Abstract
Background
Interim results from the CHER trial showed that early antiretroviral therapy (ART) was life-saving for HIV-infected infants. Given limited options and potential for toxicity with life-long ART, CHER compared early limited ART with deferred ART.
Methods
CHER was an open 3-arm trial in HIV-infected asymptomatic infants aged <12 weeks with CD4% ≥25%. Infants were randomized to deferred (ART-Def) or immediate ART for 40weeks (ART-40W) or 96weeks (ART-96W), followed by interruption. Criteria for ART initiation in ART-Def and re-initiation after interruption were CD4% <25% in infancy; otherwise <20% or CDC severe stage B or stage C disease. Lopinavir-ritonavir, zidovudine, lamivudine was the first-line regimen at ART initiation and re-initiation. The primary endpoint was time-to-failure of first-line ART (immunological/clinical/virological) or death. Comparisons were by intent-to-treat, using time-to-event methods.
Findings
377 infants were enrolled: median age 7.4weeks; CD4% 35% and HIV RNA log 5.7copies/ml. Median follow-up was 4.8 years; 34 (9%) were lost-to-follow-up. Median time to ART initiation in ART-Def was 20 (IQR 16–25) weeks. Time to restarting ART after interruption was 33 (26–45) weeks in ART-40W and 70 (35–109) weeks in ART-96W; at trial end 19% and 32% respectively, remained off ART. Proportions of follow-up time spent on ART were 81%, 70% and 69% in ART-Def, ART-40W and ART-96W arms. 48/125(38%), 32/126(25%) and 26/126(21%) children reached the primary endpoint; hazard ratio (95%CI), relative to ART-Def, was 0.59(0.38-0.93, p=0.02) for ART-40W and 0.47(0.27-0.76, p=0.002) for ART-96W. Seven children (3 ART-Def, 3 ART-40W, 1 ART-96W) switched to second-line ART.
Interpretation
Early limited ART had superior clinical/immunological outcome with no evidence of excess disease progression during subsequent interruption and less overall ART exposure than deferred ART.
Longer time on primary ART permits longer subsequent interruption with marginally better outcomes.
Introduction
Human Immunodeficiency Virus (HIV-1) infection causes high mortality and rapid disease progression in infants.1, 2 If untreated, over one third die during infancy and approximately half by two years.3, 4 Although early antiretroviral therapy (ART) is lifesaving, its duration will be life-long. ART options are limited in resource-limited settings and further restricted in infants because of formulation and pharmacokinetic limitations and the risk of resistance following exposure to drugs to prevent mother-to-child transmission (pMTCT). 5Cumulative effects of treatment in the growing child are concerning, with long-term pharmacovigilance data lacking. Therefore, we hypothesized that a strategy of early limited ART started close to primary infection, compared with deferred ART, would prevent disease progression and safely allow a subsequent period off ART, thus preserving future treatment options.
In 2007, when the median follow-up time was40 (IQR: 24 - 58) weeks, interim data showed that early ART reduced risk of death by 75% compared with deferred ART;2 subsequently becoming standard of care.6-8 We now report the 5-year results of the completed CHER trial.
Methods
Study Design and Participants
HIV-infected infants aged 6-12 weeks with confirmed HIV infection tests and CD4% ≥25% were eligible. No previous ART was permitted, apart from pMTCT. Exclusion criteria included: birth weight <2kg, Grade 3 or 4 laboratory (transaminases, neutrophil count, haemoglobin, electrolytes and creatinine) or clinically significant medical events or life-threatening congenital abnormalities. 2 Infants were randomly assigned to one of three strategies: deferred therapy (ART-Def), early limited ART for 40 weeks (ART-40W) or early limited ART for 96 weeks (ART-96W). The immunologic criterion for initiating ART in ART-Def or re-initiating continuous ART following interruption was CD4% <20% (later revised to CD4% <25% or CD4 count <1000 cells/mm3 during infancy).9 Corresponding clinical criteria were protocol-defined Centers for Disease Control and Prevention (CDC) severe stage B or stage C events, the former including oxygen-dependent lymphoid interstitial pneumonitis or bronchiectasis, nephropathy and cardiomyopathy. Failure-to-thrive not meeting CDC stage C criteria, recurrent pneumonia and severe oral candidiasis were added during the trial to promptstarting and restarting ART. (See protocol in web appendix for definitions)
The first 40 infants with baseline CD4 <25% were randomised to ART-40W or ART-96W in a parallel study designated as “Part B” and did not contribute to the results in the present study.
The trial was conducted in the Perinatal HIV Research Unit (PHRU), Soweto and the Children's Infectious Diseases Clinical Research Unit, Tygerberg (FDA application 71,494. Research Ethics Committees in South Africa and United States of America approved the trial; parents or legal guardians gave written consent for screening and, if eligible, for enrolment.
Study treatment and Randomization
First-line ART was twice dailyzidovudine (240mg/m2/dose) and lamivudine (4mg/kg/dose), provided by GlaxoSmithKline, with lopinavir–ritonavir (300mg/75mg/m2/dose) until 6 months of age,10 then 230/57·5 mg/m2/dose. Second-line ART was didanosine, abacavir, and nevirapine orefavirenz. Within class switching for tolerability and drug-specific toxicity was permitted. Criteria for second-line therapy, lopinavir-ritonavir and second-line ART were supplied by the South African Department of Health. 11
The trial statistician at the MRC Clinical Trials Unit, London prepared the randomization schedule stratified by clinical site using permuted blocks of random sizes for balancing numbers of infants allocated to arms. The data manager at the PHRU implemented the schedule through fax for each subject.
Procedures and Follow-up
HIV-exposed infants were identified from the public pMTCT programmes in the Western Cape and Gauteng provinces. HIV-1 DNA polymerase chain reaction (PCR) was performed from four weeks of age when cotrimoxazole prophylaxis commenced. HIV infection was confirmed by plasma HIV-1 RNA PCR >1,000 copies/mL. The pMTCT regimen was single dose nevirapine (sdNVP) to mother and neonate in both centers. In the Western Cape, zidovudine was also given to mothers from 34 weeks gestation and to neonates for 7 days. Breast feeding was permitted. Infants were reviewed 4-weekly to week 24, 8-weekly to week 48, and then 12-weekly. At each scheduled visit, HIV-related clinical events, full blood count with differential, biochemistry tests and T-cell subsets were undertaken. DAIDS criteria were used to grade adverse events. 12The majority of infants (349/92%) co-enrolled on a study evaluating the effect of ART strategy on response to conjugated 7-valent pneumococcal and Haemophilus influenzae B vaccines. 13
Outcomes
The primary outcome was time to death or failure of first-line ART, defined as any of: a) CD4% decrease to <20% from week 24 b) CDC severe stage B or stage C events, 14or c) regimen-limiting toxicity (more than one drug substitution within the same class, switching to a new class or requiring permanent treatment discontinuation). Virologic failure, defined as confirmed (repeat within 4 weeks) HIV-RNA viral load (VL) ≥10 000 copies/mm3 after 24 weeks on ART was included after October 2009, when VL became standard of care. Prior VL data were obtained retrospectively from stored specimens.
Secondary outcomes were time to starting or needing to start continuous ART, cumulative CDC severe stage B, C or ‘other’ significant clinical events or death at 3.5 years, grade 3 or 4 clinical/laboratory adverse events, hospitalizations and viral resistance.
A composite secondary outcome was time to death or life-threatening stage C events or HIV events associated with end-organ damage. Life-threatening stage C events were lymphoma or Kaposi sarcoma, progressive multifocal leukoencephalopathy or intercurrent infection requiring an intensive care unit. HIV events with end-organ damage included unresolved HIV encephalopathy, chronic lung disease, cardiomyopathy and nephropathy. The term ‘primary therapy’ described early limited ART from randomisation to treatment interruption in the early ART arms.
An independent clinical End-point Review Committee (ERC) adjudicated cause of all deaths and reviewed CDC severe stage B and C events, without knowledge of CD4 values, ART status, or allocated treatment group.
Statistical methods and data analysis
As detailed previously2, we calculated that 375 infants (125 per group), enrolled over 18 months and followed for minimum 3•5 years, provided 80% power to reject the null hypothesis of no difference between arms in time to primary endpoint if the average hazard ratio is 0.72 and 0.50 for ART-40W and ART-96W respectively, relative to ART-Def. This was based on a global log-rank test with two-sided alpha level of 0.05. Following a protocol amendment, which raised the CD4% threshold for ART initiation in infants from <20% to <25% in line with updated WHO guidelines,9 34 additional infants were enrolled between September 2007 and March 2008 to retain 80% power. By this time, at the second interim analysis on June 20th 2007, the independent Data Safety and Monitoring Board (DSMB) had already recommended discontinuing enrolment to ART-Def. Therefore these infants were randomized to ART-40W or ART-96W arms, contributing data only to the secondary comparison between the early limited ART arms.
Time-to-event methods (Kaplan–Meier plots and log-rank test stratified by site) compared arms for time to primary endpoint and survival. Cox proportional-hazard modeling was used to estimate summary hazard ratios for death or treatment failure for ART-40W and ART-96W compared to ART-Def.
The three arms were compared for a) percentage total follow-up time on ART and b) time from randomization to initiating continuous ART (estimated using Kaplan–Meier methods). For the latter comparison, follow-up in ART-Def was censored at 20th June 2007 when the Data Safety Monitoring Board (DSMB) recommended evaluating all children in ART-Def for ART initiation.
The frequency of grade 3 or 4 adverse events was compared across arms by chi-square test. Changes in CD4 percentage and count over time were summarized by point estimates of mean changes from baseline, documented at each visit. Follow-up data were censored either when lost to follow-up before July 1, 2011, or on that date. Lost to follow-up was defined as no return within six months of a previous study visit. All randomized comparisons were by intent-to-treat; p-values are two-sided.
Ethics review and interim monitoring
The ethics committees of participating institutions approved the trial protocol. The DSMB reviewed the trial at least annually. Per protocol, the DSMB considered early trial termination or protocol modification for proof beyond reasonable doubt of clear benefit or harm from a randomised treatment strategy using the Haybittle–Peto criterion, based on a difference of at least 3 standard deviations in the summary log relative hazard or nominal P<0.002 in any interim analysis.15
Role of the funding source
Representatives from the US National Institutes of Health were part of the study team and were involved in study design, data interpretation and writing this manuscript. The pharmaceutical company and provincial departments of health providing study drugs had no role in the study design, analysis, or preparation of the report. The corresponding authors had full access to all study data and had final responsibility for the decision to submit for publication.
Results
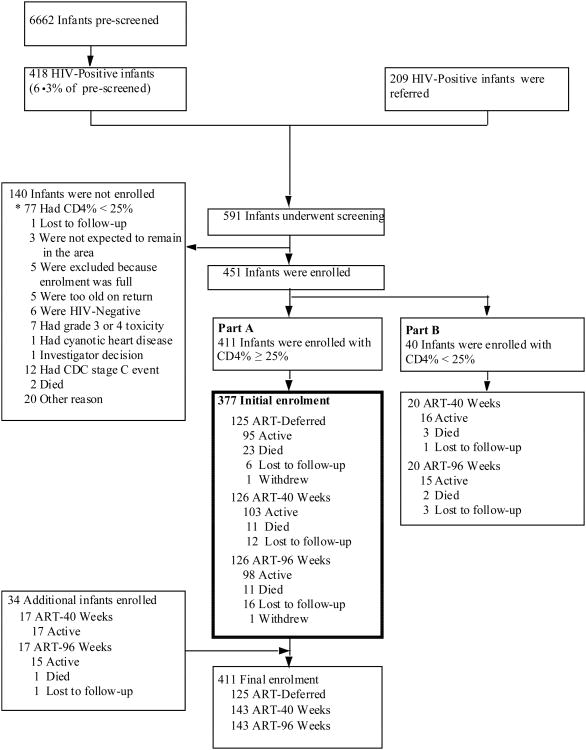
Among 591 HIV-infected infants screened, 411 had CD4% ≥25%; of these, 377 were randomized to ART-Def (125), ART-40W (126) or ART-96W (126) between July 2005 and February 2007. An additional 34 infants were randomized to ART-40W (17) or ART-96W (17) between September 2007 and March 2008 after the DSMB recommended halting recruitment to ART-Def in June 2007 (Figure 1 and appendices).
Figure 1. Screening and enrolment.
* Relevant to infants enrolled in Part A
Median age of the 377 infants at enrolment was 7.4 weeks; 85% were exposed to pMTCT, the majority (51%) to sdNVP (Table 1). No infant had CDC severe stage B or C clinical disease; median weight for age z-score was near normal (-0.7); median CD4 was 35% and mean VL was log 5.7 copies/ml. Only 54(14.3%) infants were breast-fed. 2
Table 1. Baseline Characteristics-Health Status.
| Variable | ART-Def | ART-40W | ART-96W |
|---|---|---|---|
|
| |||
| Number of participants enrolled | 125 | 126 | 126 |
| Age (weeks) at randomization | |||
| Median (IQR) | 7.1 (6.4 to 8.9) | 7.4 (6.6 to 8.7) | 7.5 (6.6 to 9.0) |
| Mother/Child receiving ART for PMTCT | |||
| No therapy (%) | 15 (12)/19 (15) | 13 (10)/18 (14) | 12 (10)/18 (14) |
| * NVP (%) | 72 (58)/67 (54) | 85 (68)/66 (52) | 80 (64)/61 (48) |
| AZT (%) | 5 (4)/1 (< 1) | 6 (5)/2 (2) | 3 (2)/3 (2) |
| AZT + NVP (%) | 26 (21)/34 (27) | 20 (16)/32 (25) | 30 (24)/37 (29) |
| HAART (%) | 7 (6)/0 (0) | 2 (2)/0 (0) | 1 (< 1)/0 (0) |
| Baseline weight (kg) | |||
| Median (IQR) | 4.5 (4.0 to 5.0) | 4.3 (4.0 to 4.8) | 4.4 (3.8 to 5.0) |
| Weight Z-score | |||
| Median (IQR) | -0.6 (-1.3 to 0.2) | -0.8 (-1.4 to-0.2) | -0.7 (-1.6 to 0.0) |
| Birth weight (kg) | |||
| < 2.5 (%) | 23 (18.4) | 18 (14.3) | 21 (16.7) |
| 2.5-3.0 (%) | 39 (31.2) | 46 (36.5) | 42 (33.3) |
| 3.0-3.5 (%) | 50 (40.0) | 47 (37.3) | 52 (41.3) |
| ≥3.5 (%) | 13 (10.4) | 15 (11.9) | 11 (8.7) |
| Median (IQR) | 3 (2.7 to 3.2) | 3 (2.7 to 3.2) | 3 (2.7 to 3.3) |
| CDC Classification | |||
| Class N (%) | 106 (84.8) | 97 (77.0) | 105 (83.3) |
| Class A (%) | 13 (10.4) | 24 (19.0) | 13 (10.3) |
| Class B (%) | 6 (4.8) | 5 (4.0) | 8 (6.3) |
| CD4% | |||
| Median (IQR) | 35.7 (29.1 to 43.6) | 35.3 (29.5 to 41.8) | 34.1 (29.0 to 39.9) |
| CD4 Count (cells/mm3) | |||
| Median (IQR) | 2039 (1585 to 2960) | 1985.5 (1476 to 2793) | 2069.5 (1537 to 2743) |
| Screening viral loads (1,000/ml) | |||
| 10-100 (%) | 9 (7) | 6 (5) | 6 (5) |
| 100-500 (%) | 27 (22) | 42 (33) | 26 (21) |
| 500-750 (%) | 15 (12) | 15 (12) | 19 (15) |
| >750 (%) | 74 (59) | 63 (50) | 75 (60) |
| Mean log10 viral load (std) | 5.7 (0.4) | 5.6 (0.3) | 5.7 (0.4) |
Single dose nevirapine
Follow-up and Use of ART
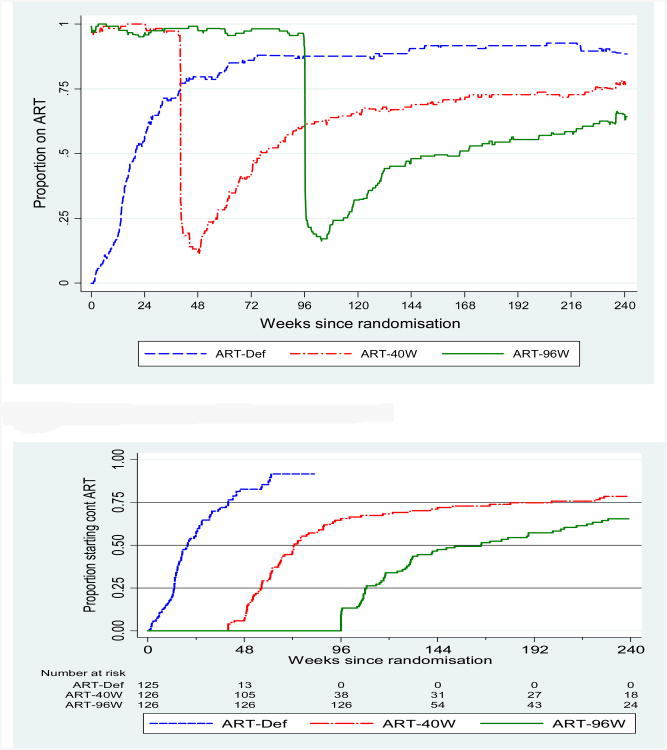
Median follow-up was 249 weeks (4.8 years; range 3.5 to 5.9 years). Thirty-four children (9%) were lost to follow-up, having been on study for a median of 67 (IQR: 29-124) weeks. The proportion of total follow-up time on ART was 81%, 70% and 69% in ART-Def, ART-40W and ART-96W arms, respectively (Figure 2a). The median time from randomisation to ART initiation in ART-Def was 20 (95% CI 16 to 25)weeks. Median time to starting continuous ART after interruption was 33(26 to 45) weeks for ART-40W and 70(35 to 109) weeks for ART-96W. This was estimated by time from randomisation to starting continuous ART minus 40 weeks (for ART-40W) and minus 96 weeks (for ART-96W) to adjust for length of early ART; thus the difference between ART-96W and ART-40W was 37 (-11 to 85) weeks (p=0.13, Figure 2b). Seven children switched to second-line therapy: three in ART-Def, three in ART-40W and one in ART-96W.
Figure 2.
(a) Proportion of participants on ART over calendar time
(b) Time to starting continuous treatment
NB: Follow-up for ART-Def is censored at the time of the DSMB meeting
Primary Outcome (Death or failure of first-line ART)
There were 32 (25%) and 26 (21%) primary endpoint events in ART-40W and ART-96W compared with 48 (38%) in ART-Def (Table 2). Death comprised nearly half the primary endpoints in ART-Def (21) versus about one third in early ART arms. Immunological and clinical failures on ART were more similar between arms, but virologic failure was more frequent in ART-Def (10) than in early ART (one in each of ART-40W and ART-96W). Relative to ART-Def, the hazard ratio (HR) for death or failure of first-line therapy in ART-40W was 0.59(95% CI 0.38 to 0.93; p=0.023), and in ART-96W, was 0.47(95% CI 0.29 to 0.76; p=0.002). The HR for time to primary endpoint in ART-96W relative to ART-40W was 0.79(95% CI 0.47 to 1.32; p=0.36).
Table 2. Primary endpoints.
| Variable | ART- Deferred | ART-40W | ART-96W |
|---|---|---|---|
| Number of Participants Enrolled | 125 | 126 | 126 |
| Total number of endpoints | 48(38.4%) | 32(25.4%) | 26(20.6%) |
| Deaths | 21(16.8%) | 11(8.7%) | 9(7.1%) |
| Immunological Failure on Therapy | 9(7.2%) | 14(11.1%) | 11(8.7%) |
| Failure to reach a level > 20% by week 24 | 4(3.2%) | 7(5.6%) | 5(4.0%) |
| CD4% fell below 20% on two occasions after 24 weeks on therapy | 5(4.0%) | 7(5.6%) | 6(4.8%) |
| Clinical Failure on Therapy | 8(6.4%) | 6(4.8%) | 5(4.0%) |
| Severe Stage B Disease | 5(4.0%) | 1(0.8%) | 2(1.6%) |
| CDC C Disease | 3(2.4%) | 5(4.0%) | 3(2.4%) |
| Virological Failure on Therapy | 10(8.0%) | 1(0.8%) | 1(0.8%) |
| Failure due to ART Limiting Toxicity | 0(0%) | 0(0%) | 0(0%) |
Secondary outcomes and end of trial status
There were 23 (18%) deaths in ART-Def and 11 (9%) in each of ART-40W and ART-96W. Relative to ART-Def, HR (95% CI) for death was 0.40 (0.19 to 0.85, p=0.02) in ART-40W and 0.45 (0.22 to 0.91, p=0.03) in ART-96W.
Over the entire follow-up, the total number of clinical disease progression events (CDC C, severe B or death) was 66, 38 and 29 in 38 (30%), 22 (17%) and 17 (13%) children in ART-Def, ART-40W and ART-96W respectively. Relative to ART-Def, the HR (95% CI) was 0.53 (CI: 0.34 to 0.82, p=0.005) in ART-40W and 0.42 (CI: 0.26 to 0.67, p=0.0003) in ART-96W. The cumulative probability of clinical disease progression or death by 3.5 years was 41% vs 28% vs 21% in the ART-Def, ART-40W and ART-96W respectively. Relative to ART-Def, the difference for ART-40W was 13% (CI: 1% to 25%, p=0.03) and 20% (CI: 9% to 31%, p=0.0006) for ART-96W.
The number of children who died or experienced HIV events associated with end-organ damage was 34 (27%), 18 (14%) and 13 (10%) in the ART-Def, ART-40W and ART-96W respectively. Relative to ART-Def, the HR (95% CI) for death or HIV events associated with end-organ damage was 0.48 (CI: 0.27 to 0.84, p=0.011) in ART-40W and 0.34 (CI: 0.18 to 0.64, p=0.0009) in ART-96W. The hospital admission rate in ART-def was double that in early limited ART (p<0.0001, Table 3a).
Table 3. (a): Clinical events during trial and status at end of trial.
| Number of events | ART-Def (N=125) |
ART-40W (N=126) |
ART-96W (N=126) |
Global P-Value |
|---|---|---|---|---|
| Deaths (Rate per 100 person years) | 23 (4.6) | 11 (2.0) | 11 (2.0) | 0.02 |
| Severe Stage B Disease Events (Rate per 100 person years) | 3 (0.6) | 3 (0.6) | 0 (0) | 0.08 |
| Other significant events (Rate per 100 person years; see definitions below) | 18 (3.6) | 15 (2.8) | 12 (2.2) | 0.41 |
| CDC Stage C Disease Events (Rate per 100 person years) | 22 (4.4) | 9 (1.7) | 6 (1.1) | 0.001 |
|
| ||||
| Total Clinical Events (Rate per 100 person years) | 66 (13.1) | 38 (7.0) | 29 (5.3) | <0.0001 |
| Total number of participants with clinical events | 38 | 22 | 17 | |
|
| ||||
| Timing of events | ||||
| Primary Therapy (Rate per 100 person years) | N/A | 15 (16.1) | 25 (11.8) | |
| ART naive (Rate per 100 person years) | 49 (55.6) | N/A | N/A | |
| Interruption (Rate per 100 person years) | N/A | 14 (8.8) | 3 (1.7) | |
| Continuous Therapy (Rate per 100 person years) | 17 (4.1) | 9 (3.1) | 1 (0.6) | |
|
| ||||
| Type of Clinical Events | ||||
| Severe Stage B Disease | 3 | 3 | 0 | |
| Cardiomyopathy | 0 | 1 | 0 | |
| Chronic Lung Disease | 3 | 2 | 0 | |
| CDC Stage C Disease | 22 | 9 | 6 | |
| CMV Disease | 2 | 0 | 0 | |
| Disseminated Tuberculosis | 1 | 0 | 3 | |
| HIV Encephalopathy | 9 | 5 | 2 | |
| HIV Wasting Syndrome | 3 | 4 | 1 | |
| Esophageal Candidiasis | 1 | 0 | 0 | |
| Pneumocystis jerovici Pneumonia | 5 | 0 | 0 | |
| Recurrent Bacterial Infections (excluding pneumonias) | 1 | 0 | 0 | |
| Other significant events | 18 | 15 | 12 | |
| Failure To Thrive; not stage C | 14 | 14 | 10 | |
| Recurrent Pneumonia | 4 | 1 | 2 | |
|
| ||||
| No. of hospitalizations (No. of children) | 139(70) | 90(50) | 78(50) | <0.0001 |
| Total number of days hospitalized | 1018 | 533 | 414 | |
|
| ||||
| End of trial status | ||||
| Median CD4% (IQR) | 32 (27 to 37) | 31 (26 to 36) | 30 (24 to 36) | |
| Median CD4 Count (IQR) | 1101 (876 to 1483) | 1112 (791 to 1418) | 945 (770 to 1441) | |
| Viral Load >1,000 copies/ml on ART | 5 | 12 | 9 | |
| Viral load <400c/ml on ART | 88/101 (87%) | 95/113 (84%) | 97/117 (83%) | 0.96 |
| Median WAZ (IQR) | -0.43 (-0.99 to 0.2) | -0.33(-0.97 to 0.02) | -0.68 (-1.2 to -0.04) | |
| Median HAZ (IQR) | -0.88 (-1.3 to -0.1) | -0.7 (-1.4 to -0.41) | -1.1 (-1.8 to -0.55) | |
| Median WHZ (IQR) | 0.35 (-0.44 to 0.78) | 0.31 (-0.49 to 0.98) | 0.21 (-0.29 to 0.59) | |
| Off ART | N/A | 24 | 40 | |
|
| ||||
| Hospitalization Rate: ART-Def 27.6/100 PY, ART-40W 16.4/100 PY, ART-96W 14.2/100 PY | ||||
| (b): Grade 3 and 4 clinical and laboratory events | ||||
|---|---|---|---|---|
|
| ||||
| Variable | ART-Def (N=125) |
ART-40W (N=126) | ART-96W (N=126) | Global P-value |
| Total Grade 3 or 4 Clinical Events | 170 | 118 | 88 | |
| Rate per 100 person years | 33.8 | 21.6 | 16.0 | <0.0001 |
| Total Study Drug Related Grade 3 or 4 Clinical Events | 0 | 3 | 2 | |
| Rate per 100 person years | 0 | 0.6 | 0.4 | 0.14 |
| Acute Renal Failure1 | 0 | 1 | 0 | |
| Gastroenteritis1,2 | 0 | 1 | 1 | |
| Lipoatrophy3 | 0 | 1 | 0 | |
| Myocarditis1 | 0 | 0 | 1 | |
| Total Grade 3 or 4 Laboratory Events | 35 | 44 | 33 | |
| Rate per 100 person years | 7.0 | 8.1 | 6.0 | 0.46 |
| Total Study Drug Related Grade 3 or 4 Laboratory Events | 10 | 17 | 16 | |
| Rate per 100 person years | 2.0 | 3.1 | 2.9 | 0.48 |
| Gamma Glutamyl Transpeptidase Increased | 2 | 0 | 2 | |
| Alanine or Aspartate Aminotransferase Increased | 4 | 3 | 1 | |
| Anaemia | 1 | 7 | 2 | |
| Neutropenia | 3 | 5 | 8 | |
| Thrombocytopenia | 0 | 1 | 3 | |
| Creatinine Increased | 0 | 1 | 0 | |
Key
Probably not related
Possibly related
Probably related
By trial-end, 24(19%) and 40(32%) children in ART-40W and ART-96W respectively had not started continuous ART. Nevertheless, the median CD4% was over 30% in all arms and anthropometric data were well within population standards and similar in all arms (Table 3a).
Among 331 children on ART for at least 24 weeks who had VL tested at the last study visit, HIV-1 RNA was <400copies/ml in 88/101(87%) in ART-Def, 95/113(84%) in ART-40W and 97/117(83%) (p=0.96) in ART-96W. Among 31 children with VL >1000 copies/ml(5 ART-Def, 13 ART-40W and 13 ART-96W), only 2 children had PI mutations, not present at baseline (one each in ART-Def and ART-40W; both V82A) and 7 (23%) had M184V mutations (1 ART-Def, 5 ART-40W and 1 ART-96W). There were no thymidine-associated mutations (TAMs) and no differences between randomized groups for any resistance. Few grade 3 or 4 clinical or laboratory events were considered ART-related. Whereas clinical event rates were significantly higher in ART-Def (p <0.0001), rates of laboratory events (particularly those considered ART-related) were similar and low in all 3 arms (Table 3b and web appendix table 1). Analyses including the additional 34 children in ART-40W and ART-96W in web appendix tables 1, 2, 3; 4a, 4b and figures 1 and 2 are similar to the main analyses.
Discussion
The results of the completed CHER trial show that early limited ART in young infants is superior to deferred ART over an extended time period. Importantly, during planned interruption after primary therapy, the rapid disease course seen with deferred ART 2was not observed; this may have been because the children given early limited ART were older when ART was discontinued than those who had not started ART in the ART-Def group. Another explanation is that starting ART shortly after infection limits both the reservoir of persisting HIV and viral diversity virus and preserves the immune response. 16, 17 In addition, no excess morbidity due to renal, cardiac or lung disease was noted during interruption. Paradoxically, the proportion of time spent on ART was highest in the deferred arm, which also had the greatest number of deaths, clinical events and hospitalisations. Thus from a health system perspective, early limited ART was less costly than deferred ART supporting the cost analysis based on early CHER trial data. 18
Three adult studies addressing early ART followed by treatment interruption showed benefit in higher CD4 count and lower viral set point, but unlike the CHER trial, did not show clinical benefit. 19-21 Of note, there have been no randomised trials in adults or children comparing early continuous ART with early limited ART. We observed marginally better outcomes with longer primary therapy. It is plausible that a longer period than in our trial, might have sustained better long-term outcomes following interruption. However, it is also plausible that the comparative benefit of early therapy is finite, and that longer periods of primary therapy will not allow increasingly longer periods off ART.
Treatment interruption following primary infection appears to be very different from interruption in chronic, well-established HIV infection, which in adults is associated with increased morbidity and mortality.22 Unexpectedly, non-infectious renal, cardiovascular and hepatic disease can contribute to poorer outcomes during interruption in adults. 23There are two pilot randomized trials, PENTA 11 in Europe and the Optimizing Pediatric HIV-1 Therapy 03 (OPH03) Study in Kenya, which evaluated CD4-driven planned treatment interruptions in older HIV-infected children. Neither showed significant morbidity.24,25 Unlike adults, greater CD4 recovery following interruption is possible in children. 24In the smaller Kenyan study, children could not interrupt therapy beyond 3 months without reaching CD4 criteria for ART re-initiation. These data suggest that interruption after ART has been initiated later in infancy than in the CHER trial is unlikely to succeed, at least in the African environment.
The neurodevelopmental consequences of deferring or interrupting ART require consideration. HIV encephalopathy was more common in the ART-Def arm in CHER, also reflecting results of the CHER infancy neurodevelopmental sub-study in Cape Town. 26Of note, no neurodevelopmental consequences were seen in long term follow-up of PENTA 11 27or among chronically HIV-infected children on deferred ART in the Pediatric Randomized Early versus Deferred Initiation in Cambodia and Thailand (PREDICT) trial. However, HIV-infected children in both trials functioned worse than HIV-uninfected peers, suggesting the possibility that early ART initiation, as in the CHER trial, could be neuroprotective. Long-term results of detailed neurodevelopmental testing are awaited from CHER sub-studies.
An important observation in CHER is the excellent long-term outcome despite interruption, using a PI-based first-line ART regimen. Very few children switched to second-line ART. Viral suppression rates were excellent after nearly 5 years. As in the PENPACT1 trial,28 PI-based ART with ongoing viral replication protects against developing TAMs as opposed to non-nucleoside reverse transcriptase-based ART. The same PI-based regimen was more effective than nevirapine-based first line therapy in P1060 at 24 weeks, regardless of previous ART exposure for pMTCT.29 Cumulatively, both studies reported few drug-related adverse events, emphasizing the safety of this regimen in children.30
There are limitations in the CHER trial. Firstly, as screening only began after 4 weeks of age, we cannot comment on in-utero versus intra-partum infections. Secondly, neither an early continuous ART arm nor one with more prolonged primary therapy were included. Thirdly, the trial was insufficiently powered to detect differences between the ART-40W and ART-96W arms, which would have required a much larger sample size.
An important consideration is the extent to which early limited ART would be feasible in resource limited settings, because of the need for close clinical and CD4 monitoring. In many high prevalence settings there is heterogeneity of resources available. For example, in South Africa, viral loads and CD4 counts are readily available, as they are in a few centers of excellence in many other African countries. ‘Point of care’ CD4 and viral load tests will hopefully become available in the near future. Finally, caregivers may stop ART and drug stock-outs are not uncommon. Understanding the risks and benefits associated with interruption at different ages is therefore important.
Conclusion
The CHER trial supports the concept and feasibility of early limited primary ART for infants without CD4 depletion provided that close clinical and CD4 monitoring is available. The trend towards better outcome with treatment for 96 rather than 40 weeks favours longer primary treatment. More critically, with ART coverage in children standing at only 28% in 2011, our results emphasise the urgency of increasing early diagnosis and ART initiation in young infants; extending to entry points other than pMTCT programmes. 31 Finally, the CHER trial results support the durability and robustness of lopinavir-ritonavir-based first-line ART for infants, which should encourage developing cheaper, more palatable formulations for infants. Further studies of immunological responses to early ART and the virological and immunological consequences of ART interruption are ongoing using stored samples and will contribute to the CURE agenda by increasing our understanding of HIV pathogenesis in young vertically HIV-infected infants.
Research in context
We searched for randomized studies comparing early limited to deferred ART during acute or recent HIV infection in children and adults in PubMed and Scopus databases.
In infants, in a small pilot study from Durban, South Africa, infants starting immediate (n = 43) compared with deferred (n = 20) ART had fewer minor illness episodes (P = 0.003), and were less likely to be hospitalized (p = 0.09) over one year but with no difference in mortality. Data on planned treatment interruption were not presented. 32 In the Optimizing Pediatric HIV-1 Therapy 03 (OPH03)study in Kenya, infants commenced ART at a median age of 5 months. Treatment was interrupted after 24 months, with 14 of 21 reaching CD4 restart criteria within 3 months. The CHER trial is the only large randomised study in young infants identified shortly after birth at a median of 7 weeks of age. Clinical endpoints, mainly death, occurred early and remained significantly higher in the deferred arm despite a longer period on continuous ART.
Adult studies are limited by an inability to accurately identify the timing of infection. Only one adult study had clinical endpoints. Here, zidovudine given for 6 months was associated with fewer opportunistic infections than a deferred strategy. 33Two relatively small studies, the Setpoint (ACTG A5217) and Primo-SHM trial showed a lower average viral load after early limited ART compared to deferred ART. 20, 34 The Short Pulse Anti-Retroviral Therapy at Sero-conversion (SPARTAC) trial, similar in design to the CHER trial, showed significantly lower viral load and higher CD4 trajectories after early limited ART for 48 weeks. 19
Interpretation
The CHER trial, whose composite primary endpoint was clinical, immunological and virological establishes the feasibility and safety of early limited ART in asymptomatic young infants and without severe CD4+ T cell depletion. Although there are still insufficient data to recommend early limited ART for infants universally, this strategy was safe and superior to deferring ART initiation, which should rather commence as soon as possible after birth. The trial also confirms the durability and lack of toxicity of the first line regimen consisting of lopinavir-ritonavir, lamivudine and zidovudine.
Supplementary Material
Acknowledgments
Support for this study was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the US National Institutes for Health (NIH), through the Comprehensive International Program of Research on AIDS (CIPRA) network, grant number U19 AI53217. The Departments of Health of the Western Cape and Gauteng, South Africa and GlaxoSmithKline plc provided additional support.
Funding: US National Institutes of Health
ClinicalTrials.gov number, NCT00102960
Footnotes
Disclaimer: The views expressed in written conference materials or publications and by speakers and moderators at HHS-sponsored conferences, do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Collaborators and Centers for CHER: South Africa: Nastassja Choonilal, Steven Masenya, Haseena Cassim, Sylvia Dittmer, Boitumelo Shezi, Jan Steyn, PHRU SDMC, Sibongile Dlamini, Valerie Kemese, Moipone Piliso, Refilwe Khumalo, (Perinatal HIV Research Unit) George Fourie, Frieda Verheye-Dua, Marietjie Bester, Wilma Orange, Ronelle Arendze, Catherine Andrea, Marlize Smuts, Kurt Smith, Alison Riddick, Sue Purchase, Camilla Wattrus, Barbara Laughton, Rehana Taliep, Thembi Mbuqe, Maria Vass, Philma Martin, Rolien Sadie, Morné Isaacs, Christine Davies, Alec Abrahams, Kenny Kelly, Edward Barnes, Natasha Samuels (Children's Infectious Diseases Clinical Research Unit) Alvina Mathee (Stellenbosch University Medical library) Glenda Gray, Ian Sanne, Christie Davies, Morna Cornell (CIPRA-SA); Wendy Stevens, Debbie Glencross (CIPRA-SA Laboratory Core), Leon Levin (End point Review Committee)
United Kingdom: Tim Peto (Chair, Endpoint Review Committee, John Radcliff Hospital, University of Oxford), Eddie Loeliger, Jean-Marc Steens, Wendy X Snowden, Navdeep Thoofer (GlaxoSmithKline).
United States of America: Karen Reese, Jeff Nadler, Carla Petinelli (DAIDS/NIAID/NIH), James McNamara (DAIT/NIAID/NIH), Rod Hoff (REDI Centre), Sandi Lehrman (Merck), Chuck Oster (Walter Reed).
DSMB - Haroon Saloojee (Chair), Susan Ellenberg, Wafaa El-Sadr, David Harrington, Carl Lombard, Mary Faith Marshall, Lucky Mokgatlhe, AlwynMwinga, Paula Munderi, Andrew Nunn, CheweLuo, Jerome Singh, Rebecca DerSimonian (Executive Secretary).
Contributors: AV, MFC, DMG and AGB designed the CHER trial. Funding from DAIDS was obtained by JAM, PI of CIPRA-SA. RP managed the trial and coordinated data for the DSMB meetings. ED managed the patients in KID-CRU and contributed to protocol development. HR supervised patient management in KID-CRU and contributed to protocol development. DJ contributed to protocol development, compiled reports for the ERC and controlled endpoint data entry. AL and EL managed patients at PHRU. AJvR and WP were study coordinators. EH and PJP contributed to protocol development, data interpretation and trial administration for the sponsor. KO undertook the analysis under supervision of AGB. AGB oversaw interim analyses for the DSMB. HT managed the database. MFC wrote the primary manuscript. AV, KO, RP, ED, HR, DJ, AL, EL, SI, AJvR, WP, HT, SAM, PJP, JAM, DMG and AGB were in the writing committee and contributed to the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23(1):101–6. doi: 10.1097/qad.0b013e32831c54bd. [DOI] [PubMed] [Google Scholar]
- 2.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinda E, Humphrey JH, Iliff PJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26(6):519–26. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 4.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 5.Church JD, Omer SB, Guay LA, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-Dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198(7):1075–82. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Department of Health and Human Services; 2007. [Google Scholar]
- 7.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2008 [Google Scholar]
- 8.PENTA Steering Committee. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009;10:591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Recommendations for a public health approach. Geneva: 2006. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. [PubMed] [Google Scholar]
- 10.Chadwick EG, Caparelli EV, Yogev R, et al. Pharmacokinetics, effacy and safety Lopinavir/Ritonavir for HIV-1-infected Infants less than 6 months of age: 24 week results. AIDS. 2008;22:249–55. doi: 10.1097/QAD.0b013e3282f2be1d. [DOI] [PubMed] [Google Scholar]
- 11.National Department of Health. Department of Health. 1st. Pinetown: Jacana Publishers; 2004. National Antiretroviral Treatment Guidelines. [Google Scholar]
- 12.Division of AIDS National Institute of Allergy and Infectious Diseases. Table for Grading the Severity of Adult and Pediatric Adverse Events. 1994 [Google Scholar]
- 13.Madhi S, Adrian P, Cotton MF, et al. Effect of HIV exposure and antiretroviral therapy on quantitative antibody concentration to pneumococcal conjugate vaccine (PCV) in infants. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town. 2009. [Google Scholar]
- 14.Centers For Disease Control and Prevention. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morb Mortal Wkly Rep. 1994;43:1–10. [Google Scholar]
- 15.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persaud D, Gay H, Ziemniak C, et al. Functional HIV Cure after Very Early ART of an Infected Infant. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, USA. 2013. [Google Scholar]
- 17.Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS Pathogens. 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer-Rath G, Violari A, Cotton MF, et al. 1st year of the CHER trial XVIII International AIDS Congress. Vienna, Austria: 2010. Cost of early vs deferred paediatric ART in RSA. [Google Scholar]
- 19.The SPARTAC Trial Investigators. Short-Course Antiretroviral Therapy in Primary HIV Infection. New England Journal of Medicine. 2013;368(3):207–17. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grijsen ML, Steingrover R, Wit FW, et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: the randomized Primo-SHM trial. PLoS Med. 2012;9(3):e1001196. doi: 10.1371/journal.pmed.1001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan CM, Degruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012;205(1):87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count–guided interruption of antiretroviral treatment. N Eng J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 23.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Eng J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 24.Paediatric European Network for Treatment of AIDS (PENTA) Response to planned treatment interruptions in HIV infection varies across childhood. AIDS. 2012;24(2):231–41. doi: 10.1097/QAD.0b013e328333d343. [DOI] [PubMed] [Google Scholar]
- 25.Wamalwa D, Benki-Nugent S, Langat A, et al. Treatment Interruption in Infants following 24 Months of Empiric ART: Kenya. 19th Conference on Retrovirology and Opportunistic Infection; Seattle. 2012. [Google Scholar]
- 26.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012 doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos J, Melvin D, Medin G, et al. Neurocognitive and Quality of Life Outcomes in Children after Planned Treatment Interruptions: The Randomized PENTA 11 Trial. 19th Conference on Retrovirology and Opportunistic Infection; Seattle, USA. 2012; p. #963. [Google Scholar]
- 28.The PENPACT (PENTA / PACTG) study team. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet. 2011;11:273–83. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. New Engl J Med. 2012;366(25):2380–9. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidari S, Mofenson LM, Hobbs CV, Cotton MF, Marlink R, Katabira E. Unresolved Antiretroviral Treatment Management Issues in HIV-Infected Children. J Acquir Immune Defic Syndr. 2012;59(2):161–9. doi: 10.1097/QAI.0b013e3182427029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UNAIDS. UNAIDS report on the global AIDS epIdemIc: 2012. 2012 [Google Scholar]
- 32.Prendergast A, Mphatswe W, Tudor-Williams G, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22(11):1333–43. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 33.Kinloch-De Loes S, Hirschel BJ, Hoen B, et al. A controlled trial of zidovudine in primary human immunodeficiency virus infection. N Engl J Med. 1995;333(7):408–13. doi: 10.1056/NEJM199508173330702. [DOI] [PubMed] [Google Scholar]
- 34.Hogan CM, Degruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012;205(1):87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.