Abstract
Objective
The goal of this study was to determine the impact of the nuclear receptor constitutive androstane receptor (CAR) on lipoprotein metabolism and atherosclerosis in hyperlipidemic mice.
Methods and Results
Low-density lipoprotein receptor–deficient (Ldlr−/−) and apolipoprotein E–deficient (ApoE−/−) mice fed a Western-type diet were treated weekly with the Car agonist 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) or the vehicle only for 8 weeks. In Ldlr−/− mice, treatment with TCPOBOP induced a decrease in plasma triglyceride and intermediate-density lipoprotein/low-density lipoprotein cholesterol levels (≈30% decrease in both cases after 2 months, P<0.01). These mice also showed a significant reduction in the production of very-low-density lipoproteins associated with a decrease in hepatic triglyceride content and the repression of several genes involved in lipogenesis. TCPOBOP treatment also induced a marked increase in the very-low-density lipoprotein receptor in the liver, which probably contributed to the decrease in intermediate-density lipoprotein/low-density lipoprotein levels. Atherosclerotic lesions in the aortic valves of TCPOBOP-treated Ldlr−/− mice were also reduced (−60%, P<0.001). In ApoE−/− mice, which lack the physiological apoE ligand for the very-low-density lipoprotein receptor, the effect of TCPOBOP on plasma cholesterol levels and the development of atherosclerotic lesions was markedly attenuated.
Conclusion
CAR is a potential target in the prevention and treatment of hypercholesterolemia and atherosclerosis.
Keywords: atherosclerosis, lipoproteins, constitutive androstane receptor
The constitutive androstane receptor (CAR) is a nuclear receptor that is primarily considered a xenobiotic sensor,1,2 and many of its target genes are involved in the hydroxylation, conjugation, and excretion of potentially harmful exogenous molecules. In addition to these well-recognized functions, CAR plays a role in the metabolism of endogenous compounds, such as bile acids. Indeed, under cholestatic conditions, CAR promotes the detoxification of bile acids through alternative pathways, distinct from those regulated by the classical bile acid receptor farnesoid X receptor.3–5 Very recently, we observed that Car activation is able to stimulate the excretion of cholesterol in the feces through its conversion into bile acids, with positive consequences on reverse cholesterol transport and whole-body cholesterol homeostasis.6 In addition, the first characterizations of atherosclerotic lesions in the aortic arch and descending aorta by en face analysis indicated that the Car agonist 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) has an atheroprotective effect in apolipoprotein E–deficient (ApoE−/−) mice.6 As well as being involved in bile acid metabolism, CAR has also been shown to play a role in other lipid-related metabolic pathways, but with somewhat contradictory reports regarding its implication in cholesterol and triglyceride (TG) metabolism. On the one hand, several studies have demonstrated that Car activation reduces plasma TG levels and liver steatosis in mice through the inhibition of hepatic lipogenesis.7–10 On the other hand, it has been reported that the rise in plasma TG levels observed in ob/ob mice fed a high-fat diet is abolished in Car-deficient animals.11 TCPOBOP, a specific Car activator, was found to increase plasma TG in the same study.11 We have recently shown that 5 days of treatment with TCPOBOP is able to markedly reduce high-density lipoprotein (HDL) cholesterol levels in WT and human apoA-I transgenic mice.12 Interestingly, and in addition to its impact on HDL, TCPOBOP was also found to induce a significant decrease in plasma apoB-containing lipoproteins, with significant reductions in plasma apoB-100 and low-density lipoprotein (LDL) cholesterol levels.12 Unlike in humans, HDL predominates in the plasma of both WT and human apoA-I transgenic mice. Because of this, the observed decreases in LDL levels did not contribute to a large extent to the TCPOBOP-mediated decrease in total plasma cholesterol levels in these mice, and the impact on apoB-containing lipoproteins might well have been underestimated. In all cases, the observed changes in lipoproteins were found to be Car specific, with no changes in either HDL or apoB-containing lipoproteins in Car-deficient mice treated with TCPOBOP.12
The aim of the present study was to explore the effect of Car activation on lipoprotein metabolism and atherogenesis in the long term, with specific emphasis on apoB-containing lipoproteins in hyperlipidemic mouse models. To this end, low-density lipoprotein receptor–deficient (Ldlr−/−) and ApoE−/− mouse homozygotes were fed a Western-type diet, and they were treated or not with a weekly injection of the Car agonist TCPOBOP. Plasma lipoprotein profiles were determined at 5 days, 4 weeks, and 8 weeks, and the development of atherosclerosis lesions was assessed after 8 weeks. It is shown here that the Car agonist TCPOBOP decreases plasma TG-rich lipoprotein and intermediate-density lipoprotein (IDL)/LDL levels, resulting in a significant reduction in the size of atherosclerotic lesions in the aortic valves of TCPOBOP-treated Ldlr−/− mice. In contrast, TCPOBOP had a reduced impact on plasma cholesterol levels in ApoE−/− mice and did not reduce the development of atherosclerotic lesions in aortic valves to a statistically significant extent in ApoE−/− mice.
Materials and Methods
A detailed Materials and Methods section is given in the supplemental material, available online at http://atvb.ahajournals.org.
Animals
Ldlr−/− and ApoE−/− mice on a homogenous C57Bl/6 background (Jackson laboratory, Bar Harbor, ME) were used in the present study. Ten-week-old female mice were used. Once a week for 2 months, 3 mg/kg of TCPOBOP (Sigma-Aldrich, St. Louis, MO) was given to the mice via an intraperitoneal injection, with corn oil as vehicle. Control animals received the vehicle only. The mice were fed a Western-type diet (21% fat and 0.2% cholesterol, Safe, Augy, France). Weight gain and food intake were monitored once a week. The mice were killed 5 days after the last injection. All of the experimental procedures were conducted in accordance with the local guidelines for animal experimentation. Protocol no. 7105 was approved by the Animal Care and Use Committee of the University of Burgundy. The plasma lipoproteins were fractionated in a Superose 6 HR 10/30 column (Amersham Pharmacia Biotech, Saclay, France) connected to a fast protein liquid chromatography system. The plasma lipid parameters were determined on a Victor2 1420 Multilabel Counter (PerkinElmer Life Science, Boston, MA). Total cholesterol and TG concentrations were measured by enzymatic methods. Relative mRNA levels were determined by real-time reverse transcription–polymerase chain reaction using a SYBR Green real-time polymerase chain reaction kit (Invitrogen, Carlsbad, CA) on a LightCycler 2.0 detection system (Roche Diagnostics, Meylan, France).
Quantification of Atherosclerosis Lesions
The hearts and proximal aortas were perfused and fixed with paraformaldehyde and excised. The tissues were serially cryosectioned (6 μm thickness) and stained with Oil Red O. The extent of Oil Red O staining was measured using color thresholding to delimit the area of staining with Photoshop software, and the surface area stained with Oil Red O was measured. The size of the aortic valve lesion of each animal was calculated as the mean lesion area of 5 sections. Two investigators, blinded to the treatment received, independently analyzed the images. For immunohistological analyses, serial sections from proximal aortas were stained with Oil Red O or Masson trichrome or immunostained with specific antibodies (anti-α-smooth muscle actin [Sigma-Aldrich] and Lamp-2 antibody [Dako] for macrophages).
Statistical Analysis
The results were expressed as mean±SD. All of the statistical data were analyzed using the Student t test except for atherosclerosis lesion areas, which were analyzed using the Mann-Whitney U test.
Results
Effect of TCPOBOP on Lipoprotein Profile in Ldlr−/− Mice
To assess the effect of Car activation on plasma lipoprotein profiles with specific emphasis on apoB-containing lipoproteins, Ldlr−/− mice were treated for up to 8 weeks with TCPOBOP. Plasma samples were collected after 5 days, 4 weeks, and 8 weeks of treatment. As previously described,9 TCPOBOP-treated mice gained slightly less weight than did vehicle-treated mice (Supplemental Figure I). As shown in Figure 1A and Supplemental Table I, Car activation induced a marked decrease in total plasma cholesterol concentration at day 5, with significant reductions in the cholesterol content of all lipoprotein subclasses, including very-low-density lipoprotein (VLDL), LDL, and HDL. The plasma TG concentration was also significantly reduced (Supplemental Table I) because of a decrease in the VLDL fraction (Figure 1B). Overall, a consistent picture was maintained throughout the 8-week study period. However, there were a few differences between the effect of TCPOBOP after 5 days and after 8 weeks of treatment. A 30% reduction in IDL/LDL (IDL+LDL fractions) cholesterol was still observed in TCPOBOP-treated mice after 8 weeks, whereas HDL and VLDL cholesterol levels no longer differed from those of untreated animals (Figure 1C and 1D). The reduction in plasma TGs was also maintained during the 8 weeks of treatment (Figure 1D). As expected, the plasma level of apolipoprotein B was significantly reduced in the TCPOBOP-treated group (Figure 1F).
Figure 1.
A to D, Plasma lipoprotein profiles of low-density lipoprotein receptor–deficient (Ldlr−/−) mice treated or not with 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP). Plasma from control and constitutive androstane receptor (Car) agonist–treated animals was fractionated by fast protein liquid chromatography. A, Cholesterol distribution after 5 days; B, triglyceride distribution after 5 days; C, cholesterol distribution after 2 months; D, triglyceride distribution after 2 months; E, cholesterol concentration in lipoprotein subclasses after 2 months (n=10 per group; *P<0.01 vs vehicle). F, Apolipoprotein B (ApoB) concentration in Ldlr−/− mice after 2 months of treatment (n=8 per group; *P<0.05). Ten-week-old female mice were used for these experiments. VLDL indicates very-low-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Effect of TCPOBOP on Hepatic Lipid Content, VLDL Production, and Lipogenic Pathways
To determine whether the alteration in VLDL production by the liver might contribute to the reduction in plasma TG concentration, the accumulation of liver-derived VLDL particles in plasma was measured in fasted Ldlr−/− mice that had been injected with poloxamer P407 (ie, a VLDL catabolism blocker).13 As shown in Figure 2A, intraperitoneal injection of poloxamer induced a time-dependent accumulation of TGs in the blood of both control and TCPOBOP-treated mice. Whereas TG secretion was substantially reduced in TCPOBOP-treated mice compared with controls receiving the vehicle, no difference in VLDL cholesterol secretion was observed (Figure 2B). As expected, the analysis of VLDL composition in TCPOBOP-treated mice after 8 weeks of treatment revealed that the particles were enriched in cholesterol compared with TG (cholesterol to TG ratio: 6.68±2.67 vs 4.38±1.59, P<0.05). Because lipid availability is a limiting step for secretion of apoB-containing lipoproteins by the liver, we measured the hepatic TG and cholesterol content. As shown in Figure 2C, the livers of TCPOBOP-treated mice showed a reduction in TG and cholesterol levels. Finally, we checked for mRNA levels of genes involved in the control of lipogenesis, such as insulin-induced gene 1 (Insig-1), sterol responsive element binding protein 1c (Srebp1c), fatty acid synthase (Fas), acetyl coenzyme A carboxylase (Acc), and steroyl coenzyme A desaturase (Scd-1) (Figure 2E). As previously described, Insig-1, which is a negative regulator of Srebp1c cleavage,14 was induced by the TCPOBOP treatment, Srebp1c and Scd-1 mRNA levels were decreased, and no changes were observed for Acc and Fas (Figure 2E). Interestingly and as previously reported, mRNA levels of peroxisome proliferator–activated receptor-α target genes, such as Cpt1 (carnitin-palmitoyl transferase 1), Cyp4a14, and Cte1 (cytosolic acyl–coenzyme A thioesterase 1) were markedly reduced in TCPOBOP-treated mice (Figure 2D). The expression level of hydroxymethyl glutaryl COA reductase, (Hmgr) (ie, a well-known cholesterol-sensitive gene) was increased in the liver of TCPOBOP-treated animals (Figure 2E), bringing further support to the decreased hepatic cholesterol content of TCPOBOP-treated mice.
Figure 2.
A, Triglyceride (TG) secretion in low-density lipoprotein receptor–deficient (Ldlr−/−) mice treated or not with 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) for 2 months. Rates of production were assessed after injection of Poloxamer 407. TG were measured by enzymatic methods before injection and after 30 minutes, 2 hours, and 4 hours (n=10 per group; *P<0.005 vs vehicle). Basal levels of TG were 1.95±0.7 g/L for vehicle-treated mice and 1.06±0.32 g/L for TCPOBOP-treated mice (P<0.005). Data are given as μg/g of body weight. B, Rate of TG and cholesterol secretion in very-low-density lipoprotein (VLDL). Cholesterol and TGs were measured in the VLDL fraction before injection and after 4 hours (n=10 per group; *P<0.01 vs vehicle). C, Hepatic lipid content. Ldlr−/− mice were treated with TCPOBOP or vehicle for 2 months. Lipids were extracted from the liver and measured as described in Materials and Methods. Values are expressed as nmol/mg of tissue (wet weight) (n=10 per group; *P<0.01 vs vehicle). D, Relative mRNA levels of peroxisome proliferator–activated receptor-α target genes. Liver mRNA levels of Cpt1, Cte, and Cyp4A14 were determined by real-time reverse transcription–polymerase chain reaction (n=10; *P<0.05 vs vehicle). E, mRNA levels of genes involved in the control of lipogenesis and cholesterol synthesis. Liver and mRNA levels of Insig-1, Srebp1c, Scd-1, Acc, Fas, and Hmgr were determined by real-time reverse transcription–polymerase chain reaction (n=10 per group; *P<0.05 vs vehicle). Ten-week-old female mice were used for these experiments.
Finally, to demonstrate further that changes in mRNA levels were a direct consequence of Car activation and not restricted to Ldlr−/− mice, WT and Car−/− mice were treated with TCPOBOP injections. As shown in Supplemental Figure II, the TCPOBOP-mediated changes were abolished in Car−/− mice for all the genes, whereas the effect was maintained in WT mice with the repression of Cpt1, Cte, Cyp4a14, and Srebp1c and the induction of Insig-1 (Supplemental Figure II).
Car Activation Increases mRNA and Protein Levels of the VLDL Receptor in the Liver
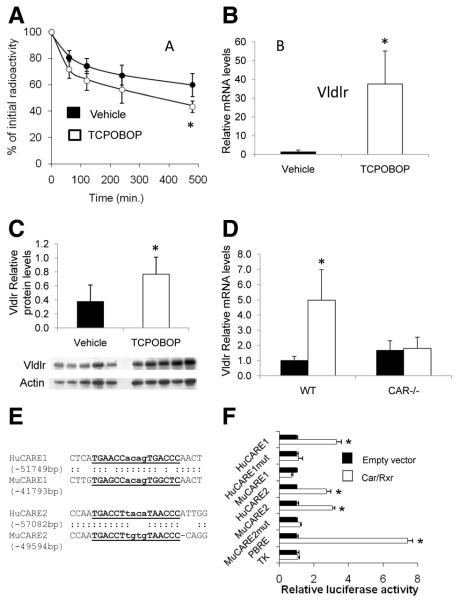
A reduction in IDL/LDL cholesterol levels in TCPOBOP-treated mice was observed throughout the 8-week period studied. To establish whether it might be related to changes in lipoprotein clearance pathways, kinetic analysis was performed with IDL/LDL labeled with 3H-cholesteryl esters. As shown in Figure 3A, plasma radioactivity was significantly lower in TCPOBOP-treated mice 8 hours after injection of the tracer, suggesting an increased clearance of apoB-containing lipoproteins after Car activation. In parallel, hepatic mRNA levels of major genes that drive recognition and catabolism of apoB-containing lipoproteins were assessed by real-time polymerase chain reaction. As shown in Figure 3B, VLDL receptor (Vldlr) mRNA levels were increased by approximately 30-fold after TCPOBOP treatment, also leading to a rise in Vldlr protein levels as shown by Western blot analysis (Figure 3C). In contrast, no changes were observed for mRNA levels of LDL receptor–related protein, hepatic lipase, lipoprotein lipase, or scavenger receptor B1 (Supplemental Figure III). Again, to demonstrate further that Vldlr overexpression was a direct consequence of Car activation, WT and Car−/− mice15 were treated with TCPOBOP injections. As shown in Figure 3D, the TCPOBOP-mediated induction of Vldlr was completely abolished in Car−/− mice.
Figure 3.
Analysis of hepatic receptors involved in apolipoprotein B (apoB)-lipoprotein catabolism in mice treated or not with the constitutive androstane receptor (Car) agonist. A, Plasma kinetics of IDL/LDL labeled with 3H-cholesteryl esters in control or 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP)–treated low-density lipoprotein receptor–deficient (Ldlr−/−) mice. Blood samples were taken at the tail vein at the indicated time points and analyzed for radioactivity on a scintillation counter. Values are the fraction of the injected dose remaining at each time point. The curves were fitted using a biexponential equation. Data are given as mean±SD; n=5 mice/group for each point; *P<0.05 vs vehicle. B, Relative very-low-density lipoprotein receptor (Vldlr) mRNA levels in the liver of Ldlr−/− mice after 2 months of treatment (n=10 per group; *P<0.001 vs vehicle). C, Western blot analysis of Vldlr in the liver. Total proteins were extracted from the liver, and Vldlr was detected with a specific antibody. Data were normalized with β-actin. D, Relative mRNA levels of Vldlr in wild-type (WT) and Car−/− mice treated or not with TCPOBOP (n=10; *P<0.05 vs vehicle). Ten-week-old female mice were used for these experiments. E and F, Identification of Car-responsive elements in the genomic area of the Vldlr gene. E, Potential Car-responsive elements were identified by chromatin immunoprecipitation sequencing in HepG2 cells and were aligned to mouse genomic sequences. F, Human 293 HEK cells were transfected with constructs containing putative Car-responsive elements placed in front of a minimal thymidine kinase promoter in pGL3 luciferase reporter vectors in the presence of pSG5-retinoid X receptor and pCMx-Car or empty vectors. Phenobarbital-responsive enhancer module (PBRE) was used as the positive control. One day after transfection, cells were treated with TCPOBOP (1 μmol/L) or dimethyl sulfoxide (control) for 24 hours, and luciferase activities were measured on cell lysates. Results are relative to the levels in untreated cells. Each bar is the mean±SD of triplicate determinations. *P<0.05 vs empty vector. Hu indicates human; Mu, murine; mut, mutated; TK, thymidine kinase.
Because the Vldlr is known to bind apoE but not apoB,16 we checked the distribution of these 2 apolipoproteins in fast protein liquid chromatography fractions (Supplemental Figure IV). We observed that apoE was present not only in VLDL but also in IDL/LDL fractions, thus supporting the hypothesis that Car-mediated induction of the Vldlr contributes to the TCPOBOP-mediated decrease in these lipoprotein fractions.
To test whether CAR/retinoid X receptor-heterodimers activate the mouse Vldlr promoter, we cloned fragments of the mouse Vldlr promoter into a pGL3 luciferase reporter vector. Although some induction was observed with Car/TCPOBOP conditions, no Car-responsive element could be characterized by mutagenesis. In addition, we could not find any evidence of Car binding to the proximal promoter of the Vldlr gene (data not shown). Based on these negative results, we searched for potential Car-binding elements in the genomic area near the Vldlr gene by chromatin immunoprecipitation sequencing in HepG2 cells, which express the murine form of CAR. With this approach, we were able to identify 3 Car-responsive elements located approximately 50 kbp upstream of the Vldlr gene in cells treated with TCPOBOP (Supplemental Figure V). Two of these elements (−51 and −57 kbp) were conserved in the mouse genome and were selected for luciferase reporter assay. For the first element (CARE1), the human sequence but not the mouse sequence induced luciferase activity in response to mouse Car. However, for the second element (CARE2), both the human and mouse sequences were fully responsive to Car/TCPOBOP conditions (Figure 3F), and mutation of the DR4 element (GGGTTAcacaAGGTCA to GGGTTAcacaATTTTT) totally abolished the response of the promoter construct to Car. Altogether, these data suggest that this element may act as an enhancer to stimulate mouse Vldlr expression in response to TCPOBOP treatment. Overall, these observations bring support to the hypothesis that TCPOBOP plays a role in increasing the catabolic pathway for the apoB-containing lipoproteins through the Car-mediated upregulation of Vldlr.
TCPOBOP Decreases Atherosclerotic Lesions in Ldlr−/− Mice
We sought to determine the consequences of TCPOBOP-mediated changes in plasma lipoprotein parameters on the susceptibility to atherosclerosis. After completion of the 8-week treatment period, the extent of atherosclerotic lesions was quantified on serial cryosections of the proximal aorta after Oil Red O staining. As shown in Figure 4A, the area of the atherosclerotic lesion in aortic valves was substantially smaller, approximately 60%, in TCPOBOP-treated animals than in controls, which received vehicle only. The immunohistological analysis of lesions revealed no major morphological differences between the 2 groups, in both cases with an accumulation of lipid-rich macrophages (Supplemental Figure VI).
Figure 4.
Quantification of atherosclerotic lesions of low-density lipoprotein receptor–deficient (Ldlr−/−) mice treated or not with 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) for 2 months. A, Quantification of atherosclerotic lesion area in the aortic valves. The areas stained with Oil Red O were quantified by color thresholding and are expressed as μm2 (n=10; *P<0.001 vs vehicle). B, Representative picture of Oil Red O–stained aortic valves. Lesions in aortic roots were stained with Oil Red O to detect the accumulation of lipids (left, control; right, TCPOBOP). Ten-week-old female mice were used for these experiments.
Car Activation Has a Reduced Impact on Plasma Cholesterol Levels and Atherosclerotic Lesions in ApoE−/− Mice
Because apoE, unlike apoB, is a ligand for the Vldlr,16,17 we sought to determine whether the impact of Car activation on plasma cholesterol levels and the development of atherosclerosis was maintained in ApoE−/− mice. The mice were treated with TCPOBOP for 8 weeks using the same protocol. As for Ldlr−/−, TCPOBOP was well tolerated throughout the 8-week study period, with no significant changes in food intake. Again, TCPOBOP-treated mice gained slightly less weight (Supplemental Figure VII). We compared the expression of Car target genes after only 1 TCPOBOP injection and after 2 months of treatment to check whether efficient Car activation was maintained throughout the treatment. In all cases, similar or even greater induction of the genes was observed in the TCPOBOP-treated group after 2 months, thus indicating efficient and persistent Car stimulation (Supplemental Figure VIII). As shown in Supplemental Table II and Figure 5A, Car activation induced only a moderate decrease in total plasma cholesterol concentration at day 5 (−10%, P<0.05) with a reduction in the cholesterol content of the VLDL fraction (Figure 5A). Plasma TG concentrations were also significantly reduced (Supplemental Table II) because of a decrease in the VLDL fraction (Figure 5A). As was the case in Ldlr−/− mice, Vldlr mRNA levels were highly increased after TCPOBOP treatment. Interestingly, a significant induction of Ldlr mRNA levels was also observed in the TCPOBOP-treated group (Supplemental Figure VIII). This was probably related to the decreased cholesterol concentration in the liver induced by Car activation. However, no significant changes in plasma cholesterol and TG levels were observed after 4 and 8 weeks, and the proportion of VLDL and IDL/LDL fractions had not changed at 8 weeks (Figure 5A).
Figure 5.

Plasma lipoprotein profiles of apolipoprotein E–deficient (ApoE−/−) mice treated or not with 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP). Shown are the fast protein liquid chromatography (FPLC) profiles after 5 days and 2 months of treatment. Plasma from control and constitutive androstane receptor (Car) agonist–treated animals were fractionated by FPLC. A, Cholesterol distribution after 5 days; B, triglyceride distribution after 5 days; C, cholesterol distribution after 2 months; D, triglyceride distribution after 2 months. Ten-week-old female mice were used for these experiments (n=9 per group). VLDL indicates very-low-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Finally, after completion of the 8-week treatment period, the extent of the atherosclerotic lesions was quantified on serial cryosections of the proximal aorta after Oil Red O staining. As shown in Figure 6A, there was only a nonsignificant trend toward a reduction in the atherosclerotic lesion of the proximal aorta in TCPOBOP-treated animals compared with controls receiving the vehicle only.
Figure 6.
Quantification of atherosclerotic lesions of apolipoprotein E–deficient (ApoE−/−) mice treated or not with 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) for 2 months. A, Quantification of atherosclerotic lesion area in the aortic valves. The area stained with Oil Red O was quantified by color thresholding, and results are expressed as μm2 (n=9; N.S. indicates not significant vs vehicle). B, Representative picture of Oil Red O–stained aortic valves. Lesions in aortic roots were stained with Oil Red O to detect the accumulation of lipids. Ten-week-old female mice were used for these experiments.
Discussion
We have previously shown in our laboratory that TCPOBOP is able to decrease both HDL and LDL cholesterol in mice.12 However, these effects were monitored over short periods of treatment in wild-type (WT) and human apoA-I transgenic mice, which, unlike humans, normally present only moderate levels of apoB-containing lipoproteins. The results of the present study demonstrate that treatment with the Car agonist TCPOBOP has the potential to decrease circulating levels of apoB-containing lipoproteins and to reduce the development of atherosclerotic lesions in the long term in Ldlr−/− mice. The beneficial effects on the lipoprotein profile are related to a decreased secretion of TG-rich lipoproteins and to an increased clearance of cholesterol-rich/apoB-containing lipoproteins, whereas the early effect on HDL level is only transient. The Car-mediated induction of the Vldlr probably accounts, at least in part, for the observed phenotype because the effects of TCPOBOP are markedly attenuated in ApoE−/− mice that lack this physiological ligand for the Vldlr.
CAR was initially described as a xenobiotic sensor that regulates genes involved in the detoxification and elimination of potentially harmful exogenous molecules. However, recent studies have underlined its role in the metabolism of endogenous molecules, including bile acids, cholesterol, and TGs.6,10,12 As observed in a recent study from our group,12 acute treatment of WT and human apoA-I transgenic mice with a Car agonist decreased HDL cholesterol and apoA-I, and it also reduced LDL cholesterol levels. We chose here to use Ldlr−/− mice fed a Western-type diet to assess the effects of Car activation on both lipoprotein metabolism and atherosclerotic lesions over a longer/2-month period. Ldlr−/− mice display high levels of apoB-containing lipoproteins and are highly susceptible to diet-induced atherosclerosis. As previously described, 5 days of treatment with the Car agonist produced a marked reduction in total plasma cholesterol levels that was related to a decrease in all lipoprotein classes, including VLDL, LDL, and HDL. A reduction in plasma TG levels was also observed. A consistent but slightly different picture was observed after longer periods of treatment. Whereas VLDL and HDL cholesterol levels were no longer reduced, ≈30% reductions in LDL cholesterol and plasma TG levels were still observed at weeks 4 and 8. These results clearly show that Car activation can exert 2 kinds of effects. On the one hand, acute effects on HDL and VLDL are only transient and disappear after a few weeks of treatment, suggesting the existence of compensatory mechanisms that antagonize Car activity. On the other hand, reductions in LDL cholesterol and TG levels were observed at every time point and might be mediated by distinct, Car-specific mechanisms.
There is an apparent controversy about the effect of Car on TG metabolism. On the one hand, Car activation has been shown to reduce plasma TG levels, to decrease VLDL secretion, and to reduce lipogenesis in mice.8–10 These effects were further associated, as in the present study, with the Car-mediated induction of the Insig-1 gene that negatively regulates the lipogenic transcription factor Srebp1c.7,8 On the other hand, treatment with TCPOBOP has been shown to increase TG levels in WT mice fed a Western-type diet,11,12 and Car-deficient mice present significantly lower plasma TG levels under a high-fat diet.11 Moreover, Car deficiency has been shown to increase fatty acid β-oxidation, suggesting that Car antagonizes some peroxisome proliferator–activated receptor-α mediated pathways (including β-oxidation). We provide here a tentative explanation for this apparent contradiction. In accordance with earlier observations,11 the Car-mediated inhibition of peroxisome proliferator–activated receptor-α–dependent pathways was supported by the decreased expression of peroxisome proliferator–activated receptor-α target genes, such as Cpt1, Cyp4a14, and Cte1. In contrast, and as previously described,7,8 the Insig-1 gene (ie, a negative regulator of Srepbp1c cleavage) was induced after Car activation, and mRNA levels of key lipogenic genes, such as Srebp1c and Scd-1, were downregulated. Thus, Car is able to exert concomitant and antagonistic actions, and the overall effect is likely to result from a subtle balance depending on the metabolic context. As far as the present study is concerned, Car activation clearly decreased liver TG content and plasma TG concentration, ie, 2 potential beneficial effects in terms of atherosclerosis. Moreover, because the effect of Car activation on plasma TG levels was more pronounced in Ldlr−/− than in ApoE−/− mice, we cannot exclude the possibility that induction of the Vldlr also contributed to the decrease in plasma TG levels.
Besides plasma TG levels, the atherogenicity of the lipoprotein profile in Ldlr−/− mice is mainly determined by the accumulation of cholesterol-rich IDL/LDL lipoproteins because of the absence of LDL-receptor expression. Importantly, we show here that TCPOBOP is able to increase the catabolism of these lipoproteins, probably through the induction of hepatic VLDL-receptor which belongs to the LDL receptor family. Although VLDLR is a peripheral receptor, which is normally expressed in muscle and adipose tissue with a very low level of expression in the liver, its hepatic expression is known to increase in Ldlr−/− mice. Its expression is detectable at the mRNA and protein levels in the liver and is subject to transcriptional regulation.18–20 This suggests that VLDLR has a potential role as a backup for the LDL receptor.18 Our results show that TCPOBOP is able to increase Vldlr expression even further, by more than 20-fold in the liver after 8 weeks of treatment. Upregulation of the Vldlr is the most relevant explanation for the increased clearance of apoB-containing lipoproteins in TCPOBOP-treated animals because we did not observe any changes for any other major apoB-containing lipoprotein receptors. In further support of the latter view, data from the literature clearly show that hepatic overexpression of Vldlr by the use of viral vectors promotes the preferential reduction of IDL/LDL particles, just as we observed in the present study.19,21–24 Most importantly, when the expression of the Vldlr ligand (ie, ApoE) was suppressed in ApoE−/− mice, the impact of TCPOBOP on plasma apoB-containing lipoproteins was markedly attenuated. Importantly, the TCPOBOP-mediated regulation is Car specific because the effect on Vldlr expression was totally abolished in Car-deficient mice (Figure 3), and Car seems to activate specific enhancer elements upstream of the Vldlr gene (Figure 3).
Finally, in accordance with these potentially beneficial changes, atherosclerosis was significantly decreased in TCPOBOP-treated animals. The mechanisms that account for this reduction are probably related to the modification of plasma lipoprotein metabolism at the systemic level. Local effects at the vascular wall are unlikely because Car is mainly expressed in the liver and intestine, with much lower levels of expression in other tissues.15 Although the decrease in plasma TG levels could also have contributed to the reduction in lesion size, the reduction in cholesterol levels in apoB-containing lipoproteins probably produced the major impact in preventing atherosclerosis. Accordingly, hepatic expression of Vldlr by different strategies efficiently reversed hypercholesterolemia and susceptibility to atherosclerosis in Ldlr−/− mice.19,22–24
Very recently, we observed that Car activation was able to stimulate the fecal elimination of cholesterol by converting it into bile acid, with positive consequences on reverse cholesterol transport and whole-body cholesterol homeostasis in Ldlr−/− mice and ApoE−/− mice.6 It is therefore likely that the decreased atherogenicity of the lipoprotein profile, as well as the stimulation of RCT, contributed in a synergistic manner to the reduction of atherosclerotic lesions in Ldlr−/− mice. In accordance with this hypothesis, the impact of TCPOBOP on atherosclerosis was less pronounced in ApoE−/− mice than in Ldlr−/− mice. Indeed, although we previously observed a decrease in atherosclerotic lesions in the aortic arch and descending aorta in ApoE−/− mice treated with TCPOBOP by en face analysis,6 the reduction in lesion size in the aortic valves of ApoE−/− mice did not reach statistical significance in the present study and was less pronounced than in Ldlr−/− mice (Figures 4 and 6).
In conclusion, the results of present study indicate that Car activation is atheroprotective in some mouse models. Whether CAR constitutes a potential target in the prevention or treatment of atherosclerosis in humans deserves further investigation.
Supplementary Material
Table SI: Plasma lipids levels in Ldlr−/− mice. Animals were fed a western diet during 2 months and were treated either with vehicle or TCPOBOP (3 mg/kg/week). Blood from fasted mice was collected after 5 days, 4 and 8 weeks of the treatment. For HDL cholesterol determination, individual plasma samples were fractioned by ultracentrifugation at d=1.21. (Values are expressed in mmol/l and as mean ± SD; *P<0.05 vs vehicle).
Table SII: Plasma lipids levels in ApoE−/− mice. Animals were fed a western diet during 2 months and were treated either with vehicle or TCPOBOP (3 mg/kg/week). Blood from fasted mice was collected after 5 days, 4 and 8 weeks of the treatment. For HDL cholesterol determination, individual plasma samples were fractioned by ultracentrifugation. (Values are expressed in mmol/l and as mean ± SD; *P<0.05 vs vehicle).
Figure SI : Body weight curve of Ldlr−/− mice treated or not with TCPOBOP. Animals were fed a western diet during 2 months and were treated either with vehicle or TCPOBOP. Body weight was evaluated weekly all along the period of treatment. Food intake per mouse was 16.9+/−1.99 g/week in the vehicle group and 17.58+/−1.27 g/week in the TCPOBOP group (N.S.).
Figure SII: Effect of TCPOBOP treatment on hepatic gene expression in WT and Car −/−mice. WT and Car−/− mice were treated by TCPOBOP or vehicle only and relative mRNA levels of selected genes were determined by real time PCR (A) Relative mRNA levels of genes involved in fatty acid catabolism. (B) Relative mRNA levels of genes involved in the control of lipogenesis. (n=5 in each cases, *: P<0.05 Vs Vehicle group)
Figure SIII Relative mRNA levels of Lrp, Lpl, Hl and Sr-BI in Ldlr−/− treated with vehicle or TCPOBOP for 8 weeks (n=10 per group).
Figure SIV: ApoE and ApoB distribution in FPLC fractions in Ldlr−/− mice. Cholesterol concentration as well as ApoB and apoE distribution in FPLC fractions from Ldlr−/− mice treated or not with TCPBOP for 8 weeks. Pooled plasma samples (n= 5 in each case) were submitted to FPLC ApoE and APOB distribution were determined by western blot as describe in materials and methods. (A): Vehicle treated mice, (B): TCPOBOP treated mice.
Figure SV. ChIP-seq peak data for part of chromosome 9 in HepG2 cells. The top lane represents peaks from cells expressing BirA only. Lane 2 to 4 lane represent peaks identified from BLRP ChIP-seq in cells expressing BirA and BLRP-TEV tagged mouse Car and treated with vehicle, TCPOBOP or androstenol respectively.
Figure SVI : Immunohistological analysis of atherosclerotic lesion in Ldlr−/− mice treated or not with TCPOBOP and fed a Western type diet for 8 weeks. (A) Immunostaining for macrophages (Lamp2) and (B) SMCs (a-SMA) of sections from the proximal aorta, (C) Masson trichrome staining (collagen content) (D) oil red O staining (lipid content)
Figure SVII Body weight curve of ApoE−/− mice treated or not with TCPOBOP.Animals were fed a western type diet during 2 months and were treated either with the vehicle or TCPOBOP. Body weight was evaluated weekly all along the period of treatment. Food intake per mouse was 21.7+/−0.45 g/week in the Vehicle group and 22.68+/−1.09 g/week in the TCPOBOP group (N.S.)
Figure SVIII: Effect of TCPOBOP on the expression of Car target genes after 1 or 8 weeks of treatment. ApoE−/− mice were sacrificed after one or 8 weeks of TCPOBOP treatment. In both cases mice were sacrificed 5 days after the last I.P. injection. Relative mRNA levels were determined by real time PCR as describe in materials and methods.
Acknowledgments
We thank Philip Bastable for manuscript editing.
Sources of Funding This work was supported by grants from the Université de Bourgogne, the Conseil Regional de Bourgogne, Institut National de la Santé et de la Recherche Médicale, the Agence Nationale de la Recherche, Fondation de France, and National Institutes of Health Grants R01-DK46546 and U19DK62434.
Footnotes
Disclosures None.
References
- 1.Qatanani M, Moore DD. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab. 2005;6:329–339. doi: 10.2174/1389200054633899. [DOI] [PubMed] [Google Scholar]
- 2.Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A. 2005;102:2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 5.Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, Gonzalez FJ, Sinal CJ. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- 6.Sberna AL, Assem M, Gautier T, Grober J, Guiu B, Jeannin A, Pais de Barros JP, Athias A, Lagrost L, Masson D. Constitutive androstane receptor activation stimulates fecal bile acid excretion and reverse cholesterol transport in mice. J Hepatol. 2011;55:154–161. doi: 10.1016/j.jhep.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Roth A, Looser R, Kaufmann M, Blattler SM, Rencurel F, Huang W, Moore DD, Meyer UA. Regulatory cross-talk between drug metabolism and lipid homeostasis: constitutive androstane receptor and pregnane X receptor increase Insig-1 expression. Mol Pharmacol. 2008;73:1282–1289. doi: 10.1124/mol.107.041012. [DOI] [PubMed] [Google Scholar]
- 8.Baskin-Bey ES, Anan A, Isomoto H, Bronk SF, Gores GJ. Constitutive androstane receptor agonist, TCPOBOP, attenuates steatohepatitis in the methionine choline-deficient diet-fed mouse. World J Gastroenterol. 2007;13:5635–5641. doi: 10.3748/wjg.v13.i42.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J, He J, Zhai Y, Wada T, Xie W. The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J Biol Chem. 2009;284:25984–25992. doi: 10.1074/jbc.M109.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, Stevens RD, Ilkayeva O, Newgard CB, Chan L, Moore DD. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci U S A. 2009;106:18831–18836. doi: 10.1073/pnas.0909731106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maglich JM, Lobe DC, Moore JT. The nuclear receptor CAR (NR1I3) regulates serum triglyceride levels under conditions of metabolic stress. J Lipid Res. 2008 doi: 10.1194/jlr.M800226-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Masson D, Qatanani M, Sberna AL, Xiao R, Pais de Barros JP, Grober J, Deckert V, Athias A, Gambert P, Lagrost L, Moore DD, Assem M. Activation of the constitutive androstane receptor decreases HDL in wild-type and human apoA-I transgenic mice. J Lipid Res. 2008;49:1682–1691. doi: 10.1194/jlr.M700374-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005;46:2023–2028. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 15.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S, Sakai J, Fujino T, Miyamori I, Yamamoto TT. The very low density lipoprotein (VLDL) receptor–a peripheral lipoprotein receptor for remnant lipoproteins into fatty acid active tissues. Mol Cell Biochem. 2003;248:121–127. doi: 10.1023/a:1024184201941. [DOI] [PubMed] [Google Scholar]
- 17.Hussain MM, Strickland DK, Bakillah A. The mammalian low-density lipoprotein receptor family. Annu Rev Nutr. 1999;19:141–172. doi: 10.1146/annurev.nutr.19.1.141. [DOI] [PubMed] [Google Scholar]
- 18.Degrace P, Moindrot B, Mohamed I, Gresti J, Du ZY, Chardigny JM, Sebedio JL, Clouet P. Upregulation of liver VLDL receptor and FAT/CD36 expression in LDLR−/− apoB100/100 mice fed trans-10,cis-12 conjugated linoleic acid. J Lipid Res. 2006;47:2647–2655. doi: 10.1194/jlr.M600140-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Chen SJ, Rader DJ, Tazelaar J, Kawashiri M, Gao G, Wilson JM. Prolonged correction of hyperlipidemia in mice with familial hypercholesterolemia using an adeno-associated viral vector expressing very-low-density lipoprotein receptor. Mol Ther. 2000;2:256–261. doi: 10.1006/mthe.2000.0122. [DOI] [PubMed] [Google Scholar]
- 20.Sirvent A, Claudel T, Martin G, Brozek J, Kosykh V, Darteil R, Hum DW, Fruchart JC, Staels B. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 2004;566:173–177. doi: 10.1016/j.febslet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Jong MC, van Dijk KW, Dahlmans VE, Van der Boom H, Kobayashi K, Oka K, Siest G, Chan L, Hofker MH, Havekes LM. Reversal of hyperlipidaemia in apolipoprotein C1 transgenic mice by adenovirus-mediated gene delivery of the low-density-lipoprotein receptor, but not by the very-low-density-lipoprotein receptor. Biochem J. 1999;338:281–287. [PMC free article] [PubMed] [Google Scholar]
- 22.Kozarsky KF, Jooss K, Donahee M, Strauss JF, III, Wilson JM. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat Genet. 1996;13:54–62. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- 23.Oka K, Pastore L, Kim IH, Merched A, Nomura S, Lee HJ, Merched-Sauvage M, Arden-Riley C, Lee B, Finegold M, Beaudet A, Chan L. Long-term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation. 2001;103:1274–1281. doi: 10.1161/01.cir.103.9.1274. [DOI] [PubMed] [Google Scholar]
- 24.MacDougall ED, Kramer F, Polinsky P, Barnhart S, Askari B, Johansson F, Varon R, Rosenfeld ME, Oka K, Chan L, Schwartz SM, Bornfeldt KE. Aggressive very low-density lipoprotein (VLDL) and LDL lowering by gene transfer of the VLDL receptor combined with a low-fat diet regimen induces regression and reduces macrophage content in advanced atherosclerotic lesions in LDL receptor-deficient mice. Am J Pathol. 2006;168:2064–2073. doi: 10.2353/ajpath.2006.051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI: Plasma lipids levels in Ldlr−/− mice. Animals were fed a western diet during 2 months and were treated either with vehicle or TCPOBOP (3 mg/kg/week). Blood from fasted mice was collected after 5 days, 4 and 8 weeks of the treatment. For HDL cholesterol determination, individual plasma samples were fractioned by ultracentrifugation at d=1.21. (Values are expressed in mmol/l and as mean ± SD; *P<0.05 vs vehicle).
Table SII: Plasma lipids levels in ApoE−/− mice. Animals were fed a western diet during 2 months and were treated either with vehicle or TCPOBOP (3 mg/kg/week). Blood from fasted mice was collected after 5 days, 4 and 8 weeks of the treatment. For HDL cholesterol determination, individual plasma samples were fractioned by ultracentrifugation. (Values are expressed in mmol/l and as mean ± SD; *P<0.05 vs vehicle).
Figure SI : Body weight curve of Ldlr−/− mice treated or not with TCPOBOP. Animals were fed a western diet during 2 months and were treated either with vehicle or TCPOBOP. Body weight was evaluated weekly all along the period of treatment. Food intake per mouse was 16.9+/−1.99 g/week in the vehicle group and 17.58+/−1.27 g/week in the TCPOBOP group (N.S.).
Figure SII: Effect of TCPOBOP treatment on hepatic gene expression in WT and Car −/−mice. WT and Car−/− mice were treated by TCPOBOP or vehicle only and relative mRNA levels of selected genes were determined by real time PCR (A) Relative mRNA levels of genes involved in fatty acid catabolism. (B) Relative mRNA levels of genes involved in the control of lipogenesis. (n=5 in each cases, *: P<0.05 Vs Vehicle group)
Figure SIII Relative mRNA levels of Lrp, Lpl, Hl and Sr-BI in Ldlr−/− treated with vehicle or TCPOBOP for 8 weeks (n=10 per group).
Figure SIV: ApoE and ApoB distribution in FPLC fractions in Ldlr−/− mice. Cholesterol concentration as well as ApoB and apoE distribution in FPLC fractions from Ldlr−/− mice treated or not with TCPBOP for 8 weeks. Pooled plasma samples (n= 5 in each case) were submitted to FPLC ApoE and APOB distribution were determined by western blot as describe in materials and methods. (A): Vehicle treated mice, (B): TCPOBOP treated mice.
Figure SV. ChIP-seq peak data for part of chromosome 9 in HepG2 cells. The top lane represents peaks from cells expressing BirA only. Lane 2 to 4 lane represent peaks identified from BLRP ChIP-seq in cells expressing BirA and BLRP-TEV tagged mouse Car and treated with vehicle, TCPOBOP or androstenol respectively.
Figure SVI : Immunohistological analysis of atherosclerotic lesion in Ldlr−/− mice treated or not with TCPOBOP and fed a Western type diet for 8 weeks. (A) Immunostaining for macrophages (Lamp2) and (B) SMCs (a-SMA) of sections from the proximal aorta, (C) Masson trichrome staining (collagen content) (D) oil red O staining (lipid content)
Figure SVII Body weight curve of ApoE−/− mice treated or not with TCPOBOP.Animals were fed a western type diet during 2 months and were treated either with the vehicle or TCPOBOP. Body weight was evaluated weekly all along the period of treatment. Food intake per mouse was 21.7+/−0.45 g/week in the Vehicle group and 22.68+/−1.09 g/week in the TCPOBOP group (N.S.)
Figure SVIII: Effect of TCPOBOP on the expression of Car target genes after 1 or 8 weeks of treatment. ApoE−/− mice were sacrificed after one or 8 weeks of TCPOBOP treatment. In both cases mice were sacrificed 5 days after the last I.P. injection. Relative mRNA levels were determined by real time PCR as describe in materials and methods.