Significance
Microbial communities associated with coral reefs influence the health and sustenance of keystone benthic organisms (e.g., coral holobionts). The present study investigated the community structure and metabolic potential of microbes inhabiting coral reefs located across an extensive area in the central Pacific. We found that the taxa present correlated strongly with the percent coverage of corals and algae, while community metabolic potential correlated best with geographic location. These findings are inconsistent with prevailing biogeographic models of microbial diversity (e.g., distance decay) and metabolic potential (i.e., similar functional profiles regardless of phylogenetic variability). Based on these findings, we propose that the primary carbon sources determine community structure and that local biogeochemistry determines finer-scale metabolic function.
Keywords: microbial biogeography, marine bacteria, metabolic potential
Abstract
Holobionts are species-specific associations between macro- and microorganisms. On coral reefs, the benthic coverage of coral and algal holobionts varies due to natural and anthropogenic forcings. Different benthic macroorganisms are predicted to have specific microbiomes. In contrast, local environmental factors are predicted to select for specific metabolic pathways in microbes. To reconcile these two predictions, we hypothesized that adaptation of microbiomes to local conditions is facilitated by the horizontal transfer of genes responsible for specific metabolic capabilities. To test this hypothesis, microbial metagenomes were sequenced from 22 coral reefs at 11 Line Islands in the central Pacific that together span a wide range of biogeochemical and anthropogenic influences. Consistent with our hypothesis, the percent cover of major benthic functional groups significantly correlated with particular microbial taxa. Reefs with higher coral cover had a coral microbiome with higher abundances of Alphaproteobacteria (such as Rhodobacterales and Sphingomonadales), whereas microbiomes of algae-dominated reefs had higher abundances of Gammaproteobacteria (such as Alteromonadales, Pseudomonadales, and Vibrionales), Betaproteobacteria, and Bacteriodetes. In contrast to taxa, geography was the strongest predictor of microbial community metabolism. Microbial communities on reefs with higher nutrient availability (e.g., equatorial upwelling zones) were enriched in genes involved in nutrient-related metabolisms (e.g., nitrate and nitrite ammonification, Ton/Tol transport, etc.). On reefs further from the equator, microbes had more genes encoding chlorophyll biosynthesis and photosystems I/II. These results support the hypothesis that core microbiomes are determined by holobiont macroorganisms, and that those core taxa adapt to local conditions by selecting for advantageous metabolic genes.
Coral reefs are complex ecosystems that provide habitats for diverse, interdependent macro- and microorganisms. A coral colony itself is a complex holobiont, each made up of a coral polyp and a suite of prokaryotic microbes, viruses, protists, endolithic fungi and algae, and other invertebrates (1–4). Some coral-associated microbes confer benefits by, for example, remineralizing nutrients that are essential for the coral holobiont (5–9). Others contribute to coral demise by causing a number of specific diseases as well as nonspecific detrimental effects (e.g., hypoxia) (10–12). On degraded reefs, where coral cover is reduced and the benthic surface is dominated by fleshy algae, the microbial community includes higher abundances of copiotrophic microbes, many of which are known pathogens (13). Higher abundances of potential pathogens on reefs are also known to correlate with higher prevalence of coral disease (14), indicating a link between the community structure of reef-associated microbes and coral health.
Previous studies have described the biogeographic distribution of pelagic microbial communities by investigating statistical relationships between pelagic microbes and environmental parameters (15–18). However, application of this approach to coral reef-associated microbes is complicated by a number of factors. First, for microbial members of specific coral holobionts, microbial biogeography is directly linked to the distribution of the coral species. Second, reef-associated microbial communities are influenced by the other benthic macroorganisms present, such as macroalgae—both calcifying and fleshy, which may vary markedly between locations. Third, these microbial communities are subject to abiotic factors— such as variable nutrient, temperature, and hydrodynamic regimes—associated with a particular geographic location. Given this complexity, understanding the drivers that influence the community structure of reef-associated microbes requires unraveling numerous interdependent factors.
The relationships between microbial community structure, the metabolic capacity of the assemblage, and their habitat are complex. Numerous taxa share core genes required for survival in the marine habitat. Supplementing these core housekeeping genes in each strain are a varied combination of metabolic genes (the pan-genome) associated with specialized pathways that contribute to fitness under particular local conditions, e.g., limited phosphate availability. These specialization genes do not respect species boundaries and may be found in multiple taxa adapted to similar environmental conditions (19, 20). Due to the mobility of these genes via horizontal gene transfer, the microbes can be considered to share a common gene pool, with specific genes being enriched within communities in particular niche habitats where they increase fitness. As a result, the similar community metabolism (i.e., functional redundancy) can be associated with high phylogenetic variability (21), and likewise communities comprised of similar taxa may differ in metabolic capabilities (22).
The mechanisms that govern community structure and gene flow in complex microbial communities, such as those associated with benthic marine habitats, remain largely unknown to the field of microbial ecology. Coral reefs are of particular interest because of their importance as centers of biodiversity, their contribution to global marine productivity, and their alarming decline. Coral reefs of the Line Islands (LIs) in the central Pacific offer a unique opportunity to investigate these questions as they span a latitudinal gradient from 6° north to 11° south. These islands and atolls (hence forth referred to as atolls) also span across the Equatorial Counter Current and Intertropical Convergence Zone, and thus experience significant variability in nutrient concentrations, temperature and precipitation.
In addition to oceanographic variability, the northern LIs also span a gradient of human disturbance where Teraina, Tabuaeran, and Kiritimati support populations of ∼1,000, 2,500, and 5,000 people, respectively. Reefs at these atolls are impacted by subsistence and commercial fishing, as well as some pollution (e.g., sewage, chemicals) and agricultural runoff. Some of the highest known biomass of the fishes for a coral reef ecosystem were observed on the unpopulated atolls (14, 23), where reefs were characterized by the high cover of reef-building corals and crustose coralline algae, abundant coral recruits, and low levels of coral disease (14). In contrast, the populated atolls, most notably Kiritimati, had reefs with as low as 2% coral cover and were associated with a higher abundance of super heterotrophs, many of which are known pathogens (13), and a higher prevalence of coral diseases (14). Because the reefs at the uninhabited atolls have been largely spared from such anthropogenic disturbances, they provide a baseline for a comparative evaluation of the effects of human activity on coral reef-associated microbes. However, to definitively attribute any observed differences to anthropogenic activities, the role of other environmental drivers that differ between atolls must also be examined. For instance, the three inhabited atolls are clustered together in a region spanning <3° latitude, inciting a counterargument that local biogeochemical factors were responsible for reef degradation rather than fishing or other local activities as had been suggested by a prior study (14).
Here we used comparative metagenomics to tease out the key environmental factors driving the composition and metabolism of reef-associated microbial communities in the LIs. Although the 11 atolls are clustered in the same oceanic region, they differ in three key environmental variables that are predicted to influence their microbial communities: nutrient levels, latitudinal distance from the equator, and the percentage of benthic surface occupied by various functional groups of macroorganisms. In this study, we collected reef-associated microbes, then extracted and sequenced the community DNA. Taxonomic and functional annotations were assigned to the resultant reads by comparison with the SEED protein database. We then quantified variation in the structure and metabolic potential of the communities in relation to the three key variables. These comparisons show that (i) the microbial taxa present and their relative abundances reflect the benthic community whose carbon-containing exudates provide the primary local energy source, and (ii) the presence of various specialized metabolic capabilities correlates with nutrient levels and other latitude-dependent factors.
Results
Studies were conducted at 22 reef sites distributed across 11 LIs spanning 18° latitude (Table S1). At each site, seawater samples were collected at the surface of the benthos for microbial metagenome preparation and from the immediately overlying water for nutrient analysis. The macroorganisms comprising the benthic cover were surveyed. Subsequent analyses assessed the relationships between three predictor variables (benthic macroorganisms, nutrient levels, and latitude) and both the structure and the metabolic capabilities of the microbial communities at these atolls.
Nutrient Concentration.
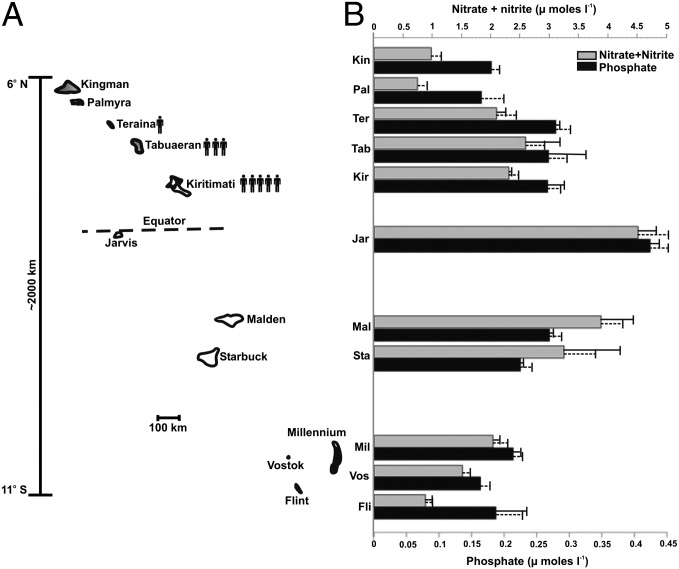
Inorganic nitrogen (nitrate + nitrite) and phosphate concentrations were generally highest near the equator and declined with increasing latitude both north and south (Fig. 1 and Table S2). Nitrate + nitrite concentrations ranged from 0.52 to 4.83 μM, whereas phosphate concentrations varied less (0.15–0.44 μM). Compared with the northernmost (Kingman) and southernmost (Flint) atolls, nitrate + nitrite and phosphate concentrations at equatorial Jarvis were approximately five- and twofold higher, respectively.
Fig. 1.
The LIs and their nutrient concentrations. (A) The 11 main atolls sampled in this study. The scale on the left indicates latitude and distance between atolls. Atoll sizes are proportionate, but not to scale. (B) Average nutrient concentrations at the 11 atolls. Nutrient concentrations were measured in triplicate for each of the 22 study sites (n = 66) and averaged; sites were then averaged for each atoll. Solid and dashed error bars show the SE for atoll and site replicates, respectively. Average values for each site are provided in Table S2.
Benthic Macroorganisms.
The benthic cover was quantified as the percentage covered by each of seven functional groups: hard coral, crustose coralline algae, calcified macroalgae, soft coral, fleshy macroalgae, fleshy turf algae, and “other” (Table S2). A list of the genera within each category is also provided (Table S3). Coral cover varied markedly from 2.2% at one site on Kiritimati to 86.7% at one site on Malden (mean = 44.4%; Table S2). In general, the uninhabited atolls were dominated by reef-building calcifiers including coral, crustose coralline algae, and calcified macroalgae (24), whereas fleshy algae, such as turf and fleshy macroalgae, dominate the inhabited atolls (14).
Reef-Associated Microbes.
DNA isolated from microbes sampled at each site was sequenced to yield 22 metagenomic libraries totaling 2.25 million quality reads (average length 389 bp; Table S1). The sequenced reads were translated in silico into predicted protein sequences; subsequent comparison with the SEED database provided taxonomic annotations for 21–47% of the reads and assignments to functional subsystems for 27–62% of the reads from each site. These annotations were the basis for comparative analyses of the microbial community structure and metabolic capabilities across the LI archipelago.
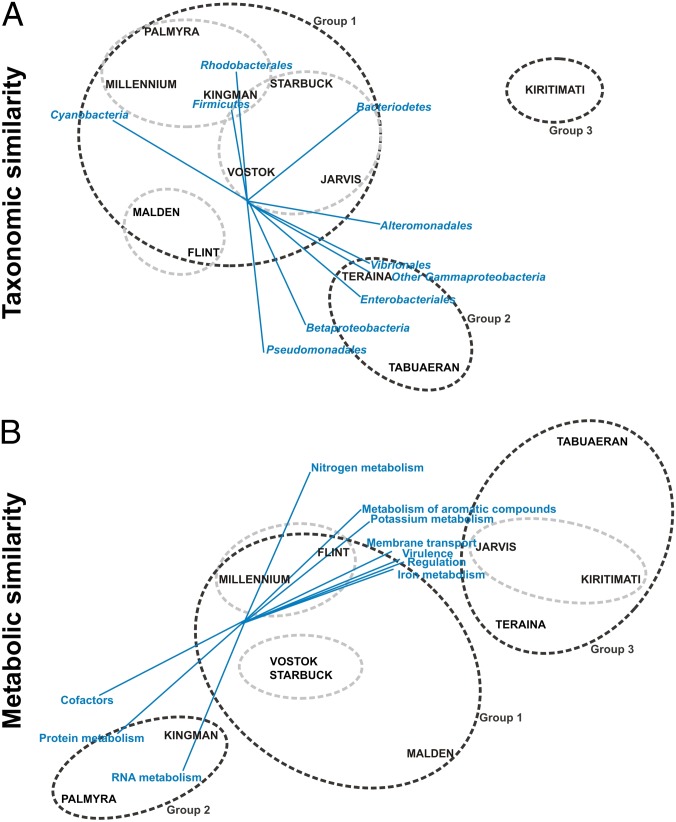
The relative abundances of the major taxonomic groups were tabulated (Fig. S1), plotted in 2D using nonmetric multidimensional scaling (nMDS; Fig. 2A), and analyzed for multivariate structure using similarity profile (SIMPROF) analysis (Fig. S2). By all measures, the geographic location of the atoll was a poor predictor of similarity of microbial community structure. For example, the two northernmost atolls, Kingman and Palmyra, are clustered with the Southern LIs in group 1 (Fig. 2A) and were most similar to Millennium, one of the southernmost atolls. Likewise Malden and Flint, separated by nearly 900 km, had similar taxonomic composition. In contrast, the metabolic capabilities (based on level 1 subsystem designations in the SEED; n = 20) of microbial communities in geographic proximity were more similar, forming three groups corresponding to the northern, middle, and southern atolls (Fig. 2B and Fig. S3). SIMPROF analyses conducted at the site level resulted in a higher number of significant groupings, although each site generally remained located within its own atoll group (Figs. S2 and S3) provided some exceptions, particularly in the metabolic groupings (e.g., Flint 2 clustered with group 3 atolls, Fig. S3). Further analyses were performed to quantify correlations between three key variables and both microbial community structure and metabolism across the LIs.
Fig. 2.
nMDS plots for the relative abundances of taxonomic similarities (A) and metabolic subsystem similarities (B). Sites were averaged for each atoll. The 2D stress values are 0.05 and 0.03 for the taxonomic and metabolic similarities, respectively. Dark gray circles indicate significant groupings from the SIMPROF analysis (Figs. S2 and S3; Bray–Curtis similarity, P < 0.01). Light gray circles cluster atolls with greatest similarity within each statistically significant group.
Community Structure.
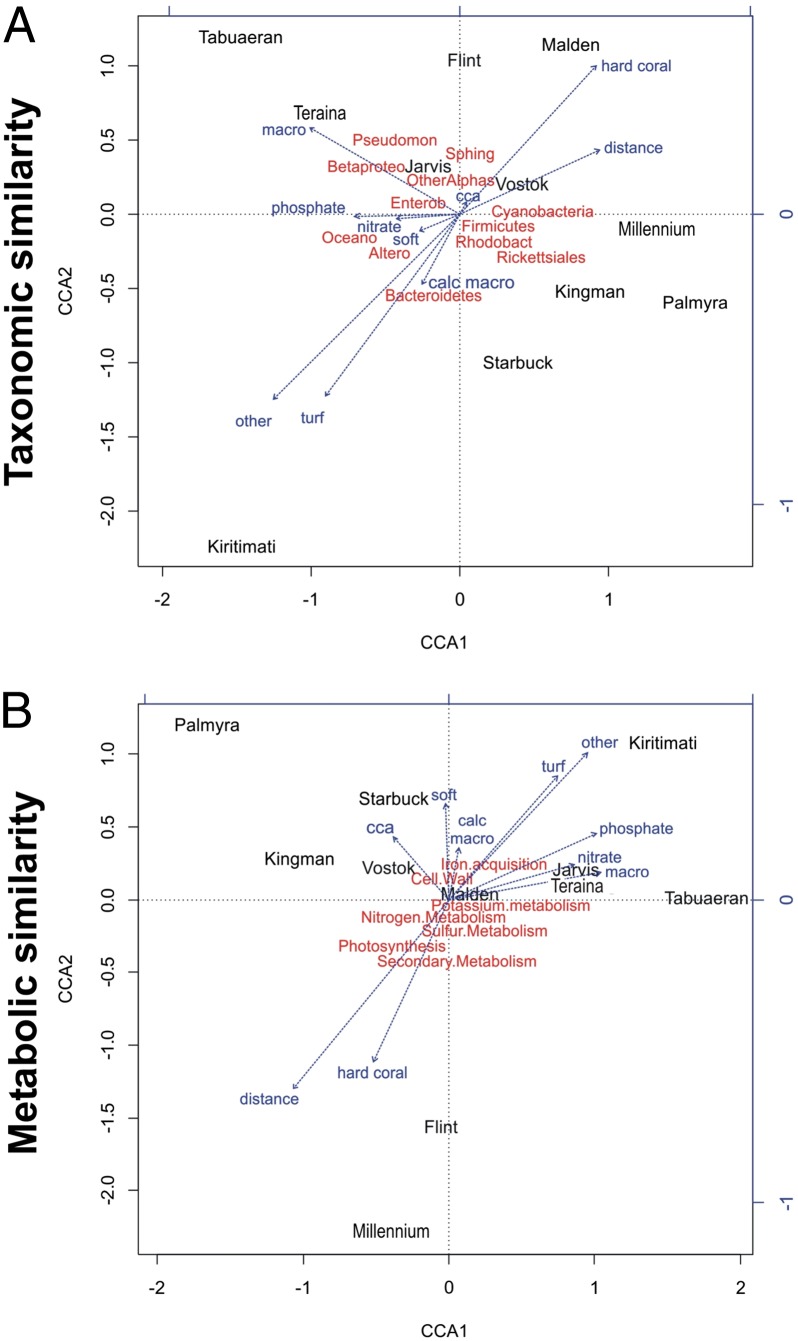
The correlations visualized by canonical correspondence analysis (CCA) (Fig. 3A) illustrate that microbial community structure on LI reefs is closely associated with benthic community composition. Reefs at all of the uninhabited LIs (group 1 in Fig. 2A and Fig. S2) associated with a higher percent cover of reef-building calcifiers were characterized by higher abundances of Cyanobacteria, Alphaproteobacteria (i.e., orders Rhodobacterales and Rickettsiales), and Firmicutes. Reefs with the highest hard coral coverage, such as Malden and Flint, had higher abundances of Sphingomonadales and Cyanobacteria (Fig. 3A). Although the abundance of the genus Synechococcus correlates positively with nutrient concentration in pelagic microbial communities, here it was positively correlated with the percentage of hard coral cover (Table 1; r = 0.665, P = 0.026). In contrast, hard coral cover showed a strong negative correlation with the abundance of Alteromonadales (r = −0.819, P = 0.002).
Fig. 3.
CCA depicting the correlations between predictor variables (blue) and the relative abundance of taxonomic similarities (A) and metabolic similarities (B) at each LI. Loading vectors for the taxa and subsystems are shown in red. Altero, Alteromonadales; Betaproteo, Betaproteobacteria; cca, crustose coralline algae; calc macro, calcified macroalgae; dist, distance from the equator in degrees latitude; Enterob, Enterobacteriales; macro, fleshy macroalgae; Oceano, Oceanospirillales; OtherAlphas, other Alphaproteobacteria; Pseudomon, Pseudomonadales; Rhodobact, Rhodobacterales; soft, soft coral; Sphing, Sphingomonadales.
Table 1.
Significance test for linear correlations between taxon abundance and specific predictor variables
| Taxon | Predictor variable | r | P |
| Flavobacteriales | Turf algae | 0.815 | 0.002 |
| Alteromonadales | Turf algae | 0.682 | 0.021 |
| Alteromonadales | Hard coral | −0.819 | 0.002 |
| Synechecoccus | Hard coral | 0.665 | 0.026 |
| Gammaproteobacteria | Macroalgae | 0.560 | 0.073 |
| Prochlorococcus | Phosphate | −0.614 | 0.045 |
| Sphinogomonadales | Nitrate | 0.758 | 0.007 |
| Erythrobacter | Nitrate | 0.674 | 0.023 |
r, Pearson’s coefficient.
The inhabited group 2 atolls associated with higher percent cover of fleshy macroalgae (Tabuaeran and Teraina; Fig. 3A) had greater abundances of Gammaproteobacteria (e.g., orders Enterobacteriales and Pseudomonadales) and Betaproteobacteria. In contrast, the reefs at populated Kiritimati were dominated by fleshy turf algae (58.9–82.4%) and supported a markedly increased abundance of Bacteriodetes (25.1 ± 4.2%, n = 2) compared with the other atolls (7.2 ± 3.5%, n = 20). Specifically, five genera within the class Flavobacteria (genera Croceibacter, Dokdonia, Gramella, Leeuwenhoekiella, and Polaribacter) were consistently overrepresented compared with sites on other atolls. Overall, the percent coverage of fleshy turf algae on LI reefs was positively correlated with bacteria from the orders Flavobacteriales and Alteromonadales (Table 1; r = 0.815, P = 0.002 and r = 0.682, P = 0.021, respectively). The CCA also depicted a correlation between the percent cover of other benthic organisms and Kiritimati reefs. Although other benthic organisms contributed to <1% of the benthic composition on most LI reefs, the two sites on Kiritimati had a higher percentage of sand, which contributed to the higher percent cover of this category (5.2 ± 0.5%).
A distance-based linear model (DistLM) was used to formally quantify which suite of predictor variables formed the best-fit model (balancing performance with complexity) for explaining variations in microbial communities across LI reefs. Hard coral alone had the largest impact on microbial community structure, explaining 15.2% of the variation between reefs (Table S4).
Community Metabolism.
Distance from the equator was the strongest predictor of community metabolism, explaining 18.4% of the variation in microbial metabolic potential (Table S4). The two northern atolls (group 2 in Fig. 2B, Kingman and Palmyra) were characterized by high abundances of genes encoding cofactors, RNA metabolism, and protein metabolism. Moving southward, the midlatitude atolls (group 3 in Fig. 2B; Jarvis, Kiritimati, Teraina, and Tabuaeran) were characterized by higher abundances of genes for aromatic compound utilization, iron metabolism, membrane transport, nitrogen metabolism, potassium metabolism, regulation, and virulence. All of the southern LIs were combined into one group and had similar community metabolism (group 1; Fig. 2B).
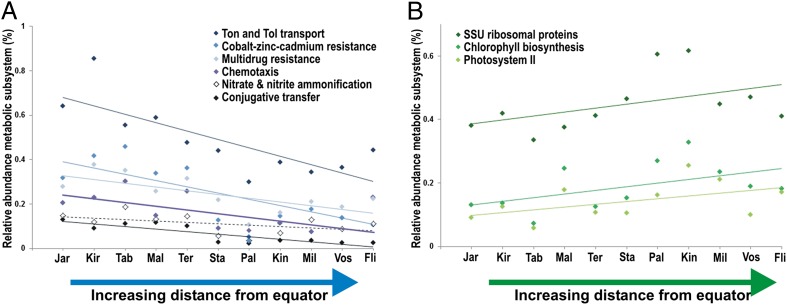
The question remained as to which environmental parameters associated with latitude were driving these variations. Nutrient levels varied across the LIs as expected due to the influence of equatorial upwelling (Fig. 1). As such, a number of metabolic pathways (SEED level 3 subsystems) demonstrated significant correlations with local phosphate concentrations across all 11 atolls. These included six pathways positively correlated with phosphate concentration: conjugative transfer, chemotaxis, nitrate and nitrite ammonification, cobalt–zinc–cadmium resistance, multidrug resistance efflux pumps, and Ton and Tol transport (Fig. 4A and Table S5). Phosphate concentration was negatively correlated with two metabolic pathways involved in photosynthesis (chlorophyll biosynthesis and photosystems I and II) (Fig. 4B and Table S5), and also with the abundance of Prochlorococcus (Table 1). Genes for ribosomal proteins were also overrepresented at oligotrophic sites (Fig. 4B).
Fig. 4.
Metabolic pathways that correlate positively (A) and negatively (B) with increasing distance from the equator (decreasing nutrient concentrations) across the LIs. Pathways are level 3 subsystem annotations from the SEED database. SSU, small subunit.
Interisland Comparison.
Atolls in close proximity were observed to have similar metabolic capabilities despite differences in their taxonomic composition. For example, microbial communities from the geographically close Jarvis and Kiritimati had similar metabolic profiles (Fig. 2), but the taxonomic profile of Jarvis was most similar to Vostok and Starbuck, whereas that for Kiritimati was the most dissimilar of all (Fig. 2). Conversely, the distant atolls of Kingman and Malden supported taxonomically similar microbial communities that encoded divergent metabolic capabilities. Hence, microbial communities composed of different taxa can encode similar functions, and vice versa.
Discussion
This study reports, to our knowledge, the first large-scale metagenomic survey of microbial communities associated with coral reefs that simultaneously characterizes both taxonomic composition and metabolic capabilities. We have demonstrated that, at the ecosystem level, benthic macroorganisms most strongly influence the taxonomic composition of the microbial community, whereas metabolic specialization genes carried by these taxa vary between locations and reflect functional adaptations to local oceanographic conditions.
For this study, microbial communities were sampled from 22 coral reef sites at 11 atolls across the LI archipelago; atolls that differed with respect to their benthic community, nutrient levels, and latitude. The microbes collected by our procedure were closely associated with the surface of the benthic macroorganisms (corals and algae). As a result, they included species-specific bacterial components of the coral holobiont (1), as well as specific bacterial taxa associated with some algal functional groups (1, 25). In addition, the microbial communities sampled on these reefs reflected selection by the adjacent benthic macroorganisms, as evidenced by the differences between reef-associated bacterioplankton communities and open ocean communities (26). There is evidence that reef-associated communities undergo selection in shallow reef environments by the locally available labile organic matter exuded by the benthic organisms (27). For example, in an empirical study, Nelson et al. demonstrated that exudates collected from coral and macroalgae selectively fostered growth of distinct bacterioplankton communities (27). Coral exudates promoted communities with higher diversity, including lineages of Alphaproteobacteria with relatively few virulence factors (e.g., Erythrobacteraceae); whereas exudates from fleshy macroalgae selected for less diverse communities with more copiotrophic Gammaproteobacteria lineages (e.g., the families Alteromonadaceae, Pseudoalteromonadaceae, and Vibrionaceae).
Community Structure.
The current study confirms and extends earlier findings (27) by demonstrating similar correlations between benthic community composition and the enrichment of specific microbial taxa on coral reefs in situ (Table 1). Consistent with the effects of individual exudates, high coral cover was associated with higher abundances of Alphaproteobacteria, whereas the abundant fleshy macroalgae at Tabuaeran and Teraina were accompanied by more Gammaproteobacteria (e.g., Enterobacteriales and Pseudomonadales). Together, these complementary research approaches indicate that coral- and algae-derived organic exudates enrich for specific types of bacteria living in close association with coral reefs.
Nutrient levels have also been postulated to influence microbial community composition. Here we tested this hypothesis using the natural nutrient gradient present across the LIs. Due to the equatorial Pacific upwelling in this region, phosphate and nitrate are elevated at the equator and decrease with latitude both north and south (Fig. 1). In high-nitrate, low-chlorophyll ecosystems such as this, iron may be the nutrient limiting primary production (28). Other unspecified biogeographic factors also vary with latitude across the LIs. In this study, neither nutrients nor other latitude-dependent variables were included in the best-fit model for determining microbial community structure. Therefore, we propose that on these geographically separate coral reefs, microbial community structure is determined by the available energy source, i.e., the dissolved organic carbon provided in the form of benthic exudates, which provides a mechanism for the correlations observed between the macro- and microbial components of reef communities.
Community Metabolism.
In contrast to community structure, the specialized and ecologically relevant metabolic capabilities of these communities reflected local nutrient concentrations. For example, six level 3 metabolic subsystems (SEED database) correlated positively with phosphate concentration across the LIs (Fig. 4A). Some of these, such as the TonB system, contribute to nutrient acquisition. The TonB system transports large molecules (e.g., polysaccharides, proteins, and siderophores) in through the outer membrane of Gram-negative bacteria. Its importance in marine environments is evidenced by the presence of these genes in marine bacterial genomes and pelagic metagenomes (29–31), their high levels of expression in metatranscriptome data (32), and the proteomic identification of their products as the predominant membrane proteins in pelagic bacteria (33). In this study, they accounted for nearly 1% of gene function annotations at some high-nutrient sites (Fig. 4A). Genes of the conjugative transfer subsystem, also overrepresented at high-nutrient sites, may function in energy and nutrient acquisition via type IV secretion of ectoenzymes and siderophores, and may support active horizontal gene transfer via conjugation. Conversely, the more oligotrophic sites exhibited overrepresentation of two photosynthesis pathways (chlorophyll biosynthesis and photosystems I and II) (Fig. 4B and Table S5), as well as greater abundance of Prochlorococcus, a key primary producer in oligotrophic oceans (Table 1).
Previous studies have shown that the anaerobic ammonification of nitrate and nitrite (also referred to as dissimilatory nitrate reduction to ammonium or DNRA) is significant for nitrogen metabolism in the diffusive boundary layer, an environment with heterogeneous distribution of dissolved oxygen during the day (12) that then becomes anoxic at night (34). That anaerobes dominate coral-associated microbial communities suggested that this anaerobic nitrogen metabolism may be important on coral surfaces (25). An interesting observation from the nutrient measurements is that atolls with higher nitrate + nitrite availability have lower ammonium concentrations, whereas low nitrate + nitrite atolls have higher ammonium. Nitrate + nitrite to ammonium ratios were 0.26, 0.29, and 0.22 on Malden, Jarvis, and Kiritimati compared with 3.23 and 1.47 on Flint and Kingman, respectively (Table S2). Therefore, the overrepresentation of DNRA may reflect the lower abundances of ammonium at these high-nutrient sites.
Reef-associated microbial communities in high-nutrient environments encoded greater metabolic complexity, suggesting that they carry more specialization genes and thus generally possess larger genomes (Fig. S4). Consistent with this hypothesis, single-copy genes encoding ribosomal proteins were overrepresented at oligotrophic sites (Fig. 4B), indicating that the community overall possessed smaller genomes compared with those at high-nutrient sites.
Although both phosphorus and nitrogen concentrations correlated with distance from the equator (r = −0.74 and −0.64, respectively; Table S6), neither was as strong a predictor of metabolism, as was latitudinal distance from the equator (as assessed by DistLM analysis). Distance from the equator may serve as a proxy for other influential but unsampled variables, such as seawater temperature, salinity, photosynthetically active radiation, or micronutrient concentrations (e.g., iron). In addition, the limited sampling (one to four sites at each atoll) may have obscured significant correlations to specific nutrients. Had the atoll averages been based on sampling of 20+ sites per atoll, significant correlation with specific nutrients might have been discernible. Nevertheless, the availability of the macronutrients nitrate + nitrite and phosphate are posited to be important factors influencing microbial community metabolism on LI reefs.
Anthropogenic Impacts on LI Reefs.
The findings of this study indicate that local human populations influence the reef-associated microbial community indirectly by influencing the composition of benthic macroorganisms. Typically activities such as fishing remove important grazing herbivore species resulting in increased cover of fleshy algae, and this in turn profoundly impacts microbial community structure at the populated atolls (Fig. 2 and Fig. S1). Increased coverage by fleshy algae selects for specific microbes that may be detrimental to coral health (27, 35), thereby opening additional benthic space for further algal colonization (36).
Discordance Between Taxa and Metabolism.
Both the abundance of specific taxonomic groups and the community metabolic capabilities of the reef-associated microbial communities varied across the LIs. Both correlated with ecological factors, but did so independent of each other. As a result, atolls as far apart as Kingman and Malden (∼1,400 km) hosted taxonomically similar communities, but these communities effectuated different metabolisms. Conversely, the different microbial communities at equatorial Jarvis and Kiritimati encoded similar metabolic specialization genes. This discordance between taxonomy and metabolism is intriguing. We hypothesize that although community structure is attributable to the core genes that classify each taxon, community metabolism reflects the particular complement of specialization genes that comprise the dynamic genome of each strain present. Previously, strain-specific adaptation to different nutrient levels had been documented in marine cyanobacteria for genes involved in phosphate acquisition. The particular genes present and their genomic organization depended on phosphate availability in each isolate’s source environment. Strains of Prochlorococcus that showed 99.9% similarity of their 16S rRNA genes nevertheless possessed different phosphate metabolism genes located in different genomic locations (19). Conversely, some more divergent strains that occupied environments with similar nutrient regimes shared similar phosphate gene content and organization. Additionally, although Prochlorococcus typically assimilates only ammonium, in regions of nitrogen limitation strains have adapted to use nitrate and nitrite by using genes acquired horizontally from Synechococcus (20).
The observed adaptation of microbial community metabolism patterns could have resulted from either gene acquisition and loss or shifts in the relative abundances of strains adapted to different conditions. Traditionally, only changes in strain abundance (i.e., beta diversity) have been considered as possible drivers of rapid adaptation in ecological time. Increased genetic diversity, i.e., evolution, by mechanisms such as horizontal movement of genes between strains or species, has been expected to require evolutionary time. We posit that in these microbial communities, evolution is rapid, occurring in ecological time.
Attempts to identify the evolutionary mechanisms active in this situation have been hampered by the limited representation of marine microbes in databases (37) such as SEED, due to our inability to culture most species (38). The availability of single-cell whole-genome amplification methods (39) promises to enable genomic characterization of unculturable marine microbes, thereby substantially accelerating resolution of this question.
Materials and Methods
Metagenomic sequence reads were compared with the SEED protein database (http://theseed.org/wiki/Main_Page) using BLASTx. For taxonomic annotation, sequences with significant similarities (E < 10−5) were assigned to the closest identified microbial representative. For functional annotation, sequences were assigned the function of the closest identified protein and these functions were then grouped into metabolic pathways according to the subsystems in the SEED database. Community structure was compared using the relative abundances of 19 higher-rank microbial taxa (see SI Materials and Methods and Table S7 for clarification of taxonomic groups). Similarly, community metabolism was determined by comparing the relative abundance of 20 level 1 subsystem categories in the SEED database.
nMDS analyses were used with the annotated metagenome data to visualize between-atoll similarity in terms of two discrete response variables: community structure and community metabolism. For an initial exploration of potential correlations between the three predictor variables and either microbial community structure or metabolism, a CCA was performed using the R package, vegan. To formally quantify how much variation in the microbial communities or their metabolism could be explained by the predictors measured (continuous variables), a permutational DistLM was used in PERMANOVA+ (www.primer-e.com/permanova.htm).
Full methods and any associated references are available in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Beltran Rodriguez-Mueller, Rob Schmieder, Bahador Nosrat, Federico Lauro, Nao Hisakawa, Jeremy Frank, Bas Dutilh, Katrine Whiteson, Barbara Bailey, and Jim Nulton for mathematical and bioinformatics support; and the Palmyra Atoll Research Consortium and the Nature Conservancy for field support. We are also grateful to Jennifer Martiny for valuable discussions and Heather Maughan for her editing expertise. The microbial samples were collected during two research expeditions to the LIs funded by the National Geographic Society, the Moore Family Foundation, the Hawaii Undersea Research Laboratory of the Coral Reef Conservation Program [a program of the National Oceanic and Atmospheric Administration (NOAA)], and several private donors and during one Reef Assessment and Monitoring Program cruise to Jarvis supported and executed by NOAA-Coral Reef Ecosystem Division. This work was carried out under research permits from the Palmyra Atoll National Wildlife Refuge, US Fish and Wildlife Service, and the Environment and Conservation Division of the Republic of Kiribati. This research was sponsored by National Science Foundation Awards OCE-0927415 and DEB-1046413 (to F.R.), OCE-0927411 (to C.A.C.), and OCE-0417412 (to Moorea Coral Reef Long-Term Ecological Research); and a Canadian Institute for Advanced Research Integrated Microbial Biodiversity Program Fellowship 141679 (to F.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the MG-RAST Metagenomics Analysis Server, http://metagenomics.anl.gov/linkin.cgi?project=9220 (project name: Pacific Reef Microbiomes).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403319111/-/DCSupplemental.
References
- 1.Rohwer F, Seguritan V, Azam F, Knowlton N. Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser. 2002;243:1–10. [Google Scholar]
- 2.Bourne DG, Munn CB. Diversity of bacteria associated with the coral Pocillopora damicornis from the Great Barrier Reef. Environ Microbiol. 2005;7(8):1162–1174. doi: 10.1111/j.1462-2920.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 3.Koren O, Rosenberg E. Bacteria associated with mucus and tissues of the coral Oculina patagonica in summer and winter. Appl Environ Microbiol. 2006;72(8):5254–5259. doi: 10.1128/AEM.00554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunagawa S, Woodley CM, Medina M. Threatened corals provide underexplored microbial habitats. PLoS ONE. 2010;5(3):e9554. doi: 10.1371/journal.pone.0009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowlton N, Rohwer F. Multispecies microbial mutualisms on coral reefs: The host as a habitat. Am Nat. 2003;162(Suppl 4):S51–S62. doi: 10.1086/378684. [DOI] [PubMed] [Google Scholar]
- 6.Lesser MP, et al. Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar Ecol Prog Ser. 2007;346:143–152. [Google Scholar]
- 7.Fiore CL, Jarett JK, Olson ND, Lesser MP. Nitrogen fixation and nitrogen transformations in marine symbioses. Trends Microbiol. 2010;18(10):455–463. doi: 10.1016/j.tim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Wegley L, Edwards R, Rodriguez-Brito B, Liu H, Rohwer F. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environ Microbiol. 2007;9(11):2707–2719. doi: 10.1111/j.1462-2920.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- 9.Raina JB, Tapiolas D, Willis BL, Bourne DG. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol. 2009;75(11):3492–3501. doi: 10.1128/AEM.02567-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JE, et al. Indirect effects of algae on coral: Algae-mediated, microbe-induced coral mortality. Ecol Lett. 2006;9(7):835–845. doi: 10.1111/j.1461-0248.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- 11.Barott K, et al. Hyperspectral and physiological analyses of coral-algal interactions. PLoS ONE. 2009;4(11):e8043. doi: 10.1371/journal.pone.0008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas AF, et al. Visualization of oxygen distribution patterns caused by coral and algae. PeerJ. 2013;1:e106. doi: 10.7717/peerj.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinsdale EA, et al. Microbial ecology of four coral atolls in the northern Line Islands. PLoS ONE. 2008;3(2):e1584. doi: 10.1371/journal.pone.0001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandin SA, et al. Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE. 2008;3(2):e1548. doi: 10.1371/journal.pone.0001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewson I, Paerl RW, Tripp HJ, Zehr JP, Karl DM. Metagenomic potential of microbial assemblages in the surface waters of the central Pacific Ocean tracks variability in oceanic habitat. Limnol Oceanogr. 2009;54(6):1981–1994. [Google Scholar]
- 16.Fuhrman JA, et al. A latitudinal diversity gradient in planktonic marine bacteria. Proc Natl Acad Sci USA. 2008;105(22):7774–7778. doi: 10.1073/pnas.0803070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pommier T, et al. Global patterns of diversity and community structure in marine bacterioplankton. Mol Ecol. 2007;16(4):867–880. doi: 10.1111/j.1365-294X.2006.03189.x. [DOI] [PubMed] [Google Scholar]
- 18.Martiny AC, Tai APK, Veneziano D, Primeau F, Chisholm SW. Taxonomic resolution, ecotypes and the biogeography of Prochlorococcus. Environ Microbiol. 2009;11(4):823–832. doi: 10.1111/j.1462-2920.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 19.Martiny AC, Coleman ML, Chisholm SW. Phosphate acquisition genes in Prochlorococcus ecotypes: Evidence for genome-wide adaptation. Proc Natl Acad Sci USA. 2006;103(33):12552–12557. doi: 10.1073/pnas.0601301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martiny AC, Kathuria S, Berube PM. Widespread metabolic potential for nitrite and nitrate assimilation among Prochlorococcus ecotypes. Proc Natl Acad Sci USA. 2009;106(26):10787–10792. doi: 10.1073/pnas.0902532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke C, Steinberg P, Rusch D, Kjelleberg S, Thomas T. Bacterial community assembly based on functional genes rather than species. Proc Natl Acad Sci USA. 2011;108(34):14288–14293. doi: 10.1073/pnas.1101591108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore LR, Rocap G, Chisholm SW. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature. 1998;393(6684):464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- 23.Williams ID, et al. Differences in reef fish assemblages between populated and remote reefs spanning multiple archipelagos across the central and western Pacific. J Mar Biol. 2011;2011:826234. [Google Scholar]
- 24.Williams GJ, et al. Benthic communities at two remote Pacific coral reefs: Effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ. 2013;1:e81. doi: 10.7717/peerj.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barott KL, et al. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ Microbiol. 2011;13(5):1192–1204. doi: 10.1111/j.1462-2920.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- 26.Nelson CE, Alldredge AL, McCliment EA, Amaral-Zettler LA, Carlson CA. Depleted dissolved organic carbon and distinct bacterial communities in the water column of a rapid-flushing coral reef ecosystem. ISME J. 2011;5(8):1374–1387. doi: 10.1038/ismej.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson CE, et al. Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. ISME J. 2013;7(5):962–979. doi: 10.1038/ismej.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin JH, et al. Testing the iron hypothesis in ecosystems of the equatorial Pacific-Ocean. Nature. 1994;371(6493):123–129. [Google Scholar]
- 29.Hopkinson BM, Barbeau KA. Iron transporters in marine prokaryotic genomes and metagenomes. Environ Microbiol. 2012;14(1):114–128. doi: 10.1111/j.1462-2920.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 30.Tang K, Jiao N, Liu K, Zhang Y, Li S. Distribution and functions of TonB-dependent transporters in marine bacteria and environments: Implications for dissolved organic matter utilization. PLoS ONE. 2012;7(7):e41204. doi: 10.1371/journal.pone.0041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupont CL, et al. Genomic insights to SAR86, an abundant and uncultivated marine bacterial lineage. ISME J. 2012;6(6):1186–1199. doi: 10.1038/ismej.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ottesen EA, et al. Metatranscriptomic analysis of autonomously collected and preserved marine bacterioplankton. ISME J. 2011;5(12):1881–1895. doi: 10.1038/ismej.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris RM, et al. Comparative metaproteomics reveals ocean-scale shifts in microbial nutrient utilization and energy transduction. ISME J. 2010;4(5):673–685. doi: 10.1038/ismej.2010.4. [DOI] [PubMed] [Google Scholar]
- 34.Shashar N, Cohen Y, Loya Y. Extreme diel fluctuations of oxygen in diffusive boundary-layers surrounding stony corals. Biol Bull. 1993;185(3):455–461. doi: 10.2307/1542485. [DOI] [PubMed] [Google Scholar]
- 35.Kelly LW, et al. Black reefs: Iron-induced phase shifts on coral reefs. ISME J. 2012;6(3):638–649. doi: 10.1038/ismej.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barott KL, Rohwer FL. Unseen players shape benthic competition on coral reefs. Trends Microbiol. 2012;20(12):621–628. doi: 10.1016/j.tim.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Yooseph S, et al. Genomic and functional adaptation in surface ocean planktonic prokaryotes. Nature. 2010;468(7320):60–66. doi: 10.1038/nature09530. [DOI] [PubMed] [Google Scholar]
- 38.Rappé MS, Giovannoni SJ. The uncultured microbial majority. Annu Rev Microbiol. 2003;57:369–394. doi: 10.1146/annurev.micro.57.030502.090759. [DOI] [PubMed] [Google Scholar]
- 39.Stepanauskas R, Sieracki ME. Matching phylogeny and metabolism in the uncultured marine bacteria, one cell at a time. Proc Natl Acad Sci USA. 2007;104(21):9052–9057. doi: 10.1073/pnas.0700496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.