Significance
Sodium is an essential micronutrient that is often limited in animal diets. It is important in the development of neural tissue and thought to have driven the evolution of specific foraging behavior. Human activity is drastically altering patterns of sodium availability, particularly through road salt application, but we know little about the consequences of such anthropogenic change on the development and evolution of wild animals. Here, we show that road salt runoff affects sodium concentrations of roadside plants, which in turn, have significant, (sometimes positive) effects on neural and muscular development of herbivores. These results suggest that anthropogenic changes in sodium can have major consequences for both behavioral development and selection on foraging behavior.
Keywords: nutritional ecology, Danaus plexippus, Pieris rapae, ecological stoichiometry
Abstract
The development of organisms is changing drastically because of anthropogenic changes in once-limited nutrients. Although the importance of changing macronutrients, such as nitrogen and phosphorus, is well-established, it is less clear how anthropogenic changes in micronutrients will affect organismal development, potentially changing dynamics of selection. We use butterflies as a study system to test whether changes in sodium availability due to road salt runoff have significant effects on the development of sodium-limited traits, such as neural and muscle tissue. We first document how road salt runoff can elevate sodium concentrations in the tissue of some plant groups by 1.5–30 times. Using monarch butterflies reared on roadside- and prairie-collected milkweed, we then show that road salt runoff can result in increased muscle mass (in males) and neural investment (in females). Finally, we use an artificial diet manipulation in cabbage white butterflies to show that variation in sodium chloride per se positively affects male flight muscle and female brain size. Variation in sodium not only has different effects depending on sex, but also can have opposing effects on the same tissue: across both species, males increase investment in flight muscle with increasing sodium, whereas females show the opposite pattern. Taken together, our results show that anthropogenic changes in sodium availability can affect the development of traits in roadside-feeding herbivores. This research suggests that changing micronutrient availability could alter selection on foraging behavior for some roadside-developing invertebrates.
The development of fitness-related traits is closely tied to nutrition—from fecundity being influenced by protein availability (1, 2) to ornament coloration being linked to carotenoid abundance (3, 4). However, humans are having a major impact on the availability of many nutrients important in the development of these traits. For instance, nitrogen and phosphorus availability has increased dramatically because of fertilizer application (5, 6), with drastic consequences for biomass and nutrient content of producers and consumers (7–9). Although the effects of changing macronutrients have been well-studied, the importance of human-induced changes in micronutrients is less established. Are anthropogenic changes in once-limited micronutrients enough to drive differences in trait development, potentially altering selection dynamics?
This research focuses on changing availability of an important micronutrient: sodium. Sodium is a key component of animal development, important for the function of neural and muscle tissue (10–12) and affecting the development of traits, such as brain size (13–16). However, sodium availability is limited in most ecosystems (17–19), which is thought to have led to the evolution of sodium cravings (20, 21) and specific foraging behavior to acquire sodium (22–25). Humans are increasing sodium availability, particularly through the application of road salt (26–29) but also, through agricultural activity (30). In the metropolitan area of Minneapolis and St. Paul, Minnesota, ∼300,000 tons of sodium chloride are applied to roads each winter (31). Research on the ecological impact of road salt has mostly focused on the negative effects of chloride entering waterways (32–34). However, road salt application can also increase the availability of dietary sodium for animals. A handful of studies suggest that road salt application may affect sodium foraging in animals from ants to moose (35, 36). We know little about whether local increases in sodium along roadsides have significant effects on development of fitness-related traits for species feeding along roadsides, thus altering evolutionary dynamics in the anthropocene.
Butterflies are an excellent study system to test the consequences of changing sodium availability. Sodium availability has been shown to affect the development and activity of flight muscle in male Lepidoptera (37–39). Many adult male Lepidoptera actively forage for sodium through puddling, transferring much of this sodium to females during mating (23, 40–43). In addition, host plants of many butterfly species commonly grow along roadsides and would be affected by roadside runoff. Butterfly larvae also have limited movement (44, 45), such that the spatially restricted effects of salt runoff are biologically relevant. This work starts by documenting the effects of roadside salt runoff on sodium availability in common butterfly host plants. Two rearing experiments—one using roadside-collected and control host plants and the other using a controlled artificial diet manipulation—show the importance of changing sodium availability on trait development. In particular, we focus on two fitness-related traits—muscle and neural tissue—where sodium availability has a shown importance in trait development and function.
Results
Sodium Concentration in Plants and Control Butterfly Tissue.
Sodium of roadside plants.
For two of four plant species collected, specimens collected adjacent to roadways tended to have higher sodium concentrations in leaves relative to specimens collected at control sites 100 m away. There were significant differences between sites for the milkweed and the oak species but not the grass or the mustard (Table 1). There were no significant differences in leaf nitrogen concentration between the sites (Table 1).
Table 1.
Sodium concentration in roadside plants
| Species | Sodium (ppm) |
Nitrogen (%) |
||
| Roadside (control) | F1,4 | Roadside (control) | F1,4 | |
| Milkweed | 2,065 (62) | 11.1* | 4.87 (4.75) | 0.03 |
| Mustard | 115 (112) | 0.07 | 3.73 (3.35) | 1.65 |
| Oak | 50.9 (35.8) | 33.9† | 2.60 (2.37) | 1.06 |
| Grass | 71.6 (54.7) | 0.65 | 3.21 (2.97) | 0.20 |
Shown are nutrient concentrations in plants collected along roadsides or from control sites over 100 m away. For sodium values, statistics were performed on log-transformed data (raw values are shown as means).
P < 0.05.
P < 0.01.
Sodium in control butterfly tissue.
Sodium was the most concentrated micronutrient measured in butterfly tissue relative to plant tissue, being more limited in availability than potassium, calcium, and phosphorus (Table 2). Pieris rapae reared on bok choy had, on average, 5,096 ppm sodium in their tissue relative to 112 ppm in wild-collected mustards (Table 2). Similarly, sodium bioaccumulated in monarch tissue was, on average, six times more concentrated than in control host plant tissue (Table 2). Element concentration varied somewhat with tissue type (e.g., head or abdomen) and sex (Tables S1–S3).
Table 2.
Element concentration in butterfly tissue and their respective host plants
| Tissue | Calcium | Potassium | Sodium | Phosphorus |
| Pieris tissue | 755 | 12,024 | 5,097 | 11,579 |
| Mustard leaves* | 14,208 | 28,870 | 112 | 9,657 |
| Monarch tissue | 2,055 | 18,084 | 383 | 8,852 |
| Milkweed leaves | 14,208 | 33,955 | 62 | 9,020 |
Shown are mean concentrations (in milligrams per kilogram or parts per million) of different elements in cabbage white (Pieris; n = 38) and monarch tissue (mean concentration across head, thorax, and abdomen tissue; n = 27). Leaf samples (n = 3) came from control sites at Cedar Creek Ecosystem Science Reserve. Full analysis (by sex and tissue) is in Tables S1–S3.
For further measures of sodium leaf concentration of mustards, see Table S4.
Experiment 1: Host Plant Diet Manipulation with Monarchs.
Survival.
Survival rates of monarch caterpillars were significantly lower on the roadside-collected leaves (40.5%) than the prairie-collected leaves (58.2%; χ2 = 5.2, P = 0.02); sex ratio did not differ on the two diet types (χ2 = 0.03, P = 0.85).
Eye and muscle investment.
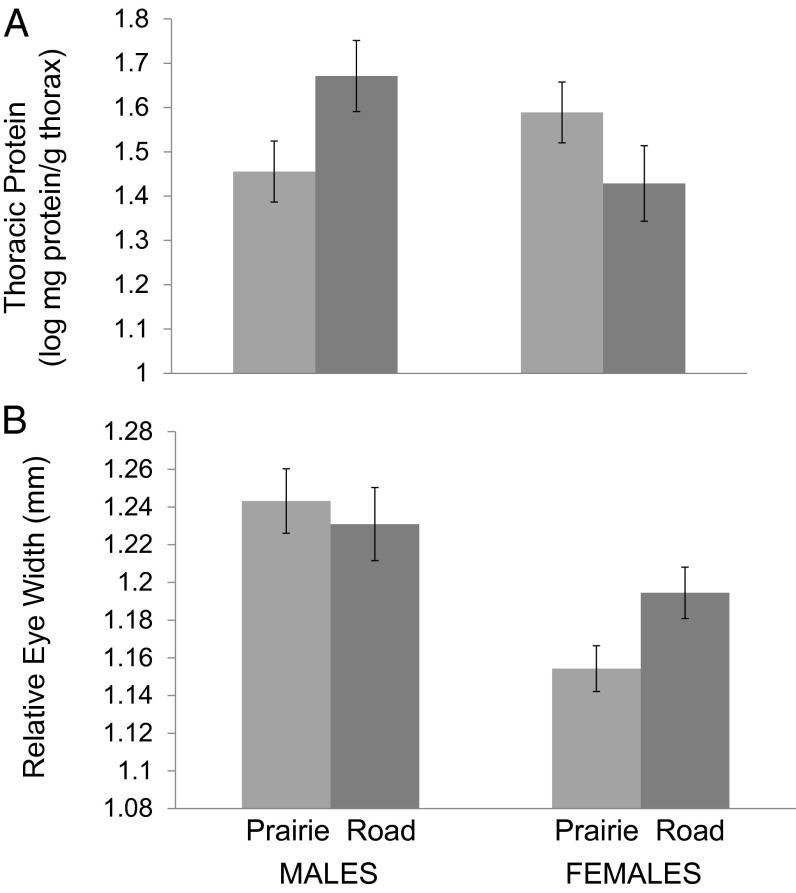
Because there were significant interactions between sex and diet, we analyzed male and female monarchs separately. There was a significant effect of diet on thoracic muscle investment in males but not females (Fig. 1A and Table 3). Males reared on roadside-collected milkweed had greater thoracic protein investment than those reared on prairie-collected milkweed; this pattern was reversed in females but was not significant. There was a significant effect of diet on relative eye size in females but not males (Fig. 1B and Table 3): females reared on roadside milkweed had significantly larger eyes (controlling for body size) than those reared on prairie-collected milkweed.
Fig. 1.
Effects of sodium manipulation on eye size and thoracic protein in monarchs. Monarchs reared on roadside-collected milkweed had 16 times the amount of sodium in their larval diet than those reared on milkweed collected from prairies 500 m away. Shown are differences in (A) thoracic protein and (B) relative eye width for adult males and females. For eye width, least square means (and SEs) are plotted from a model that also included body size (wing length) and head alignment accuracy (statistics in Table 3).
Table 3.
Effects of roadside vs. prairie plants on monarch development
| Males | Females | |
| Thoracic protein | ||
| Diet | t29 = 2.06* | t12 = −1.32 |
| Eye width | ||
| Diet | F1,16 = 0.18 | F1,14 = 4.75* |
| Wing length | F1,16 = 0.23 | F1,14 = 10.1† |
| Alignment | F1,16 = 0.01 | F1,14 = 2.61 |
Shown are results from t tests and ANOVAs testing for effects of diet on thoracic protein (mass protein per mass dry thorax) and eye width. The model for eye width also included a measure of body size (wing length) and head alignment error.
P < 0.05.
P < 0.01.
Tissue sodium.
Monarchs reared on roadside-collected milkweed contained significantly more sodium in their abdomens than prairie-reared monarchs, regardless of sex [mean (SE): prairie-reared = 129.7 ppm (89.7); roadside-reared = 636.6 ppm (82.5); diet: F1,22 = 18.3, P = 0.0003; sex: F1,22 = 0.44, P = 0.5]. Concentrations of other elements are reported in Table S3.
Experiment 2: Artificial Diet Manipulation with Cabbage Whites.
Survival.
Cabbage white butterfly survival was significantly lower on the high-sodium artificial diet than the medium- or low-sodium diet, although there was no survival difference between the latter two diet types (high: 10.9%; medium: 34.3%; low: 41.7%; χ22,525 = 48.8, P < 0.0001; difference between medium and low: χ21,351 = 2.05, P = 0.15). Because of the low survival on the high-sodium diet, all subsequent analyses contrast the low and medium diet types only. Development time was longer by 1 d on the medium-sodium diet relative to the low-sodium diet, but this difference was only marginally significant (low: 36 d; medium: 37.1 d; F1,131 = 3.23, P = 0.07 in a model controlling for sex).
Neural and muscle investment.
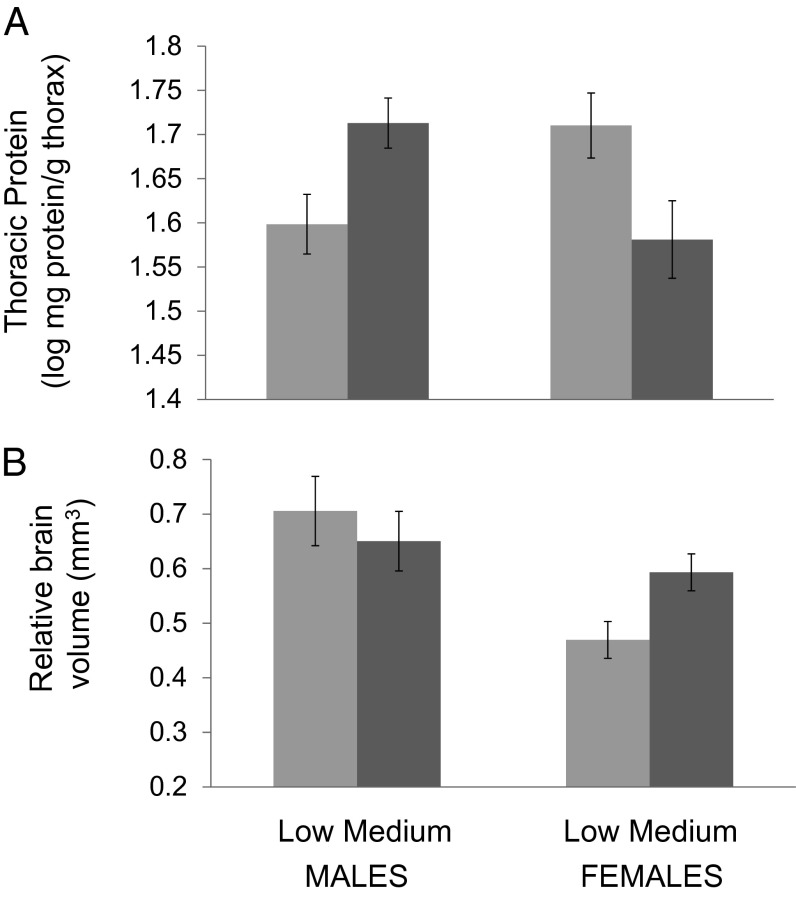
Because there were significant sex by diet interactions, males and females were analyzed separately. There was a significant effect of diet on thoracic protein for both males and females, but the direction of that effect differed: males on the medium-sodium diet had greater thoracic protein than males on the low-sodium diet, whereas females on the low-sodium diet had greater thoracic protein than females on the medium-sodium diet (Fig. 2A and Table 4).
Fig. 2.
Effects of sodium manipulation on brain size and thoracic protein in cabbage whites. Cabbage white butterflies were reared on low- or medium-sodium artificial diets (high-sodium diets resulted in high mortality and were not analyzed further). Shown are differences in (A) thoracic protein and (B) relative brain volume for adult males and females. For brain volume, least square means (and SEs) are plotted from a model that also included body size (wing length) (statistics in Table 4).
Table 4.
Effects of low- and medium-sodium artificial diets on Pieris development
| Males | Females | |
| Thoracic protein | ||
| Diet | t19 = 2.45* | t23 = 2.24* |
| Brain size | ||
| Diet | F1,4 = 0.41 | F1,5 = 6.73* |
| Wing length | F1,4 = 0.57 | F1,5 = 1.16 |
Shown are results from t tests and ANOVAs testing for effects of diet (low vs. medium sodium in an artificial diet) on thoracic protein (mass protein per mass dry thorax) and brain size. The model for brain size also included a measure of body size (wing length).
P < 0.05.
Females raised on the low-sodium diet had significantly smaller total brain volume than those raised on the medium-sodium diet in a model that included body size (Fig. 2B and Table 4). There was no difference in brain size of males reared on the two diets (Fig. 2B and Table 4).
Discussion
Road Salt Runoff Affects Sodium Nutrition in Diverse Ways.
We found that some species of plants—but not all—had higher leaf sodium levels along roadsides than in nearby control sites. In two of four species assayed (milkweed and oak), sodium concentration of roadside plants was significantly higher relative to plants at least 100 m from the road (Table 1) (1.5–30 times greater concentration). This effect on sodium concentration of roadside leaves is likely conservative given that we were focused on an area with sandy, rapid-leaching soil and a county road with only moderate levels of traffic and salt application. We might see more pronounced differences along interstates or in areas with clay-rich soil. Sodium from road salt runoff did not seem to stress the plants in this study, because nitrogen content in leaves did not differ between sites.
There were no significant effects of road salt runoff on sodium levels in the mustard and grass species that we measured. The lack of a response in the mustard was particularly surprising, because several existing laboratory studies have shown that salt treatments significantly elevate sodium concentration in Brassica leaf tissue (from 1.3 to 20 times) (Table S4). It is possible that the lack of an effect is specific to the mustard species that we measured, which tends to have lower sodium levels than other mustards (Table S4) and is not a preferred host of Pieris butterflies (46, 47). The variation that we observed between plant species in sodium bioaccumulation could stem from phylogenetic variation in sodium physiology (48, 49). Alternatively, because we did not wash collected leaves (as we were interested in what a caterpillar would consume), it is also possible that differences stem from variation in leaf stickiness. For instance, common milkweed has pubescent leaves covered with many soft short hairs (50), which could potentially trap salt precipitate. Regardless, these results suggest that salt application will affect the nutrition of at least some roadside herbivores, but more research is needed to determine exactly how changing sodium translates into nutritional differences for specific herbivores.
Changes in Sodium Affect Butterfly Muscle and Neural Development.
Sodium was the most limited micronutrient in the diet of butterflies relative to its concentration in butterfly tissue (Table 2 and Tables S1–S3). Increases in sodium availability from road salt runoff affected the development of neural and muscle tissue in butterflies. Monarchs reared on roadside-collected milkweed, which had 16 times more sodium than control milkweed, developed greater flight muscle in males and greater relative eye size in females (Fig. 1 and Table 3). We were interested in whether trait differences were being driven specifically by variation in sodium. We used an artificial diet manipulation with cabbage white butterflies to specifically measure the effects of sodium, contrasting two diets where sodium was limited (with respect to butterfly tissue) but fell within the range of variation in host plant sodium values (Tables 1 and 2 and Table S4) (51). Results of this artificial diet experiment paralleled the results from the plant-rearing experiment: males in the enriched sodium treatment invested significantly more in thoracic protein, and females invested significantly more in neural tissue (Fig. 2 and Table 4). These data are consistent with observations from both vertebrates and invertebrates that sodium is a key micronutrient for brain (14) and muscular development (38). Although our manipulations were unable to eliminate the importance of chloride, other diet manipulations have determined the importance of sodium over chloride in driving insect foraging behavior (18, 52). In addition, as a cation, sodium in road salt runoff is more likely than chloride to be retained in negatively charged soils (33).
Somewhat unexpectedly, female butterflies reared on relatively higher-sodium diets invested less in thoracic protein than those reared on lower-sodium diets. Although this difference was significant only for cabbage whites reared on artificial diet, the same pattern was seen in monarchs reared on field-collected plants (Figs. 1 and 2 and Tables 3 and 4). It is possible that this sex-specific effect reflects differences between males and females in what levels of sodium are stressful (see below). Alternatively, it is possible that females are plastically altering dispersal strategies depending on the quality of their larval diet, which has been seen in other species of butterflies (53, 54). If females reared on higher-sodium diets perceive these resources as higher quality, it may pay to invest less in flight and dispersal and instead, invest more in searching locally (55, 56). However, experiments are needed to determine whether the pattern in females is adaptive or simply a stress response.
Although increasing sodium availability can assuage the need for a limited micronutrient, it is also clear that increasing nutrients beyond some level can be stressful. Rearing cabbage whites on a high-sodium diet (6,000 ppm) resulted in significantly lower survival than the medium- and low-sodium levels. Monarch survival was also lower on roadside-collected milkweed, but it is unclear whether that was because of differences in sodium or some other factor (e.g., car exhaust contamination). Thus, it is possible that more extreme road salt runoff (e.g., along interstates) could result in more negative impacts on herbivores feeding alongside the road. Although caterpillars may sometimes be able to move away from host plants with toxic sodium levels and choose more nutritious host plants (57), their ability to disperse and make adaptive diet choices is likely quite limited (44, 45, 58, 59). Studies on the movement and food preferences of caterpillars will be necessary to determine the extent to which corridors of roadside plants with very high sodium levels may negatively affect caterpillar survival.
Implications: Changes in Limited Nutrients and Selection on Foraging Behavior.
Taken together, these data suggest that anthropogenic changes in nutrient availability may affect trait development in some species. Our data suggest that increases in sodium from road salt runoff have the potential to affect diverse species (e.g., both monarchs and cabbage whites), but the effects will depend on whether salt runoff affects the diet of a given species. Even within plant families, we see variation in the effects of salt on plant sodium levels (Table 1 vs. Table S4).
Our results leave open the extent to which road salt runoff affects the net fitness of roadside-feeding herbivores. Monarchs reared on roadside milkweed had significantly more sodium in their abdomen than those reared on control milkweed. Given the importance of sodium in Lepidopteran mating (41–43) and egg production (60), it is possible that the increased abdominal sodium may directly translate into differences in fitness, such as spermatophore size or egg number (61). Although the levels of sodium that we measured in butterfly tissue were similar to those reported in other studies (60), the results of these studies emphasize that sodium concentrations vary significantly in butterfly tissue, and we do not fully understand the fitness consequences of such variation (if any). Future experiments will have to determine the net effects of sodium on fitness given that we also observed a decline in survival with high dietary sodium.
If increased sodium does, indeed, impact fitness, it is possible that anthropogenic changes in nutrient availability will change selection on foraging behavior in animals. We already see evidence of altered foraging behavior in some species—ants that live closer to roads forage less actively for sodium than those farther from the road (36), and moose also show some preference for roadside ponds because of salt runoff (35). Given that butterflies and moths can detect sodium concentration through contact chemoreceptors (62, 63), it is possible that they are also showing changes in foraging behavior. This observation raises the question of whether a thirst for a micronutrient, such as sodium, could lead to increased preference for roadside habitats. This preference might come with other changes, such as increased mortality because of car collisions [as seen in moose (64)] and subsequent selection on flight patterns to avoid cars [as seen in birds (65)]. Animals, including insects and humans, can choose to feed on sodium to the point of toxicity (66, 67), suggesting that roadside sodium runoff may function as an evolutionary trap (68) or select for resistance to high-sodium levels (69, 70). However, consistent preferences for roadside plants with higher sodium levels could potentially lead to decreases in adult puddling behavior or even evolutionary changes in brain size (71).
Overall, this work adds to a growing body of research suggesting that humans are changing the nutritional ecology of species, which results in changes in competition and community ecology, life history traits and population cycles, and selection on foraging behavior. This work highlights that changes in micronutrients may be just as important as changes in macronutrients, such as carbon, nitrogen, and phosphorus, where the majority of the research has been focused. Humans are changing the availability of other micronutrients other than sodium, and it is likely that similar effects hold true for those micronutrients. For instance, given that calcium affects the abundance and development of birds, snails, and worms (72–74), anthropogenic changes in calcium (75, 76) may be particularly relevant for these taxa. Our results highlight the need for more comprehensive descriptions of how nutrition is changing in the face of humans and the diverse responses across traits and species to such changes.
Materials and Methods
Sodium in Roadside Plants.
To determine how road salt runoff affects sodium availability in plant tissue, we collected plant samples from Cedar Creek Ecosystem Science Reserve, a 2,200-ha field site just north of Minneapolis and St. Paul, Minnesota. We focused on four common roadside species that represent four common host plant families of butterflies: a perennial grass (Poaceae: Panicum oligosanthes), a mustard (Brassicaceae: Berteroa incana), an oak (Fagaceae: Quercus ellipsoidalis), and a milkweed (Apocynaceae: Asclepias syriaca). At least three samples of each species were taken from roadside ditches (within 5 m of a county road), and at least three samples were taken from open field sites (at least 100 m from the paved road). Plant material was harvested on the same day about 6 wk into the growing season of these plants (June 14, 2013). Leaf material was dried in a drying oven (at 60 °C), and at least 1 g was submitted for analysis to the University of Minnesota Research Analytical Laboratory. Inductively coupled plasma atomic emission spectrometry (ICP-AES), dry ash method, was used to determine sodium concentration in leaves (77). Leaves were not washed before drying, because we were interested in the nutrients that a caterpillar would consume. Given that sodium can result in plant stress and lower nitrogen concentrations (78), a key macronutrient for herbivores (79, 80), we also assayed total nitrogen in these samples using the Dumas method (81).
Butterfly Rearing.
Experiment 1: Rearing on roadside-collected plants.
We compared monarchs (Nymphalidae: Danaus plexippus) reared on roadside- and prairie-collected host plants [in this case, common milkweed (Apocynaceae: A. syriaca)]. Monarchs were chosen, because milkweed is a common roadside plant, and investment in sodium-rich muscle should be important for a migratory species like monarchs. In 2011, milkweed was collected from the same locations as the plant measurements (see above). ICP-AES analysis of these leaves found that the roadside-collected milkweed was 16 times richer in sodium than prairie-collected milkweed (764 relative to 47.5 ppm) but that nitrogen levels were comparable (3.24% relative to 3.13%). Monarch eggs were obtained from mating cages of wild-derived monarchs that had been reared in the laboratory for one generation. Monarchs were reared in the laboratory (one or two per 15-oz cup) on a 14-h photoperiod at 24 °C. Field-collected milkweed was refreshed every 1–2 d. Adults were frozen at emergence and stored in sealed containers at −20 °C for additional analysis.
Experiment 2: Rearing on artificial diets.
We used an artificial diet to vary the concentration of dietary sodium while holding other nutrients constant. We focused on cabbage white butterflies (Pieridae: Pieris rapae), because this species is easy to rear in large numbers on an artificial diet (82, 83). We used an existing diet recipe but varied the concentration of sodium chloride. We used ICP-AES to test the concentration of sodium in a set of pilot diet manipulations. Based on these values, we constructed NaCl diets that were ∼400, 3,000, and 6,000 ppm sodium. These levels were chosen based on values from field and laboratory studies of sodium in Brassicaceae leaves, which show concentrations ranging from 69 to 14,900 ppm (median = 2,800 ppm) (Table S4). To make the artificial diet, we first constructed a base Wesson salt mix without NaCl. We based our salt mix on established mixes but used less calcium, because this mix has more calcium than is needed by insects (84, 85). The base salt mix (wt/wt) included 43% potassium phosphate monobasic, 17% potassium chloride, 14% calcium carbonate, 11% tricalcium phosphate, 13% magnesium sulfate, 1.5% ferric phosphate, and trace amounts (<0.05%) of copper sulfate, manganese sulfate, potassium aluminum sulfate, potassium iodide, and sodium fluoride. Per 800 mL water, the artificial diet was made with 60 g wheat germ, 15 g cabbage flour, 27 g casein, 24 g sucrose, 6.44 g base salt mix, 12 g torula yeast, 3.6 g cholesterol, 10.5 g vitamin mix, 0.75 g methyl paraben, 1.5 g sorbic acid, 3 g ascorbic acid, and 0.2 g streptomycin mixed with 15 g agar and 6 mL linseed oil. The low-, medium-, and high-sodium diets were constructing by adding 0.42, 6.1, and 12.65 g NaCl, respectively, to the base diet ingredients.
Gravid female P. rapae were obtained from wild populations in Minnesota and Virginia. Eggs were collected from females on cabbage plants in greenhouse cages. Within 7–10 d of eggs being laid, larvae were transferred from cabbage to one of three artificial diets (randomly assigned). Larvae were reared in groups of three per 4-oz cup in climate chambers at 23 °C with a 14-h photoperiod. On emergence, the head of each individual was fixed for brain histology (see below). The rest of the butterfly was stored in sealed containers at −20 °C until additional analysis. No significant differences were seen between populations, and therefore, they were pooled for analyses. An additional set of P. rapae from Minnesota populations was reared (three to six per rearing cup) in the laboratory on a host plant (bok choy; changed daily) to test levels of sodium in butterfly tissue reared on plants. Adult butterfly tissue was analyzed using ICP-AES (three to four individuals per sample to increase measurement accuracy).
Trait Measurements.
Development time, survival, and body size.
Development time was measured as the number of days between the date that an egg was laid and the date that the adult butterfly emerged. Survival was measured across all individuals transferred from the original egg-laying substrate to their respective diet manipulation. Body size was measured as forewing length (from removed wings) from the articulation of the wing with the thorax to the wing apex.
Muscle tissue.
A Bradford assay was used to determine the amount of protein in the thorax, which is primarily composed of flight muscle. Thoraxes of individual butterflies were separated and dried in a drying oven (at 60 °C) for at least 24 h. Individual thoraxes were weighed to the nearest 0.1 mg and then processed using established methods (86). Briefly, 2% sodium sulfate was added to the sample (800 μL for monarchs and 400 μL for Pieris), which was then finely ground before a 2- or 1-μL aliquot was taken from each Pieris and monarch sample, respectively. Samples (two technical replicates each) were compared against eight BSA standards (average R2 of standard curve = 0.974). We controlled for variation across batches and measurement error by taking the least square mean for each individual in a model across all replicate measurements that included batch as a fixed effect. An individual’s thoracic protein was quantified as the total protein from the Bradford assay divided by the dry mass of their thorax.
Neural investment—eye size and brain size.
We used two methods to measure neural investment. First, we used standard histological techniques to measure the brain size of P. rapae reared on artificial diets (87) (n = 8 females and n = 7 males). Briefly, heads were fixed in formalin, stained with 1% osmium tetroxide, embedded in plastic, and sectioned using a carbide tungsten knife at 15-μm thickness. Brains were imaged on a Leica DM 2500 microscope using differential interference contrast and measured in ImageJ (National Institutes of Health). Total brain volume was measured as neuropil from the appearance of the antennal lobes (anterior) to the end of the medulla (posterior). Second, given that brain processing is labor-intensive (5 h/individual) with high probability of sample loss because of damage, we used eye size as a measure of neural investment for monarchs. About 75% of the butterfly brain is dedicated to visual processing (88, 89), and relative eye size is correlated with visual processing areas and brain size in vertebrates and invertebrates (90–92). To measure eye size, we took frontal images of each individual’s head (93) after orienting heads (with labial palps removed) in a Styrofoam holder. Any individuals with eye damage were eliminated. Eye width was measured for each eye. Measurement error (the absolute value of the difference in width between each eye) was included in each model to correct for alignment errors.
Sodium in butterfly tissue.
We used ICP-AES to measure sodium in monarch abdomens. We focused on monarch abdomens, because they were large enough to accurately measure sodium in individual samples and given that they contain reproductive tissues, are presumably relevant for fitness.
Analyses.
We used JMP 9.0 (SAS Institute, Cary, NC) for all statistical analyses. ANOVA and t tests were used to test for effects of diet on focal traits. For traits that were correlated with body size (e.g., eye size), we included body size (wing length) in the model. Thoracic protein, leaf sodium, and eye alignment measures were log-transformed for normality.
Supplementary Material
Acknowledgments
We thank Susan Kenzie, Aaron Dahl, Nate Fremling, Rob Kulhanek, Isaac Bolduc, and Naomi Wick, who helped with aspects of rearing and measurements of specimens. Eli Swanson and Sarah Jaumann helped get brain sectioning working in the laboratory; Sofia Casasa helped get protein extractions working in the laboratory. Thanks to Meredith Steck and Alex Eilts, who helped with plant collection and identification. We also thank the laboratory of Karen Oberhauser for giving us male and female laboratory-reared wild lines of monarchs for use in experiment 1. We thank two anonymous reviewers for comments and insights. This research was funded by a Grant-in-Aid-of-Research from the Office of the Vice President for Research at the University of Minnesota.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the DRYAD database, http://datadryad.org (doi:10.5061/dryad.v2t58).
See Commentary on page 10033.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323607111/-/DCSupplemental.
References
- 1.Lee KP, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105(7):2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler D. The role of nourishment in oogenesis. Annu Rev Entomol. 1996;41:407–431. doi: 10.1146/annurev.en.41.010196.002203. [DOI] [PubMed] [Google Scholar]
- 3.Hill GE. Proximate basis of variation in carotenoid pigmentation in male house finches. Auk. 1992;109(1):1–12. [Google Scholar]
- 4.Morales J, Velando A, Torres R. Fecundity compromises attractiveness when pigments are scarce. Behav Ecol. 2009;20(1):117–123. [Google Scholar]
- 5.Galloway JN, et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science. 2008;320(5878):889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 6.Vitousek PM, et al. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol Appl. 1997;7(3):737–750. [Google Scholar]
- 7.Chen YZ, Lin L, Wang CW, Yeh CC, Hwang SY. Response of two Pieris (Lepidoptera: Pieridae) species to fertilization of a host plant. Zoolog Stud. 2004;43(4):778–786. [Google Scholar]
- 8.Hwang SY, Liu CH, Shen TC. Effects of plant nutrient availability and host plant species on the performance of two Pieris butterflies (Lepidoptera: Pieridae) Biochem Syst Ecol. 2008;36(7):505–513. [Google Scholar]
- 9.Prudic KL, Oliver JC, Bowers MD. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia. 2005;143(4):578–587. doi: 10.1007/s00442-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 10.Hodgkin A. The ionic basis of electrical activity in nerve and muscle. Biol Rev Camb Philos Soc. 1951;26(4):339–409. [Google Scholar]
- 11.Katz B. The transmission of impulses from nerves to muscle, and the subcellular unit of synaptic action. Proc R Soc Lond B Biol Sci. 1962;155(961):455–477. [Google Scholar]
- 12.Chapman R. The Insects: Structure and Function. Cambridge, United Kingdom: Cambridge Univ Press; 1998. [Google Scholar]
- 13.Al-Dahhan J, Jannoun L, Haycock GB. Effect of salt supplementation of newborn premature infants on neurodevelopmental outcome at 10-13 years of age. Arch Dis Child Fetal Neonatal Ed. 2002;86(2):F120–F123. doi: 10.1136/fn.86.2.F120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bursey RG, Watson ML. The effect of sodium restriction during gestation of offspring brain development in rats. Am J Clin Nutr. 1983;37(1):43–51. doi: 10.1093/ajcn/37.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier RL. The moth and the aspen tree: Sodium in early postnatal development. Kidney Int. 2001;59(5):1617–1625. doi: 10.1046/j.1523-1755.2001.0590051617.x. [DOI] [PubMed] [Google Scholar]
- 16.Haycock GB. The influence of sodium on growth in infancy. Pediatr Nephrol. 1993;7(6):871–875. doi: 10.1007/BF01213376. [DOI] [PubMed] [Google Scholar]
- 17.Kaspari M, Yanoviak SP, Dudley R. On the biogeography of salt limitation: A study of ant communities. Proc Natl Acad Sci USA. 2008;105(46):17848–17851. doi: 10.1073/pnas.0804528105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaspari M, Yanoviak SP, Dudley R, Yuan M, Clay NA. Sodium shortage as a constraint on the carbon cycle in an inland tropical rainforest. Proc Natl Acad Sci USA. 2009;106(46):19405–19409. doi: 10.1073/pnas.0906448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seastedt TR, Crossley DA. Sodium dynamics in forest ecosystems and the animal starvation hypothesis. Am Nat. 1981;117(6):1029–1034. [Google Scholar]
- 20.Dudley R, Kaspari M, Yanoviak SP. Lust for salt in the Western Amazon. Biotropica. 2012;44(1):6–9. [Google Scholar]
- 21.Leshem M. Biobehavior of the human love of salt. Neurosci Biobehav Rev. 2009;33(1):1–17. doi: 10.1016/j.neubiorev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Belovsky GE, Jordan PA. Sodium dynamics and adaptations of a moose population. J Mammal. 1981;62(3):613–621. [Google Scholar]
- 23.Smedley SR, Eisner T. Sodium uptake by puddling in a moth. Science. 1995;270(5243):1816–1818. doi: 10.1126/science.270.5243.1816. [DOI] [PubMed] [Google Scholar]
- 24.Rothman JM, Van Soest PJ, Pell AN. Decaying wood is a sodium source for mountain gorillas. Biol Lett. 2006;2(3):321–324. doi: 10.1098/rsbl.2006.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brightsmith DJ, Munoz-Najar RA. Avian geophagy and soil characteristics in southeastern Peru. Biotropica. 2004;36(4):534–543. [Google Scholar]
- 26.Findlay SEG, Kelly VR. Emerging indirect and long-term road salt effects on ecosystems. Ann N Y Acad Sci. 2011;1223:58–68. doi: 10.1111/j.1749-6632.2010.05942.x. [DOI] [PubMed] [Google Scholar]
- 27.Jackson RB, Jobbágy EG. From icy roads to salty streams. Proc Natl Acad Sci USA. 2005;102(41):14487–14488. doi: 10.1073/pnas.0507389102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaushal SS, et al. Increased salinization of fresh water in the northeastern United States. Proc Natl Acad Sci USA. 2005;102(38):13517–13520. doi: 10.1073/pnas.0506414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelting DL, Laxson CL, Yerger EC. Regional analysis of the effect of paved roads on sodium and chloride in lakes. Water Res. 2012;46(8):2749–2758. doi: 10.1016/j.watres.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 30.Rengasamy P. World salinization with emphasis on Australia. J Exp Bot. 2006;57(5):1017–1023. doi: 10.1093/jxb/erj108. [DOI] [PubMed] [Google Scholar]
- 31.Sander A, Novotny E, Mohseni O, Stefan H. Inventory of Road Salt Use in the Minneapolis/St. Paul Metropolitan Area Report. Minneapolis: St. Anthony Falls Laboratory, University of Minnesota; 2007. [Google Scholar]
- 32.Forman RTT, Alexander LE. Roads and their major ecological effects. Annu Rev Ecol Syst. 1998;29:207. [Google Scholar]
- 33.Ramakrishna DM, Viraraghavan T. Environmental impact of chemical deicers - A review. Water Air Soil Pollut. 2005;166(1-4):49–63. [Google Scholar]
- 34.Sanzo D, Hecnar SJ. Effects of road de-icing salt (NaCl) on larval wood frogs (Rana sylvatica) Environ Pollut. 2006;140(2):247–256. doi: 10.1016/j.envpol.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Laurian C, et al. Behavioral adaptations of moose to roadside salt pools. J Wildl Manage. 2008;72(5):1094–1100. [Google Scholar]
- 36.Kaspari M, Chang C, Weaver J. Salted roads and sodium limitation in a northern forest ant community. Ecol Entomol. 2010;35(5):543–548. [Google Scholar]
- 37.Molleman F, Grunsven RHA, Liefting M, Zwaan BJ, Brakefield PM. Is male puddling behaviour of tropical butterflies targeted at sodium for nuptial gifts or activity? Biol J Linn Soc Lond. 2005;86(3):345–361. [Google Scholar]
- 38.Xiao K, Shen K, Zhong JF, Li GQ. Effects of dietary sodium on performance, flight and compensation strategies in the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) Front Zool. 2010;7:11. doi: 10.1186/1742-9994-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall JPW, Willmott KR. Patterns of feeding behaviour in adult male riodinid butterflies and their relationship to morphology and ecology. Biol J Linn Soc Lond. 2000;69(1):1–23. [Google Scholar]
- 40.Boggs CL, Dau B. Resource specialization in puddling Lepidoptera. Environ Entomol. 2004;33(4):1020–1024. [Google Scholar]
- 41.Molleman F. Puddling: From natural history to understanding how it affects fitness. Entomol Exp Appl. 2010;134(2):107–113. [Google Scholar]
- 42.Smedley SR, Eisner T. Sodium: A male moth’s gift to its offspring. Proc Natl Acad Sci USA. 1996;93(2):809–813. doi: 10.1073/pnas.93.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pivnick KA, McNeil JN. Puddling in butterflies – sodium affects reproductive success in Thymelicus lineola. Physiol Entomol. 1987;12(4):461–472. [Google Scholar]
- 44.Jones RE. Search behavior – study of 3 caterpillar species. Behaviour. 1977;60(3-4):236–259. [Google Scholar]
- 45.Mauricio R, Bowers MD. Do caterpillars disperse their damage – larval foraging behavior of 2 specialist herbivores, Euphydryas phaeton (Nymphalidae) and Pieris rapae (Pieridae) Ecol Entomol. 1990;15(2):153–161. [Google Scholar]
- 46.Forsberg J. Size discrimination among conspecific hostplants in 2 pierid butterflies – Pieris napi and Pontia daplidice L. Oecologia. 1987;72(1):52–57. doi: 10.1007/BF00385044. [DOI] [PubMed] [Google Scholar]
- 47.Leimar O, Karlsson B, Wiklund C. Unpredictable food and sexual size dimorphism in insects. Proc Biol Sci. 1994;258(1352):121–125. doi: 10.1098/rspb.1994.0151. [DOI] [PubMed] [Google Scholar]
- 48.Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot (Lond) 2003;91(5):503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horie T, Schroeder JI. Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiol. 2004;136(1):2457–2462. doi: 10.1104/pp.104.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agrawal AA, et al. Phylogenetic ecology of leaf surface traits in the milkweeds (Asclepias spp.): Chemistry, ecophysiology, and insect behavior. New Phytol. 2009;183(3):848–867. doi: 10.1111/j.1469-8137.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe T, et al. Evolutionary control of leaf element composition in plants. New Phytol. 2007;174(3):516–523. doi: 10.1111/j.1469-8137.2007.02078.x. [DOI] [PubMed] [Google Scholar]
- 52.Pizarro LC, McCreery HF, Lawson SP, Winston ME, O'Donnell S. Sodium-specific foraging by leafcutter ant workers (Atta cephalotes, Hymenoptera: Formicidae) Ecol Entomol. 2012;37(5):435–438. [Google Scholar]
- 53.Merckx T, Van Dyck H. Landscape structure and phenotypic plasticity in flight morphology in the butterfly Pararge aegeria. Oikos. 2006;113(2):226–232. [Google Scholar]
- 54.Pellegroms B, Van Dongen S, Van Dyck H, Lens L. Larval food stress differentially affects flight morphology in male and female speckled woods (Pararge aegeria) Ecol Entomol. 2009;34(3):387–393. [Google Scholar]
- 55.Hern A, Edwards-Jones G, McKinlay RG. A review of the pre-oviposition behaviour of the small cabbage white butterfly, Pieris rapae (Lepidoptera: Pieridae) Ann Appl Biol. 1996;128(2):349–371. [Google Scholar]
- 56.Snell-Rood EC, Papaj DR. Patterns of phenotypic plasticity in common and rare environments: A study of host use and color learning in the cabbage white butterfly Pieris rapae. Am Nat. 2009;173(5):615–631. doi: 10.1086/597609. [DOI] [PubMed] [Google Scholar]
- 57.Schultz J. Habitat selection and foraging tactics of caterpillars in heterogeneous trees. In: Denno R, McClure M, editors. Variable Plants qnd Herbivores in Natural and Managed Systems. New York: Academic; 1983. pp. 61–90. [Google Scholar]
- 58.Bierzychudek P, Warner KA, McHugh A, Thomas L. Testing the host-finding ability of a monophagous caterpillar in the field. Ecol Entomol. 2009;34(5):632–637. [Google Scholar]
- 59.Despland E, Noseworthy M. How well do specialist feeders regulate nutrient intake? Evidence from a gregarious tree-feeding caterpillar. J Exp Biol. 2006;209(Pt 7):1301–1309. doi: 10.1242/jeb.02130. [DOI] [PubMed] [Google Scholar]
- 60.Adler PH, Pearson DL. Why do male butterflies visit mud puddles. Can J Zool. 1982;60(3):322–325. [Google Scholar]
- 61.Molleman F, Zwaan BJ, Brakefield PM. The effect of male sodium diet and mating history on female reproduction in the puddling squinting bush brown Bicyclus anynana (Lepidoptera) Behav Ecol Sociobiol. 2004;56(4):404–411. [Google Scholar]
- 62.Mitchell BK, Seabrook WD. Electrophysiological investigations on tarsal chemoreceptors of the spruce budworm, Choristoneura fumiferana (Lepidoptera) J Insect Physiol. 1974;20(7):1209–1218. doi: 10.1016/0022-1910(74)90227-3. [DOI] [PubMed] [Google Scholar]
- 63.Inoue TA, et al. Japanese Papilio butterflies puddle using Na+ detected by contact chemosensilla in the proboscis. Naturwissenschaften. 2012;99(12):985–998. doi: 10.1007/s00114-012-0976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laurian C, et al. Behavior of moose relative to a road network. J Wildl Manage. 2008;72(7):1550–1557. [Google Scholar]
- 65.Brown CR, Bomberger Brown M. Where has all the road kill gone? Curr Biol. 2013;23(6):R233–R234. doi: 10.1016/j.cub.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 66.Hernandez LMA, Todd EV, Miller GA, Frederickson ME. Salt intake in Amazonian ants: Too much of a good thing? Insectes Soc. 2012;59(3):425–432. [Google Scholar]
- 67.Battarbee HD, Meneely GR. The toxicity of salt. CRC Crit Rev Toxicol. 1978;5(4):355–376. doi: 10.3109/10408447809081011. [DOI] [PubMed] [Google Scholar]
- 68.Robertson BA, Rehage JS, Sih A. Ecological novelty and the emergence of evolutionary traps. Trends Ecol Evol. 2013;28(9):552–560. doi: 10.1016/j.tree.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Brady SP. Road to evolution? Local adaptation to road adjacency in an amphibian (Ambystoma maculatum) Sci Rep. 2012;2(2012):235. doi: 10.1038/srep00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hopkins GR, French SS, Brodie ED. Potential for local adaptation in response to an anthropogenic agent of selection: Effects of road deicing salts on amphibian embryonic survival and development. Evol Appl. 2013;6(2):384–392. doi: 10.1111/eva.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leonard WR, Robertson ML. Evolutionary perspectives on human nutrition: The influence of brain and body size on diet and metabolism. Am J Hum Biol. 1994;6(1):77–88. doi: 10.1002/ajhb.1310060111. [DOI] [PubMed] [Google Scholar]
- 72.Reich PB, et al. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol Lett. 2005;8(8):811–818. [Google Scholar]
- 73.Skeldon MA, Vadeboncoeur MA, Hamburg SP, Slum JD. Terrestrial gastropod responses to ecosystem-level calcium manipulation a northern hardwood forest. Can J Zool. 2007;85(9):994–1007. [Google Scholar]
- 74.Wilkin TA, Gosler AG, Garant D, Reynolds SJ, Sheldon BC. Calcium effects on life-history traits in a wild population of the great tit (Parus major): Analysis of long-term data at several spatial scales. Oecologia. 2009;159(2):463–472. doi: 10.1007/s00442-008-1222-8. [DOI] [PubMed] [Google Scholar]
- 75.Wright IA, Davies PJ, Findlay SJ, Jonasson OJ. A new type of water pollution: Concrete drainage infrastructure and geochemical contamination of urban waters. Mar Freshw Res. 2011;62(12):1355–1361. [Google Scholar]
- 76.Branquinho C, et al. Biomonitoring spatial and temporal impact of atmospheric dust from a cement industry. Environ Pollut. 2008;151(2):292–299. doi: 10.1016/j.envpol.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Munter R, Halverson T, Anderson R. Quality assurance for plant tissue analysis by ICP-AES. Commun Soil Sci Plant Anal. 1984;15(11):1285–1322. [Google Scholar]
- 78.Debouba M, Gouia H, Suzuki A, Ghorbel MH. NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato “Lycopersicon esculentum” seedlings. J Plant Physiol. 2006;163(12):1247–1258. doi: 10.1016/j.jplph.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 79.Mattson WJ. Herbivory in relation to plant nutrogen content. Annu Rev Ecol Syst. 1980;11:119–161. [Google Scholar]
- 80.Scriber JM, Slansky F. The nutritional ecology of immature insects. Annu Rev Entomol. 1981;26:183–211. [Google Scholar]
- 81.Matejovic I. Total nitrogen in plant material determined by means of dry combustion: A possible alterntive to determination by Kjedahl digestion. Commun Soil Sci Plant Anal. 1995;26(13-14):2217–2229. [Google Scholar]
- 82.Troetschler RG, Malone CM, Bucago ER, Johnston MR. System for rearing Pieris rapae (Lepidoptera: Pieridae) on a noncruciferous artificial diet developed for Manduca sexta (Lepidoptera: Sphingidae) J Econ Entomol. 1985;78(6):1521–1523. [Google Scholar]
- 83.Webb S, Shelton A. Laboratory rearing of the imported cabbageworm. N Y Food Life Sci Bull. 1988;122:1–6. [Google Scholar]
- 84.Beck S, Chippendale G, Swinton D. Nutrition of the European corn borer, Ostrinia nubilalis. VI. A larval rearing medium without crude plant fractions. Ann Entomol Soc Am. 1968;61(2):459–462. [Google Scholar]
- 85.Medici JC, Taylor MW. Mineral requirements of the confused flour beetle, Tribolium confusum (Duval) J Nutr. 1966;88(2):181–186. doi: 10.1093/jn/88.2.181. [DOI] [PubMed] [Google Scholar]
- 86.Snell-Rood EC, Davidowitz G, Papaj DR. Plasticity in learning causes immediate and trans-generational changes in allocation of resources. Integr Comp Biol. 2013;53(2):329–339. doi: 10.1093/icb/ict030. [DOI] [PubMed] [Google Scholar]
- 87.Snell-Rood EC, Papaj DR, Gronenberg W. Brain size: A global or induced cost of learning? Brain Behav Evol. 2009;73(2):111–128. doi: 10.1159/000213647. [DOI] [PubMed] [Google Scholar]
- 88.Ali F. Structure and metamorphosis of the brain and suboesophageal ganglion of Pieris brassicae (L.) (Lepidoptera: Pieridae) Trans R Entomol Soc Lond. 1974;125(4):363–412. [Google Scholar]
- 89.Heinze S, Reppert SM. Anatomical basis of sun compass navigation I: The general layout of the monarch butterfly brain. J Comp Neurol. 2012;520(8):1599–1628. doi: 10.1002/cne.23054. [DOI] [PubMed] [Google Scholar]
- 90.Garamszegi LZ, Møller AP, Erritzøe J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc Biol Sci. 2002;269(1494):961–967. doi: 10.1098/rspb.2002.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gronenberg W, Hölldobler B. Morphologic representation of visual and antennal information in the ant brain. J Comp Neurol. 1999;412(2):229–240. doi: 10.1002/(sici)1096-9861(19990920)412:2<229::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 92.Pearce E, Dunbar R. Latitudinal variation in light levels drives human visual system size. Biol Lett. 2012;8(1):90–93. doi: 10.1098/rsbl.2011.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rutowski RL. Variation of eye size in butterflies: Inter- and intraspecific patterns. J Zool. 2000;252(2):187–195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.