Abstract
Elevated Specific Absorption Rate (SAR) associated with increased main magnetic field strength remains as a major safety concern in ultra-high-field (UHF) Magnetic Resonance Imaging (MRI) applications. The calculation of local SAR requires the knowledge of the electric field induced by radiofrequency (RF) excitation, and the local electrical properties of tissues. Since electric field distribution cannot be directly mapped in conventional MR measurements, SAR estimation is usually performed using numerical model-based electromagnetic simulations which, however, are highly time consuming and cannot account for the specific anatomy and tissue properties of the subject undergoing a scan. In the present study, starting from the measurable RF magnetic fields (B1) in MRI, we conducted a series of mathematical deduction to estimate the local, voxel-wise and subject-specific SAR for each single coil element using a multi-channel transceiver array coil. We first evaluated the feasibility of this approach in numerical simulations including two different human head models. We further conducted experimental study in a physical phantom and in two human subjects at 7T using a multi-channel transceiver head coil. Accuracy of the results is discussed in the context of predicting local SAR in the human brain at UHF MRI using multi-channel RF transmission.
Keywords: SAR, B1-mapping, Electrical Properties Tomography (EPT), Magnetic Resonance Imaging (MRI), ultrahigh-field (UHF), parallel transmission
I. INTRODUCTION
Ultra-high-field (UHF) Magnetic Resonance Imaging (MRI) systems (7T and higher) have been pursued with increasing interest [1]. Their main advantages include higher signal-to-noise ratio (SNR), higher spatial resolution, and increased sensitivity for intrinsic contrast mechanisms such as blood oxygenation level-dependent (BOLD) contrast. However, the magnitude of electric fields generated for a given strength of the transmit magnetic field increases with the operating radio frequency (RF) (proportional to the main magnetic field strength). The electric fields induce eddy currents and hence dissipate heat into the human body, resulting in elevated energy deposition as the frequency increases [2],[3]. Possible local overheating due to the latter poses a serious safety concern at UHF. Consequently, when using a multiple element transmit coil which has been recognized as a powerful tool for B1 inhomogeneity compensation and has been widely utilized at UHF [4–6], energy deposition in the body must be carefully controlled and kept under international safety guidelines. Although attempts to address this overheating concern have been made by constraining B1 shimming or parallel transmission RF pulse design solutions with worst-case conditions derived from electromagnetic (EM) simulations [7–9], in some situations this may excessively limit the achievable SNR and contrast at UHF.
Although various approaches have been proposed for the assessment of RF heating at UHF [10–12], current guidelines (International Standard IEC 60601-2-33 2010) impose upper limits on global specific absorption rate (SAR) as well as on local SAR averaged through 10 gram of tissue. In practice, local averaged SAR often reaches maximum upper limits before global SAR at UHF [11]. While global SAR estimation can be estimated by real-time measurement of forward and reflected RF power at the coil ports [13], the estimation of local SAR for human MR experiments typically relies on highly time-consuming computational electromagnetic simulations. Such extensive computations have been conducted using a variety of commercial software, based on the numerical models of EM properties of the human body [14–19]. However, beside the time-consuming constraint of these simulation approaches, it remains to be shown whether such findings based on a very limited number of different individuals' anatomy could reliably provide a quantitative prediction of local SAR characteristics of a given specific subject undergoing a scan. As such, real-time and subject-specific local SAR estimation is highly desired in UHF MRI applications.
Recently, B1-mapping based electric field and SAR estimation approaches have been developed by post-processing the measured complex B1 fields (rotating RF field induced by MRI RF coils, consisting of transmit B1 and receive B1 [20]) through a series of mathematical deduction, which involves the retrieval of complex B1 information and the reconstruction of electrical properties (EPs) of the subject (known as Electrical Properties Tomography, EPT) [21–24]. This approach overcomes the limitation of conventional simulation methods relying on a generic human body model, and allows for real-time calculation [23],[25]. Most previous EPT studies were typically restricted to conventional quadrature birdcage coils, relying only on the spatial transmit B1 phase which was approximated as one half of the measured total phase in a single transmit and receive (transceiver) element [22]. However, for multiple-element coils (e.g. transceiver coil array) at UHF, the shorter operating RF wavelength yields significant spatial phase and magnitude discrepancies between transmit and receive B1 fields for each coil element, hindering the use of this simplification. Complex information for both transmit and receive B1 is, however, essential for a reliable estimation of the electric field distributions that will subsequently be used for SAR calculation. In order to address this need at UHF with multi-channel RF coils, a new approach has recently been introduced to measure transmit and receive complex B1 fields that includes the retrieval of absolute B1 phases from measured relative B1 phases between coil elements [26],[27]. In addition, most existing EPT inverse methods utilize the Helmholtz equation by assuming negligible local spatial gradients of the electrical properties [21],[22],[25],[27]. It can be anticipated that, when using this assumption in biological tissues with complex anatomical structures, artifacts may arise in the vicinity of tissue boundaries in reconstructed EPs maps, resulting in an inaccurate SAR estimation. Nevertheless, the Dual-Excitation EPT algorithm, that has been previously proposed [24], takes the spatial variation of EPs distribution into account and produced reasonable simulation results for head tissues, indicating that this method could improve EPs reconstruction accuracy.
As described in our recent studies [24],[26],[27], three assumptions have been proposed and employed throughout the mathematical deduction and data analysis, from complex B1-mapping to EPs reconstruction: (1) a fairly left-right symmetric structure of the human brain about coronal plane through the brain center, (2) the 16-fold elliptical symmetric head coil structure about the long axis, and (3) negligible spatial variations of the z-axis component of induced RF magnetic fields. Besides quantitatively and separately evaluating the performance of above assumptions in the calculated intermediate quantities (e.g., reconstructed EPs in [24], and extracted proton density in [27]), it is necessary to rigorously investigate the resulted error propagation altogether along the whole data post-processing towards the ultimate local SAR estimation.
In the present study, subject-specific local SAR estimation is demonstrated for human brain imaging, at 7T, for individual coil element in a 16-channel transceiver coil, based on complex transmit and receive B1 maps acquired from MR data acquisition. For this purpose, EPs reconstruction was obtained with a modified, B1-based Dual-Excitation inverse algorithm. The performance of the proposed method is assessed and evaluated by computer simulations using two human head models, and with experiments in a physical phantom and on two human subjects.
II. THEORY
A. Complex B1 Mapping for Individual Coil Element
Using a N-channel microstrip transceiver array coil [28–30] and a hybrid B1-mapping technique [5],[31],[32], the magnitude of transmit B1 field (labeled with “~” denoting complex quantity), the magnitude of receive B1 field biased by proton density (PD) (labeled ), and the relative phase distributions between coil elements can be measured for each coil element, where k ∈[1,2,…N] and j ∈[1,2,…N] refer to the transmit and receive components of each coil element, respectively. Based on the approximately elliptical symmetry of the human brain in a transverse plane, the PD distribution and the magnitude of receive B1 field for each coil element are extracted and estimated separately. Then, knowing the relative phase information between coil elements for both transmit and receive B1 fields, assuming negligible spatial variation of RF-coil-induced B̃z component along z-axis direction, as |∂B̃z / ∂z|<< |∂B̃x / ∂x| & |∂B̃y / ∂y|, the absolute transmit and receive B1 phases of each coil element can be calculated by applying Gauss’s Law for magnetism [26],[27].
B. Modified Dual-excitation Algorithm – the Inverse Problem
Considering the magnetic permeability inside biological tissues to be equal to that in a vacuum, assuming isotropic EPs distribution, the previously proposed dual-excitation algorithm reads [24]
| (1) |
in which B̃x and B̃y are induced RF magnetic field components in Cartesian frame, μ0 the free space permeability, ω the operating angular frequency, ε̃c the complex permittivity ε̃c = εrε0 − iσ / ω with εr as the relative permittivity and ε0 as the free space permittivity. Negligible spatial variations of RF coil induced B̃z are assumed as |∂B̃z / ∂x| << |∂B̃x / ∂z|, |∂B̃z / ∂y| << |∂B̃y / ∂z| and |∂B̃z / ∂z| << |∂B̃x / ∂x| or |∂B̃y / ∂y|.
The principle of reciprocity [20] links magnetic field components in Cartesian and rotating frames via
| (2) |
where * denotes complex conjugation. Based on Gauss’s Law for magnetism (Eq. 3), due to much smaller magnitude of the z-component of Cartesian magnetic field B̃z when compared with that of B̃x and B̃y within RF head coil [24],[27], by ignoring the B̃z gradient term in Eq.(3)
| (3) |
Eq. (4) is derived as follows
| (4) |
By combining the two expressions in Eq. (1) with Eq. (4), the following equation is re-written in the form of
| (5) |
Eq. (5) is the central equation of the modified dual-excitation algorithm, in which ε̃c, (∂ε̃c / ∂z) / ε̃c and (∂ε̃c / ∂x + i∂ε̃c / ∂y) / ε̃c are unknowns. Thus, at least three sets of data are needed to solve εc. In the present study, three datasets were arbitrarily formed via the summation of the retrieved complex of individual coil element in the following three groups of channels: {1,4,7,10,13}, {2,5,8,11,14} and {3,6,9,12,15}. (Note that, similar to Eq. (5), an equation can also be written in the form of ). Here, compared to the original dual-excitation algorithm, the calculations are directly performed over the B1 field, avoiding excessive calculations of the Cartesian transverse magnetic components of the induced RF field.
C. Local SAR Estimation
Local SAR is defined by [14]:
| (6) |
where Ẽx, Ẽy and Ẽz are Cartesian components of the induced electric field, and ρ is material mass density. Under the assumption that |Ẽz| dominates the electric field [33],[34], only the |Ẽz| component is considered in local SAR estimation, as shown in Eq. (9) (this issue will be further investigated in the following sections).
| (7) |
The Ampere’s Law is
| (8) |
From Eq. (8) and Eq. (2), the following can be derived:
| (9) |
Therefore, through measuring and estimating the complex B1 distribution which involves receive B1 magnitude estimation and absolute phase retrieval as described in [27], EPs values can be reconstructed based on Eq. (5), allowing for computing |Ẽz| via Eq. (9); and local SAR can then be estimated via Eq. (7).
It has been shown in previous simulation studies [14],[19] that, some local SAR hot spots as well as local temperature rise can be localized in the skin. However, in experimental conditions, weak MRI signals observed in bone tissues are expected to significantly deteriorate B1-mapping results and the subsequent EPs reconstructions within scalp and skull regions [27]. For this reason, the anatomical targets of the present study, including B1 mapping, EPs reconstruction and local SAR estimation, were spatially confined within brain tissues. Addressing the local SAR estimation in the scalp is an important concern, which will require additional acquisition and reconstruction strategies that are beyond the scope of the present work.
RF safety standards as defined by the International Electrotechnical Commission (IEC) rely on SAR averaged over 10 grams of tissues. In practice, several computing difficulties arise for averaged SAR calculation that include the type of meshing approaches used to spatially distribute the Ẽ field, B̃ field and EPs, as well as the type of averaging algorithm used to produce 10g averaged SAR in certain locations (e.g. edge voxels at air/tissue interface). As a result, in the current study, non-averaged, voxel-wise SAR distribution is investigated in order to facilitate direct result comparison, avoiding possible interference or misleading interpretation that may arise due to the non-linear relationship between voxel-wise and 10g averaged local SAR.
III. MATERIALS AND METHODS
A. Simulations
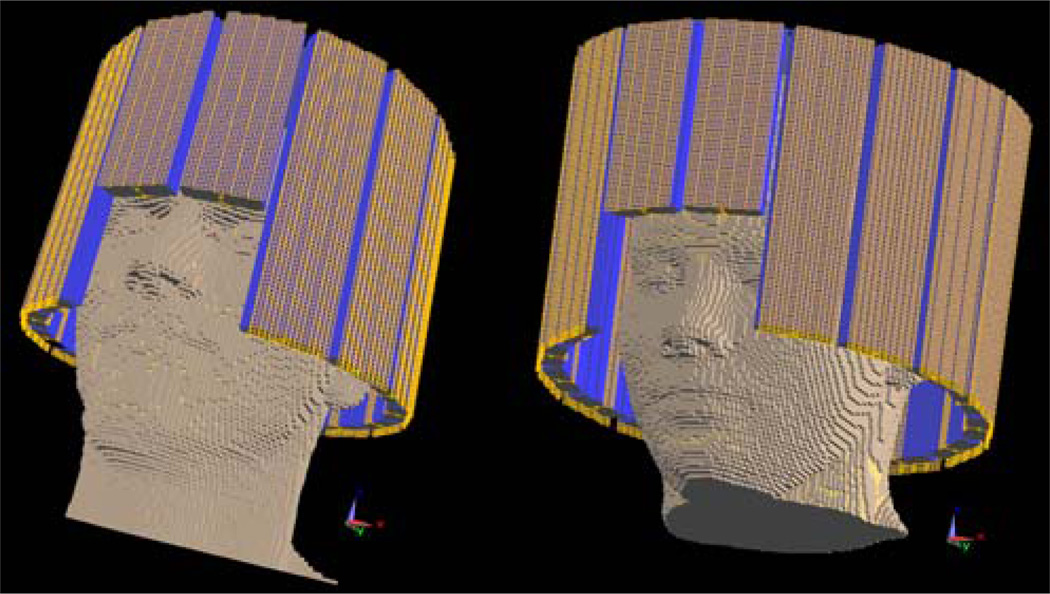
Simulation data were utilized to test the above methods. Finite-Difference Time-Domain (FDTD) based electromagnetic (EM) simulation software SEMCAD (Speag, Switzerland) was used to perform the simulation of B1 distributions in the human head. The coil model design reproduced the elliptical 16-channel RF microstrip transceiver coil that was utilized in experiments [30]. The heads and necks of Duke and Ella models from Virtual Family (25.6cm for Duke model and 22.4cm for Ella model in length along the z-axis direction), with 2×2×2mm3 resolution, were utilized to load the RF coil in EM simulations as illustrated in Fig. 1. The coil was tuned to 300MHz (7T) when loaded.
Fig. 1.
The reproduced 16-channel elliptical microstrip transceiver head coil loaded with SEMCAD Duke (left) and Ella (right) head models.
Electromagnetic fields were calculated with the same RF power applied to a single coil element at a time, assuming that all RF coil elements were ideally decoupled. The final sixteen simulated complex and maps were derived from the simulated complex Cartesian magnetic field components by Eq. (2). Each individual magnitude image was then multiplied by typical proton density values, i.e. PDCSF:PDGM:PDWM = 1:0.8:0.65 for CSF, gray matter and white matter, respectively [35], in order to mimic the experimentally measured PD-biased receive B1 magnitude maps [27]. In the following, according to the parametric maps effectively measured in experimental conditions, the magnitude of the sixteen fields, the sixteen PD-biased fields, and the relative phase maps of complex B1 field (transmit and receive) between each coil element will be the only simulation results utilized as known variables to subsequently generate simulation-based EPs and SAR maps. First-order derivatives were computed over adjacent voxels, while the Laplacian operator was applied over a 3×3×3 kernel. Note that known mass density values reported in the literature for different head tissues [14] were utilized in the local SAR calculation in this simulation study.
In addition, using the Ella head model, an offset of 6mm, 10mm and 20mm along x-, y- and z- axis, respectively, was introduced to the model position at one time, to evaluate the differences of local SAR distributions with respect of the head position within the coil.
For a reconstructed quantity q (e.g. EPs, SAR, etc.) in a specific region of interest, the relative error (RE) and correlation coefficient (CC) are defined to evaluate the performance of the present method as follows:
| (10) |
where qi,tar is the target (simulated) value in the ith voxel, qi the estimated (reconstructed) value in the ith voxel, and N the total number of voxels within the specified region of interest.
Electromagnetic fields and the following reconstructions were computed in a similar manner using the numerical design of a cylindrical phantom which will be described in the following section.
B. Experimental Studies
Imaging experiments were performed on a 7T magnet (Magnex Scientific, UK) driven by a Siemens console (Erlangen, Germany). The 16-channel transceiver head coil, as described above, was used for both RF transmit and receive operations, with the transmit channels powered by 16×1kW amplifiers (CPC, Hauppauge, NY) interfaced with a remotely controlled phase/amplitude gain unit. A single-compartment phantom, made of an acrylic cylinder with a diameter of 8.7cm and a length of 20cm, built for the validation purpose, was filled with a gel composed of distilled water, NaCl, Gelatin and CuSO4·5H2O (mass ratios 100:0.12:3:0.025) with a conductivity of 0.34 S/m and a relative permittivity of 77. For MR measurements, the phantom was placed in the head coil with its long axis parallel to B0.
In vivo data were acquired in two healthy human volunteers who had signed a consent form approved by the University of Minnesota Institutional Review Board, and laid in supine position with the head centered in the head coil.
A similar imaging protocol was used in both phantom and in vivo human experiments as follows (acquisition parameters are detailed in [27]). A series of 16 small flip angle 2D gradient-recalled echo (GRE) images were acquired with only one channel transmitting at a time while receiving signal on 16 independent channels. Relative phase maps between coil elements were calculated for the transmit and receive fields for each coil elements, with k=1,2,…,16 and j=1,2,…,16, as described in [5]. A 3D map of the excitation flip angle was obtained with the Actual Flip Angle (AFI) technique [36], by all channels transmitting together, to be merged with previously acquired small flip angle GRE images in order to calculate the magnitude map of for each of the sixteen coil elements as indicated in [31]. Finally, a 2D GRE image was acquired, by all channels transmitting together, using a large flip angle (high SNR), a long TR (longitudinal magnetization approximately at equilibrium) and a short TE (negligible T2* relaxation); each of the sixteen images (one per receive channel) of this data set was normalized by the sinus of the excitation flip angle to produce sixteen PD-biased magnitude maps [30].
Data were acquired in axial views at a spatial resolution of 1.5×1.5×1.5 mm3 in phantoms and of 1.5×1.5×5 mm3 in humans with, in both cases, an in-plane FOV of 288×189 mm2. B1 data (axial slices) were filtered in plane with a 2D Hanning window, 7×7 in size, to reduce Gibbs ringing artifact and thermal noise. First-order derivatives were computed over adjacent voxels, while the Laplacian was calculated within a kernel of 3×3×3.
For SAR estimation in the human experiments, average values for brain conductivity and relative permittivity [37] were assigned for those regions where negative or very large values occurred in the reconstructed EPs maps [27]; meanwhile, the mass density was assumed to be constant through the whole head region.
Experimental results for phantom and in vivo studies were compared against simulations results. For the human brains, the axial slices exhibiting the largest structural similarities between the simulation model and experimental anatomical images were chosen for this comparison. Computations were performed using MATLAB 2011b (Mathworks Inc., MA, USA) with its Parallel Computing Toolbox. The computation time on a single axial slice for each individual simulation or experiment subject was within 5 minutes.
IV. RESULTS
A. Simulations
With channel #13 arbitrarily chosen as a reference for all relative B1 maps, Fig. 2 depicts, on the axial slice of interest as indicated in (a1) and (b1) (slice #33 of 65 total slices for the Duke model, and slice #33 of 60 total slices for the Ella model), for coil element #13, the target and reconstructed distributions of: (a2)&(b2) absolute phase of transmit B1, (a3)&(b3) the absolute phase of receive B1, (a4)&(b4) the relative phase between transmit and receive B1, and (a5)&(b5) conductivity and (a6)&(b6) relative permittivity for both head models, respectively. The retrieved phase exhibits a CC of 0.990 on both models when compared with the target distribution, while reconstructed EPs for CSF, GM and WM showed −32.3%, −3.3%, +5.9% (Duke) / −28.1%, −2.6%, +10.7% (Ella) REs for conductivity, and −16.6%, −8.2%, +4.5% (Duke) / −15.9%, −7.4%, +2.0% (Ella) REs for relative permittivity, respectively. Note that the present approach is able to retrieve the relative phase distribution between transmit and receive B1 for each coil element, which is essential for Ẽz calculation using the closed-form Eq. (9).
Fig. 2.
Simulation results. (a1) Sagittal view of the Duke head model (colors represent different tissue types) with the axial slice of interest indicated; (a2)–(a4) absolute phase of transmit B1, absolute phase of receive B1, and relative phase between transmit and receive B1 for coil element #13 (the reference channel), (a5) conductivity map, and (a6) relative permittivity map; 1st row: target (simulated) results, 2nd row: estimated (reconstructed) results. (b1) Sagittal view of the Ella head model with the axial slice of interest indicated; (b2)–(b4) absolute phase of transmit B1, absolute phase of receive B1, and relative phase between transmit and receive B1 for coil element #13, (b5) conductivity map, and (b6) relative permittivity map; 3rd row: target results, 4th row: estimated results.
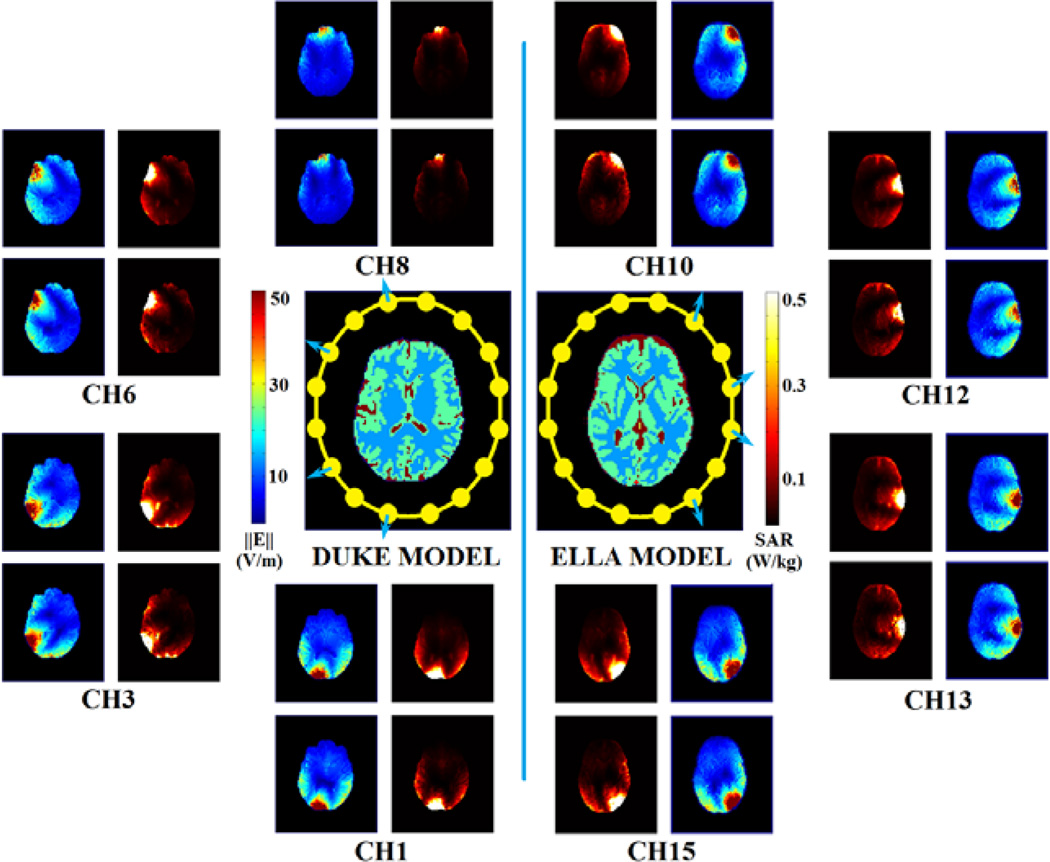
Fig. 3 shows in pairs: for both head models, on the aforementioned axial slice of interest (shown in the central maps), the target and estimated electric field and voxel-wise SAR results for selected channels, in which the target electric field intensity ‖Ẽtotal‖ includes all three components (Ẽx, Ẽy and Ẽz), while only z component electric field intensity |Ẽz| was estimated. The estimated |Ẽz| and SAR show reasonably similar distributions with the target distribution: exhibiting CCs of 0.922±0.030 (electric field) / 0.951±0.023 (SAR) for Duke model, and 0.931±0.018 (electric field) / 0.962±0.013 (SAR) for Ella model.
Fig. 3.
Simulation results. In the center: the axial view of the Duke (left) and Ella (right) head models (colors represent different tissue types) on the slice of interest with the arrangement of all coil elements. Surrounding inset maps: electric field intensity (colormaps range from blue to red) and voxel-wise SAR (colormaps range from black to white) distributions for selected coil element. In each inset map, top row: target (simulated) results, and bottom row: estimated (reconstructed) results.
Fig. 4 (a1)&(b1) show, on the slice of interest for each head model, the distribution of voxel-wise relative errors of estimated |Ẽz| compared with the target full electric field intensity (considering all three components). In addition, diagrams (a2)&(b2) show the relative error of the estimated voxel-wise local SAR compared with the target local SAR, all in blue bars, respectively (REs were calculated by summarizing the results for each individual coil element used for transmission at a time). Since hot spots with relatively higher SAR values draw more attention in terms of safety concern at UHF MRI, a threshold of 0.5 W/kg was arbitrarily chosen to further summarize local SAR results, restricted to SAR values above the threshold, for both models in (a3)&(b3). As it was empirically observed that these histograms consistently exhibited a Gaussian-like shape, they were fitted with a Gaussian distribution (red line) in order to facilitate their comparisons. Each Gaussian curve shows a mean relative error and a standard deviation of 6.43%±26.68% (electric field), −13.31±40.01% (SAR) −8.06±29.27% (SAR with threshold) for Duke model, and 1.75%±26.76% (electric field), −5.27±37.40% (SAR) −4.12±31.27% (SAR with threshold) for Ella model. The quadratic dependency of SAR upon |Ẽz| (see Eq. (7)) explains the wider distribution for local SAR estimation seen in Fig. 4(a2)(b2), whereas omitting |Ẽx| and |Ẽy| components may explain the fact that, in Fig. 4(b), the corresponding Gaussian distributions of RE for local SAR are now shifted towards larger, negative mean values.
Fig. 4.
Simulation results. Top row: using Duke head model, bottom row: using Ella head model. The number of voxels versus the relative error of estimated (a1)&(b1) electric field intensity, (a2)&(b2) local SAR, and (a3)&(b3) local SAR (above 0.5 W/kg) on the axial slice of interest, respectively. The original distributions (plotted in blue bars) are fitted against with a Gaussian distribution (in red lines). This is a summarization of all the results while each of sixteen coil elements were used for transmission.
For each axial slice (slices numbered along head→foot direction), RE distributions of estimated |Ẽz| and of local SAR (with and without threshold) were calculated and fitted against by Gaussian curves (data not shown). The corresponding and its standard deviation of the latter are plotted in Fig. 5(a1)–(a3)&(b1)–(b3), respectively, for both head models. An illustration of the total number of voxels with the target SAR value above threshold is summarized on each axial slice for both models, when each individual coil element used for transmission at a time, as in Fig. 5 (a4)&(b4), implying a larger SAR distribution within central transverse slices. Another direct illustration of local SAR spatial pattern is also provided for channel #16 with a comparison, in a sagittal view (on the central sagittal slice), between target and estimated SAR maps in Fig. 5 (a5)&(b5), respectively, with estimated SAR results exhibiting CCs of 0.9513 (Duke) and 0.9443 (Ella). The estimated SAR distributions reproduced the main pattern characteristics observed in the target maps, including, for a particular position along y-axis, faster spatial variation of SAR along the z-axis at the top and bottom of the head compared with the coil center. It can also be seen that, as the slice position along the z-axis moves away from the coil center and reaches the physical extremities of the RF coil, reconstructed SAR values are more and more underestimated compared with target values. This can easily be appreciated in Fig. 5(a2)&(b2), where reaches around −80% for the top slices. It is speculated that, as slice position gets closer to the upper end of the coil element along z, Ẽz magnitude becomes comparable to, or smaller than that of the transverse components of the electric field in those regions; in this situation, the dominant |Ẽz| approximation utilized in Eq. (7) does not hold anymore.
Fig. 5.
Simulation results. Top row: using Duke head model, bottom row: using Ella head model. The mean relative error (in red dots) and its standard deviation (in blue lines) of the fitted Gaussian distribution for (a1)&(b1) the electric field intensity, (a2)&(b2) local SAR, and (a3)&(b3) local SAR (above 0.5 W/kg) for each brain axial slice (along z-axis, from head to foot), respectively. (a4)&(b4) Summarized total number of voxels with the target SAR value above 0.5 W/kg for each axial slice when each individual coil element used for transmission at a time. (a5)&(b5) Target (upper) and estimated (lower) voxel-wise SAR on the central sagittal slice of interest when coil element #16 as the transmitter.
In Fig. 6, on an axial slice of interest, for channel #1 (a1) and #10 (a2), the previously demonstrated target (simulated) local SAR distributions (upper row) are plotted against the simulated local SAR distributions considering the aforementioned position offset (lower row). Notable differences are observed: the position offset has introduced an overall under-estimation with an average RE of −70.1% for channel #1, and an over-estimation with an average RE of +150.7% for channel #10. Selected SAR profiles along two vertical axes as denoted in (a1) and (a2) are shown in (b) as a comparison.
Fig. 6.
Simulated voxel-wise target SAR on an axial slice of interest of Ella head model. For channel #1 (a1) and #10 (a2), target SAR distribution (in dB, 0 dB = 1.6 W/kg) without (upper row) and with (lower row) a head model position offset within the coil, respectively. Selected SAR profiles (in W/kg) along vertical axes denoted in (a1) and (a2) are plotted in (b).
B. Phantom Experiment
Channel #5 was arbitrarily chosen as the reference for absolute phase retrieval. The values of the reconstructed EPs are 0.32±0.21 S/m for conductivity and 78±15 for permittivity; these mean values were used for subsequent SAR calculation. Simulated SAR distributions and estimated experiment results are shown for five channels (#2, #3, #4, #11 and #12) on an axial slice in Fig. 7, in which an agreement (including the localization of two hot spots) between simulated and experiment results are observed. The small shift of hot spots localization observed in Fig. 7 between experimental and simulation results may be due to some residual errors when assigning the experimental phantom position within the RF coil during FDTD modeling.
Fig. 7.
Phantom results. (a) Estimated axial proton density image of the phantom within the 16-channel microstrip head coil. (b) The simulated voxel-wise SAR (top row) and estimated voxel-wise SAR of experiment results (bottom row).
C. Human Experiment
Fig. 8 shows, for two human subjects, normalized T1-weighted images on the slice of interest [38], extracted proton density images, and, for two selected coil elements in each case, voxel-wise SAR as calculated based on simulated and experiment results. The two axial slices taken from the Ella head model, used as a comparison reference for SAR distribution, exhibit structure patterns with overall reasonable similarity with those observed in the T1-weighted images of the two human experiments. Note that, with RF wavelengths equivalent or shorter than the size of the human head at UHF, B1 distribution is more sensitive to head geometry, head position within the coil, as well as actual current distribution along coil conductors. Noticeable differences can be observed in Fig. 8, which may result from such factors in numerical modeling.
Fig. 8.
Human results. Subject I: (a1) The reference axial slice from Ella head model (colors represent different tissue types) with coil elements arrangement, (a2) simulated voxel-wise local SAR for coil element #4, and (a3) for coil element #11; (b1) normalized T1-weighted image of the slice of interest, (b2) extracted proton density image, (b3) estimated voxel-wise local SAR for coil element #4, and (b4) for coil element #11. Subject II: (c1) The reference axial slice from Ella head model with coil elements arrangement, (c2) simulated voxel-wise local SAR for coil element #5, and (c3) for coil element #12; (d1) normalized T1-weighted image of the slice of interest, (d2) extracted proton density image, (d3) estimated voxel-wise local SAR for coil element #5, and (d4) for coil element #12.
V. DISCUSSION
Unlike external model-based SAR simulation methods that are based on a few human models (i.e. not derived on a per-subject basis) and are highly time-consuming, EPs mapping methods derived from measured B1 maps may allow for fast, subject specific SAR calculation. Our present simulation and experimental results are encouraging, which suggest that real-time local SAR prediction for a specific human subject may be eventually feasible, relying only on measurable B1 information (i.e. magnitude and relative phase). Indeed, simulation studies conducted with electromagnetic numerical models of a 16 channel transceiver coil, loaded with a phantom and human heads at 7T, were in general consistent with experimental results; further development of EPT methods would result in acceptable accuracy of SAR estimation based on complex transmit B1 and/or receive B1 distributions. The present method can be applied to any MRI system equipped with a multi-channel transceiver coil; furthermore, B1 measurements using as few as five coil elements are sufficient to retrieve the necessary information for subsequent computation [27].
Recently, several EPs reconstruction methods have been proposed with multi-element coil arrays [39],[40], utilizing the homogeneous Helmholtz equation as the fundamental equation. Differing from these approaches, the phase retrieval of the proposed method originates from the Gauss’s Law for magnetism, utilizing the continuously differentiable nature of the magnetic field vector in space. Furthermore, the present modified Dual-Excitation algorithm, which also offers a more convenient implementation than in a previously introduced formalism [24], takes into account the spatial variation of EPs distribution as Eq.(5) indicates. Therefore, the proposed approach is theoretically capable of dealing with objects with inhomogeneous EPs distribution, and further work will be dedicated to demonstrate that this can indeed provide a practical way to study complex anatomical structures, such as the human brain. Note that, in a recent simulation study, it has been shown that simplifying the head model into a more homogeneous object produces a more uniform local SAR pattern, leading to lower SAR peaks [41], which suggests that an elevated safety concern exists at transitions between dielectrics due to increased local SAR values, which necessitates a more accurate EPs reconstruction in the vicinity of tissue boundaries. Another property, specific to the proposed method, is a phase retrieval approach capable of deriving the relative phase between transmit and receive B1 for each channel directly, without direct knowledge of transceive phase which requires redundant phase removal (e.g. ΔB0 and eddy currents phases) from the raw MRI image phase [25]. Retrieving this relative phase information, however, is necessary for accurate closed-form Ẽz calculation as Eq. (9) indicates.
In the current stage of this study, the proposed approach still relies on two assumptions. First, a fairly left-right symmetric structure of the human brain about the coronal plane through the brain center and the 16-fold elliptical symmetric head coil structure about the long axis were both utilized to estimate receive B1 magnitude for each coil element. As previous studies suggested [32],[27], even considering the residual coupling between coil elements, by examining the extracted proton density distribution of human subjects, the mirroring symmetry between coupled transmit and receive B1 along the vertical axis still preserves. While such an approximate symmetry nature is expected to exhibit in healthy human brains, however, further investigations are needed to overcome this limitation in order to extend its application to human anatomical structures where such symmetry does not apply, either because of pathological processes, or simply because of anatomical variations. Secondly, B1-mapping techniques have been well established to measure the transverse RF magnetic field components that contribute to MR signal; however, there is currently no standard MR method to measure the longitudinal RF magnetic field B̃z. Thus, the spatial variations of B̃z were neglected throughout the whole approach, from absolute phase retrieval, via EPs reconstruction, up to SAR calculation. Specifically, B̃z is essential for the calculation of transverse induced electric field components Ẽx and Ẽy, as the Ampere's Law indicates. As a result, in ours and others' related studies [23],[25],[33],[34],[39], SAR computation has been dictated to only considering Ẽz component (Eq. (7)), thus restricted to spatial regions where Ẽz is assumed to be dominant. Consistent with this observation, on both head models, the present simulated SAR-estimation results were judged satisfactory ( around −10%) when examining slices at the level of the coil center, exhibiting spatial patterns similar to those derived from experiments in locations where induced currents are anticipated to flow mostly along z-axis direction. This assumption, however, does not stand in regions close to the upper end of the coil elements (close to the apex) where Ẽx and Ẽy are not anymore minor components of the electric field: by observing simulation data, |Ẽz| usually 7~20 times higher than |Ẽx| and |Ẽy| on central brain slices, but 1~3 times less than |Ẽx| and |Ẽy| close to coil upper end. This rationale provides a direct explanation to the apparent drop in calculated SAR close to the upper end of the coil elements, as shown in Fig. 5 (a5)&(b5). Considering the whole brain for all coil elements, the relative error increases rapidly as a function of the distance of a given transverse slice upper to the central slice along z-axis, as seen in Fig. 5, with about −60% (Duke model) and −50% (Ella model) in |Ẽz| estimation close to the top of the brain, and with corresponding drop of about −90% (Duke and Ella models) in estimated SAR value. When examining voxels with target SAR value above 0.5 W/kg (taken as an arbitrary threshold), a flatter curve is seen for both head models as in Fig. 5(a3)&(b3), with of −40%~−80% on the uppermost slices, while within −40%~0% for the other slices; on the twenty central slices, nevertheless, was within −20%~0%. Moreover, as is depicted in Fig. 5 (a4)&(b4), a larger target SAR distribution is observed on central transverse slices, within which a dominant |Ẽz| is expected and an accuracy of 40%~0% of SAR estimation can be reached. Thus, on one hand, it will be worth investigating when using similar microstrip head coils, whether a stronger energy deposition pervades in such a region, in which a more reliable SAR estimation can be attained due to a dominant |Ẽz|. On the other hand, further work remains to be pursued to amend the absence of Ẽx and Ẽy in SAR computation via any possible B̃z measurement or estimation techniques, aiming for a more accurate SAR prediction as well as an extensive application by using various multi-channel RF coils designs.
In addition, computational EM simulation, which has been regarded as one of the standard tools for SAR prediction in current UHF MRI applications, computes SAR distribution each time for a specific position the model is placed at. However, despite accurate subject modeling, position offset would occur because it is less straightforward to capture the exact position of the subject with respect to the RF coil. As documented in Fig. 6 and in agreement with [41], significant SAR estimation discrepancies can be introduced with a trivial position offset. Therefore, it is believed that the B1 based approach, which does not require or rely heavily on accurate 3D modeling (in terms of model structure and position), preserves certain merits in subject-specific local SAR quantification. Also note that an acquisition time of more than 1 hour was needed to obtain the B1 maps with satisfactory SNR; further efforts should be mainly dedicated to improving the B1-mapping technique with a much shorter acquisition time, enabling the proposed B1 based approach to be a substantially real-time local SAR prediction scenario.
Although differences in mass densities through different human tissues have been reported [14], e.g. ρCSF = 1000 kg/m3, ρGM = 1036 kg/m3 and ρWM = 1027 kg/m3, reflecting negligible ≈3% differences, it is assumed, for simplicity, a homogeneous mass density in SAR calculation in the present human experiment. Note that the resulting SAR inaccuracies do not impact the conclusions with regards to electric field mapping, EPs mapping or SAR result comparisons, considering that mass density is only utilized at the last SAR computational step and has no role in electric field or EPs mapping.
Also note that, while there are other approaches being pursued for the estimation of EPs of biological tissues, including Electrical Impedance Tomography (EIT) [42],[43], Magnetic Resonance Electrical Impedance Tomography (MREIT) [44–46], and Magnetoacoustic Tomography with Magnetic Induction (MAT-MI) [47–49], these approaches are operated at much lower frequency so not able to estimate EPs during frequency range of MRI.
In summary, the ultimate goal of this work is to rapidly estimate local SAR, during an in vivo scanning session, based on B1-mapping technique. The present results suggest that electric fields for each coil element and electric properties for each voxel of tissue can be derived from measured complex B1 maps for individual coil element. The present approach holds promises for enabling the use of subject-specific local SAR computation which in turn can be used as explicit constraint in B1 shimming calculation and parallel transmission RF pulse design.
ACKNOWLEDGMENT
The authors would like to thank Dr. Gregor Adriany for providing details of RF coil design, Dr. Daniel Sodickson, Dr. Christopher Collins, Dr. Jinfeng Tian, Mr. Leo Mariappan, Dr. Xiaoping Wu and Dr. Wen Ling for useful discussions.
This work was supported in part by NIH RO1EB006433, RO1EB007920, R21EB009133, R21EB014353-01A1, NSF CBET-0933067, NIH P41 RR008079, P30 NS057091, and WM KECK Foundation, and in part by the Supercomputing Institute of the University of Minnesota.
Contributor Information
Xiaotong Zhang, Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN 55455 USA.
Sebastian Schmitter, Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN 55455 USA.
Pierre-François Van de Moortel, Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN 55455 USA.
Jiaen Liu, Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN 55455 USA.
Bin He, Department of Biomedical Engineering and Institute for Engineering in Medicine, University of Minnesota, Minneapolis, MN 55455 USA (binhe@umn.edu).
REFERENCES
- 1.Ugurbil K, Adriany G, Andersen P, Chen W, Garwood M, Gruetter R, Henry PG, Kim SG, Lieu H, Tkac I, Vaughan T, Van De Moortele PF, Yacoub E, Zhu XH. Ultrahigh field magnetic resonance imaging and spectroscopy. Magn. Reson. Imaging. 2003;21:1263–1281. doi: 10.1016/j.mri.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn. Reson. Med. 2001;46:24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- 3.Lattanzi R, Sodickson DK, Grant AK, Zhu Y. Electrodynamic constraints on homogeneity and radiofrequency power deposition in multiple coil excitations. Magn. Reson. Med. 2009;61:315–334. doi: 10.1002/mrm.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adriany G, Van de Moortele PF, Wiesinger F, Moeller S, Strupp JP, Andersen P, Snyder C, Zhang X, Chen W, Pruessmann KP, Boesiger P, Vaughan T, Ugurbil K. Transmit and Receive Transmission Line Arrays for 7 Tesla Parallel Imaging. Magn. Reson. Med. 2005;53:434–445. doi: 10.1002/mrm.20321. [DOI] [PubMed] [Google Scholar]
- 5.Van de Moortele PF, Akgun C, Adriany G, Moeller S, Ritter J, Collins CM, Smith MB, Vaughan JT, Ugurbil K. B1 destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn. Reson. Med. 2005;54:1503–1518. doi: 10.1002/mrm.20708. [DOI] [PubMed] [Google Scholar]
- 6.Metzger GJ, Snyder C, Akgun C, Vaughan T, Ugurbil K, Van de Moortele PF. Local B1+ shimming for prostate imaging with transceiver arrays at 7T based on subject-dependent transmit phase measurements. Magn. Reson. Med. 2008;59(2):396–409. doi: 10.1002/mrm.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Vaughan J, Ugurbil K, Van de Moortele PF. Parallel Excitation in the Human Brain at 9.4 T Counteracting k-Space Errors With RF Pulse Design. Magn. Reson. Med. 2010;63:524–529858. doi: 10.1002/mrm.22247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopanoglu E, Erturk VB, Atalar E. Analytic Expressions for the Ultimate Intrinsic Signal-to-noise Ratio and Ultimate Intrinsic Specific Absorption Rate in MRI. Magn. Reson. Med. 2011;66:846–858. doi: 10.1002/mrm.22830. [DOI] [PubMed] [Google Scholar]
- 9.Schmitter S, Bock M, Johst S, Auerbach E, Ugurbil K, Van de Moortele PF. Contrast Enhancement in TOF Cerebral Angiography at 7 T Using Saturation and MT Pulses Under SAR Constraints: Impact of VERSE and Sparse Pulses. Magn. Reson. Med. 2012;68:188–197. doi: 10.1002/mrm.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh S, Webb AG, Neuberger T, Park B, Collins CM. Experimental and Numerical Assessment of MRI-Induced Temperature Change and SAR Distributions in Phantoms and In Vivo. Magn. Reson. Med. 2010;63:218–223. doi: 10.1002/mrm.22174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrivastava D, Hanson T, Kulesa J, DelaBarre L, Iaizzo P, Vaughan JT. Radio frequency heating at 9.4T (400.2 MHz): in vivo thermoregulatory temperature response in swine. Magn. Reson. Med. 2009;62:888–895. doi: 10.1002/mrm.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Lier ALHMW, Kotte ANTJ, Raaymakers BW, Lagendijk JJW, van den Berg CAT. Radiofrequency heating induced by 7T head MRI: Thermal assessment using discrete vasculature or pennes' bioheat equation. J. Magn. Reson. Imaging. 2012;35:795–803. doi: 10.1002/jmri.22878. 2011. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Alon L, Deniz CM, Brown R, Sodickson DK. System and SAR Characterization in Parallel RF Transmission. Magn. Reson. Med. 2012;67:1367–1378. doi: 10.1002/mrm.23126. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins CM, Liu W, Wang J, Gruetter R, Vaughan JT, Ugurbil K, Smith MB. Temperature and SAR Calculations for a Human Head Within Volume and Surface Coils at 64 and 300 MHz. J. Magn. Reson. Imaging. 2004;19:650–656. doi: 10.1002/jmri.20041. [DOI] [PubMed] [Google Scholar]
- 15.van den Bergen B, van den Berg CAT, Klomp DWJ, Lagendijk JJW. SAR and Power Implications of Different RF Shimming Strategies in the Pelvis for 7T MRI. J. Magn. Reson. Imaging. 2009;30:194–202. doi: 10.1002/jmri.21806. [DOI] [PubMed] [Google Scholar]
- 16.Eichfelder G, Gebhardt M. Local specific absorption rate control for parallel transmission by virtual observation points. Magn. Reson. Med. 2011;66:1468–1476. doi: 10.1002/mrm.22927. [DOI] [PubMed] [Google Scholar]
- 17.Homann H, Börnert P, Eggers H, Nehrke K, Dössel O, Graesslin I. Toward Individualized SAR Models and In Vivo Validation. Magn. Reson. Med. 2011;66:1767–1776. doi: 10.1002/mrm.22948. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Pang Y, Wang C, Wu B, Vigneron D, Zhang X. Evaluation of Common RF Coil Setups for MR Imaging at Ultrahigh Magnetic Field: A Numerical Study. Proceedings of the 4th International Symposium on Applied Sciences in Biomedical and Communication Technologies (ISABEL) 2011 doi: 10.1145/2093698.2093768. article No. 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian J, Shrivastava D, Strupp J, Zhang J, Vaughan T. Proceedings of the 20th Annual Meeting ISMRM. Melbourne, Australia: Barcelona, Spain; 2012. From 7T to 10.5T: B1+, SAR and Temperature Distribution for Head and Body MRI; p. 2666. [Google Scholar]
- 20.Hoult DI. The principle of reciprocity in signal strength calculations - A mathematical guide. Conc. Mag. Res. 2000;12(4):173–187. [Google Scholar]
- 21.Haacke EM, Petropoulos LS, Nilges EW, Wu DH. Extraction of conductivity and permittivity using magnetic resonance imaging. Phys. Med. Biol. 1991;38:723–734. [Google Scholar]
- 22.Wen H. Noninvasive quantitative mapping of conductivity and dielectric distributions using RF wave propagation effects in high-field MRI. Proc. SPIE. 2003;5030:471–477. [Google Scholar]
- 23.Katscher U, Voigt T, Findeklee C, Vernickel P, Nehrke K, Doessel O. Determination of electric conductivity and local SAR via B1 mapping. IEEE Trans. Med. Imaging. 2009;28:1365–1374. doi: 10.1109/TMI.2009.2015757. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Zhu S, He B. Imaging electric properties of biological tissues by RF field mapping in MRI. IEEE Trans. Med. Imaging. 2010;2:474–481. doi: 10.1109/TMI.2009.2036843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voigt T, Homann H, Katscher U, Doessel O. Patient-Individual Local SAR Determination: In Vivo Measurements and Numerical Validation. Magn. Reson. Med. 2012;68:1117–1126. doi: 10.1002/mrm.23322. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Van de Moortele PF, Schmitter S, He B. Proceedings of the 19th Annual Meeting ISMRM. Canada: Montreal; 2011. Imaging Electrical Properties of the Human Brain using a 16-channel Transceiver Array Coil at 7T; p. 126. [Google Scholar]
- 27.Zhang X, Van de Moortele PF, Schmitter S, He B. Complex B1 Mapping and Electrical Properties Imaging of the Human Brain using a 16-channel Transceiver Coil at 7T. Magn. Reson. Med. doi: 10.1002/mrm.24358. early view (online). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Ugurbil K, Chen W. Microstrip RF surface coil design for extremely high-field MRI and spectroscopy. Magn. Reson. Med. 2001;46:443–450. doi: 10.1002/mrm.1212. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Ugurbil K, Chen W. A microstrip transmission line volume coil for human head MR imaging at 4 T. J. Magn. Reson. 2003;161:242–251. doi: 10.1016/s1090-7807(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 30.Adriany G, Van de Moortele PF, Ritter J, Moeller S, Auerbach EJ, Akgun C, Snyder CJ, Vaughan T, Ugurbil K. A geometrically adjustable 16-channel transmit/ receive transmission line array for improved RF efficiency and parallel imaging performance at 7 Tesla. Magn. Reson. Med. 2008;59:590–597. doi: 10.1002/mrm.21488. [DOI] [PubMed] [Google Scholar]
- 31.Van de Moortele PF, Snyder C, DelaBarre L, Adriany G, Vaughan JT, Ugurbil K. Proceedings of the 15th Annual Meeting ISMRM. Berlin, Germany: 2007. Calibration tools for RF shim at very high field with multiple element RF coils: from ultra fast local relative phase to absolute magnitude B1+ mapping; p. 1676. [Google Scholar]
- 32.Van de Moortele PF, Ugurbil K. Proceedings of the 17th Annual Meeting ISMRM. Honolulu, Hawaii: USA; 2009. Very Fast Multi Channel B1 Calibration at High Field in the Small Flip Angle Regime; p. 367. [Google Scholar]
- 33.Cloos MA, Bonmassar G. Proceedings of the 17th Annual Meeting of ISMRM. Honolulu, Hawaii: USA; 2009. Towards Direct B1 based local SAR estimation; p. 3037. [Google Scholar]
- 34.Buchenau S, Haas M, Hennig J, Zaitsev M. Proceedings of the 19th Annual Meeting of ISMRM. Honolulu, Hawaii: USA; 2009. Quality assessment of B1-based local SAR estimation as a function of position within a parallel transmit coil at 3T; p. 493. [Google Scholar]
- 35.Haacke EM, Brown PW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Principles and Sequence Design. Wiley: New York; 1999. [Google Scholar]
- 36.Yarnykh YL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn. Reson. Med. 2009;57:192–200. doi: 10.1002/mrm.21120. [DOI] [PubMed] [Google Scholar]
- 37.Radio Frequency Safety, Federal Communications Commission; http://transition.fcc.gov/oet/rfsafety/dielectric.html. [Google Scholar]
- 38.Van de Moortele PF, Auerbach EJ, Olman C, Yacoub E, Uğurbil K, Moeller S. T1 weighted brain images at 7 Tesla unbiased for Proton Density, T2* contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization. Neuroimage. 2009;46:432. doi: 10.1016/j.neuroimage.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katscher U, Findeklee C, Voigt T. B1-Based Specific Energy Absorption Rate Determination for Nonquadrature Radiofrequency Excitation. Magn. Reson. Med. 2012;68:1911–1918. doi: 10.1002/mrm.24215. [DOI] [PubMed] [Google Scholar]
- 40.Sodickson DK, Alon L, Deniz CM, Brown R, Zhang B, Wiggins GC, Cho GY, Eliezer NB, Novikov DS, Lattanzi R, Duan Q, Sodickson LA, Zhu YD. Proceedings of the 20th Annual Meeting of ISMRM. Melbourne, Australia: 2012. Local Maxwell Tomography Using Transmit-Receive Coil Arrays for Contact-Free Mapping of Tissue Electrical Properties and Determination of Absolute RF Phase; p. 387. [Google Scholar]
- 41.Wolf S, Diehl D, Gebhardt M, Mallow J, Speck O. SAR simulations for high field MRI: - How much detail, effort, and accuracy is needed? Magn. Reson. Med. doi: 10.1002/mrm.24329. early view (online) [DOI] [PubMed] [Google Scholar]
- 42.Metherall P, Barber DC, Smallwood RH, Smallwood RH, Brown BH. Three-dimensional electrical impedance tomography. Nature. 1996;380(6574):509–512. doi: 10.1038/380509a0. [DOI] [PubMed] [Google Scholar]
- 43.Paulson K, Lionheart W, Pidcock M. Optimal experiments in electrical-impedance tomography. IEEE Trans. Med. Imaging. 1993;12:681–686. doi: 10.1109/42.251118. [DOI] [PubMed] [Google Scholar]
- 44.Joy M, Scott G, Henkelman M. In vivo detection of applied electric currents by magnetic resonance imaging. Magn. Reson. Imaging. 1989;7:89–94. doi: 10.1016/0730-725x(89)90328-7. [DOI] [PubMed] [Google Scholar]
- 45.Woo EJ, Seo JK. Magnetic resonance electrical impedance tomography (MREIT) for high-resolution conductivity imaging. Physiol. Meas. 2008;29:R1–R26. doi: 10.1088/0967-3334/29/10/R01. [DOI] [PubMed] [Google Scholar]
- 46.Gao N, Zhu SA, He B. A New Magnetic Resonance Electrical Impedance Tomography (MREIT) Algorithm: RSM-MREIT Algorithm with Applications to Estimation of Human Head Conductivity. Phys. Med. Biol. 2006;51(12):3067–3083. doi: 10.1088/0031-9155/51/12/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, He B. Magnetoacoustic Tomography with Magnetic Induction (MAT-MI) Phys. Med. Biol. 2005;50:5175–5187. doi: 10.1088/0031-9155/50/21/015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Xu Y, He B. Imaging Electrical Impedance of Biological Tissue from Acoustic Measurements by means of Magnetoacoustic Tomography with Magnetic Induction (MAT-MI): A Model Study. IEEE Trans. Biomed. Eng. 2007;54(2):323–330. doi: 10.1109/TBME.2006.883827. [DOI] [PubMed] [Google Scholar]
- 49.Mariappan L, Li X, He B. B-Scan Based Acoustic Source Reconstruction for Magnetoacoustic Tomography with Magnetic Induction (MAT-MI) IEEE Trans. Biomed. Eng. 2011;58(3):713–720. doi: 10.1109/TBME.2010.2094618. [DOI] [PMC free article] [PubMed] [Google Scholar]