Abstract
This review will examine existing results on the postoperative treatment of women with high-risk and advanced stage endometrial cancer. Preliminary data suggests that response to treatment is highly dependent on both grade and stage. It is hoped that this discussion will highlight deficiencies in our collective knowledge base to be addressed in future clinical trials for the benefit of women with endometrial cancer.
Keywords: Endometrial cancer, radiotherapy, chemotherapy, uterine serous carcinoma
Introduction
Controversy surrounding surgical staging and the role of lymphadenectomy in patients with endometrial cancer has occupied center stage at clinical congresses nationally and internationally. While we remain in need of a surgical standard of care, this decades-old preoccupation has in some ways distracted us from crucial considerations necessary to improve oncologic outcomes: namely, which patients are most likely to die of disease as opposed to co-morbid conditions, and how are they most effectively treated? This review will explore these fundamental questions by assessing investigations of patients who received post-operative treatment for high-risk or advanced stage disease. It is hoped that this discussion will highlight deficiencies in our collective knowledge base that will be addressed in future clinical trials for the benefit of women with endometrial cancer.
As a starting point for discussion, figure 1 is a graphic representation of 1303 consecutive patients surgically treated for endometrial cancer at a single institution. To account for inconsistencies in staging techniques around the world, in this figure patients are stratified by uterine risk factors alone. Considering the potential for trial enrollment, the low and low intermediate risk groups are a tempting cohort to study given they represent 60% of all women with endometrial cancer and 70% of women with endometrioid lesions. However, overall survival (OS) was 93% and disease specific survival (DSS) 99%, indicating that these women are far more likely to die of comorbidities than of endometrial cancer itself (only 16% of deaths in low-risk patients are cancer related) [1]. In other words, in the Unites States, endometrial cancer is most commonly not an oncologic threat, but a public health dilemma most effectively addressed with interventions aimed at promoting an active lifestyle and healthy diet. In stark contrast, the 40% of patients with high-risk and stage IV disease have an appreciable risk of treatment failure and death. While this represents a smaller cohort, the need to improve their oncologic outcomes is more urgent. In fact, only 8% of endometrial cancer-related deaths are in low and low intermediate risk patients, while 86% of recurrences and 90% of cancer-related deaths occur in the remaining risk groups.
Figure 1.
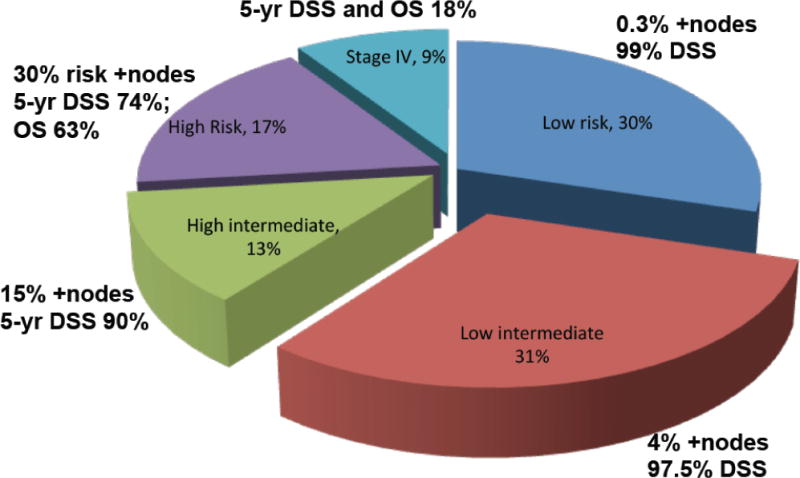
Representation of 1303 consecutive patients with endometrial cancer treated surgically at Mayo Clinic, Rochester, with accompanying risk of lymphatic metastases and survival. Note that in the Mayo risk classification system patients are stratified by uterine risk factors alone, not stage. Low risk: endometrioid grade 1 or 2, ≤50% myometrial invasion (MI), and primary tumor diameter (PTD) ≤2cm; endometrioid without MI; Low intermediate risk: low risk grade 1 or 2 cases, but PTD >2cm or PTD unknown; High intermediate risk: endometrioid grade 1 or 2 and >50% MI; endometrioid grade 3 and ≤50% MI; High risk: non-endometrioid; endometrioid grade 3 and >50% MI; adnexal, vaginal, or parametrial involvement; Stage IV: FIGO stage IV.
High risk endometrial cancer
Considering the high risk group, a substantial proportion will have positive lymph nodes (Fig 1). However, irrespective of lymph node status, 30% will develop hematogenous recurrences with accompanying 5-year survival of less than 70% [2, 3]. A number of investigators have therefore attempted to improve outcomes through the use of adjuvant therapies. ASTEC/EN.5 randomized 905 women with high grade and any MI or low grade and >50% MI to external beam irradiation therapy vs. observation [4]. While not a pure cohort, 72% of patients were of endometrioid histology with >50% MI, providing useful information on the high-risk group. Given their high underlying risk of hematogenous dissemination, it is not surprising that regional radiation did not improve outcomes, even when stratified by intermediate or high risk of recurrence. To address this problem of distant metastases, JGOG randomized patients with stage I-III endometrial cancer, all with MI >50%, to either pelvic radiation therapy or cyclophosphamide–doxorubicin–cisplatin (CAP) chemotherapy [5]. No difference in outcomes was found for the entire cohort. However, significant improvements were seen for specific risk categories. Notably, the 5-year progression free survival (PFS) (84% vs. 66%, HR 0.44; p=0.02) and overall survival (OS) (90% vs. 74%, HR 0.24; p<0.01) favored CAP for high intermediate risk patients (HIR, n=120). The investigators defined HIR as follows (all with >50% MI): patients over age 70 years, grade 3 of any age, stage II, or IIIA (positive cytology). The observed difference in OS is convincing, with a few caveats. First, type II histologies were excluded in this trial (note: for the purposes of this review, type I is defined as endometrioid histology irrespective of grade; type II refers to serous or clear cell carcinomas). Second, only 14% of patients were grade 3. We can therefore conclude from these two trials that 1) radiation therapy does not appear to appreciably impact disease specific, recurrence free, or overall survival in patients with high risk endometrial cancer; 2) chemotherapy may improve OS in a subset of patients with deep MI, recognizing that little data exists for high grade and serous lesions. The nuance that heterogeneous carcinomas require tailored therapies should not be lost and is purposely repeated throughout this review.
At first read, the second caveat appears unnecessarily conservative given that poorly differentiated lesions are generally thought to have better initial responses to cytotoxic therapy than their well-differentiated counterparts. But histologic subtype, and more precisely grade (discussed later), appears to be a critical consideration when predicting response to therapy, as further supported by trials in advanced stage patients. Hogberg, et al., pooled 540 patients with high-risk stage I/II and stage III endometrial cancer randomly assigned to pelvic radiotherapy with or without sequential chemotherapy from NSGO and MaNGO [6]. Half of patients were grade 3, 70% type I, and 56% were stage IB or IC (FIGO 2009 stage IB). The combination resulted in a 37% reduction in the risk for relapse or death (p<0.01), a 45% reduction in DSS (p=0.01) and a 31% reduction in OS that approached statistical significance (p=0.07). However, there was no difference in PFS for patients with serous or clear cell carcinomas (HR for PFS and OS was 0.83 (p=0.59) and 0.94 (p=0.88), respectively). GOG 122 randomized 396 patients with stage III/IV endometrial cancer to whole abdominal irradiation therapy vs. doxorubicin and cisplatin [7]. Grade 3 patients accounted for 53%, and 26% were type II. Distinct from previously reviewed investigations, this was not a pure adjuvant trial as 16% harbored gross residual disease at the time of treatment. The use of chemotherapy was associated with improved 5-year PFS and OS (HR 0.68; p<0.01). However, an infrequently quoted finding from this trial was that while chemotherapy appeared to be efficacious for endometrioid patients, PFS and OS were not statistically different for patients with type II histologies (HR 0.91 and 1.03, respectively). A third investigation pooled over 1200 patients from 4 randomized GOG trials [8]. Although response rates were similar between histologic subtypes (44% response rate for both endometrioid and serous; 32% for clear cell), 70% of patients were recurrent and 55% had received prior radiation therapy limiting extrapolation of these responses to high risk stage I or untreated stage III/IV patients. Furthermore, type I patients were predominately grade 3, so the investigation was in effect a comparison of high-grade patients of differing histologies. In GOG 184, histology was associated with PFS on multivariable analysis, but only when grade 1 was the reference group. PFS was no different between serous, clear cell, and grade 3 endometrioid lesions [9]. In a review of 4180 high-grade cases from the Surveillance, Epidemiology, and End Results (SEER) database, improved outcomes were demonstrated for endometrioid carcinomas compared to type II histologies after controlling for stage [10]. However, SEER is a poor tool to assess outcomes given the rarity of definitive staging, lack of central pathology review, and most importantly, absence of information on adjuvant treatment. Other studies have failed to demonstrate significant differences in survival among high-grade subtypes [11–14]. The important distinction to be made is that irrespective of histology, response and outcomes for patients with high-grade endometrial cancers is similarly poor.
We recently reported on the outcomes of 450 patients with stage I/II endometrial cancer, limited to type II or type I grade 3 [15]. As shown in Figure 2, OS (and DSS, not shown) were no different between histologies. The distant failure rate was 10%, and the use of chemotherapy in these high-grade lesions did not confer a significant survival advantage, even on univariate analysis. The absence of treatment effect in this retrospective assessment is notable given that treatment in the adjuvant setting is most likely to be withheld from the sickest patients, presumably inflating potential survival differences between groups. The potential risk for treatment bias is particularly evident for retrospective series of early stage uterine serous cancer (USC), which tend to be diagnosed in elderly patients. While some series have demonstrated apparent reductions in recurrence rates with chemotherapy, others have not, with wide variations in outcome ([15–19]). For example, Nickles Fader reported 88% DSS for 142 stage I USC treated with chemotherapy, while Ayeni reported 90% DSS in 96 stage I USC despite the fact that 72% did not receive chemotherapy [15, 16]. Boll, et al., examined outcomes for 25,804 patients with endometrial cancer between 1989 and 2009 [20]. Although treatment was not described in this investigation, the study period corresponds with increasing rates of chemotherapy administration. A statistically significant improvement in survival was seen for grade 1 and 2 (89% to 93%), but not grade 3 (60% to 61%) endometrial cancer. Collectively, these data add to the randomized results from GOG 122 and from Hogberg et al., suggesting that grade 3 endometrial cancer, whether type I or type II, may be relatively resistant to platinum-based chemotherapy. This conclusion is far from definitive given the inclusion of varying chemotherapeutic regimens in heterogeneous cohorts. Furthermore, this question has not been a primary endpoint, but rather studied as an unplanned and underpowered analysis. But until we are able to advance our molecular subclassification to an extent that it reliably informs chemosensitivity, this question deserves investigation. At this time we may conclude that node negative patients with >50% MI may derive benefit from platinum based chemotherapy. Evidence also exists that this effect may be limited to patients with low-grade lesions and should be a subject of future study.
Figure 2.
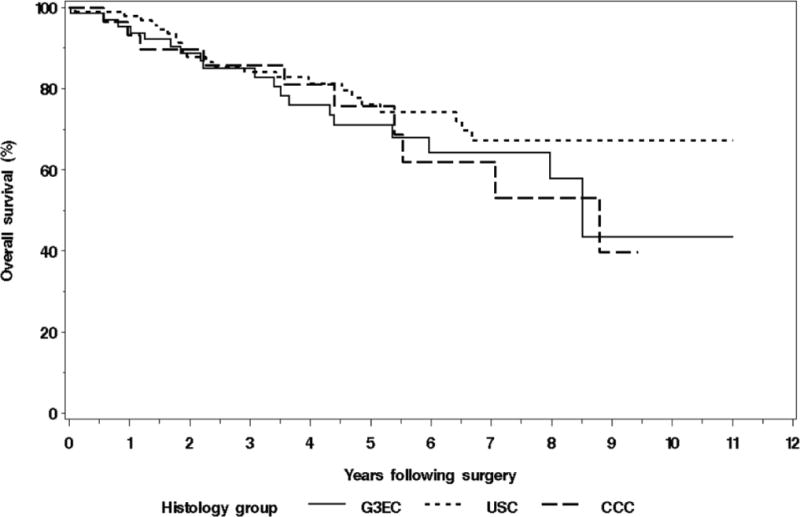
Kaplan-Meier survival curves for stage I/II patients with endometrioid, serous, and clear cell carcinoma of the uterine corpus, all high grade. Note absence of difference in overall survival (or disease specific survival, not shown)(14).
The European Network of Gynaecological Oncological Trial Groups (ENGOT) has been tremendously successful and demonstrated a willingness to take on a number of important questions. One example is a trial randomizing patients with stage I/II, grade 3 (including type II) tumors (all lymph node negative) to 6 cycles of carboplatin and paclitaxel vs. observation (ENGOT-EN2-DGCG/EORTC 55102). To date 28 of a planned 678 patients have enrolled (personal communication), with an estimated primary completion date of January 2018 (Table 1). This investigation will provide important data on this subject, although a subanalysis or separate trial will be necessary to investigate patients with deeply invasive, high-grade lesions, and the relative impact of histologic subtype. GOG 249 randomized patients with high risk, early stage endometrial cancer to pelvic radiotherapy versus vaginal cuff brachytherapy followed by carboplatin and paclitaxel. It will be interesting to learn if chemotherapy reduces the rate of distance recurrences, although responses may be difficult to evaluate given the heterogeneous nature of the cohort: GOG 99 high intermediate risk factors (itself a heterogeneous group), occult stage II, or stage I/II type II histology [21]. This study completed enrollment in February 2013.
Table 1.
Ongoing randomized trials of particular interest for patients with endometrial cancer. Note wide variation in inclusion criteria.
| Trial | Treatment | Patient Cohort | Primary Completion Date |
|---|---|---|---|
| ENGOT-EN2-DGCG/EORTC55102 | Chemotherapy vs. observation | Stage I/II (grade 3 or type II histology) | January 2018 |
| GOG 249 | Pelvic radiation vs. vaginal brachytherapy and chemotherapy | GOG 99 high intermediate risk, stage II, or stage I/II with type II histology | Completed February 2013 |
| PORTEC 3 | Pelvic radiation with or without concomitant and post-radiation chemo | Stage I (grade 3 with LVSI or >50% MI; type II histology), stage II, stage IIIA, IIIC | Completed; Results December 2018 |
| GOG 258 | Chemo alone vs. pelvic radiation with concomitant and post-radiation chemo | Stage I/II (type II with positive cytology), stage III–IVA | February 2016 |
Advanced disease
Treatment options for patients with positive lymph nodes or stage IV disease at diagnosis are severely limited at present. Five-year survival rates of less than 50% for patients with lymph node metastases, and less than 20% for patients with peritoneal or distant disease are unfavorable even compared to advanced ovarian cancer. Relatively few investigations have been completed and as reviewed above have been plagued by heterogeneous cohorts. High risk stage I, stage III, and stage IV patients are commonly studied in aggregate despite clear difference in outcome and in patterns of recurrence [2]; hence our naivety to the nuances of treatment efficacy. For example, a patient at high risk for distant or peritoneal failure (stage IV) would not be expected to obtain significant benefit from a loco-regional therapy such as pelvic radiation, while radiotherapy may be extremely effective for patients at high risk of local failure (stage IIIC).
Maggi, et al, randomized patients with high grade, deep MI, or positive nodes to pelvic radiotherapy vs. chemotherapy; radiation improved local control while chemotherapy reduced distant recurrences, but there were no differences in PFS or OS [22]. However, only 22% were stage IIIC and so the efficacy of radiation in this particular cohort remains unknown. The JGOG trial was also underpowered to answer this question, although a non-significant advantage was seen in the RT arm compared to CAP when limited to stage III patients (5-year PFS 79% vs. 64%; p=0.169)[5]. Primary stage IV patients are rarely investigated in isolation, and so the true efficacy of chemotherapy for patients with peritoneal disease remains unanswered. GOG 122 demonstrated improved PFS, but not OS, for stage IV patients treated with chemotherapy [7]. Table 2 outlines the most noteworthy randomized trials comparing radiotherapy to chemotherapy (with or without radiotherapy). Differences in inclusion criteria and chemotherapy regimen are highlighted which limit inter-trial comparisons and prohibit more definitive recommendations at this time.
Table 2.
Randomized trials comparing radiotherapy vs. chemotherapy (with or without radiotherapy) in patients with stage I–IV endometrial cancer. Chemotherapy was platinum-based in all investigations. MI: myometrial invasion; RT: external beam radiotherapy; WAI: whole abdominal irradiation; OS: overall survival; PFS: progression free survival.
| Author | Treatment | Ml >50% | Stage I/II | Stage IIIC | Stage IV | Grade 3 | Residual Disease | Results |
|---|---|---|---|---|---|---|---|---|
| Susumu; JGOG (5) | RT vs. chemo | 100% | 35% | 22% | 0% | 56% | 0% | Chemo superior OS for high intermediate risk patients |
| Maggi; Italy (15) | RT vs. chemo | 72% | 78% | 22% | 0% | 57% | 0% | No change in PFS or OS |
| Randall; GOG 122 (7) | RT (WAI) vs. chemo | – | 0% | 73% | 27% | 52% | 16% | Chemo superior OS; No change for type II or stage IIIC |
| Hogberg; NSGO, MaNGO (6) | RT vs. RT + chemo | – | 55% | 45% | 0% | 50% | 0% | RT + chemo superior DSS: No change for type II |
In an attempt to identify recurrence patterns and to further investigate the absence of treatment effect in regards to high-grade lesions in GOG 122 and the Hogberg trial, we retrospectively analyzed response in patients with grade 3, stage IIIC and IV endometrial cancer [15]. While radiation therapy (with or without chemotherapy) were predictive of survival on multivariate analysis for stage IIIC patients, chemotherapy alone was not. In contrast, the use of chemotherapy was predictive of outcome on multivariate analysis for stage IV patients, but radiotherapy was not. A recent multicenter study limited to stage IIIC patients provides analogous results [23]. Three-year PFS was significantly worse for patients treated with chemotherapy alone vs. radiation alone or radiation with sequential chemotherapy (PFS 56%, 73%, and 72%, respectively). In fact, patients treated with chemotherapy alone were at a 2.2 fold increased risk of recurrence and 4 fold increased risk of death compared to patients whose treatment included radiation. But as discussed above in the context of high-risk stage I patients, grade appears to be equally important for predicting response to treatment for stage IIIC disease. In a separate investigation we analyzed response in stage IIIC cases by grade; chemotherapy was independently associated with DFS for grade 1/2 cases, but not for grade 3 cases [24]. Extra-pelvic 5-yr DFS was 93% and 52% for low-grade patients treated with and without chemotherapy, respectively (p=0.01). In contrast, chemotherapy did not appear to impact extra-pelvic recurrences for grade 3 patients (extra-pelvic 5-yr DFS of 43% and 42% for patients treated with and without chemotherapy, respectively). Similarly, stratification of patients by grade in the Hogberg trial demonstrated improved PFS for grade 1/2 patients who received chemotherapy with radiation compared to radiation alone, but no difference was observed for grade 3 [6]. This leads to an important point: the importance of radiotherapy for stage IIIC patients cannot be discounted. Recent evolutions in practice, particularly in parts of Europe, have omitted pelvic radiation in favor of chemotherapy alone for stage III patients. But it is important to recognize that GOG 122 demonstrated that at best, response to chemotherapy in stage IIIC patients was inferior to that seen for stage IV. Evidence of varying responses to chemotherapy by grade, reviewed above, cast further doubt on the wisdom of omitting radiation in favor of chemotherapy alone outside of clinical trials.
The retrospective data and subanalyses reviewed here should be used with caution to direct clinical decision-making, yet should be seriously considered when designing future trials. The available literature demonstrates clear dissimilarities in treatment effect and patterns of failure between stage III and IV patients; combing these disparate cohorts in clinical trials therefore cannot be justified by convenience alone. Two important investigations in progress include cohorts heterogeneous with respect to stage and grade, but may provide data to confirm or refute potential concerns raised above (Table 1). Despite deficiencies in study design, ongoing QOL corollaries in these investigations will provide greatly needed data. PORTEC 3 randomized patients with stage I (grade 3 with LVSI or >50% MI; type II histology), stage II, and stage III endometrial cancer to pelvic radiation vs. pelvic radiation with concomitant chemotherapy and post-radiation chemotherapy. Enrollment has closed with an estimated study completion date of late 2018 (Table 1). GOG 258 randomizes patients with stage I/II (type II with positive cytology) to IVA endometrial cancer to radiation with concomitant chemotherapy and postradiation chemotherapy vs. chemotherapy alone, with an estimated primary completion date of February 2016.
Conclusions
Just as concerns about the short and long-term morbidity of surgical interventions (i.e. lymphadenectomy) are valid for patients unlikely to derive benefit, so are concerns about the side effects of non-surgical interventions (i.e. chemotherapy or radiotherapy) for patients unlikely to respond. One-third of patients with advanced endometrial cancer will not complete the recommended course of chemotherapy due to toxicity and in GOG 122, 88% of patients had at least one grade 3/4 toxicity [7]. Long term follow-up of patients who received whole pelvic irradiation in the Post-Operative Radiation Therapy in Endometrial Carcinoma (PORTEC) trial demonstrated a 20% absolute increase in incontinence, more diarrhea, fecal urgency and leakage leading to limitations of daily activities, worse sexual function for patients treated with either brachytherapy or pelvic radiation, and nearly double the risk of developing a secondary malignancy [25, 26]. While long-term assessments of the side effects of combination modalities are poorly studied, they are unlikely to be better tolerated than either modality alone. The old adage remains true today: Poor prognosis does not justify ineffective treatment, particularly when toxic. We must pursue improvements in the value and quality of our care with as much vigor as we pursue improvements in survival.
In total, these data are sufficiently compelling to suggest that varying treatment approaches may be needed based on both stage and grade: more simply, endometrial cancer is heterogeneous and will require specific therapy based on risk factors. This conclusion should come as no surprise to anyone in this emerging age of genomics. Grade and stage are simply surrogates for molecular subclassifications that will reveal themselves once our capacity to interpret sequencing and methylation data matches our ability to collect it. For now, using the centuries-old tools of surgery and light microscopy, we can infer that low-grade lesions may be more chemosensitive than high-grade lesions, that discounting radiotherapy for patients with isolated lymphatic metastases may be premature, and that chemotherapy appears to be beneficial for patients with stage IV disease. The corollary is that we should not miss the opportunity to question the efficacy of chemotherapy in high-grade patients, and radiotherapy for stage IV patients in ongoing clinical trials. Despite the apparent benefit of chemotherapy in patients with peritoneal disease, a 2-year failure rate of 80%, a 5-yr OS of 8–12% for high-grade lesions, and a lack of substantive improvement in outcome over decades of clinical trials highlight the desperate need for innovative approaches to treatment [15]. Continued investigations of old cytotoxic therapies in various permutations of dose and schedule with a dash of Bevacizumab will guarantee at best incremental rather than revolutionary progress. Given current outcomes, high-grade stage IV patients should be enrolled into phase I trials at diagnosis, the cohort in most urgent need of novel therapies.
Highlights.
Existing and planned trials in high risk and advanced stage endometrial cancer have included heterogenous cohorts
Preliminary evidence exists that response to chemotherapy may be correlated with both stage and grade
Consideration should be given to enrolling stage IV patients into phase I trials at diagnosis given their poor prognosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no conflicts of interest for this manuscript.
References
- 1.Dowdy SC, Borah BJ, Bakkum-Gamez JN, Weaver AL, McGree ME, Haas LR, Keeney GL, Mariani A, Podratz KC. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012;127:5–10. doi: 10.1016/j.ygyno.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 2.Mariani A, Dowdy SC, Keeney GL, Long HJ, Lesnick TG, Podratz KC. High-risk endometrial cancer subgroups: candidates for target-based adjuvant therapy. Gynecol Oncol. 2004;95:120–6. doi: 10.1016/j.ygyno.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 3.Mariani A, Webb MJ, Keeney GL, Calori G, Podratz KC. Hematogenous dissemination in corpus cancer. Gynecol Oncol. 2001;80:233–8. doi: 10.1006/gyno.2000.6058. [DOI] [PubMed] [Google Scholar]
- 4.Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, Eisenhauer E, Bacon M, Tu D, Parmar MK, Amos C, Murray C, Qian W. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–46. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, Kudo R. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226–33. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, Andersson H, Grenman S, Lundgren C, Rosenberg P, Boman K, Tholander B, Scambia G, Reed N, Cormio G, Tognon G, Clarke J, Sawicki T, Zola P, Kristensen G. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer–results from two randomised studies. Eur J Cancer. 2010;46:2422–31. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, Thigpen JT, Benda JA. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 8.McMeekin DS, Filiaci VL, Thigpen JT, Gallion HH, Fleming GF, Rodgers WH. The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;106:16–22. doi: 10.1016/j.ygyno.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 9.Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, Morris RT, DeGeest K, Lee R, Montag A. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:543–52. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, Powell MA, Hendrickson MR, Kapp DS, Chan JK. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–6. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alektiar KM, McKee A, Lin O, Venkatraman E, Zelefsky MJ, McKee B, Hoskins WJ, Barakat RR. Is there a difference in outcome between stage I–II endometrial cancer of papillary serous/clear cell and endometrioid FIGO Grade 3 cancer? Int J Radiat Oncol Biol Phys. 2002;54:79–85. doi: 10.1016/s0360-3016(02)02913-9. [DOI] [PubMed] [Google Scholar]
- 12.Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95:593–6. doi: 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Fader AN, Starks D, Gehrig PA, Secord AA, Frasure HE, O’Malley DM, Tuller ER, Rose PG, Havrilesky LJ, Moore KN, Huh WK, Axtell AE, Kelley JL, Zanotti KM. An updated clinicopathologic study of early-stage uterine papillary serous carcinoma (UPSC) Gynecol Oncol. 2009;115:244–8. doi: 10.1016/j.ygyno.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, Wei W, Sundar S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol. 2012;124:15–20. doi: 10.1016/j.ygyno.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Ayeni TA, Bakkum-Gamez JN, Mariani A, McGree ME, Weaver AL, Haddock MG, Keeney GL, Long HJ, 3rd, Dowdy SC, Podratz KC. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecol Oncol. 2013;129:478–85. doi: 10.1016/j.ygyno.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Fader AN, Drake RD, O’Malley DM, Gibbons HE, Huh WK, Havrilesky LJ, Gehrig PA, Tuller E, Axtell AE, Zanotti KM. Platinum/taxane-based chemotherapy with or without radiation therapy favorably impacts survival outcomes in stage I uterine papillary serous carcinoma. Cancer. 2009;115:2119–27. doi: 10.1002/cncr.24247. [DOI] [PubMed] [Google Scholar]
- 17.Kelly MG, O’Malley DM, Hui P, McAlpine J, Yu H, Rutherford TJ, Azodi M, Schwartz PE. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98:353–9. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Slomovitz BM, Burke TW, Eifel PJ, Ramondetta LM, Silva EG, Jhingran A, Oh JC, Atkinson EN, Broaddus RR, Gershenson DM, Lu KH. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol. 2003;91:463–9. doi: 10.1016/j.ygyno.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MB, Mariani A, Cliby WA, Keeney GA, Podratz KC, Dowdy SC. Role of systematic lymphadenectomy and adjuvant therapy in stage I uterine papillary serous carcinoma. Gynecol Oncol. 2007;107:186–9. doi: 10.1016/j.ygyno.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Boll D, Karim-Kos HE, Verhoeven RH, Burger CW, Coebergh JW, van de Poll-Franse LV, van Doorn HC. Increased incidence and improved survival in endometrioid endometrial cancer diagnosed since 1989 in The Netherlands: a population based study. Eur J Obstet Gynecol Reprod Biol. 2013;166:209–14. doi: 10.1016/j.ejogrb.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 22.Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, Colombo A, Fossati R. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–71. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secord AA, Geller MA, Broadwater G, Holloway R, Shuler K, Dao NY, Gehrig PA, O’Malley DM, Finkler N, Havrilesky LJ. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013;128:65–70. doi: 10.1016/j.ygyno.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Bakkum-Gamez JN, Mariani A, Dowdy SC, Weaver AL, McGree ME, Martin JR, Keeney GL, Jatoi A, Gostout BS, Podratz KC. Efficacy of contemporary chemotherapy in stage IIIC endometrial cancer: A histologic dichotomy. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creutzberg CL, Nout RA, Lybeert ML, Warlam-Rodenhuis CC, Jobsen JJ, Mens JW, Lutgens LC, Pras E, van de Poll-Franse LV, van Putten WL. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e631–8. doi: 10.1016/j.ijrobp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Nout RA, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A, Stenfert Kroese MC, Nijman HW, van de Poll-Franse LV, Creutzberg CL. Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer. 2012;48:1638–48. doi: 10.1016/j.ejca.2011.11.014. [DOI] [PubMed] [Google Scholar]


