Significance
Species’ distributions result from dispersal and physiological constraints, interactions with other species, and ultimately, evolution. Biological invasions result from the deliberate or accidental movement of species between regions they would not reach through natural dispersal and can cause major conservation, economic, and human health issues. However, invasions also provide fascinating insights into species’ distribution limits. We investigate the invasion of the cane toad from South America to Australia by comparing the results of two modeling approaches: one considering physiological constraints and the other considering the joint influences of physiology, dispersal, and biotic interactions. Our findings demonstrate that the cane toad is limited in its native distribution by biotic interactions but, in Australia, is free to fill its climatic potential.
Keywords: biophysical model, Bufo marinus, Maxent, range shift, species distribution model
Abstract
Accurate forecasts of biological invasions are crucial for managing invasion risk but are hampered by niche shifts resulting from evolved environmental tolerances (fundamental niche shifts) or the presence of novel biotic and abiotic conditions in the invaded range (realized niche shifts). Distinguishing between these kinds of niche shifts is impossible with traditional, correlative approaches to invasion forecasts, which exclusively consider the realized niche. Here we overcome this challenge by combining a physiologically mechanistic model of the fundamental niche with correlative models based on the realized niche to study the global invasion of the cane toad Rhinella marina. We find strong evidence that the success of R. marina in Australia reflects a shift in the species’ realized niche, as opposed to evolutionary shifts in range-limiting traits. Our results demonstrate that R. marina does not fill its fundamental niche in its native South American range and that areas of niche unfilling coincide with the presence of a closely related species with which R. marina hybridizes. Conversely, in Australia, where coevolved taxa are absent, R. marina largely fills its fundamental niche in areas behind the invasion front. The general approach taken here of contrasting fundamental and realized niche models provides key insights into the role of biotic interactions in shaping range limits and can inform effective management strategies not only for invasive species but also for assisted colonization under climate change.
Understanding the factors that limit species’ geographic ranges has long stood as a fundamental goal in ecology (1) and is critical for making robust predictions of species’ range shifts as a result of climate change and biotic exchange. Niche theory (2) argues that species’ ranges are limited by physiological tolerances (which define the fundamental niche), as well as biotic interactions and dispersal barriers (which further constrain the fundamental niche to the realized niche), but the relative roles of these factors in shaping range limits remain poorly understood. Standard approaches to range prediction are based on correlations between species' observed distributions and climate (i.e., the realized niche) (3, 4), and thus confound the influences of abiotic and biotic constraints on species’ ranges.
Range shift projections based on correlative models also assume that species’ niches (both realized and fundamental) are conserved through space and time (3, 5, 6). However, there is growing evidence to suggest that species can undergo rapid niche shifts in novel environments (7–9) through either evolved environmental tolerances (fundamental niche shifts) (10, 11) or release from dispersal barriers or biotic constraints (realized niche shifts) (12, 13). Understanding whether such niche shifts are widespread in nature not only is important for validating the use of correlative models in climate change and invasive species impact assessments but also has implications for understanding patterns of community assembly and speciation (14, 15).
Invasive species frequently experience release from biotic interactions and dispersal barriers in their invaded ranges (4, 12, 16), and thus provide model systems for investigating the degree to which niches are spatially and temporally conserved. Current approaches for examining niche shifts in invasive species primarily rely on comparisons of climates occupied by species in their native and invaded ranges (6, 7, 17, 18). However, such correlative comparisons fail to differentiate between the influences of adaptation after introduction and biotic interactions and dispersal barriers that are absent in a species’ invaded range. Here we present an approach that helps resolve this issue by integrating correlative niche models with mechanistic biophysical predictions of the fundamental niche (19). Biophysical models incorporate links between climate and an organism’s functional traits and are developed independent of a species’ current distribution. The biophysical approach thus provides a prediction of where a species can survive and reproduce in the absence of biotic interactions and dispersal limitations (19). We apply this mechanistic approach to investigate whether the invasion of Australia by the cane toad (Rhinella marina, formerly Bufo marinus) has been facilitated by a shift in the species’ realized or fundamental niche. Since its introduction to Australia in 1935 as a biological control agent, R. marina has expanded its range to include more than 1.2 million km2 of the continent (20). This large-scale invasion has been facilitated by thermal acclimation (21, 22), as well as evolutionary shifts in locomotor performance (23). Have the environmental tolerances of toads evolved as well?
Results and Discussion
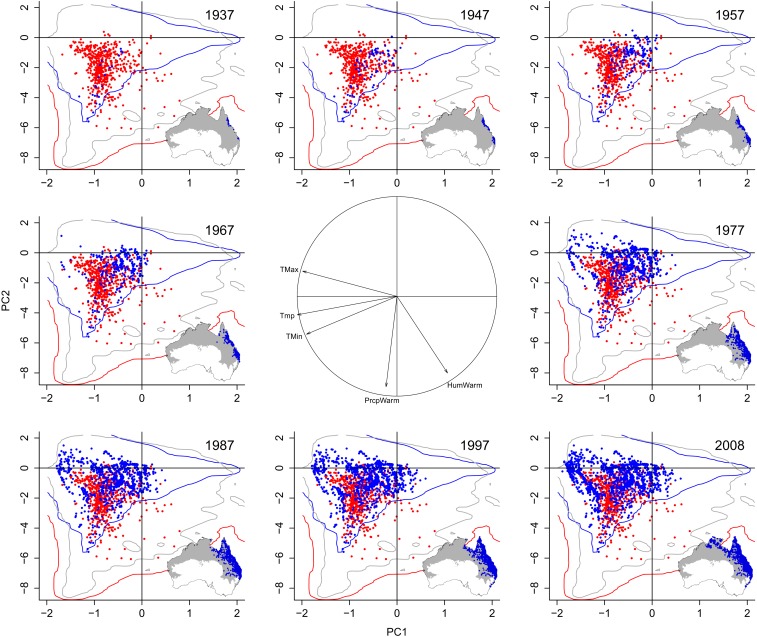
We first compared the climatic conditions occupied by R. marina in its native and Australian ranges, using a weighted principal components analysis (PCA) based on available climates in the species’ native and Australian ranges (18). The first two axes of this PCA were statistically significant (based on the broken stick criterion) (24) and accounted for 88.9% of the variation in the data. The first PCA axis was negatively related to mean, minimum, and maximum temperatures, whereas the second axis was negatively associated with precipitation and humidity of the warmest quarter of the year (Fig. 1). Examining the position of the native and Australian realized niches of R. marina along these two PCA axes (18) revealed that the species’ niches were not equivalent (Schoener’s D = 0.28; niche equivalency test, P = 0.020). The Australian niche was more similar to the native niche than would be expected by chance (niche similarity test, P = 0.020), which is expected, given the high prevalence of extremely arid environments within Australia relative to the species’ native realized niche (5). In contrast, the native niche was not similar to the Australian niche (niche similarity test, P = 0.15), plausibly because of the considerable variation in climate across the native range background (25). However, focusing solely on changes in niche overlap ignores alternative niche dynamics that can occur via changes in niche variances (25, 26). Our results suggest increased variance in the Australian realized niche of R. marina relative to the species’ native niche (13% niche expansion, 0% niche unfilling), as well as a shift in the density of occurrences within the overall niche envelope (Fig. 1 and Fig. S1).
Fig. 1.
Projections of the native (red points) and Australian (blue points) ranges of R. marina onto the first two axes of a PCA. Red and blue contours represent the climatic conditions available in the two ranges, whereas the gray contour represents climates within the species’ native fundamental niche. Maps illustrate how R. marina has filled its geographically projected fundamental niche (gray shading) over time. The correlation circle in the middle of the figure indicates the relative importance of each variable on the PCA axes. See Materials and Methods for variable descriptions.
To investigate whether our results were similar when more than two environmental dimensions were included in our analyses, we also used a recently developed method (27) to estimate the overlap between the species’ native and Australian niches in five-dimensional climate space (i.e., using raw climate variables instead of PCA axes). This analysis demonstrated that only 7% of the combined hypervolume of both niches was shared, providing additional evidence for a shift in the species’ niche between its native and Australian ranges (Fig. S2). Previous studies have also reported niche changes in invasive species (7–9), although a recent review of 180 case studies suggested that only ∼50% of these have found evidence for niche shifts (26). However, comparative analyses of niche conservatism are difficult because of the varied ways in which niche changes have been quantified.
Analyses with different temporal aggregations of data suggest that R. marina was introduced to climates in Australia that were similar to those occupied in its native range, but that the species quickly expanded its range into drier climates, with more extreme temperatures, in Australia. Similarly, Urban and colleagues (20) found that R. marina has increasingly colonized areas with higher maximum temperatures in Australia over time. Importantly, our findings demonstrate that novel climates colonized by R. marina in Australia were available, but unoccupied, in the species’ native range (union of the gray and blue outlines in Fig. 1). Thus, the observed niche shift between the native and Australian ranges of R. marina is not a result of climatic availability in the species’ native range.
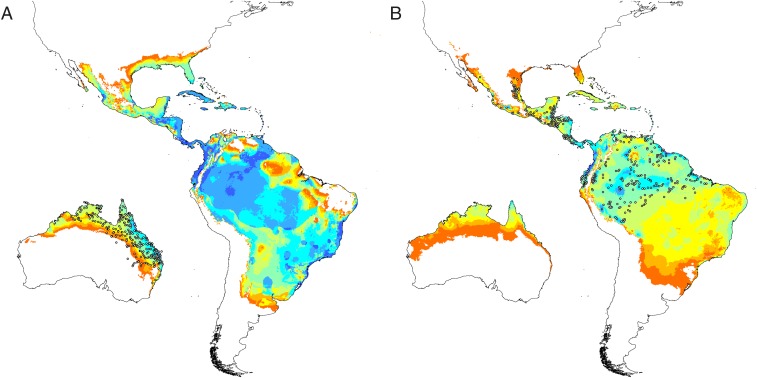
To investigate the implications of this niche shift on correlative models based on the realized niche, we modeled the native and Australian ranges of R. marina separately, using a maximum entropy modeling algorithm (28) and the same occurrence records and climate variables that were used in our PCA analysis. Reciprocal projections of these models illustrated that the correlative model trained on the Australian realized niche accurately predicted the Australian range of R. marina (area under the receiver operating characteristic curve, mean ± SD = 0.85 ± 0.019) but predicted a broader latitudinal distribution in the species’ native range than is currently observed (Fig. 2A). The Maxent model trained on the native realized niche successfully predicted the species’ native range (area under the receiver operating characteristic curve = 0.87 ± 0.011), as well as portions of its invaded range in northern Australia, but failed to predict the existence of invasive populations in drier and cooler regions of Queensland and northern New South Wales (Fig. 2B). The native-range model therefore underpredicted the species’ invaded range in exactly the types of climates in which there was evidence of niche expansion. Maps of multivariate environmental similarity demonstrated that this gross underprediction was not a result of extrapolation of fitted response curves into unsampled environmental space (Fig. S3).
Fig. 2.
Predictions of correlative Maxent models calibrated using data from either the Australian (A) or native (B) realized niche of R. marina. Predictions are depicted in 10% suitability classes ranging from white to orange to yellow to green to blue. White dots represent occurrence records of R. marina and have been thinned to improve visibility.
Was the shift between the native and Australian niches of R. marina a result of adaptive changes postintroduction, or simply a consequence of the absence of biotic interactions or dispersal limitations that are present in the species’ native range? To investigate these two hypotheses, we used a previously parameterized mechanistic model to make predictions of the fundamental niche of R. marina (29). This model links microclimatic condition estimates derived from long-term weather station data with biophysical and physiological constraints on the eggs, larvae, and adults of R. marina to predict the potential number of breeding months per year. Physiological parameters were derived from R. marina populations spanning the species’ invasion history in Australia. Importantly, comparisons of R. marina populations from across the species’ invaded range in Australia (29), and between the species’ native and Australian ranges, indicate there have been no evolutionary shifts in the physiological parameters (thermal sensitivity of locomotor performance, and water requirements for breeding) that constrain the species’ range limit in the mechanistic model (Fig. S4; see Materials and Methods).
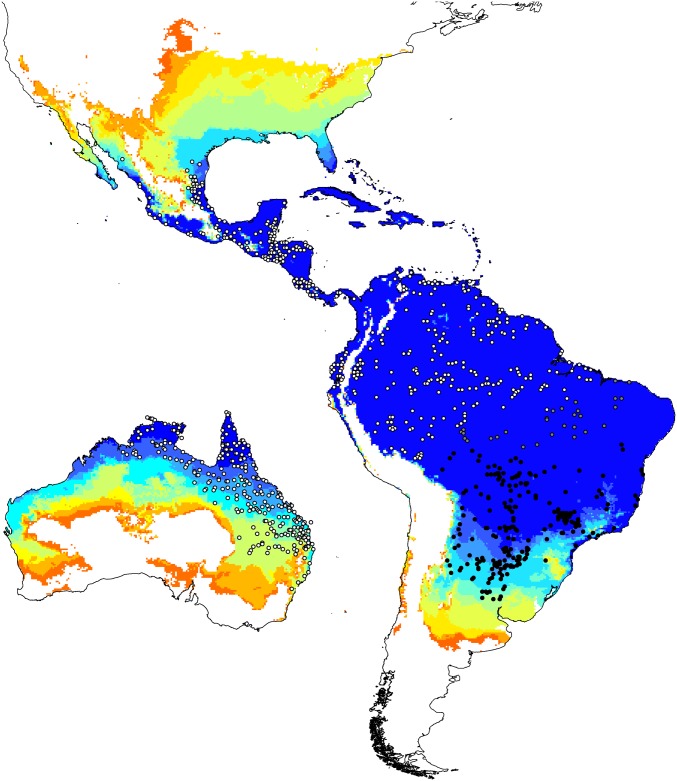
The mechanistic model correctly classified 100% of the occurrence records in the species’ native range but predicted a wider latitudinal distribution than is presently observed in South America (Fig. 3), suggesting that biotic interactions and/or dispersal barriers constrain R. marina to a narrower realized niche than is physiologically possible. Conversely, in Australia, where dispersal constraints and coevolved taxa are absent, R. marina is beginning to fill its fundamental niche in areas behind the invasion front (99% of occurrence records correctly classified). Interestingly, the correlative model trained on the species’ Australian realized niche also identified suitable climatic conditions south of the species’ native range (Fig. 2A). Mechanistic predictions in these areas are therefore not simply a result of differences in the structure and parameterization of the correlative and mechanistic models. The mechanistic model also predicted a broader potential range in the Neotropical, Afrotropical, and Indo-Malayan realms than did the correlative model based on native-range data (Fig. S5). Collectively, these results provide evidence for a shift in the realized, as opposed to the fundamental, niche between the species’ native and Australian ranges.
Fig. 3.
Prediction of the fundamental niche (potential number of breeding months per year) of R. marina according to a mechanistic model. Predictions are depicted in 10 equal interval classes, with the highest class depicting 9–12 breeding months per year and the white area representing no breeding months per year. White dots represent occurrence records of R. marina, whereas black dots represent occurrence records of a congener (R. schneideri). Gray dots in Central Brazil lie within a hybrid zone and could not be confidently assigned to either species.
Why does R. marina fail to fill its fundamental niche in its native range? One possibility is that the presence of a closely related species (R. schneideri) in cooler and drier regions of Southern Brazil may be preventing R. marina from colonizing suitable environments south of its present range. Indeed, these two species hybridize at the southern range edge of R. marina (30), and even low rates of interspecific hybridization can enforce stable parapatric range boundaries (31). In addition, the ranges of R. marina and R. schneideri collectively fill the native fundamental niche of R. marina (Fig. 3), and the climates occupied by R. marina in Australia (where the species is closer to filling its fundamental niche) are similar to those occupied by R. schneideri in South America (Fig. 2A and Fig. S3). However, all niche models, whether correlative or mechanistic, should be viewed as tools for generating hypotheses and elucidating knowledge gaps (32, 33). The fact that the mechanistic model and the correlative model based on the Australian realized niche both predicted suitable climatic conditions south of the observed native range of R. marina suggests that interspecific hybridization may be important; however, future studies could usefully test this hypothesis, using laboratory or field experiments.
Our results demonstrate that contrasting fundamental and realized niche models can provide novel insights into the role of biotic interactions in shaping species’ range limits. Such comparisons should contribute greatly to our understanding and management of range shifts and, thus, ultimately lead to the development of more effective management strategies in the face of climate change and increasing rates of biotic exchange. For example, detecting realized niche shifts could help identify potential biocontrol agents for invasive species, whereas identifying fundamental niche shifts could provide insight into the ability of native species to adapt to climate change. Our general approach can be applied to any taxon or environment (19), and thus holds great promise for identifying generalities in the proximate causes of niche shifts through space and time.
Materials and Methods
Occurrence Data.
Data on the native distributions of R. marina (n = 585 grid squares, 10’ in size) and R. schneideri (n = 230 squares) were collated from our own surveys, HerpNET (www.herpnet.org), The Global Biodiversity Information Facility (www.gbif.org), speciesLink (http://splink.cria.org.br), the Biodiversity and Environmental Resource Data System of Belize (www.biodiversity.bz), the World Biodiversity Information Network (www.conabio.gob.mx/remib_ingles/doctos/remibnodosdb.html), the Smithsonian Museum, and Museu de História Natural de São Paulo. To avoid taxonomic misidentifications, we excluded occurrence records that were within the hybrid zone of R. schneideri and R. marina (30) and only included records that were within 5 km of the native ranges of R. schneideri and R. marina (based on extent of occurrence range maps available from the Global Amphibian Assessment: www.iucnredlist.org/initiatives/amphibians).
Australian range data for R. marina (as of 2009; n = 1,249 grid squares, 10’ in size) were sourced from researchers and from the following organizations: FrogWatch, Department of Environment and Conservation, National Parks and Wildlife Service, Forests New South Wales, WildNet (Queensland Environmental Protection Agency), and Northern Territory Parks and Wildlife Service. Most of these data have strict release policies but are freely available from the above sources.
Climate Data.
Climate data were taken from the CL 1.0 (34) and CL 2.0 (35) data sets. From these data sets, we extracted five climate variables (1961–1990 normal, 10’ resolution) related to heat and water balance: minimum temperature of the coldest month (Tmin), maximum temperature of the warmest month (Tmax), mean annual temperature (Temp), mean humidity of the warmest quarter (HumWarm), and precipitation of the warmest quarter (PrcpWarm). These variables were selected because they have a direct influence on the physiological performance of R. marina (5) and because they were uncorrelated with one another (Pearson’s correlation coefficients within both geographic ranges < 0.85).
Measuring Realized Niche Shifts.
We tested for a shift in the realized niche of R. marina in R© 3.0.1 (36) by subjecting climate variables at all grid cells within the species’ native and Australian ranges to a weighted principal components analysis PCA. Species occurrence records were weighted in the PCA so that the species’ native and invaded ranges were equally represented. The first two axes of this PCA were then used to examine the overlap between the species’ native and Australian niches, taking into account the densities of occurrence records and climatic conditions within the species’ ranges (18). To measure niche overlap, we used Schoener’s D (37), a metric that varies between 0 (for no overlap between niches) and 1 (complete overlap). We then used this metric to test for niche equivalency and niche similarity (18, 38). The niche equivalency test compares the observed niche overlap with the overlap estimated when occurrence records are randomly reallocated to the two niches. In contrast, the niche similarity test examines whether the observed overlap between the two niches differs from the overlap between the observed niche in one range and randomly selected niches in the other range. In this case, the center of the simulated density grid is randomly selected from the available climate space in the opposite range (18). For both tests, statistical significance was assessed on the basis of 100 randomizations (α = 0.05).
To provide a more complete depiction of niche changes, we also calculated niche unfilling and niche expansion (6, 26). Niche unfilling represents the proportion of the native niche that does not overlap with the invaded niche, whereas niche expansion is the proportion of the invaded niche that does not overlap with the native niche. These metrics were calculated using the 75th percentile of environments available in each range to remove marginal climates.
To examine niche overlap in more than two dimensions, we also used the kernel density estimation procedure proposed by Blonder and colleagues (27). Specifically, we used a Silverman bandwidth estimator and a threshold that included 100% of the total probability density to estimate native and Australian hypervolumes, based on the five climate variables described above. All five climate variables were centered and scaled before analysis.
Modeling the Realized Niche.
We modeled the native and Australian ranges of R. marina, using Maxent (version 3.3.3k), a machine learning algorithm that uses environmental covariates to discriminate presence records from random background points (28). We used the default settings of Maxent with two exceptions: we used only hinge features, and we increased the regularization multiplier (beta) to 1.5. These modifications were made to produce smoother, more general response curves (5). For our model parameterized in the native range, background records were drawn from all areas in the New World that hosted confamilial species (according to range maps taken from the Global Amphibian Assessment). For the Australian-range model, we reduced the chance of including background locations that were inaccessible because of dispersal limitations by taking samples from all areas that R. marina could potentially have invaded, given the species’ current distribution and rate of spread (i.e., the “reachable background”) (5). To reduce the effects of sampling bias on model predictions, presence records were randomly thinned so that each location was separated by a minimum distance of 27 km (grid cell resolution was ∼18 km at the equator). Predictive performance of each model was assessed using fivefold cross-validation, and the area under a receiver operating characteristic curve, which measures a model’s ability to discriminate presence from background records (0.5 = random, 1 = perfect). Niche models calibrated on data from each range were then projected onto the opposite range.
To investigate whether reciprocal projections of our Maxent models involved extrapolating to climates outside of the range of model calibration, we calculated multivariate environmental similarity surfaces (5). These surfaces estimate the similarity of locations to a set of reference points for a given suite of environmental variables. Positive multivariate environmental similarity surface values represent areas that are environmentally similar to the supplied reference points, whereas negative values indicate novel environments.
Modeling the Fundamental Niche.
Our prediction of the fundamental niche of R. marina was derived from a previously parameterized biophysical model implemented in NicheMapper software (29). This model couples a microclimate model (39) driven by long-term weather station data and an animal energy/mass balance model that predicts physiological constraints on the eggs, larvae, and adults of R. marina. For each month, a grid cell was considered suitable for breeding if the following conditions were met: there was >1 cm of water in the pond, the eggs could survive and complete development, the larvae could develop within 3 mo, and toads could move a minimum of 5 m each night (see ref. 26 for further details). To estimate how well the mechanistic model predicted the ranges of R. marina, we used a threshold of 3 breeding months (5, 29).
The biophysical predictions generated by Kearney and colleagues (29) were produced using Australian climate surfaces derived from the ANUCLIM package (http://fennerschool.anu.edu.au/research/products/anuclim-vrsn-61), but in the present study we used global data on precipitation, relative humidity, cloud cover, wind speed, and minimum and maximum temperatures from the same climate data sets that were used in our correlative modeling. Global estimates of microclimatic conditions based on these data are available from Kearney and coworkers (40). NicheMapper is not publicly available at present but is due to be released as a library for the open-source platform R© (36) in 2015.
Our mechanistic model was calibrated on R. marina from Australia, but there is no evidence to suggest there have been evolutionary shifts in the physiological parameters (thermal sensitivity of locomotor performance, water requirements for breeding) that constrain the species’ range limit. Indeed, locomotor performance is constrained entirely by low temperatures in our model (see earlier), and there has been no shift in locomotor performance at cold temperatures at both the western (29) and southern (21) invasion fronts in Australia. To further test whether R. marina has adapted to colder environments in Australia, we compared the effect of body temperature on hopping speed between the four Australian populations (n = 44 individuals) used to parameterize our mechanistic model and four populations from southern Brazil (n = 24 individuals). These analyses revealed that the relationship between temperature and locomotor performance did not differ among native and invasive populations (two-way repeated measures ANOVA, temperature × location interaction: F = 1.207; P = 0.309; Fig. S4). However, strict importation regulations in both countries prohibited us from conducting these experiments in a common environment. We therefore also compared the results of our racing trials with those of a previous study of two additional populations from northern and southern Australia (21). Hopping speeds of toads from these two populations were similar to those observed in our Australian and Brazilian populations at low temperatures, and thus these comparisons also suggest that our findings are not a result of evolutionary changes in Australian R. marina.
Supplementary Material
Acknowledgments
J. J. Kolbe and B. L. Phillips kindly provided data on locomotor performance of Australian R. marina used in Fig. S4. B. L. Phillips, R. Shine, and J. Elith provided distribution data and made comments on an earlier draft of this manuscript. O. Broennimann provided scripts for calculating niche changes. Funding was provided by a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarship, an Endeavour International Postgraduate Research Scholarship, a University of Sydney International Postgraduate Award, and the Australian Research Council Centre of Excellence for Environmental Decisions (to R.T.); a postdoctoral grant (SFRH/BPD/87721/2012) from Fundação para a Ciência e a Tecnologia under the Programa Operacional Potencial Humano-Quadro de Referência Estratégico Nacional funds from the European Social Fund and Portuguese Ministério da Educação e Ciência (to F.S.); a researcher fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (to M.V.); and an Australian Research Fellowship from the Australian Research Council (to M.R.K.). Additional funding was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil; project (Ministério de Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil 14-2010 and 473313/2013-8), and Fundação para a Ciência e a Tecnologia through the research project PTDC/BIA-BEC/105093/2008 (funded by Fundo Europeu de Desenvolvimento Regional through the Programa Operacional Factores de Competitividade program and Portuguese national funds).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1405766111/-/DCSupplemental.
References
- 1.MacArthur RH. Geographical ecology. Patterns in the distribution of species. New Jersey: Princeton University Press; 1984. [Google Scholar]
- 2.Hutchinson GE. Concluding Remarks. Cold Spring Harb Symp Quant Biol. 1957;22:415–427. [Google Scholar]
- 3.Pearman PB, Guisan A, Broennimann O, Randin CF. Niche dynamics in space and time. Trends Ecol Evol. 2008;23(3):149–158. doi: 10.1016/j.tree.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Jiménez-Valverde A, Peterson A. Use of niche models in invasive species risk assessments. Biol Invasions. 2011;13(12):2785–2797. [Google Scholar]
- 5.Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Methods Ecol Evol. 2010;1:330–342. [Google Scholar]
- 6.Petitpierre B, et al. Climatic niche shifts are rare among terrestrial plant invaders. Science. 2012;335(6074):1344–1348. doi: 10.1126/science.1215933. [DOI] [PubMed] [Google Scholar]
- 7.Broennimann O, et al. Evidence of climatic niche shift during biological invasion. Ecol Lett. 2007;10(8):701–709. doi: 10.1111/j.1461-0248.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 8.Alexander JM, Edwards PJ. Limits to the niche and range margins of alien species. Oikos. 2010;119(9):1377–1386. [Google Scholar]
- 9.Mata RA, Tidon R, Côrtes LG, Marco P, Diniz-Filho JAF. Invasive and flexible: Niche shift in the drosophilid Zaprionus indianus (Insecta, Diptera) Biol Invasions. 2010;12(5):1231–1241. [Google Scholar]
- 10.Müller-Schärer H, Schaffner U, Steinger T. Evolution in invasive plants: Implications for biological control. Trends Ecol Evol. 2004;19(8):417–422. doi: 10.1016/j.tree.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Huey RB, Pascual M. Partial thermoregulatory compensation by a rapidly evolving invasive species along a latitudinal cline. Ecology. 2009;90(7):1715–1720. doi: 10.1890/09-0097.1. [DOI] [PubMed] [Google Scholar]
- 12.Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. Introduced species and their missing parasites. Nature. 2003;421(6923):628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell CE, et al. Biotic interactions and plant invasions. Ecol Lett. 2006;9(6):726–740. doi: 10.1111/j.1461-0248.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 14.Peterson A, Soberón J, Sánchez-Cordero V. Conservatism of ecological niches in evolutionary time. Science. 1999;285(5431):1265–1267. doi: 10.1126/science.285.5431.1265. [DOI] [PubMed] [Google Scholar]
- 15.Losos JB, et al. Niche lability in the evolution of a Caribbean lizard community. Nature. 2003;424(6948):542–545. doi: 10.1038/nature01814. [DOI] [PubMed] [Google Scholar]
- 16.Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. Is invasion success explained by the enemy release hypothesis? Ecol Lett. 2004;7(8):721–733. [Google Scholar]
- 17.Fitzpatrick M, Weltzin J. The biogeography of prediction error: Why does the introduced range of the fire ant over-predict its native range? Glob Ecol Biogeogr. 2007;16(1):24–33. [Google Scholar]
- 18.Broennimann O, et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob Ecol Biogeogr. 2012;21(4):481–497. [Google Scholar]
- 19.Kearney M, Porter W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol Lett. 2009;12(4):334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 20.Urban MC, Phillips BL, Skelly DK, Shine R. The cane toad’s (Chaunus [Bufo] marinus) increasing ability to invade Australia is revealed by a dynamically updated range model. Proc Biol Sci. 2007;274(1616):1413–1419. doi: 10.1098/rspb.2007.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolbe JJ, Kearney M, Shine R. Modeling the consequences of thermal trait variation for the cane toad invasion of Australia. Ecol Appl. 2010;20(8):2273–2285. doi: 10.1890/09-1973.1. [DOI] [PubMed] [Google Scholar]
- 22.McCann S, Greenlees MJ, Newell D, Shine R. Rapid acclimation to cold allows the cane toad to invade montane areas within its Australian range. Funct Ecol. 2014 in press. [Google Scholar]
- 23.Phillips BL, Brown GP, Shine R. Evolutionarily accelerated invasions: The rate of dispersal evolves upwards during the range advance of cane toads. J Evol Biol. 2010;23(12):2595–2601. doi: 10.1111/j.1420-9101.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 24.Legendre P, Legendre L. Numerical Ecology. Amsterdam: Elsevier Science; 1998. [Google Scholar]
- 25.Glennon KL, Ritchie ME, Segraves KA. Evidence for shared broad-scale climatic niches of diploid and polyploid plants. Ecol Lett. 2014;17(5):574–582. doi: 10.1111/ele.12259. [DOI] [PubMed] [Google Scholar]
- 26.Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol Evol. 2014;29(5):260–269. doi: 10.1016/j.tree.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Blonder B, Lamanna C, Violle C, Enquist BJ. The n-dimensional hypervolume. Glob Ecol Biogeogr. 2014;23(5):595–609. [Google Scholar]
- 28.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190(3-4):231–259. [Google Scholar]
- 29.Kearney M, Phillips B, Tracy C. Modelling species distributions without using species distributions: The cane toad in Australia under current and future climates. Ecography. 2008;31(4):1–12. [Google Scholar]
- 30.Sequeira F, et al. Hybridization and massive mtDNA unidirectional introgression between the closely related Neotropical toads Rhinella marina and R. schneideri inferred from mtDNA and nuclear markers. BMC Evol Biol. 2011;11(1):264. doi: 10.1186/1471-2148-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg EE, Lande R. Ecological and reproductive character displacement on an environmental gradient. Evolution. 2006;60(7):1344–1357. [PubMed] [Google Scholar]
- 32.Dormann CF, et al. Correlation and process in species distribution models: Bridging a dichotomy. J Biogeogr. 2012;39(12):2119–2131. [Google Scholar]
- 33.Araújo MB, Thuiller W, Pearson RG. Climate warming and the decline of amphibians and reptiles in Europe. J Biogeogr. 2006;33(10):1712–1728. [Google Scholar]
- 34.New M, Hulme M, Jones P. Representing twentieth-century space – time climate variability. Part I: Development of a 1961 – 90 mean monthly terrestrial climatology. J Clim. 1999;12(3):829–856. [Google Scholar]
- 35.New M, Lister D, Hulme M, Makin I. A high-resolution data set of surface climate over global land areas. Clim Res. 2002;21(1):1–25. [Google Scholar]
- 36.R Core Team. (2013) R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna). Available at http://www.R-project.org/. Accessed May 28, 2014.
- 37.Schoener TW. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology. 1970;51(3):408–418. [Google Scholar]
- 38.Warren DL, Glor RE, Turelli M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution. 2008;62(11):2868–2883. doi: 10.1111/j.1558-5646.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 39.Kearney MR, et al. Microclimate modelling at macro scales: A test of a general microclimate model integrated with gridded continental-scale soil and weather data. Methods Ecol Evol. 2014;5(3):273–286. [Google Scholar]
- 40.Kearney MR, Isaac AP, Porter WP. microclim: Global estimates of hourly microclimate based on long-term monthly climate averages. Sci Data. 2014;1:140006. doi: 10.1038/sdata.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.