Abstract
Introduction
Crohn's disease (CD) often affects women during the reproductive years. While several studies have examined the impact of pregnancy on luminal disease, limited literature exists in those with perianal CD. Decision regarding mode of delivery is a unique challenge in such patients due to concerns regarding the effect of pelvic floor trauma during delivery on pre-existing perianal involvement.
Methods
We performed a retrospective chart review of CD patients with established perianal disease undergoing either vaginal delivery or Caesarean section (C-section) at our institutions. We examined the occurrence of symptomatic perianal disease flares within 5 years after delivery in such women compared to non-pregnant CD controls. We also compared the occurrence of such flares between the two modes of delivery in women with established perianal CD.
Results
We identified 61 pregnant CD patients with established perianal disease (11 vaginal delivery, 50 via C-section) and 61 non-pregnant CD controls with perianal disease. One-third of the C-sections were primarily for obstetric indications. Six of the vaginal deliveries were complicated. Approximately 36% of cases had a symptomatic perianal flare within 1 year after delivery. This was similar across both modes of delivery (p=0.53), and similar to non-pregnant CD patients. There was no difference in the rates of perianal surgical intervention or luminal disease flares in our population based on mode of delivery, or between pregnant CD patients and non-pregnant CD controls.
Conclusion
We observed no difference in risk of symptomatic perianal flares in patients with established perianal CD delivering vaginally or via C-section.
Keywords: perianal fistula, Crohn's disease, pregnancy, C-section
Introduction
Crohn's disease (CD) is a chronic recurrent inflammatory bowel disorder, with a peak incidence between 15 and 35 years of age. It is characterized by remissions and relapses, frequently requiring hospitalization and surgery. Perianal involvement is common in CD, occurring in up to 50% of patients by 20 years after diagnosis1-3. The most common presentations of perianal disease are perianal abscess, fissures, or anal fistulae, with fistula formation to adjacent organs (such as rectovaginal fistulae) occurring in a subset with more severe disease1-5. Given its onset often during a woman's peak reproductive years, the impact of CD on pregnancy and conversely the impact of pregnancy on CD are important concerns for patients and providers6-11.
Numerous studies have evaluated the effect of Crohn's disease on pregnancy6-11. However, there are few studies to specifically guide choice regarding the mode of delivery in this cohort. The choice of mode of delivery has important implications for both the current and subsequent pregnancies. Caesarean sections are associated with increased risk of complications and adhesion formation which may complicate subsequent bowel surgery that may be required in up to two-thirds of patients with CD12. On the other hand, vaginal delivery may be associated with perineal trauma and sphincter injury. The choice of optimal mode of delivery is particularly pertinent in patients with established perianal CD where the consequences of perineal trauma may be greater. In addition, though there is no objective physiologic evidence, pelvic pressure during delivery has been hypothesized to be associated with perianal fistulae13. The current practice is to pursue vaginal delivery if patients have quiescent disease, encourage Cesarean sections for patients with active perianal disease, and avoid episiotomies if possible. However, the literature in this field is largely limited13-17. Thus, there is an important unmet need for continued examination of the impact of mode of delivery on outcomes of patients with established perianal CD.
Thus, this aim of our study was to address whether vaginal trauma during delivery induces clinical recurrence of perianal disease and whether cesarean section can prevent the progression of perianal disease in women with established perianal CD. We also examined the effect of pregnancy on the course of perianal disease.
Methods
Study population
This study was a retrospective cohort study of patients located at two major academic referral hospitals, Massachusetts General Hospital (MGH) and Brigham and Women's Hospital (BWH). Both hospitals, together with their affiliated medical centers, serve nearly 3 million patients in the greater Boston area and are referral centers for patients from New England and elsewhere. Potential cases were identified through a query of the Partners Research Practice Database Registry (RPDR)18. The RPDR is a centralized clinical data repository and query system that is automatically and continuously populated with patient data from a variety of data sources, including billing, encounter scheduling, medications, laboratory, radiology, endoscopy, and surgery databases. For diseases and procedures, encounters have been coded with International Classification of Diseases, 9th edition (ICD-9-CM) or Current Procedural Terminology (CPT) codes since the inception of RPDR. The use of this data repository in identifying potential cases has been described in previous publications from our center19-22.
Definition of cases, controls, and mode of delivery
For this study, the population comprised of all patients with a confirmed diagnosis of IBD using ICD-9-CM codes for CD (ICD-9-CM 555.x) in combination with diagnosis codes for perianal disease (anal fissure ICD-9-CM 565.0), anal fistula (ICD-9-CM 565.1, 619.0, 619.1, 619.2, 619.8, 619.9) or procedures potentially associated with perianal disease. These included anal fistulotomy (ICD-9-CM 49.11, 49.12), rectal endoscopic ultrasound examination (CPT 45990), anal sphincter dilation/rectal stricture treatment (CPT 45905, 45910), incision and drainage of rectal or pelvic abscess (CPT 45020, 45005, 45000, 46040), intervention on anal fistula (CPT 46288, 46280, 46285, 46270, 46275), and seton placement or removal (CPT 46030, 46020, 46040, 46060). A chart review of the electronic medical records was then performed for every potential case to confirm diagnosis of Crohn's disease, prior perianal involvement, as well as pregnancy and delivery method. A diagnosis of Crohn's disease was made using standard clinical, radiographic, endoscopic, and histological criteria. For each case, the presence of perianal disease was verified by chart review with compatible physical exam, radiologic findings, and interventions preceding the index pregnancy date. Only patients with established perianal disease prior to the index pregnancy were included in our study.
Modes of delivery were defined as delivery via C-section (CPT 59510, 59515, 59525, ICD9 669.7x) or vaginally (CPT 59409, 59510, 59612, 59614, ICD-9-CM 650.0). Confirmation of pregnancy and delivery was based on obstetric or primary care documentation within the chart. To identify controls, the RPDR was queried for a set of random controls with a diagnosis of Crohn's disease with perianal involvement. A chart review was done to ascertain the date of perianal disease diagnosis. For each case, we identified one randomly selected control from this population, matched to occurrence of perianal disease in or prior to the year of pregnancy of the cases. This avoided a potential temporal bias owing to cases and controls occurring at different calendar time periods, for example cases occur in the pre-biologic era and controls being selected from a later time period. As the time of diagnosis of perianal CD was variably documented, we did not match for duration of perianal CD but accepted the reasonable assumption that duration of CD would serve as a proxy for this
Study Variables and Outcome Measurements
Electronic medical records were reviewed for age, gender, ethnicity, age at diagnosis, location and duration of CD. Chart review was performed specifically for this study by the physician researchers and could be up to a few years after the pregnancy, allowing for sufficient duration of follow-up. The Montreal classification was used to categorize disease behavior (inflammatory, stricturing, or penetrating), location (ileal, colonic, ileocolonic), and severity (documentation of hospitalization or procedure for IBD)23. Therapy modalities were classified as oral corticosteroid (prednisone), antibiotics (ciprofloxacin, metronidazole, amoxicillin/clavulanate), immunomodulator therapy (methotrexate, azathioprine, 6-mercaptopurine), and biologics (infliximab, adalimumab, certolizumab, and natalizumab). Smoking was classified as never/ever smoked in lifetime. Perianal disease was classified as either perirectal or rectovaginal fistulae, perianal abscess, or anorectal stricture. Anal fissures alone were not considered to be adequate evidence of perianal CD due to frequent occurrence in the setting of pregnancy even in the absence of perianal CD involvement and difficulty in distinguishing such disease from CD related perianal involvement. Patients were assigned the most severe phenotype of their perianal disease if they had more than one type of perianal complication. Perianal disease was considered severe if the patient had required any of the following procedures: exam under anesthesia, abscess drainage, seton placement, stricture dilatation, or fistulotomy. Perianal disease was considered to have recurred following delivery if the patient had evidence of clinically active recurrent disease on physical exam (evidence of abscess, stricture, draining fistula), endoscopy, or radiology. CD luminal flares were included based on history (diarrhea, abdominal pain, weight loss, rectal bleeding), physical exam (abdominal tenderness or mass), endoscopy, or imaging. Endoscopically active disease included presence of erythema and ulcerations with friability while radiological evidence of active disease included wall thickening, increased vascularity and mucosal enhancement. Recurrence of surgery following delivery was counted if the patient had a related surgical procedure for perianal disease.
Mode of delivery was classified as either Cesarean or vaginal delivery. Any complications during labor were recorded, including second degree or greater laceration, episiotomy, preterm premature rupture of membranes (PPROM), premature rupture of membranes (PROM), and non-reassuring fetal heart tones (NRFHT).
Statistical Analysis
The primary outcomes were perianal disease flare defined as clinical documentation of active perianal disease, escalation of treatment based on perianal disease, or requirement for perianal surgical intervention following delivery. The primary outcomes for gravid and nongravid IBD cases were recorded in an excel spreadsheet and data were analyzed used Stata 12.0 (StataCorp, College Station, TX). Continuous variables were summarized with means and standard deviations, while categorical variables were described using proportions. The t -test was used to compare continuous variables. Categorical variables were compared using the χ2 -test with the use of the Fisher's exact test when appropriate. Multivariate logistic regression analysis was used to determine the independent association of our outcomes of interest adjusting for potential confounders. All p-values < 0.05 were considered statistically significant.
The study was approved by the Institutional Review Board of Partners Healthcare.
Results
Patient characteristics
We identified a total of 61 patients with established perianal disease and subsequent pregnancy and delivery. Of these, 50 (82%) delivered via C-section (mean age 33+5 years), and 11 (18%) via vaginal delivery (mean age 29+5 years). Eight women in the vaginal delivery group were in their first pregnancy (73%) compared to twenty-four women in the c-section group (48%, p=0.137). These were matched to 61 non-pregnant CD controls with perianal disease (mean age 40+18 years). The median duration of Crohn's disease at the date of index pregnancy was 9.5 years (± 6.7 years) for the gravid group and 14.4 years (± 11.9 years) for the control group. Following the index pregnancy, 10 women had one subsequent pregnancy and another 10 had 2 or more following pregnancies. The median age at time of delivery in the index pregnancies was 32 years. Three women were noted to have active perianal disease during pregnancy; however the quality of documentation quantifying degree of perianal CD activity was variable.
Table 1 compares the characteristics between the cases and controls. There was no significance between the groups in terms of CD location or phenotype. Both groups had similar severity of CD, with no difference in requiring hospitalization and surgery. Both groups had similar distribution of perianal disease, with the predominant type being fistulae, followed by abscess, then stricture. Although there was a trend towards higher usage rates for immunomodulators for cases as compared to controls (78% vs. 57%, p = 0.01), there was no difference in the requirement for anti-TNF therapy (36% vs. 43%, p = 0.46).
Table 1. Comparison of characteristics of pregnant CD patients with perianal disease and non-pregnant perianal CD controls.
| Characteristic | Cases (n=61), N (%) | Controls (n=61), N (%) | p-value |
|---|---|---|---|
| Age at Diagnosis (in years) (mean (SD)) | 22.5 (6.1) | 22.1 (16.6) | 0.86 |
| Duration of disease (in years) (mean (SD)) | 9.5 (5.7) | 14.5 (11.9) | 0.004 |
| Age at Pregnancy (in years) (mean (SD)) | 32.4 (5.5) | 33.5(16.8) | 0.62 |
| Crohn's Disease Location | |||
| Ileal | 1 (1.7) | 0 (0.0) | 0.244 |
| Colonic | 16 (26.7) | 23 (37.7) | |
| Ileocolonic | 43 (71.7) | 38 (62.3) | |
| Crohn's Disease Phenotype | |||
| Inflammatory | 11 (18.3) | 15 (24.6) | 0.582 |
| Stricturing | 18 (30.0) | 32 (26.5) | |
| Penetrating | 31 (51.7) | 63 (52.1) | |
| Ever smoker | 28 (45.9) | 24 (39.3) | 0.39 |
| IBD Hospitalizations | |||
| Yes | 44 (73.3) | 41 (67.2) | 0.552 |
| IBD Surgery | |||
| Yes | 39 (65) | 31 (50.8) | 0.142 |
| Perianal Disease Type | |||
| Fistula | 48 (78.7) | 43 (70.1) | 0.516 |
| Abscess | 7 (11.5) | 12 (19.7) | |
| Stricture | 6 (9.8) | 6 (9.8) | |
| Severity of Perianal Disease | |||
| Required procedure | 48 (78.7) | 44 (72.1) | 0.529 |
| Medications Prior to Pregnancy (ever use) | |||
| Antibiotics | 52 (85.3) | 46 (76.7) | 0.255 |
| Immunomodulators | 48 (78.7) | 34 (56.7) | 0.012 |
| Steroids | 44 (72.1) | 39 (63.9) | 0.438 |
| Anti-TNF | 22 (36.1) | 26 (43.3) | 0.46 |
| Medications Following Delivery | |||
| Antibiotics | 36 (62.1) | 34 (55.7) | 0.577 |
| Steroids | 32 (55.2) | 11 (18.33) | 0 |
| Anti-TNF | 31 (53.5) | 34 (56.7) | 0.853 |
| Flare of perianal Disease Following Delivery | 36 (59.0) | 33 (54.1) | 0.715 |
| Surgery Following Delivery | 26 (42.6) | 26 (42.6) | 1 |
| Luminal CD Flare Following Delivery | 30 (49.2) | 26 (42.6) | 0.586 |
Perianal disease following delivery did not differ between the two groups. Within the gravid group, approximately 36% and 56% of cases had a symptomatic perianal flare within 1 and 2 years after delivery, respectively. Thirty percent experienced a luminal flare and 26% required a surgical procedure within 2 years post-delivery. The rate of perianal flare was also similar in non-pregnant CD controls (24% at 1 year, 36% at 2 years) (p=0.89, Figure 1) (univariate hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.54 – 1.38). Other variables associated with perianal flares at p < 0.1 were age (HR 0.99, 95% CI 0.97 – 1.00) and type of perianal disease (HR for abscess 2.38, 95% CI 1.35 – 4.19). On multivariate analysis, case-control status was not associated with an increased risk of perianal flares (HR 0.77, 95% CI 0.48 – 1.25). Similarly the occurrence of luminal flares and surgery were comparable as well. Both groups seemed to progress towards requiring anti-TNF therapy, though this would be expected given the natural course of CD, especially in the cohort of Crohn's patients with documented perianal disease.
Figure 1. Mode of delivery and risk of perianal flares in established perianal Crohn's Disease.
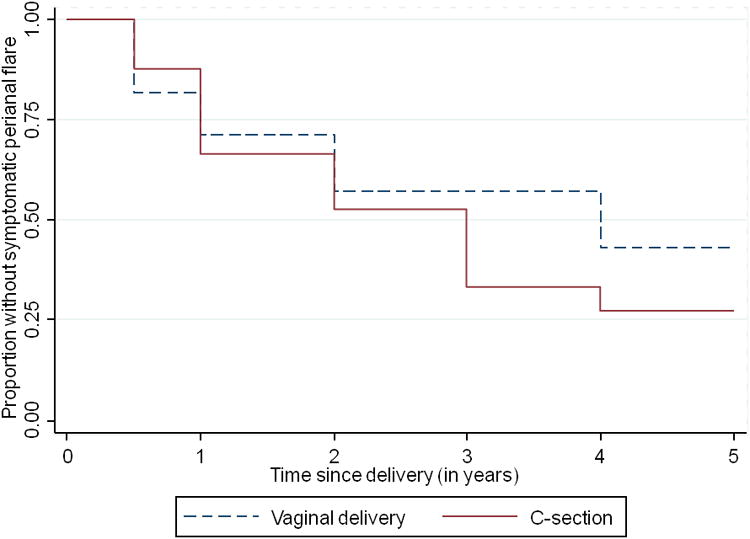
Table 2 shows the differences between the C-section and vaginal delivery groups. Both groups had ileocolonic-dominant CD. The C-section group had significantly increased occurrence of penetrating disease, however both groups required similar percentages of procedures and hospitalizations. There was no difference between the groups in their requirements for immunomodulators or anti-TNF inhibitors. Notably, most of the patients within the C-section group received a procedure for IBD related reasons, followed by obstetrical reasons, and amongst this group, 8 patients experienced complications during the labor that necessitated C-section; thus one-third (32%) of the C-sections were primarily for obstetric indications. Within the vaginal delivery group, the majority did not experience any complications during labor, however six of the vaginal deliveries were complicated by perineal laceration (n=2), need for episiotomy (n=3), or instrumental delivery (n=1).
Table 2. Comparison of characteristics of pregnant CD patients with perianal disease stratified by mode of delivery.
| Characteristic | Vaginal delivery (n=11), N (%) | C-section delivery (n=50), N (%) | p-value |
|---|---|---|---|
| Age at Diagnosis (in years) (mean (SD)) | 21.5 (6.0) | 22.7 (6.1) | 0.589 |
| Duration of disease (in years) (mean (SD)) | 7.7 (4.9) | 9.9 (5.9) | 0.264 |
| Age at Pregnancy (in years) (mean (SD)) | 29.4 (4.6) | 33.1 (5.5) | 0.04 |
| Crohn's Disease Location | |||
| Ileal | 1 (9.1) | 0 (0.0) | 0.175 |
| Colonic | 2 (18.8) | 14 (28.6) | |
| Ileocolonic | 8 (72.7) | 35 (71.4) | |
| Crohn's Disease Phenotype | |||
| Inflammatory | 4 (36.4) | 7 (14.3) | 0.112 |
| Stricturing | 4 (36.4) | 14 (28.6) | |
| Penetrating | 3 (27.3) | 28 (57.1) | |
| Ever smoker | 4 (36.4) | 24 (48.0) | 0.526 |
| IBD Hospitalizations | |||
| Yes | 8 (72.7) | 36 (73.5) | 1 |
| IBD Surgery | |||
| Yes | 8 (72.7) | 31 (63.3) | 0.731 |
| Perianal Disease Type | |||
| Fistula | 10 (90.9) | 38 (76.0) | 0.514 |
| Abscess | 0 (0.0) | 7 (14.0) | |
| Stricture | 1 (9.1) | 5 (10.0) | |
| Severity of Perianal Disease | |||
| Required procedure | 9 (81.8) | 39 (78.0) | 1 |
| Medications Prior to Pregnancy (ever use) | |||
| Antibiotics | 10 (90.9) | 42 (84.0) | 1 |
| Immunomodulators | 9 (81.8) | 39 (78.0) | 1 |
| Steroids | 10 (90.9) | 34 (68.0) | 0.159 |
| Anti-TNF | 2 (18.8) | 20 (40.0) | 0.299 |
| Medications during pregnancy | |||
| Immunomodulators | 4 (36.4) | 16 (32.0) | 1 |
| Steroids | 3 (27.3) | 5 (10.0) | 0.148 |
| Anti-TNF | 2 (18.8) | 10 (20.0) | 1 |
| First pregnancy | 8 (72.7) | 24 (48.0) | 0.137 |
| Indication for C-section | N/A | ||
| IBD-related | 33 (66.0) | ||
| Obstetrical | 16 (32.0) | ||
| Patient preference | 1 (2.0) | ||
| Delivery Complications | N/A | ||
| None | 5 (45.5) | 42 (84.0) | |
| Perineal laceration | 2 (18.8) | ||
| Episiotomy | 3 (27.3) | ||
| Conversion to C-section | 0 (0.0) | 2 (4.0) | |
| PPROM | 0 (0.0) | 4 (8.0) | |
| Instrumental delivery | 1 (9.1) | ||
| Medications Following Delivery | |||
| Antibiotics | 6 (54.5) | 30 (63.8) | 0.732 |
| Steroids | 6 (54.5) | 26 (55.3) | 1 |
| Anti-TNF | 7 (63.6) | 24 (51.1) | 0.518 |
| Flare of perianal Disease Following Delivery | 5 (45.5) | 31 (62.0) | 0.333 |
| Surgery Following Delivery | 4 (36.4) | 22 (44.0) | 0.745 |
| Luminal CD Flare Following Delivery | 6 (54.5) | 24 (48.0) | 0.749 |
| Bowel obstruction during follow-up | 0 (0.0) | 7 (14.0) | 0.332 |
Importantly, post-delivery, there was no difference between the C-section and vaginal birth groups in terms of recurrence of perianal disease, requirement for surgery, or occurrence of CD flare. Approximately 66% of C-section delivery cases had a symptomatic perianal flare within 2 years after delivery, compared to 45% for Vaginal delivery (p = 0.33, Table 2) (HR 1.31, 95% CI 0.51 – 3.39). Adjusting for other risk factors for a flare (age, perianal CD phenotype, presence of penetrating disease, duration of disease) did not materially change this estimate (HR 1.30, 95% CI 0.48 – 3.60). Perineal trauma during vaginal delivery was not predictive of subsequent flare of perianal disease. Seven patients in the Cesarean group were noted to have developed small bowel obstruction like symptoms subsequently and none within the vaginal delivery group; however this difference was not statistically significant.
Discussion
As CD often affects women during the reproductive years, the impact of pregnancy on disease course has often been studied6-11. However, few have examined the effect of mode of delivery on subsequent course of CD, particularly with reference to those with established perianal disease who represent a subgroup with potentially the greatest impact from this decision13-17. In this study from two large tertiary referral centers, we sought to address whether pregnancy or mode of delivery would affect likelihood of flare of perianal disease after pregnancy. Our results indicate that pregnancy does not influence flare rates or alter the course of perianal disease. Furthermore, mode of delivery did not influence subsequent perianal complications in those with established CD.
There are a few important findings from our study that may provide reassurance for women with perianal disease contemplating pregnancy. First, pregnancy itself did not appear to impact subsequent course of perianal CD and we did not observe an increase in perianal disease complications during pregnancy. Several prior studies have examined the impact of pregnancy on disease course in CD, however most restricted analyses to flares of luminal CD13-17. In women who are in remission at the time of conception, a subsequent disease flare during pregnancy occurs infrequently particularly in the setting of continued medical therapy. In contrast, women with active disease at the time of conception are more likely to experience continued active luminal disease or worsening of their luminal symptoms during pregnancy10, 11. It is likely that a similar association holds in perianal CD though such nuanced studies are more difficult to perform retrospectively owing to inadequate documentation regarding the activity of perianal CD at each encounter, and infrequent use of standard disease activity indices such as the perianal disease activity index. However, the findings from our cohort suggest that in a broad group of women with established perianal CD, there was no difference in rate of perianal complications within 5 years after delivery when compared to female non-pregnant CD patients.
The second set of key findings from our study stem from the examination of the mode of delivery in women with perianal CD. First, we found that vaginal delivery was used very infrequently in such women, accounting for only one-sixth of all full-term deliveries in such women. This is consistent with a recent study using the Nationwide inpatient sample by Hatch et al.14. Reassuringly, there was no difference in outcomes who delivered vaginally when compared to those who delivered via C-section. There is limited published literature to guide practice in this area. An often cited study is by Ilnyckyji et al. who examined 50 patients with and without prior perianal disease and found that vaginal delivery may exacerbate perianal disease in those with active perianal disease at the time of delivery15. We found this distinction hard to replicate as documentation regarding the activity of perianal disease at the time of delivery was often poor. However three women were noted to have some perianal disease activity during pregnancy and our findings did not change excluding such women from the analysis. Other attempts to examine the impact of mode of delivery on perianal CD have been through surveys which carry a number of limitations regarding accuracy, bias from self-report, and generalizability13. Rogers et al. performed a retrospective chart review of 17 pregnant CD patients among whom 5 had perianal disease. One delivered vaginally and had no subsequent flare while among the four who delivered via C-section, three developed recurrent perianal disease16. A larger Dutch study found no difference in perianal disease flares between vaginal and C-section delivery though that study included only 27 women with established perianal CD before pregnancy17. Though Hatch et al. used a national database to examine this question, it is difficult to draw firm conclusions from administrative data where accuracy of coding is suboptimal and there is no information on prior or subsequent disease course14. Indeed in our chart review, we found the accuracy of codes for perianal disease to be less than satisfactory, particularly with regards to anal fissures where often it wasn't clear that the fissures were CD related.
There are a few implications for our findings. Although our study and prior ones were performed in demographically distinct cohorts with large variation in IBD severity and treatment, they showed that cesarean delivery did not reduce subsequent perianal disease flares in the gravid IBD population. Conversely, potential perineal trauma during vaginal delivery did not influence subsequent flares of perianal CD. There is almost certainly a bias in the selection of women for vaginal delivery as those with severe disease may be electively scheduled for a C-section. Using a broad definition of severe disease, we did not notice this distinction between the groups and in repeating our analysis using a propensity score adjustment (for selection of mode of delivery), we arrived at similar results. Our results in conjunction with the prior studies suggest that in women with inactive perianal disease during pregnancy, in the absence of obstetric indications, vaginal delivery may be a safe option without increasing subsequent complications within 5 years of delivery, and with a reduced likelihood of complications from a C-section such as bowel obstruction. While our findings cannot be extrapolated to the small group of women with active perianal CD at the time of delivery, there does seem to be a higher risk of perianal complications in such women and further study is needed to guide selection of appropriate mode of delivery. However, at this time, in women with active perianal CD characterized by draining fistulae or abscesses during pregnancy, particular in late pregnancy, delivery via C-section may be prudent pending availability of rigorous high quality data. Our study also highlights the challenges in providing high quality data regarding this important clinical question, and the need for large multicenter collaborative studies to specifically answer this question. Routine use of standard perianal disease activity description or indices would facilitate ability to more accurately quantify the changes in perianal disease activity during pregnancy and following childbirth.
We readily acknowledge several limitations to our study. As the data source was from a cohort of patients in the US drawn from a large tertiary care IBD population, these patients have more severe disease. However as this is indeed the cohort with a higher risk of complications, the null associations in our cohort is reassuring. Second, the number of patients was small, but yet larger than most prior studies. We only included patients with prior well characterized perianal disease and used stringent clinical criteria for evidence of perianal flare including documented office visit with perianal pathology on exam, imaging evidence of fistula or abscess, or surgical intervention of perianal disease. Inclusion of other forms of perianal CD, particularly fissures, may have significantly increased our numbers but would have reduced confidence in our findings as some such pathologies may be unrelated to CD. Our results are thus less likely to be influenced by recall bias as all cases and outcomes were confirmed by medical record review. In addition, this was a retrospective study so our results could be affected by the quality of documentation; specifically, we risk underestimating the number and severity of perianal disease flare due to our stringent chart review criteria. Three women were noted to have active perianal disease during pregnancy and their exclusion did not alter our findings. However, as one would surmise, much of the care during later pregnancy is at their obstetric provider and the level of detail in documenting activity of perianal disease is variable at each of the follow-up visits. Thus, our findings may be broadly applicable to the group without active perianal CD during pregnancy and highlights the important need for a prospective study with detailed quantification of perianal disease activity using accepted indices.
In conclusion, our findings indicate that in our cohort, perianal CD was not exacerbated by pregnancy or delivery mode. However, trial of vaginal delivery remains infrequent in clinical practice. The growing, albeit limited, data suggest that in women with inactive perianal CD, vaginal delivery is unlikely to result in exacerbation of disease though more rigorous high quality data is needed. Multi-center collaborative studies will provide important clinical data that can guide us in the management of our patients.
Acknowledgments
Source of funding: Ananthakrishnan is supported in part by a grant from the National Institutes of Health (K23 DK097142). This work is also supported by the National Institutes of Health (NIH) (P30 DK043351) to the Center for Study of Inflammatory Bowel Diseases.
Footnotes
Financial Conflicts of Interest: None
Disclosures: Alice Cheng – no conflict of interest exists
Emily Oxford – no conflict of interest exists
Deanna D Nguyen – no conflict of interest exists
Jenny Sauk – no conflict of interest exists
Vijay Yajnik - no conflict of interest exists
Sonia Friedman - no conflict of interest exists
Ashwin Ananthakrishnan - Scientific advisory board for Cubist pharmaceuticals, Abbvie
Author Contributions: A Cheng: study design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content
E Oxford: data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content
D Nguyen: data collection, critical revision of the manuscript for important intellectual content.
J Sauk: data collection, critical revision of the manuscript for important intellectual content.
V Yajnik: data collection, critical revision of the manuscript for important intellectual content.
S Friedman: data collection, critical revision of the manuscript for important intellectual content.
A Ananthakrishnan: study design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, supervision for the study
References
- 1.American Gastroenterological Association Clinical Practice C. American Gastroenterological Association medical position statement: perianal Crohn's disease. Gastroenterology. 2003;125:1503–7. doi: 10.1016/j.gastro.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz DA, Loftus EV, Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–80. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 3.Rankin GB, Watts HD, Melnyk CS, et al. National Cooperative Crohn's Disease Study: extraintestinal manifestations and perianal complications. Gastroenterology. 1979;77:914–20. [PubMed] [Google Scholar]
- 4.Platell C, Mackay J, Collopy B, et al. Anal pathology in patients with Crohn's disease. Aust N Z J Surg. 1996;66:5–9. doi: 10.1111/j.1445-2197.1996.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 5.Safar B, Sands D. Perianal Crohn's disease. Clin Colon Rectal Surg. 2007;20:282–93. doi: 10.1055/s-2007-991027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agret F, Cosnes J, Hassani Z, et al. Impact of pregnancy on the clinical activity of Crohn's disease. Aliment Pharmacol Ther. 2005;21:509–13. doi: 10.1111/j.1365-2036.2005.02384.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrero S, Ragni N. Inflammatory bowel disease: management issues during pregnancy. Arch Gynecol Obstet. 2004;270:79–85. doi: 10.1007/s00404-003-0489-6. [DOI] [PubMed] [Google Scholar]
- 8.Friedman S, Regueiro MD. Pregnancy and nursing in inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:265–73. doi: 10.1016/s0889-8553(01)00015-2. , xii. [DOI] [PubMed] [Google Scholar]
- 9.Kornfeld D, Cnattingius S, Ekbom A. Pregnancy outcomes in women with inflammatory bowel disease--a population-based cohort study. Am J Obstet Gynecol. 1997;177:942–6. doi: 10.1016/s0002-9378(97)70298-9. [DOI] [PubMed] [Google Scholar]
- 10.Mahadevan U, Cucchiara S, Hyams JS, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214–23. doi: 10.1038/ajg.2010.464. quiz 224. [DOI] [PubMed] [Google Scholar]
- 11.Ng SW, Mahadevan U. Management of inflammatory bowel disease in pregnancy. Expert Rev Clin Immunol. 2013;9:161–73. doi: 10.1586/eci.12.103. quiz 174. [DOI] [PubMed] [Google Scholar]
- 12.Caprilli R, Gassull MA, Escher JC, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: special situations. Gut. 2006;55(Suppl 1):i36–58. doi: 10.1136/gut.2005.081950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt LJ, Estabrook SG, Reinus JF. Results of a survey to evaluate whether vaginal delivery and episiotomy lead to perineal involvement in women with Crohn's disease. Am J Gastroenterol. 1995;90:1918–22. [PubMed] [Google Scholar]
- 14.Hatch Q, Champagne BJ, Maykel JA, et al. Crohn's disease and pregnancy: the impact of perianal disease on delivery methods and complications. Dis Colon Rectum. 2014;57:174–8. doi: 10.1097/DCR.0b013e3182a41381. [DOI] [PubMed] [Google Scholar]
- 15.Ilnyckyji A, Blanchard JF, Rawsthorne P, et al. Perianal Crohn's disease and pregnancy: role of the mode of delivery. Am J Gastroenterol. 1999;94:3274–8. doi: 10.1111/j.1572-0241.1999.01537.x. [DOI] [PubMed] [Google Scholar]
- 16.Rogers RG, Katz VL. Course of Crohn's disease during pregnancy and its effect on pregnancy outcome: a retrospective review. Am J Perinatol. 1995;12:262–4. doi: 10.1055/s-2007-994469. [DOI] [PubMed] [Google Scholar]
- 17.Smink M, Lotgering FK, Albers L, et al. Effect of childbirth on the course of Crohn's disease; results from a retrospective cohort study in the Netherlands. BMC Gastroenterol. 2011;11:6. doi: 10.1186/1471-230X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nalichowski R, Keogh D, Chueh HC, et al. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc. 2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 19.de Silva PS, Nguyen DD, Sauk J, et al. Long-term outcome of a third anti-TNF monoclonal antibody after the failure of two prior anti-TNFs in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:459–66. doi: 10.1111/j.1365-2036.2012.05214.x. [DOI] [PubMed] [Google Scholar]
- 20.Desai A, Zator ZA, de Silva P, et al. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:309–15. doi: 10.1002/ibd.23026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxford EC, Nguyen DD, Sauk J, et al. Impact of coexistent celiac disease on phenotype and natural history of inflammatory bowel diseases. Am J Gastroenterol. 2013;108:1123–9. doi: 10.1038/ajg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zator ZA, Cantu SM, Konijeti GG, et al. Pretreatment 25-Hydroxyvitamin D Levels and Durability of Anti-Tumor Necrosis Factor-alpha Therapy in Inflammatory Bowel Diseases. JPEN J Parenter Enteral Nutr. 2013 doi: 10.1177/0148607113504002. [DOI] [PubMed] [Google Scholar]
- 23.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]


