Abstract
Virtual reality (VR) cue environments have been developed and successfully tested in nicotine, cocaine, and alcohol abusers. Aims in the current article include the development and testing of a novel VR cannabis cue reactivity assessment system. It was hypothesized that subjective craving levels and attention to cannabis cues would be higher in VR environments merits with cannabis cues compared to VR neutral environments. Twenty nontreatment-seeking current cannabis smokers participated in the VR cue trial. During the VR cue trial, participants were exposed to four virtual environments that contained audio, visual, olfactory, and vibrotactile sensory stimuli. Two VR environments contained cannabis cues that consisted of a party room in which people were smoking cannabis and a room containing cannabis paraphernalia without people. Two VR neutral rooms without cannabis cues consisted of a digital art gallery with nature videos. Subjective craving and attention to cues were significantly higher in the VR cannabis environments compared to the VR neutral environments. These findings indicate that VR cannabis cue reactivity may offer a new technology-based method to advance addiction research and treatment.
Keywords: cannabis, craving, cue reactivity, olfactory cues, marijuana, virtual reality
Marijuana is believed to be the most commonly used illicit substance in the United States (Compton et al. 2004; SAMHSA 2002), and cannabis use and dependence have been increasing in both adults and children since the early 1970s (SAMHSA 2002). According to the 2003 National Survey on Drug Use and Health, approximately 40% of Americans over the age of 12 have used marijuana (SAMHSA 2004; NIDA 2005). In 2002, Compton and colleagues (2004) found that 30% of college students had used marijuana within the previous year. Due to the increasing population of cannabis users, research is warranted to further understand cannabis craving and the abuse liability (cognitive and behavioral consequences) of chronic use in order to inform assessment and treatment approaches.
GENERAL DRUG ADDICTION AND CUE EXPOSURE
Drug craving, cue exposure, and assessment methods for substances such as nicotine, alcohol, and cocaine are phenomena that have been discussed extensively in the literature and are believed to be related to the abuse liability of drugs. Drug craving has been reported to occur weeks to years after abstinence from a drug (Gawin & Kleber 1986; Manschreck 1993; Prakash & Das 1993) and is believed to be the most prominent and disturbing of the withdrawal symptoms (Prakash & Das 1993). While the mechanism of craving is not fully understood, ii has been hypothesized that drug craving is conditioned through drug use and elicited by specific cues (i.e. cigarettes, beer, bongs) and environmental cues (i.e. bar setting, pot party; concert) related to past substance use (Carter & Tiffany 1999; Childress et al. 1993; O’Brien et al. 1993; Prakash & Das 1993; Satel 1992; Wallace 1989; Washton 1987).
Laboratory-based cue exposure is the most common method used to assess craving and reactivity to drug cues. Traditional cue exposure research has utilized the following stimuli; imagery scripts, videos, still photographs, olfactory scents, and tactile drug materials (i.e. pipes, bongs, beer cans, syringes). Typically, participants are exposed to cues in a laboratory environment and subjective (e.g. craving or desire to use) and objective (e.g. physiology) measurements are recorded. Traditional cue exposure studies demonstrate that when chronic substance users are exposed to visual, auditory, olfactory, or tactile cues, physiological arousal (Tiffany, Cox & Flash 2000; Niaura et al. 1998; Drobes & Tiffany 1997; Niaura et al. 1989) and subjective reports of urges and craving to use increase compared to neutral (non-drug-related) cues (Budney et al, 2001; Lundahl, Borden & Lukas 2001). While traditional methods involving simple cue presentation elicit craving and physiological reactivity, these methods can be improved by more thoroughly incorporating the environmental contexts in which the exposure occurs.
CANNABIS DEPENDENCE, CRAVING, AND CUE REACTIVITY
In theory, cannabis, like other drugs of abuse, when used repeatedly should lead to similar conditioned craving responses in those who become addicted. The presence or absence of cannabis craving and withdrawal symptoms has been the subject of debate since the early 1970s. Even with the ongoing debate, there has been a lack of research focused on cannabis craving specifically and related reactivity (Heishman & Singelton 2006). From a scientific point of view there appears to be little doubt that cannabis is similar to other illicit drugs of abuse on biological, behavioral, and psychosocial dimensions (Budney et al. 2004, 1998; Gardner 2002; Budney, Novy & Hughes 1999), A traditional cue reactivity laboratory study of cannabis-dependent adolescent inpatients supports the role of chronic cannabis cues in increasing craving (Lundahl, Borden & Lukas 2001), Lundahl and colleagues (2001) exposed participants to tactile, olfactory; and visual cannabis cues and neutral cues. Cannabis cues significantly increased craving and desire to use in participants compared to neutral cues. The authors concluded that the assessment of cue reactivity and identification of triggers may provide treatment professionals with valuable information on tailoring therapy sessions. Overall, laboratory and treatment research data on cannabis dependence supports the continued exploration of cues, craving, and behavioral symptoms related to use and relapse. Findings in cannabis research suggest that environmental cues and social interactions can lead to conditioned craving, and may subsequently play a role in relapse (Budney et al. 2001; Lundahl, Borden & Lukas 2001). These studies form the basis for our exposure of chronic cannabis users to complex cues comprised of social interactions and contextualized inanimate objects in virtual reality environments.
VIRTUAL REALITY CUE REACTIVITY
A novel paradigm, virtual reality (VR) based cue reactivity has been tested successfully for nicotine (Bordnick et al, 2005b, c, 2004), alcohol (Bordnick et al. 2008), and cocaine (Saladin el al. 2006) cue reactivity assessment. Building upon success in other VR-based cue programs (Bordnick et al. 2005a; Bordnick & Graap 2004), the present authors developed the virtual reality cannabis cue reactivity assessment (VR-CCRAS; Bordnick, Graap & Ferrer 2006) system, which integrates a visual head mounted display, tracking device, and directional audio, vibrotactile, and olfactory stimuli. The VR-CCRAS offers the potential of greater clinical utility and the ability to present specific drug cues and social interactions in environmental contexts that are congruent with the users’ experiences. Specifically; the VR-CCRAS contains multiple cannabis-related cues, such as visual 3D paraphernalia (pipes, bongs, marijuana plants, incense) and complex social interactions (seeing people al party smoking marijuana and responding to an offer to join them) and accompanying olfactory cues situated in appropriate contexts (i.e. party) designed to emulate a real world experience. The current study was conducted to determine the effects of VR cannabis-based cues on reactivity in current cannabis smokers. In addition, the level of attention to the sight, smell, and thoughts towards cues was explored. It was hypothesized that cannabis smokers who were exposed to VR cannabis related-cues and social interactions would report increases in subjective craving and attention to cues compared to VR neutral stimuli.
METHODS
Participants
Twenty nontreatment-seeking male (16 or 80%) and female (4 or 20%) adult cannabis smokers, aged 19 to 44 (M = 26.8, SD = 6.7) participated in the cue reactivity trial. Participants were thirteen (65%) African Americans, five (25%) Caucasians, one (5%) Hispanic, and one (5%) of another race. The participants met the following criteria: DSM-IV-TR (APA 2000) current diagnosis of cannabis abuse or dependence; self-reported good physical health; willingness to participate in a nontreatment study; ability to read and understand English at or above the sixth grade level; and ability to wear a VR helmet for up to 40 minutes. Participants were excluded from the study based on the following criteria: DSM-IV-TR diagnosis of severe mental illness or substance dependence other than marijuana or nicotine dependence; pregnancy or self-reported significant health issues; history of seizures or seizure disorder; or use of illicit drugs (not including cannabis) within the past month.
Enrolled participants smoked cannabis on average 25.8 (SD = 4.3) days out of the past 30 days and reported smoking 3.5 (SD = 2.8) limes per day. The average age when participants began regularly smoking cannabis was 16.2 (SD = 3.8). The preferred manner of smoking was blunt (9 or 45%) Joint (6 or 30%), and pipe (5 or 25%).
Design and Procedures
In 2006, potential participants responding to a newspaper advertisement recruiting current cannabis smokers telephoned the Virtual Reality Clinical Research Center (VRCRC) and were provided with basic information about the study. Individuals were asked for verbal consent to complete an initial telephone assessment designed to screen out ineligible participants. Eligible participants were scheduled for an in-depth assessment at the VRCRC laboratory and were instructed not to smoke cannabis for 12 hours prior to their appointment. Additionally, participants were informed that they would be tested for alcohol and other drug use upon arrival at the center, and would not be allowed to participate if recent drug use, other than cannabis, was detected.
Upon arriving at the VRCRC, participants were provided information regarding the study, including rationale, risks, benefits, and 1RB involvement (this study was approved by the University of Georgia Committee for the Protection of Human Subjects). Participants were then asked to provide verbal consent (IRB: approved waiver of written consent to protect identity) to continue with the study. After obtaining consent, each participant was asked to provide a breath alcohol sample to ensure abstinence. In addition, participants were asked to provide a saliva sample that was tested using a rapid screen (ORATECH III™, Branan Medical, Irvine, CA) to verify the absence of drugs other than cannabis. No participants were excluded for other drug use. Participants then completed screening assessment interviews and instruments to determine full study eligibility. After completing the screening assessment, eligible participants underwent a 15-minule VR session in a VR environment unrelated to cannabis cues or the craving environment to familiarize them with the VR experience, visual analog scales, input system, and study procedures. After completing this session, participants were provided a mandatory 20-minute break. Participants remained either within the building or directly outside in view of campus security and administration offices.
Upon returning to the laboratory, the participants were seated in a chair placed on a vibration platform (which provided tactile sensations from the music and various audio sounds in the environments; Virtually Belter, Inc., Decatur, GA) and outfitted with the VR display and tracker helmet (HMD; VFX-3D Interactive Imaging Systems, Rochester, NY customized with optics from the Emagin z800 HMD, Emagin, Belleuve, WA), which was adjusted for comfort and tested for proper operation. Participants then were given a wireless game pad device (P3000, Sailek, Torre nee, CA) to input subjective responses to the visual analog scales (VAS) at the end of each VR environment. Instructions for VR cue trial were read from a standard script and participants were given the opportunity to ask questions. The participants were asked to sit quietly as the overhead lights were turned off and the researcher selected the VR experimental path (stimulus order), musical preference (rock, pop, hip hop or new age), and smoking preference (pipe or joint) through a graphical interface. The VR-CCRAS program was started, beginning with a five-minute introduction period during which participants listened to classical jazz music and the HMD screens remained dark. Following the completion of this phase, participants were asked to stand and begin the cue reactivity trial. The trial including the introduction period was 17 minutes total, with three minutes exposure in each of the four VR environments. The VR-CCRAS program moved participants along a preprogrammed Hack at intervals timed to standardize exposure to visual, auditory, and olfactory cannabis-related cues and social interactions. Cue environments included neutral room I, paraphernalia room, social interaction party room, and neutral room 2. To control for order effects and time in the VR environments, participants were randomized into one of two paths that allowed the presentation of the social interaction party room or inanimate paraphernalia room to be switched. The neutral cue rooms were identical. After experiencing each cue environment (neutral 1, paraphernalia, social interaction party, and neutral 2), participants rated their cannabis craving intensity on a 100-point visual analog scale (CCVAS) and their attention to the sight, smell, and thoughts related to cannabis on an 11 -point cannabis attention scale (CAS) VAS. Scales were displayed in the VR environment in order to maintain immersion.
After the VR-CCRAS trial all participants were debriefed by a Ph.D.-level clinician to assess any problems related to elevated craving or other clinical issues. In addition, literature on health effects of cannabis use and referrals for treatment were provided upon request. Participants were paid $50.00 for their participation.
Measures and VR environments
CCVAS
The CCVAS was projected into the VR environment on a white background and consisted of a single item designed to assess craving. Participants rated their craving for cannabis at the current lime on a scale that ranged from 0 (not at all) to 100 (more than ever). The VAS has been used to measure craving in both VR (Bordnick et al. 2008, 2005a, b, c, 2004) and traditional cue studies (Cutler 2005; Johnson et al 1998; Preston & Jasinski 1991).
CAS
The CAS is a three-item Liken scale used to measure attention to VR cannabis cues. Participants rate their level of attention to cannabis on a scale ranging from 0 (didn’t notice al all) to 10 (completely paid attention) for the following two items: “How much did you pay attention to the sight of marijuana in the room” and “How much did you pay attention to the smell of marijuana in the room.” A third question addressed thoughts about smoking on a scale anchored by 0 (didn’t think about smoking at all) and 10 (thought about smoking all the time) using the following item: “How much did you think about smoking marijuana while you were in the room.” The CAS is a modified version of the three item alcohol attention scale (AAS) that has been used to measure attention to actual alcohol beverages in cue studies (Hutchison et al. 2001; Monti et al. 1993).
Olfactory Cue Scent System
The Scent Palette™ system (Envirodine Studios/Virtually Better 2004), designed to present olfactory cues in virtual environ merits, was utilized for this study. The Scent Palette is controlled by the VR-CCRAS, allowing scents to be presented with preprogrammed environmental triggers (i.e. seeing someone smoking and smelling cannabis). Scents are delivered through a system of fans and compressed air and lingering smells are avoided as the fans move the air continuously. Thus, smells are provided only when needed, with no carryover effect. The following scents were used in the VR-CCRAS environments: vanilla (neutral), raw cannabis (buds), cannabis smoke, pizza, incense-frankincense, beer, popcorn, and outdoors (pine/dirt).
VR-CCRAS Environments
The neutral cue rooms were identical and consisted of a room resembling an art gallery. On two of the walls in the room flat panel video screens displayed nature videos accompanied by audio and vanilla scent when approached, The neutral rooms contained no cannabis cues or references and the vanilla scent was used to control for the effect of scent presentation in the cannabis cue rooms. Participants were guided to each nature scene at preprogrammed intervals. The paraphernalia room consisted of a room with no social cues (no other people), but with visual, auditory, and olfactory stimuli related to cannabis. Participants wore moved around the room at preprogrammed intervals observing inanimate cues such as bongs, Joints, rolling papers, incense burners, black light posters, and cannabis plants (with grow lights). The music choice (rock, pop, hip hop, or new age) selected by the participant played in the background and scents were presented. The party environment provided exposure to complex social cues. The party consisted of indoor and outdoor areas of a home in which people were eating, drinking, and smoking cannabis on a patio. During the party, participants observed a person rolling a joint. Participants were encouraged to interact with people within the virtual environment. An offer to smoke a joint or “take a hit” on a marijuana pipe (based on the participants preferred method of smoking) was initiated by other party guests. Olfactory cannabis cues were presented when participants encountered people smoking cannabis at a table with pizza and popcorn. The final room was another neutral room that contained the identical visual, auditory, and olfactory stimuli as the first neutral room. Screenshots of the party room, deck, and cannabis paraphernalia are depicted in Figure 1.
FIGURE 1.
Screenshots of the VR Cannabis Cue Exposurt Rooms
Data Analysis Plan
Separate multivariate analysis of variance (MANOVA) tests were used to compare the effect of VR cue rooms (4) on subjective craving and each of the three cannabis attention scales (sight, smell, thoughts) with path (2) as the between-subjects factor. Following significant main effects on the ANOVAs, planned post hoc pairwise tests were conducted.
RESULTS
Initial MANOVAs were performed on all four dependent variables (craving, sight, smell, thoughts) and there were no main effects nor interactions involving path for any of these variables, so subsequent analyses were collapsed across path. In addition, there were no significant differences between the party and paraphernalia rooms on any of these variables so data from these rooms were collapsed into a single variable (marijuana environment). The following analyses used a one-way ANOVA with neutral room 1, the marijuana environment, and neutral room 2 as within-subject factors. Follow-up tests used Bonferroni adjustments for multiple comparisons.
Craving
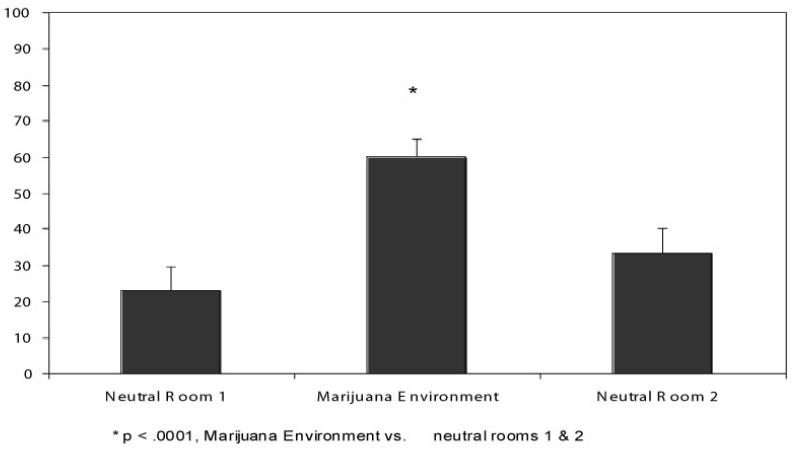
There was a Significant main effect of room/environment on craving level, F(2,38) = 27.03, p< .0001, with post hoc analyses revealing that craving response to the marijuana environment was significantly greater than neutral room 1, p < .0001, and neutral room 2, p< .0001. There was no significant difference in craving level between the two neutral rooms (see Figure 2).
FIGURE 2.
Average Craving Level (SE) across VR Cue Rooms
Attention Scales
There was a significant main effect of room/environment on attention to the sight of marijuana cues, F(2,38) = 122.0, p < .0001, with post hoc analyses revealing that attention to marijuana cues was significantly greater in the marijuana environment than neutral room 1, p < .0001, and neutral room 2, p < .0001. There was no significant difference in attention to marijuana cues between the two neutral rooms (see Table 1).
TABLE 1. Average Scores (SE) on the Cannabis Attention Scale across VR Cue Rooms.
| CAS Item | Neutral Room 1 M (SE) |
Paraphernalia Room M (SE) |
Party Room M (SE) |
Neutral Room 2 M (SE) |
|---|---|---|---|---|
| Sight of Marijuana | 0.2(.08)a | 8.1(.49)b | 8.4(.52)b | 1.6(.55)a |
| Smell of Marijuana | 0.5 (.35)a | 6.9 (.78)b | 7.3(.74)b | 1.0(.43)a |
| Thoughts about Smoking | 2.5(.65)a | 7.7(.64)b | 8.0(.60)b | 4.3(.83)a |
Note: Scores on the CAS items sight and smell ranges from 0 (didn’t notice at all) to 10 (completely paid attention) and from 0 (didn’t think about smoking at all) to 10 (thought about smoking all the time).
VR rooms in each row that do not share the same letter differ at p < .05.
There was a significant main effect of room/environment on attention to the smell of marijuana cues, F(2,38) = 58.1, p < .0001, with post hoc analyses revealing that attention to the smell of marijuana cues was significantly greater in the marijuana environment than neutral room 1, p < .0001, and neutral room 2, p < .0001. There was no significant difference in attention to the smell of marijuana cues between the two neutral rooms (see Table 1).
There was a significant main effect of room/environment on attention to marijuana thoughts, F(2,38) = 35.1, p < .0001, with post hoc analyses revealing that attention to marijuana thoughts was significantly greater in the marijuana environment than neutral room 1, p < .0001, and neutral room 2, p < .0001 There was no significant difference in attention to marijuana thoughts between the two neutral rooms (see Table 1).
Effect Sizes
Because VR is a relatively new method for examining craving in marijuana smokers, we calculated effect sizes using Cohen’s d, which is calculated as the difference between the mean of the experimental variable (e.g., party room) and the control variable (e.g., neutral room), divided by the pooled standard deviation (Cohen 1988), We obtained effect sizes for craving comparisons between the marijuana rooms and the neutral rooms. The largest effect size was between the first neutral room and the party room (d = 1.29) the smallest effect size was between the second neutral room and the paraphernalia room (d=.85), with an average effect size across both neutral and marijuana rooms (d = 1.07).
DISCUSSION
In a novel VR-based cue program, reactivity to VR cannabis cues were compared to neutral cues on measures of subjective craving and attention to stimuli. The main hypothesis was supported and current cannabis smokers experienced higher levels of craving and attention to stimuli for VR cannabis cues compared to VR neutral cues. Specifically, craving levels and attention to stimuli (sight, smell, thoughts of using) were significantly higher in the VR party and VR paraphernalia rooms compared to VR neutral rooms. We believe our results rest in the strength of the VR based cue system, in that the VR cannabis rooms were designed to go beyond simple presentation of photos or video stimuli often used in cue studies. The VR cannabis rooms allowed exposure to complex cues involving sight, smell, auditory: vibrotactile, social interactions and environment settings similar to real world situations that typical cannabis smokers might encounter. We believe that the strength of the complex cues offered led to the overall increase in craving from neutral room 1 to paraphernalia and party rooms. Interestingly, both craving and attention to smell of cannabis returned to baseline level after the final VR neutral room. This finding suggests that the VR neutral cues utilized in the current study provide enough immersion in an unrelated (neutral—non drug) environment to decrease craving, post exposure to drug cues. The neutral rooms were also designed to present a media rich neutral experience incorporating control stimuli for the visual, audio, vibrotactile, olfactory, and drug cue environments; however, these did not include a control experience for the social interactions.
The current study represents a milestone in the use of VR for cue reactivity in that this is the first study using VR-based cannabis cues to elicit craving The results of this study are similar to other VR cue studies in alcohol, nicotine and cocaine, all Of which have demonstrated VR as a suitable tool for increasing craving (Bordnick et al. 2005a, b, c, 2004; Baumann et al. 2003). More importantly, VR cue reactivity methods have produced strong cue responses as evidenced by large effect sizes (Bordnick et al. 2008). Cohen (1988) indicates that larger effects sizes are generally .8 or higher. An effect size of 1.07 found in the current study is therefore considered large, making the effects found here suitable for future studies that may attempt to manipulate this craving effect. In addition, these larger effect sizes provide an increased ability to detect smaller differences as a result of other experimental manipulations (e.g. craving context, anticraving medication effects).
Going beyond the large effect sizes found here, what are the advantages of using VR methods compared to currently used tools? This is a difficult question to answer since cannabis cue reactivity has been seldom studied. However, VR methods can offer several advantages over current approaches. VR can provide increased research and clinical utility allowing clinicians and researchers to have environmental stimuli and social interactions (i.e. drug offers, party settings) in their lab or office wilhoul having to arrange mock-social interactions using actors or other staff. For example, the clinician can have a participant immersed in a virtual party, with all of the stimuli experienced in a real world party, with just a few mouse clicks on a computer. VR–based approaches increase safety by providing settings/environments (i.e. marijuana party or crack house) for exposure that would be difficult to create realistically in an office setting, and for recreation of real world environments that may pose safety concerns and potential threats to confidentiality for the clinician or participant if encountered using real world exposure. In addition to the above, this type of VR system standardizes the presentation of complex cues in contextually congruent settings across research settings and trials, thus allowing repeated exposure to stimuli/cues with zero variance between presentations. We do not contend that non-VR approaches are necessarily Hawed with regards to experimental standardization; however, VR uses stimuli that are presented and timed via computer control versus that in which a human is controlling the time and stimuli presentations, thereby ensuring consistent exposure across participants.
Results of the current study should be interpreted with caution due to the following limitations. First, the sample is small and comprised of twenty participants who were moderate to heavy cannabis smokers without any other psychological disorders or drug use. Larger, more diverse samples are needed to determine if the current findings can be replicated and extended to other populations. Second, subjective measures of craving and attention (three items) were the primary dependent variables. The current results could be explained by potential prompting, bias, or expectation effects of experience by participants. In order to decrease this potential effect, future studies need to include additional objective measures, such as psychophysiological effects (heart rate, skin conductance), salivation, and neuro-imaging. Third, the VAS craving assessment although widely accepted in addiction research is composed of a single item and may not fully represent the stale known as drug craving or urge to use. However, we believe that craving, as with other cognitive and behavioral phenomena, is comprised of multidimensional factors and future studies need to incorporate more robust assessment of craving beyond a single item. The fourth and final limitation is lack of assessment of contextual factors and impact of VR on participants. We believe that VR-based cue reactivity offers a novel experience similar to real world situations, so new modified assessment tools may be needed to assess both the quantitative and qualitative data related to the experiences in VR environments.
Future research using technologies such as VR can offer new tools and potentially expand knowledge in the field of addictions treatment and research. The authors encourage additional studies to validate this system and compare VR to other non-VR approaches prior to widespread adoption. If future studies demonstrate that VR cue systems are equal to traditional approaches, then VR cue systems can be offered as an alternative, providing the added benefits cited. In addition, studies utilizing advanced neuro-imaging techniques (i.e. fMRI, SPECT) should be conducted to determine the utility of incorporating VR cue systems for stimuli presentation into protocols in addiction and other fields of research. In summary, VR-based cue systems are gaining support in addictions research through controlled trial sand impressive data; however, continued exploration is needed to evaluate and demonstrate the advances professed in this new technology.
Footnotes
This research was supported by National Institute on Drug Abuse Grant# 1R41 DA018454-01. Dr. Bordnick, Dr. Walton, Dr. Carter, Dr. Traylor, and Dr. Copp do not have conflicts of interest or financial interests in Virtually Better, Inc. The collaboration between Virtually Better, Inc. and Dr. Bordnick was completed under NIH/NIDA STTR grant #1R41 DA018454-01.
REFERENCES
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders (Text Revision) Fourth, Text Revision Ed. APA; Washington, DC: 2000. [Google Scholar]
- Baumann S, Neff C, Fetzick S, Stangl G, Basler L, Vereneck R, Schneider W. A virtual reality system for neurobehavioral and functional MRI studies. CyberPsychology & Behavior. 2003;6(3):259–66. doi: 10.1089/109493103322011542. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Graap K, Virtual Reality Nicotine Cue Reactivity Assessment System (VR-NCRAS) (Version 1.0) [PC] Virtually Better, Inc.; Decatur, GA: 2004. [Google Scholar]
- Bordnick PS, Graap KM, Ferrer M. Virtual Reality Cannabis Cue Reactivity Assessment System (VR-CCRAS) (Version 1.0) [PC] Virtually Better, Inc.; Decatur: 2006. [Google Scholar]
- Bordnick PS, Copp HL, Traylor A, Graap KM, Ferrer M, Brooks J. Assessing reactivity to virtual reality alcohol based cues including olfactory stimuli. Addictive Behaviors. 2008;33:743–56. doi: 10.1016/j.addbeh.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Graap KM, Brooks J, Ferrer M. Virtual Reality Alcohol Cue Reactivity Assessment System (VR-ACRAS) (Version 1.0) [PC] Virtually Better Inc; Decatur, GA: 2005a. [Google Scholar]
- Bordnick PS, Graap KM, Copp HL, Brooks J, Ferrer M. Virtual reality cue reactivity assessment in cigarette smokers. Cyberpsychology and Behavior. 2005b;8(5):487–92. doi: 10.1089/cpb.2005.8.487. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Traylor AC, Graap KM, Copp HL, Brooks J. Virtual reality cue reactivity assessment: A case study ia a teen smoker. Applied Psychophysiology & Biofeedback. 2005c;30(3):187–93. doi: 10.1007/s10484-005-6376-0. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Graap KM, Copp HL,, Brooks JS, Ferrer M, Logue B. Utilizing virtual reality to standardize nicotine craving research: A pilot study. Addictive Behaviors. 2004;29:1889–94. doi: 10.1016/j.addbeh.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94(9):131121. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes IR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004;161(11):1967–77. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Archives of General Psychiatry. 2001;58:917–24. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Radonovich KJ, Higgins ST, Wong CJ. Adults seeking treatment for marijuana dependence: A comparison with cocaine-dependent treatment seekers. Experimental and Clinical at Psychopharmacology. 1998;6(4):419–26. doi: 10.1037//1064-1297.6.4.419. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. In: Onken LS, Blaine JD, Boren JJ, editors. Behavioral Treatment for Drug Dependence. NIH; Washington: 1993. NIDA Research Monograph 137. [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second Ed. Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United Slates: 1991 - 1992 and 2001-2002. Journal of the American Medical Association. 2004;291(17):2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Cutler R. Abatement of craving in recovering alcoholics: A descriptive analysis. Addiction Research and Theory. 2005;13(2):111–27. [Google Scholar]
- Drobes DJ,, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106(1):15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Addictive potential of cannabinoids: The underlying neurobiology. Chemistry and Physics of Lipids. 2002;121:267–90. doi: 10.1016/s0009-3084(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Archives of General Psychiatry. 1986;43:107–13. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singelton EG. Assessment of cannabis craving using the Marijuana Craving Questionnaire. In: Onaivi ES, editor. Methods in Molecular Medicine: Marijuana and Cannabinoid Research: Methods and Protocols. Humana Press; Totowa. N.J: 2006. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacohgy (Berlin) 2001;155(1):27–34. doi: 10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Chen YA, Schmitz J, Bordnick P, Shafer A. Cue reactivity in cocaine-dependent subjects: Effects of cue type and cue modality. Addictive Behaviors. 1998;23(1):7–15. doi: 10.1016/s0306-4603(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Lundahl LH, Borden KN, Lukas SE. Marijuana cue-induced craving in cannabis dependent adolescents in psychiatric treatment; Paper presented at the College on Problems of Drug Dependence; Scottsdale. AZ. 2001. [Google Scholar]
- Manschreck TC. The treatment of cocaine abuse. Psychiatric Quarterly. 1993;64:183–97. doi: 10.1007/BF01065869. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Abrams DB. Alcohol cue-rcactivity: Effects of detoxification and extended exposure. Journal of Studies on Alcohol. 1993;54(2):235–45. doi: 10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenovv DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addictive Behaviors. 1998;23(2):209–24. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to Smoking-related stimuli and early relapse to smoking. Addictive Behaviors. 1989;14(4):419–28. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA) Marijuana Abuse. National Institutes of Health; Bethesda, MD: 2005. NtH Publication Number 05-3859. [Google Scholar]
- O’Brien CP;, Childress AR, McLellan AT, Ehrman R. Developing treatments that address classical conditioning. In: Tims FM, Leukefeld CG, editors. Cocaine Treatment: Research And Clinical Perspectives. NIH; Washington: 1993. NIDA Research Monograph 135. [PubMed] [Google Scholar]
- Prakash A, Das G. Cocaine and the nervous system. International journal of Clinical Pharmacology, Therapy, and Toxicoiogy. 1993;31:575–81. [PubMed] [Google Scholar]
- Preston KL, Jasinski DR. Abuse liability studies of opioid agonist-antagonists in humans. Drug and Alcohol Dependence. 1991;28:49–82. doi: 10.1016/0376-8716(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Brady KT, Graap KM, Rothbaum BO. VR to evaluate craving and cue reactivity in cocaine dependent individuals. Addictive Behaviors. 2006;31(10):1881–94. doi: 10.1016/j.addbeh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Satel S. Craving for and fear of cocaine. In: Kosten TR, Kleber HD, editors. Clinician’s Guide to Cocaine Addiction. Guilford Press; New York: 1992. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2003 National Survey on Drug Use and Health: National Findings. DHHS; Rockville, MD: 2004. NSDUH Series H-25. DHHS Pub. No. (SMA) 04-3964. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Summary of Findings from the 2001 National Household Survey on Drug Abuse: Volume II. DHHS; Rockvilie, MD: 2002. (No. (SMA) 02-03759) [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarettc smokers. Journal of Consulting and Clinical Psychology. 2000;68(2):233–40. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Wallace BC. Crack Cocaine. Brunner/Mazel; New York: 1989. [Google Scholar]
- Washton AM. Outpatient treatment techniques. In: Washton AM, Gold MS, editors. Cocaine: A Clinician’s Handbook. Guilford Press; New York: 1987. [Google Scholar]