer L, Bally M. Surface-associated serum proteins inhibit the uptake of phosphatidylserine and poly(ethylene glycol) liposomes by mouse macrophages. Biochim Biophys Acta. 2001;1513:25–37. doi: 10.1016/s0005-2736(01)00292-9. [DOI] [PubMed] [Google Scholar]
- [48].Price M, Cornelius R, Brash J. Protein adsorption to polyethylene glycol modified liposomes from fibrinogen solution and from plasma. Biochim Biophys Acta. 2001;1512:191–205. doi: 10.1016/s0005-2736(01)00330-3. [DOI] [PubMed] [Google Scholar]
- [49].Sabin J, Prieto G, Ruso J, Messina P, Salgado F, Nogueira M, et al. Interactions between DMPC liposomes and the serum blood proteins HSA and IgG. J Phys Chem B. 2009;113:1655–61. doi: 10.1021/jp804641e. [DOI] [PubMed] [Google Scholar]
- [50].Malmsten M. Studies of serum protein adsorption at phospholipid surfaces in relation to intravenous drug delivery. Colloid Surf A. 1999;159:77–87. [Google Scholar]
- [51].Sim R, Sim E. Autolytic fragmentation of complement components C3 and C4 under denaturing conditions, a property shared with α2-macroglobulin. Biochem J. 1981;93:129–41. doi: 10.1042/bj1930129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Andersson J, Ekdahl KN, Lambris JD, Nilsson B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–85. doi: 10.1016/j.biomaterials.2004.05.011. [DOI] [PubMed] [Google Scholar]
- [53].Hamad OA, Nilsson PH, Wouters D, Lambris JD, Ekdahl KN, Nilsson B. Complement component C3 binds to activated normal platelets without preceding proteolytic activation and promotes binding to complement receptor 1. J Immunol. 2010;184:2686–92. doi: 10.4049/jimmunol.0902810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pekna M, Nilsson L, Ekdahl KN, Nilsson UR, Nilsson B. Evidence for iC3 generation during cardiopulmonary bypass as the result of blood-gas interaction. Clin Exp Immunol. 1993;91:404–9. doi: 10.1111/j.1365-2249.1993.tb05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nilsson UR, Storm K, Elwing H, Nilsson B. Conformational epitopes of C3 reflecting its mode of binding to an artificial polymer surface. Mol Immunol. 1993;30:211–9. doi: 10.1016/0161-5890(93)90050-l. [DOI] [PubMed] [Google Scholar]