Abstract
Increasing evidence suggests the important role of metabolic reprogramming in the regulation of the innate inflammatory response, but the underlying mechanism remains unclear. Here, we provide evidence to support a novel role for the pyruvate kinase M2 (PKM2)-mediated Warburg effect, namely aerobic glycolysis, in the regulation of high mobility group box 1 (HMGB1) release. PKM2 interacts with hypoxia-inducible factor 1α (HIF1α) and activates the HIF-1α-dependent transcription of enzymes necessary for aerobic glycolysis in macrophages. Knockdown of PKM2, HIF1α, and glycolysis-related genes uniformly decreases lactate production and HMGB1 release. Similarly, a potential PKM2 inhibitor, shikonin, reduces serum lactate and HMGB1 levels and protects mice from lethal endotoxemia and sepsis. Collectively, these findings shed light on a novel mechanism for metabolic control of inflammation by regulating HMGB1 release and highlight the importance of targeting aerobic glycolysis in the treatment of sepsis and other inflammatory diseases.
Introduction
Severe sepsis and septic shock represent a major clinical problem and are the leading causes of death in patients within intensive care units worldwide1. This medical problem results from an exuberant and excessive host response associated with a deleterious and non-resolving systemic inflammatory response syndrome, often caused by Gram-negative bacterial infection2. As the major component of Gram-negative bacteria, lipopolysaccharide (LPS) can induce the secretion and release of multiple proinflammatory cytokines such as tumor necrosis factor-α (TNF-α)3, interleukin-1β (IL-1β)4, and high mobility group box 1 (HMGB1)5. In contrast to early proinflammatory cytokines (e.g., TNF-α and IL-1β), HMGB1 is released in a delayed manner and functions as a late mediator of lethal sepsis5 and thus as a therapeutic target has a wider time window for clinical intervention6.
In resting cells, most of the HMGB1 resides in the nucleus and functions as a DNA chaperone to regulate chromosomal structure and DNA biology7. When actively secreted by immune cells or passively leaked from necrotic or injured cells5,8,9, HMGB1 functions as a damage-associated molecular pattern to mediate innate immune responses10. The secretion of HMGB1 from activated immune cells is a highly-regulated process involving multiple steps and mechanisms. For instance, post-transcriptional modification (e.g., acetylation) in the nuclear localization signal promotes HMGB1 translocation from the nucleus to the cytoplasm11. Subsequently, secretory lysosome-mediated exocytosis and other unknown pathways facilitate HMGB1 release into the extracellular space12. Therefore, a more comprehensive understanding of the mechanisms underlying the regulation of HMGB1 release by activated immune cells may ultimately lead to better treatments for patients with various inflammation-associated diseases13.
Metabolism includes processes in which biochemicals are degraded to produce energy (in the form of adenosine triphosphate). Intermediate metabolites may serve as signaling molecules to regulate cellular processes. This metabolism-driven reprogramming dictates the progression of many cellular functions and even cellular fate. For instance, tumor cells can strategically generate adenosine triphosphate through aerobic glycolysis (in addition to oxidative phosphorylation), even when oxygen is abundant14, enabling exaggerated tumor cell proliferation. This phenomenon has been termed the “Warburg Effect” and represents one of the key hallmarks of cancer15.
Growing evidence suggests that activated immune cells (e.g., macrophages, dendritic cells, and T cells) also have the ability to switch from oxidative phosphorylation to aerobic glycolysis in a fashion similar to tumor cells. This alteration may contribute to the regulation of innate immune functions16–19 and represents a novel target for inflammatory diseases. One key mediator of the Warburg effect is the transcription factor hypoxia inducible factor 1α (HIF1α), a master regulator of metabolism that is upregulated in response to low oxygen or hypoxia20. Remarkably, a bacterial constituent, LPS, can also induce a switch from oxidative phosphorylation to aerobic glycolysis in dendritic cells and macrophages21–23 and consequently results in cell activation and release of proinflammatory cytokines, including IL-1β21–23.
Although the Warburg effect similarly occurs in activated macrophages, its possible role in the regulation of HMGB1 release is unknown. In the current study, we demonstrate that pyruvate kinase M2 (PKM2), a protein kinase and transcriptional coactivator, functions as an essential mediator of aerobic glycolysis24 and an important regulator of HMGB1 release in activated macrophages. Our findings shed light on a novel mechanism for metabolic control of inflammation by regulating HMGB1 release and highlight the possibility of targeting the switch to aerobic glycolysis in the treatment of sepsis and other inflammatory diseases.
Results
Glycolysis promotes HMGB1 release
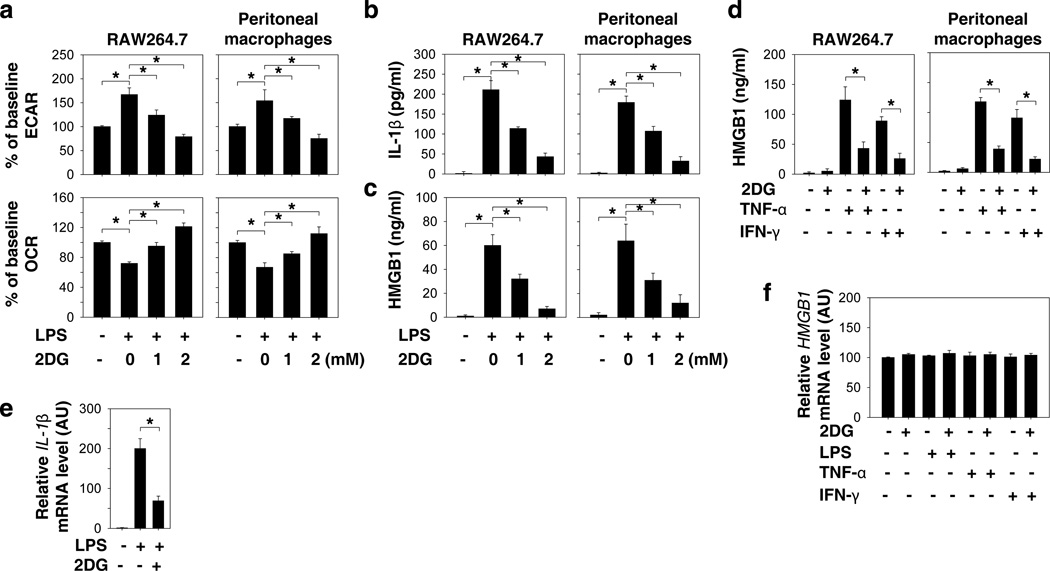
To investigate whether aerobic glycolysis plays a role in the regulation of HMGB1 release, we used a glucose analog, 2-deoxy-d-glucose (2-DG), a widely-used competitive inhibitor of the first hexokinase of the glycolytic pathway. 2-DG is phosphorylated by hexokinase to 2-DG-P, which cannot be further metabolized by phosphoglucose isomerase. 2-DG (IC50 ≈ 1 mM) significantly inhibited the LPS-induced switch from oxidative phosphorylation (as measured by oxygen consumption rate [OCR]) to glycolysis (as measured by extracellular acidification rate [ECAR]) in a concentration-dependent manner (Fig. 1a). In parallel, 2-DG treatment also concentration-dependently inhibited the release of early (e.g., IL-1β at two hours) and late (e.g., HMGB1 at 24 hours) mediators in LPS-stimulated macrophages (Fig. 1b and 1c). Several immunoregulatory cytokines such as TNF-α5 and IFN-γ25 can induce HMGB1 release in macrophages. Similarly, 2-DG also inhibited TNF-α and IFN-γ-induced HMGB1 release in macrophages (Fig. 1d), suggesting a general role for glycolysis in the regulation of HMGB1 release. Although 2-DG significantly inhibited LPS-induced upregulation of IL-1β mRNA (Fig. 1e), it did not affect the HMGB1 mRNA levels in response to LPS, TNF-α, and IFN-γ in macrophages (Fig. 1f). These results are consistent with those from previous reports; namely, LPS induced IL-1β release in macrophages through transcriptional-dependent mechanisms, whereas LPS induced HMGB1 release in macrophages through transcriptional-independent mechanisms5,22. Collectively, our results therefore indicate a possible role for aerobic glycolysis in the regulation of HMGB1 release through post-transcriptional mechanisms.
Figure 1. Glycolytic inhibitor 2DG attenuates HMGB1 release by activated macrophages.
(a–c) Murine RAW 264.7 macrophage cell line and primary peritoneal macrophages were pretreated with 2DG for three hours and then stimulated with lipopolysaccharide (LPS) (100 ng ml−1) for two-24 hours. The oxygen consumption rates (OCR, indicative of oxidative phosphorylation) and extracellular acidification rates (ECAR, indicative of glycolysis) were monitored at 24 hours using the Seahorse Bioscience Extracellular Flux Analyzer (a). The levels of secreted IL-1β at two hours (b) and HMGB1 at 24 hours (c) in the cell culture medium were measured by ELISA (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). (d) 2DG (2 mM) inhibits TNF-α (5 ng ml−1) and IFN-γ (10 ng ml−1)-induced HMGB1 release at 24 hours (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). (e) IL-1β mRNA in RAW 264.7 macrophages after treatment with LPS (100ng/ml) for two hours with or without 2DG (2 mM) (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). (f) HMGB1 mRNA in RAW264.7 macrophages after treatment with LPS (100 ng ml−1), TNF-α (5 ng ml−1), and IFN-γ (10 ng ml−1) for 24 hours with or without 2DG (2 mM) (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). AU= arbitrary units.
PKM2 is required for active HMGB1 release
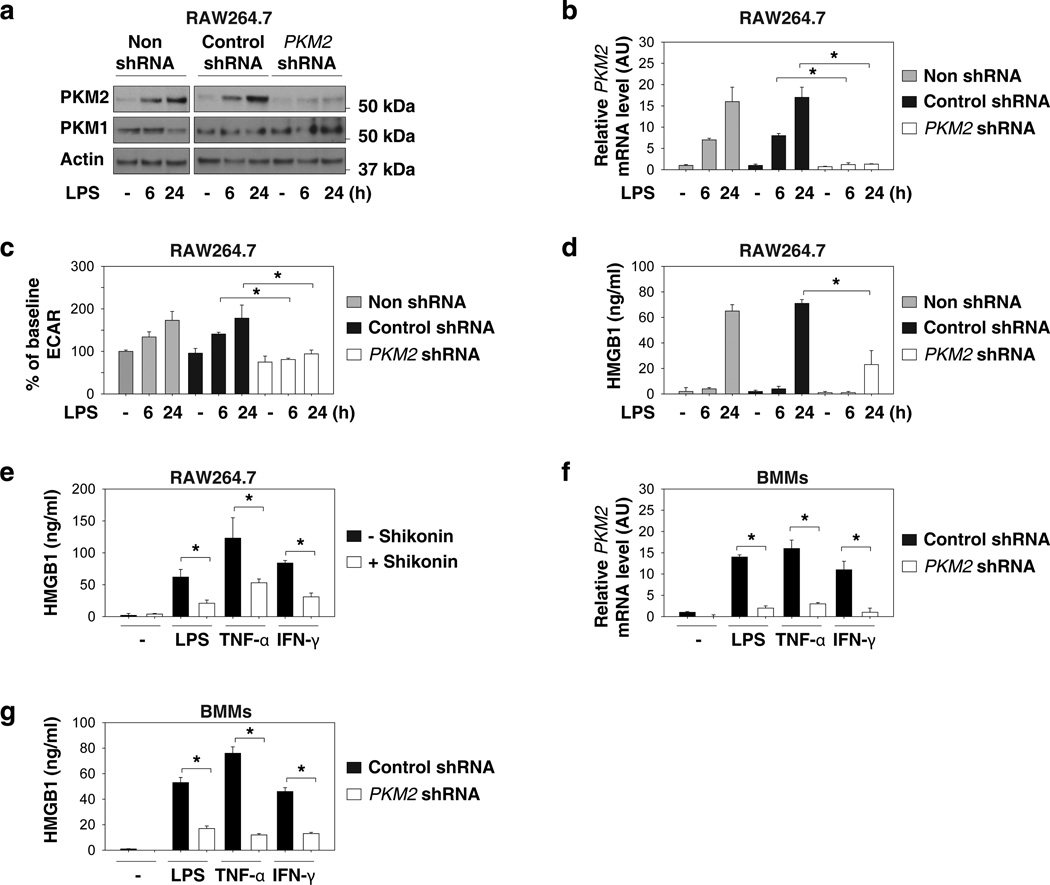
The M2 isoform of PKM2 catalyzes the final and rate-limiting reaction step of the glycolytic pathway and functions as the central regulator of cancer metabolism via regulating the Warburg effect26. It remains unclear, however, whether PKM2 is also important for the Warburg effect in activated innate immune cells. Surprisingly, LPS induced a time-dependent increase in the protein and mRNA levels of PKM2 (Fig. 2a and 2b), but not PKM1 in macrophages (Fig. 2a), suggesting a possible role for PKM2 in the regulation of the Warburg effect as well as HMGB1 release. To test this possibility, a target-specific shRNA against PKM2 was transfected into RAW 264.7 macrophages. The transfection of PKM2-shRNA led to a significant decrease in PKM2 (Fig. 2a and 2b), but not PKM1 protein levels (Fig. 2a). In parallel, the knockdown of PKM2 was associated with a decrease in LPS-induced glycolysis (Fig. 2c) and accompanying reduction of HMGB1 release (Fig. 2d). Treatment with a potential PKM2 inhibitor, shikonin27, also resulted in significant inhibition of LPS-, TNF-α-, and IFN-γ-induced HMGB1 release (Fig. 2e). Furthermore, knockdown of PKM2 by lentiviral shRNA in primary bone marrow macrophages (BMMs) inhibits LPS-, TNF-α-, and IFN-γ-induced HMGB1 release (Fig. 2f and 2g). Collectively, these results support an important role for PKM2-mediated glycolysis in the regulation of HMGB1 release in activated macrophages.
Figure 2. Upregulated PKM2 promotes aerobic glycolysis and HMGB1 release in activated macrophages.
(a–d) RAW 264.7 cells were transfected with or without indicated shRNA for 48 hours and then stimulated with LPS (100 ng ml−1) for six-24 hours. The level of indicated protein (a), mRNA (b), ECAR (c), and HMGB1 release (d) were assayed as described in the methods (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). AU= arbitrary units. (e) RAW 264.7 cells were pretreated with shikonin (5 µM) for three hours and then stimulated with lipopolysaccharide (LPS) (100 ng ml−1), TNF-α (5 ng ml−1), and IFN-γ (10 ng ml−1) for 24 hours. HMGB1 release was assayed by ELISA (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). (f, g) Bone marrow macrophages (BMMs) were transfected with or without indicated shRNA for 48 hours and then stimulated with LPS (100 ng ml−1), TNF-α (5 ng ml−1), and IFN-γ (10 ng ml−1) for 24 hours. The mRNA levels of PKM2 (f) and HMGB1 release (g) were assayed (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). AU= arbitrary units.
PKM2-mediated HIF1α activation is required for HMGB1 release
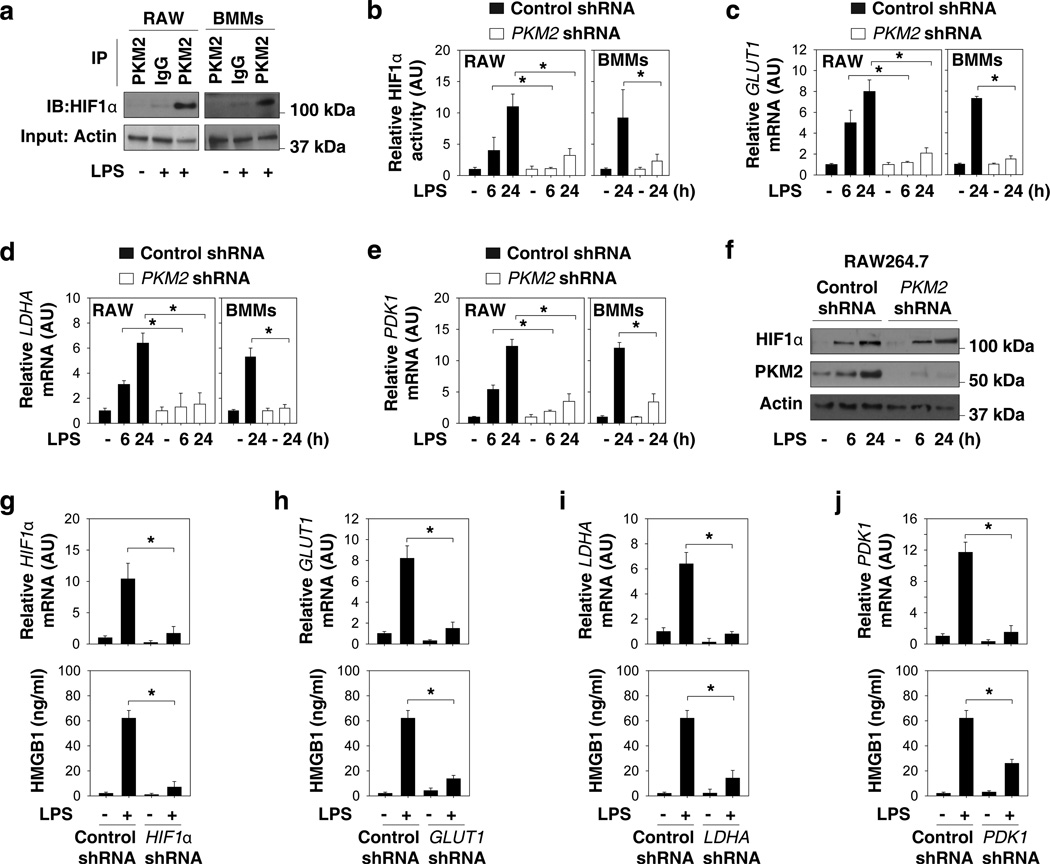
In cancer cells, PKM2 physically interacts with HIF1α in the nucleus and activates the transcription of glycolysis-related genes such as GLUT1 (glucose transporter 1, which increases glucose uptake), LDHA (lactate dehydrogenase A, which increases lactate production), and PDK1 (pyruvate dehydrogenase kinase 1, which blocks the entry of pyruvate into the tricarboxylic acid cycle)28. In LPS-stimulated RAW264.7 cells and primary BMMs, the interaction between PKM2 and HIF1α was markedly elevated, as determined by immunoprecipitation assays (Fig. 3a). However, the knockdown of PKM2 by RNAi impaired LPS-induced HIF1α transcriptional activation (Fig. 3b), as well as the consequent expression of GLUT1 (Fig. 3c), LDHA (Fig. 3d), and PDK1 mRNAs (Fig. 3e). Notably, the knockdown of PKM2 did not affect LPS-induced HIF1α protein expression (Fig. 3f), suggesting an important role of PKM2-HIF1α interaction in the regulation of the transcriptional activity, but not the stability of HIF1α protein in activated macrophages. To confirm the roles of HIF1α as well as its target glycolysis-related genes in the regulation of HMGB1 release, various shRNA constructs were transfected into cultured macrophages. Interestingly, knockdown of HIF1α (Fig. 3g), GLUT1 (Fig. 3h), LDHA (Fig. 3i), and PDK1 (Fig. 3i) by RNAi uniformly impaired LPS-induced HMGB1 release. Together, these findings suggest a novel role for PKM2-mediated HIF1α activation in the regulation of HMGB1 release by activated macrophages.
Figure 3. PKM2-mediated HIF1α activation is required for HMGB1 release in activated macrophages.
(a) RAW 264.7 cells (RAW) and bone marrow macrophages (BMMs) were stimulated with lipopolysaccharide (LPS) (100 ng ml−1) for 24 hours. The interaction between PKM2 and HIF1α was assayed by immunoprecipitation (IP) and immune-blot (IB). (b–f) RAW 264.7 cells and BMMs were transfected with control shRNA or PKM2 shRNA for 48 hours and then stimulated with LPS (100 ng ml−1) for six-24 hours. The mRNA levels of indicated genes (b–e) were assayed by real time PCR (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). AU= arbitrary units. The protein level of HIF1α was assayed by Western blot (f). (g–j) RAW 264.7 cells were transfected with indicated shRNA for 48 hours and then stimulated with LPS (100 ng ml−1) for 24 hours. The mRNA levels of indicated genes were assayed by real time PCR. In parallel, the extracellular levels of HMGB1 were assayed by ELISA (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). AU= arbitrary unit.
Lactate promotes HMGB1 acetylation and release
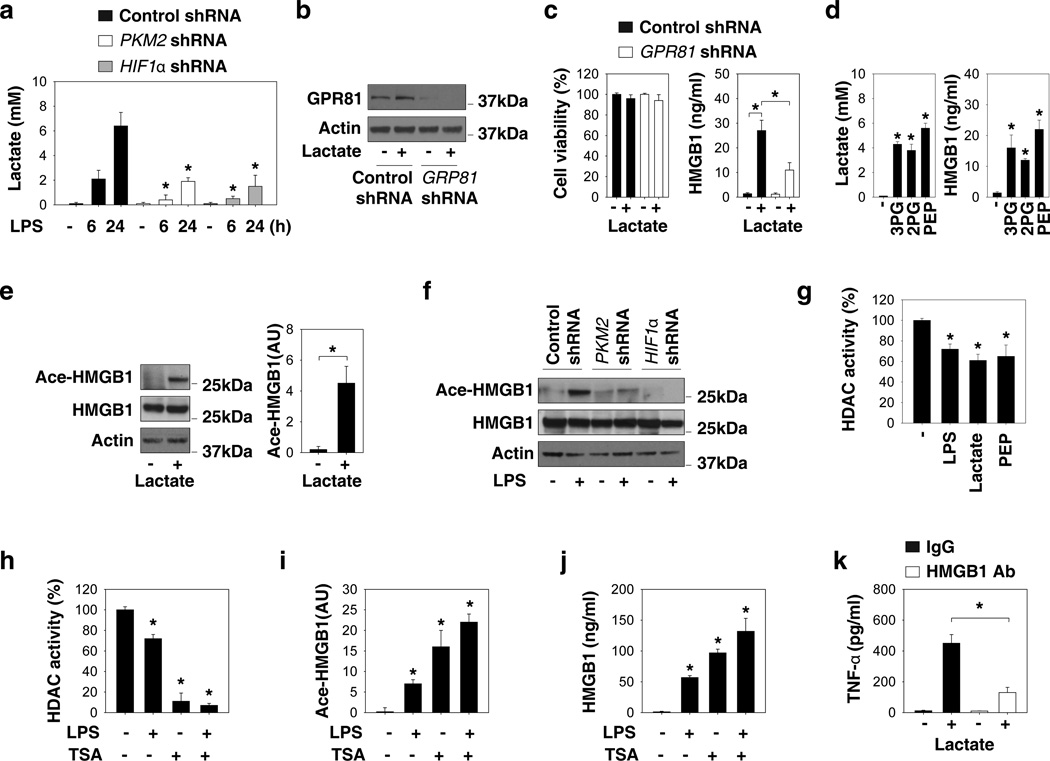
As the end product of glycolysis, lactate accumulated at increased levels (5–7 mM) in the culture medium of LPS-stimulated macrophages (Fig. 4a). In contrast, the knockdown of PKM2 and HIF1α prevented LPS-induced lactate accumulation in the culture medium (Fig. 4a). To understand the role of lactate in the regulation of HMGB1 release, we tested whether supplementation of exogenous lactate could induce HMGB1 release. Indeed, exposure of macrophage cultures to lactate (5 mM for six hours) triggered a marked HMGB1 release without reducing cell viability (Fig.4b and 4c). The recently identified lactate receptor GPR8129 was responsible for this effect (Fig.4b and 4c). In addition, intermediate metabolites of glycolysis such as 3-phosphoglycerate (3PG), 2-phosphoglycerate (2PG), and phosphoenolpyruvate (PEP) can induce lactate production and HMGB1 release in macrophages (Fig. 4d). Acetylation is a post-translational modification that can promote HMGB1 cytoplasmic translocation and subsequent release in response to various inflammatory stimuli as well as hypoxia11,30. Lactate increased HMGB1 acetylation (Fig. 4e), whereas LPS-induced HMGB1 acetylation was impaired by the knockdown of PKM2 and HIF1α (Fig. 4f). To gain insight into the mechanisms underlying glycolysis-mediated HMGB1 hyperacetylation, we assayed the activity of histone deacetylases (HDACs) which have been shown to acetylate both histone and non-histone nuclear proteins, including HMGB111,30. Like LPS and HDAC inhibitors (e.g., trichostatin A [TSA]), lactate and PEP also decreased the activity of HDACs in macrophage cultures (Fig. 4g–j). Collectively, these findings suggest that LPS-induced lactate production from PKM2-mediated aerobic glycolysis may act as an inhibitory signal for HDAC activity, which in turn enables HMGB1 hyperacetylation and subsequent release by LPS-stimulated macrophages.
Figure 4. Lactate, a product of aerobic glycolysis, promotes HMGB1 acetylation and release.
(a) RAW 264.7 cells were transfected with indicated shRNA for 48 hours and then stimulated with lipopolysaccharide (LPS) (100 ng ml−1) for six-24 hours. The lactate level in the culture medium was assayed (n=3, means ± SEM, *P < 0.05 versus control shRNA group by ANOVA LSD test). (b–c) RAW 264.7 cells were transfected with GRP81 shRNA and control shRNA for 48 hours and then stimulated with lactate (5 mM) for six hours. GRP81 expression (b), cell viability (c), and HMGB1 release (c) were assayed (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). (d) RAW 264.7 cells were stimulated with 3-phosphoglycerate (3PG, 2 mM) and 2-phosphoglycerate (2PG, 2 mM), phosphoenolpyruvate (PEP, 2 mM) for six hours and levels of lactate and HMGB1 release were assayed (n=3, means ± SEM, *P < 0.05 versus untreated group by ANOVA LSD test). (e) RAW 264.7 cells were stimulated with lactate (5 mM) for six hours and levels of acetylated HMGB1 were assayed. (f) RAW 264.7 cells were stimulated with LPS (100 ng ml−1) for 24 hours and the levels of acetylated HMGB1 were assayed. (g) RAW 264.7 cells were stimulated with LPS (100 ng ml−1), lactate (5 mM) and PEP (2 mM) for 24 hours. Histone deacetylase (HDAC) activity was assayed (n=3, means ± SEM, *P < 0.05 versus untreated group by ANOVA LSD test). (h-j) RAW 264.7 cells were stimulated with Trichostatin A (TSA) (2 µM) and/or LPS (100 ng ml−1) for 24 hours. HDAC activity (h) and acetylated HMGB1 (i) and HMGB1 release (j) were assayed (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test). (k) RAW 264.7 cells were stimulated with lactate (5 mM, six hours) with or without anti-HMGB1 neutralizing antibody (2 µg ml−1) and levels of TNF-α release were assayed (n=3, means ± SEM, *P < 0.05 by ANOVA LSD test).
A previous study demonstrated that lactate stimulates Toll-like receptor (TLR)-4 signaling activation in macrophages31. In addition, HMGB1 can stimulate TNF-α release through TLR432. We demonstrated that HMGB1-neutralizing antibody significantly inhibits lactate-induced TNF-α release (Fig. 4k). These findings suggest that lactate promotes macrophage activation via an autocrine HMGB1-dependent pathway.
Shikonin protects mice from endotoxic shock and sepsis
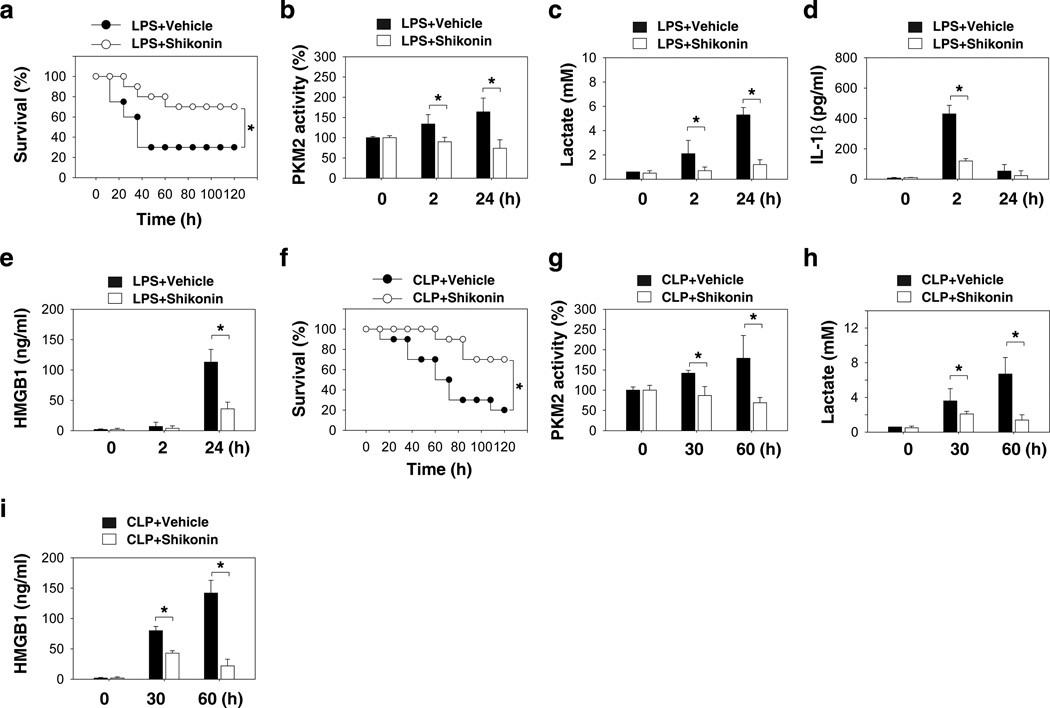
Given the capacity of the PKM2 inhibitor shikonin to attenuate LPS-induced IL-1β and HMGB1 release in vitro, we determined whether shikonin protects mice against lethal endotoxemia by inhibiting cytokine release. Repetitive administration of shikonin at −0.5, +12, and +24 hours following the onset of endotoxemia (5 mg kg−1 LPS, intraperitoneally [i.p.]) conferred significant protection against lethal endotoxemia (Fig. 5a) and simultaneously reduced the PKM2 activity in peritoneal macrophages (Fig. 5b) and the serum levels of lactate (Fig. 5c), IL-1β (Fig. 5d), and HMGB1 (Fig. 5e) in endotoxemic mice. All of these mice were observed for at least two weeks and no late deaths occurred. These findings suggest that shikonin does not merely delay animal lethality by attenuating the production of lactate and cytokines, but also provides long-lasting protection. Although endotoxemia is a useful animal model for investigating the complex network of cytokines, a more clinically relevant experimental model for sepsis is bacterial infection induced by cecal ligation and puncture (CLP). At +24, +48, +72 hours following the onset of sepsis, animals were given shikonin or vehicle i.p.. Shikonin rescued mice from CLP-induced lethal sepsis, even if given after the onset of sepsis (Fig. 5f). All of these mice were observed for at least two weeks and no late deaths occurred. The PKM2 activity in peritoneal macrophages (Fig. 5g) and the serum levels of lactate (Fig. 5h) and HMGB1 (Fig. 5i) were significantly decreased in the group receiving shikonin treatment for CLP. Collectively, these data suggest that the PKM2 inhibitor shikonin protects against experimental lethal endotoxic shock and sepsis partly by inhibiting PKM2 activity, lactate production, and HMGB1 release.
Figure 5. A potential PKM2 inhibitor shikonin protects mice from lethal endotoxemia and sepsis.
(a) Mice (n=20 group−1) were injected with a single dose of shikonin (8 mg kg−1), followed 30 minutes later by an infusion of endotoxin (LPS, 5 mg kg−1, intraperitoneally), and were then re-treated with shikonin 12 and 24 hours later. The Kaplan-Meyer method was used to compare the differences in survival rates between groups (*, P<0.05). (b–e) In parallel experiments, the PKM2 activity in peritoneal macrophages (b) and serum levels of lactate (c), IL-1β (d), and HMGB1 (e) at indicated time points were measured (n=3–5 animals group−1, values are mean ± SEM, *, P<0.05 by ANOVA LSD test). (f) The cecal ligation and puncture (CLP) technique was used to induce intraabdominal sepsis in mice (n=20 group−1). Repeated administration of shikonin (8 mg kg−1) at 24, 48, and 72 hours after CLP significantly increased survival, as compared with vehicle group (*, P<0.05), as measured by Kaplan-Meyer test. (g–i) In parallel, the PKM2 activity in peritoneal macrophages (g) and serum levels of lactate (h) and HMGB1 (i) at indicated time points were measured (n=3–5 animals group−1, values are mean ± SEM, *, P<0.05 by ANOVA LSD test).
Discussion
To ensure a timely response to infection, effective innate recognition systems that rapidly detect various microbial products such as LPS have evolved in mammals. The effective recognition of pathogen associated molecular patterns by innate immune cells triggers the sequential release of early (e.g., TNF-α and IL-1β) and late (e.g., HMGB1) proinflammatory cytokines. Appropriate inflammatory responses facilitate innate immunity against infections, but excessive inflammation may also result in sepsis and even death1,2. The molecular mechanisms underlying the innate immune system response to LPS are not completely understood. In this study, we demonstrate that PKM2-mediated aerobic glycolysis contributes to macrophage activation and the resultant inflammatory response. As a co-activator, PKM2 interacts with HIF-1α and activates the transcription of glycolysis-related genes, leading to excessive lactate production and HMGB1 hyperacetylation and its subsequent release. Moreover, genetic and/or pharmacological inhibition of PKM2 significantly impairs LPS-induced HMGB1 release in macrophages and protects mice from lethal endotoxic shock and sepsis. These findings provide novel insight into the mechanisms underlying the metabolic control of inflammation and highlight the importance of targeting aerobic glycolysis in the treatment of patients with sepsis and other inflammatory diseases.
The Warburg effect, first observed in various tumor cells, is also increasingly recognized as an essential regulator of innate and adaptive immunity. For instance, increased aerobic glycolysis contributes to the maturation of dendritic cells in response to TLR4, TLR2, and TLR9 ligands21 and influences the differentiation of both anti-inflammatory Treg cells and pro-inflammatory Th17 cells33,34. There are two major types of macrophages35: the classical M1 phenotype, which can be activated by LPS and IFN-γ to orchestrate proinflammatory responses, and the alternate M2 phenotype, which is activated by IL-4, IL-13, IL-10, and TGF-β to fulfill anti-inflammatory functions. LPS can induce the metabolic switch to aerobic glycolysis only in M1 macrophages, which contributes to transcriptional expression and release of early proinflammatory cytokines (e.g., IL-1β)22. In this study, we found that increased aerobic glycolysis also contributes to LPS-induced release of late mediators (e.g., HMGB1) by modulating its post-translational modification (rather than transcriptional expression). It now appears that the Warburg effect can participate in the regulation of proinflammatory cytokines at both the transcriptional and post-transcriptional levels.
Although PKM2 is expressed at extremely low levels (if any at all) in quiescent and normally differentiated cells, its expression levels are markedly upregulated in rapidly proliferating tumor cells as well as activated macrophages. Elevated PKM2 expression in cancer cells26 and activated immune cells enables the metabolic switch from oxidative phosphorylation to aerobic glycolysis. In cancer cells, PKM2 contributes to the Warburg effect by interacting and activating HIF-1α to induce the expression of glycolytic genes28. Here, we demonstrate that PKM2 fulfills similar functions by interacting with HIF1α to regulate the expression of LPS-induced glycolytic genes and release of proinflammatory cytokines in macrophages. Consistently, the knockdown of PKM2, HIF1α, as well as aerobic glycolysis-related genes (e.g., GLUT1, LDHA, and PDK1) significantly impaired LPS-induced HMGB1 release, suggesting an important role of the PKM2-HIF1α glycolysis pathway in the regulation of HMGB1 release by activated macrophages.
Intriguingly, it has been suggested that hyperglycemia aggravates LPS-induced HMGB1 release, whereas insulin can decrease serum HMGB1 levels in septic animals36, implying a possible role of glucose metabolism in the regulation of HMGB1 release. The mechanisms underlying the regulation of PKM2 expression in macrophages remains a subject of future investigation. In cancer cells, however, the expression of PKM2 is controlled by several transcription factors such as Sp137, NF-κB38, and Myc39, as well as HIF1α28. In addition, activation of the extracellular signal-regulated kinase (ERK) is required for PKM2 expression and nuclear translocation during aerobic glycolysis40. Given the essential role of ERK in regulating active HMGB1 secretion41, elucidating the mechanism underlying the regulation of PKM2 upregulation in activated immune cells will be important.
Hypoxia and inflammation are often intimately associated under various conditions. Hypoxia is not only a prominent feature in inflamed and injured tissues, but also an effective inducer of inflammatory responses42,43. Under hypoxic conditions, HIF1α, the master regulator of the cellular response to hypoxia, can escape degradation by the ubiquitin-proteasome pathway, thereby accumulating at high levels44. Similarly, bacterial products (e.g., LPS)45 and proinflammatory cytokines (e.g., TNF-α and IFN-γ)46,47 can also stabilize HIF1α and activate relevant signaling pathways, even under normoxic conditions. In the present study, we found that knockdown of HIF1α impairs LPS-induced HMGB1 release by macrophages under normoxic conditions. Similarly, others have found that conditional knockout of HIF1α in myeloid cells (including macrophages) decreased inflammatory responses in vivo48 and protected animals against LPS-induced lethality49.
Activated macrophages/monocytes acetylate HMGB1 at its nuclear localization sequences, leading to sequestration of HMGB1 within cytoplasmic vesicles and subsequent release into the extracellular milieu. Extracellular HMGB1 functions as a late mediator of lethal endotoxemia and sepsis because HMGB1-neutralizing antibodies or inhibitors reverse the course of established experimental sepsis even when these antidotes are given up to 24 hours after induction of experimental sepsis5,50,51. These findings support the idea of HMGB1 as a promising therapeutic target to allow rescue from lethal sepsis. It is thus important to fully understand the intricate mechanisms underlying the regulation of HMGB1 release under relevant pathological conditions. Our present study reveals a crucial role for the PKM2 pathway in the regulation of HMGB1 release and suggests another possible therapeutic target for sepsis. Indeed, a potential PKM2 inhibitor27, shikonin, conferred significant protection against lethal endotoxemia and sepsis partly by attenuating systemic accumulation of lactate and proinflammatory cytokines. Of note, increased serum lactate levels have been suggested as a biomarker of organ failure and mortality in patients with sepsis, trauma, and other critical illnesses52–54. Our findings that lactate can effectively stimulate macrophages to release HMGB1 provide a logical explanation for lactate clearance as a therapy for sepsis and other inflammatory diseases. As a component of Chinese herbal medicine, shikonin also has antioxidant properties55 and has been tested for efficacy in the treatment of microbial infections and cancer in experimental and clinical settings56. It is essential to determine whether other HMGB1 inhibitors (e.g., EGCG, tanshinone, carbenoxolone, and quercetin) confer protection against lethal sepsis similarly by inhibiting the PKM2-mediated Warburg effect in innate immune cells.
Collectively, our findings reveal a novel PKM2-dependent mechanism for the metabolic control of inflammation. Upregulation of PKM2 expression enables a metabolic switch to aerobic glycolysis, leading to excessive production of lactate. By inhibiting HDAC activity57, lactate in turn increases HMGB1 hyperacetylation, translocation to the cytoplasm, and subsequent release into the extracellular milieu. Thus, novel therapeutic strategies targeting aerobic glycolysis for the treatment of patients with sepsis and other inflammatory diseases are in order.
Methods
Reagents
LPS (Escherichia coli LPS 0111:B4; #L4391), 2DG (#D8375), TSA (#8552), 3-phosphoglycerate (#P8877), 2-phosphoglycerate (#19710), and phosphoenolpyruvate (#860077) were obtained from Sigma (St. Louis, MO, USA). Shikonin (#CAS 517-89-5) was obtained from EMD Millipore Corporation (Billerica, MA, USA). Mouse TNF-α (#410-MT-010) and IFN-γ (485-MI-100) were obtained from R&D Systems (Minneapolis, MN, USA). Mouse anti-HMGB1 2G7 neutralizing antibody was a generous gift from Kevin J. Tracey (The Feinstein Institute for Medical Research, Manhasset, NY, USA)32.
Cell culture
Murine macrophage-like RAW 264.7 cells were obtained from the American Type Culture. Mouse peritoneal and bone marrow macrophages were isolated from Balb/C mice58,59. These cells were cultured in Dulbecco's Modified Eagle's Medium (supplemented with 10% heat-inactivated fetal bovine serum and 100 unites of penicillin and 100µg ml−1 streptomycin) at 37 °C, 95% humidity, and 5% CO2.
Animal model of endotoxemia and sepsis
This study was approved by the Xiangya Hospital Institutional Ethics Committee and performed in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines (http://www.aaalac.org/). Endotoxemia was induced in Balb/C mice (male, seven to eight weeks old, 20–25 g) by i.p. injection of bacterial endotoxin (LPS, 5 mg/kg)5,51. Sepsis was induced in male Balb/C mice (male, 7–8 weeks old, 20–25 g) by cecal ligation and puncture5,51. Shikonin was dissolved in vehicle (10% DMSO, 20% Cremophor:ethanol [3:1] and 70% phosphate buffered saline) and administered i.p. to mice at the indicated time points. Blood was collected at indicated time points, allowed to clot for two hours at room temperature, and then centrifuged for 15 minutes at 1,500×g. Serum samples were stored at −20°C before analysis. Mortality was recorded for up to two-three weeks after the onset of lethal endotoxemia or sepsis to ensure that no additional late deaths occurred.
Cytokine measurements
Commercially available enzyme linked immunosorbant assay (ELISA) kits were used to measure the concentrations of HMGB1 (Shino Test Corporation, Tokyo, Japan, #ST51011), IL-1β (R&D Systems, Minneapolis, MN, USA, # MLB00C) and TNF-α (R&D Systems, Minneapolis, MN, USA, # MTA00B) in serum or the culture medium according to the manufacturer’s instructions.
Activity assay
Lactate in serum or the culture medium was measured with colorimetric L-Lactate Assay Kit (Abcam, Cambridge, MA, USA, #ab65331) according to the manufacturer’s instructions. HDAC activity was measured with the fluorometric HDAC Activity Assay kit (Abcam, #ab156064) according to the manufacturer’s instructions. HIF1α DNA binding activity in nuclear extracts was measured with HIF1α Transcription Factor Assay Kit (Cayman, Ann Arbor, MI, USA, #10006910) according to the manufacturer’s instructions. PKM2 activity was measured by a lactate dehydrogenase-coupled enzyme assay27,60. The assay was carried out with 1 µg of cell lysates with an enzyme buffer (50 mM tris-HCl [pH 7.5], 100 mM KCl, 10 mM MgCl2, 0.9 mM adenosine diphosphate, 0.6 mM phosphoenolpyruvate, 0.12 mM β-nicotinamide adenine dinucleotide and 4.8 U ml−1 lactate dehydrogenase). Enzyme activity can be measured at 340 nm absorbance by spectrophotometry.
RNAi
All shRNA constructs were in the pLKO.1 backbone (Sigma). Transfection with indicated shRNA (Supplementary Table 1) and control empty shRNA (#SHC001) from Sigma were performed using FuGENE® HD Transfection Reagent (Roche Applied Science) or MISSION shRNA Lentiviral Transduction (Sigma) according to the manufacturer’s instructions.
Western blot analysis
After extraction, proteins in cell lysates were first resolved by SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membrane and subsequently incubated with the primary antibody. The antibodies to HMGB1 (#ab18256; 1:1000) and HIF1α (#ab16066; 1:1000) were obtained from Abcam (Cambridge, MA, USA). The antibodies to PKM1 (#7067; 1:1000), PKM2 (#4053; 1:1000), acetylated-Lysine (#9441; 1:1000), and actin (#3700; 1:1000) were obtained from Cell Signaling Technology (Danvers, MA, USA). The antibody to GPR81 (#SAB1300089; 1:200) was obtained from Sigma (St. Louis, MO, USA).). After incubation with peroxidase-conjugated secondary antibodies, the signals were visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA, # 32106) according to the manufacturer's instruction. The relative band intensity was quantified using the Gel-pro Analyzer® software (Media Cybernetics, Bethesda, MD, USA).
Quantitative real time polymerase chain reaction
Total RNA was extracted using TRI reagent (Sigma) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 µg of RNA using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). cDNA from various cell samples were amplified by real-time quantitative PCR with specific primers (Supplementary Table 2).
Immunoprecipitation analysis
Cells were lysed at 4°C in RIPA buffer (Millipore, Billerica, MA, USA). Prior to immunoprecipitation, samples containing equal amounts of proteins were pre-cleared with Protein A sepharose (Millipore) and subsequently incubated with various irrelevant IgG or specific antibodies [PKM2 (#4053; 2 µg ml−1) and HMGB1 (#ab18256; 2 µg ml−1)] in the presence of protein A sepharose beads. The beads were washed three times with RIPA buffer and the immune complexes were eluted from the beads and subjected to SDS-PAGE and immunoblot analysis as previously described61,62.
Oxidative phosphorylation and glycolysis assay
Cellular OXPHOS and glycolysis were monitored using the Seahorse Bioscience Extracellular Flux Analyzer (XF24, Seahorse Bioscience Inc., North Billerica, MA, USA) by measuring the OCR (indicative of respiration) and ECAR (indicative of glycolysis)63,64. Briefly, 30,000–50,000 cells were seeded in 24-well plates designed for XF24 in 150 µl of appropriate growth media and incubated overnight. Prior to measurements, cells were washed with unbuffered media once, then immersed in 675 µl unbuffered media and incubated in the absence of CO2 for one hour. OCR and ECAR were then measured in a typical eight-minute cycle of mix (two-four minutes), dwell (two minutes), and measure (two-four minutes) as recommended by Seahorse Bioscience.
Statistical analysis
Data are expressed as means ± SEM of two or three independent experiments. Significance of differences between groups was determined by ANOVA LSD test. The Kaplan-Meier method was used to compare the differences in mortality rates between groups. A p-value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Christine Heiner (Department of Surgery, University of Pittsburgh) for her critical reading of the manuscript. This work was supported by grants from The National Natural Sciences Foundation of China (31171328 to L.C.; 81270616 to Y.Y.; 81100359 to M.Y.) and a grant from the National Institutes of Health (R01CA160417 to D.T.). H.W. is supported by grants from the National Center of Complementary and Alternative Medicine (R01AT005076) and the National Institute of General Medical Sciences (R01GM063075).
Footnotes
Author contributions
L.Y., L.C., and D.T. designed the research; L.Y., M.X., M.Y., Y.Y., S.Z., W.H., and R.K. performed the experiments; L.Y., L.C., and D.T. analyzed the results and wrote the paper; H.W. provided important experimental reagent; M.T.L., T.R.B., and H.W. edited and commented on the manuscript.
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 7.Malarkey CS, Churchill ME. The high mobility group box: the ultimate utility player of a cell. Trends Biochem Sci. 2012;37:553–562. doi: 10.1016/j.tibs.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 9.Tsung A, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 11.Bonaldi T, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardella S, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 17.Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 19.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–353. doi: 10.1101/sqb.2011.76.010678. [DOI] [PubMed] [Google Scholar]
- 21.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everts B, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab. 2012;23:560–566. doi: 10.1016/j.tem.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rendon-Mitchell B, et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 26.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, et al. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297–4306. doi: 10.1038/onc.2011.137. [DOI] [PubMed] [Google Scholar]
- 28.Luo W, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai TQ, et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun. 2008;377:987–991. doi: 10.1016/j.bbrc.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 30.Evankovich J, et al. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285:39888–39897. doi: 10.1074/jbc.M110.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol. 2009;182:2476–2484. doi: 10.4049/jimmunol.0802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 36.Hagiwara S, Iwasaka H, Hasegawa A, Koga H, Noguchi T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model. Crit Care Med. 2008;36:2407–2413. doi: 10.1097/CCM.0b013e318180b3ba. [DOI] [PubMed] [Google Scholar]
- 37.Discher DJ, Bishopric NH, Wu X, Peterson CA, Webster KA. Hypoxia regulates beta-enolase and pyruvate kinase-M promoters by modulating Sp1/Sp3 binding to a conserved GC element. J Biol Chem. 1998;273:26087–26093. doi: 10.1074/jbc.273.40.26087. [DOI] [PubMed] [Google Scholar]
- 38.Yang W, et al. EGFR-induced and PKCepsilon monoubiquitylation-dependent NF-kappaB activation upregulates PKM2 expression and promotes tumorigenesis. Mol Cell. 2012;48:771–784. doi: 10.1016/j.molcel.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JW, et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang W, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14:1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang D, et al. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilcox I, Chan KH, Lattimore JD. Hypoxia and inflammation. N Engl J Med. 2011;364:1976–1977. doi: 10.1056/NEJMc1103019. author reply 1977. [DOI] [PubMed] [Google Scholar]
- 43.Eltzschig HK. Targeting Hypoxia-induced Inflammation. Anesthesiology. 2011;114:239–242. doi: 10.1097/ALN.0b013e3182070c66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kallio PJ, Wilson WJ, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 45.Nishi K, et al. LPS induces hypoxia-inducible factor 1 activation in macrophage-differentiated cells in a reactive oxygen species-dependent manner. Antioxid Redox Signal. 2008;10:983–995. doi: 10.1089/ars.2007.1825. [DOI] [PubMed] [Google Scholar]
- 46.Regueira T, et al. Hypoxia inducible factor-1 alpha induction by tumour necrosis factor-alpha, but not by toll-like receptor agonists, modulates cellular respiration in cultured human hepatocytes. Liver Int. 2009;29:1582–1592. doi: 10.1111/j.1478-3231.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 47.Liu H, Wang P, Cao M, Li M, Wang F. Protective role of oligomycin against intestinal epithelial barrier dysfunction caused by IFN-gamma and TNF-alpha. Cell Physiol Biochem. 2012;29:799–808. doi: 10.1159/000188076. [DOI] [PubMed] [Google Scholar]
- 48.Cramer T, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peyssonnaux C, et al. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 50.Sappington PL, et al. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 51.Yang M, et al. Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochem Pharmacol. 2013;86:410–418. doi: 10.1016/j.bcp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakker J, Coffernils M, Leon M, Gris P, Vincent JL. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest. 1991;99:956–962. doi: 10.1378/chest.99.4.956. [DOI] [PubMed] [Google Scholar]
- 53.Regnier MA, et al. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology. 2012;117:1276–1288. doi: 10.1097/ALN.0b013e318273349d. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro NI, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Jin R, Bai Y. Theoretical investigation of the radical scavenging activity of shikonin and acylshikonin derivatives. J Mol Model. 2012;18:1401–1408. doi: 10.1007/s00894-011-1170-9. [DOI] [PubMed] [Google Scholar]
- 56.Andujar I, Rios JL, Giner RM, Recio MC. Pharmacological Properties of Shikonin - A Review of Literature since 2002. Planta Med. 2013 doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- 57.Latham T, et al. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012;40:4794–4803. doi: 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andujar I, Rios JL, Giner RM, Miguel Cerda J, Recio Mdel C. Beneficial effect of shikonin on experimental colitis induced by dextran sulfate sodium in BALB/c mice. Evid Based Complement Alternat Med. 2012;2012:271606. doi: 10.1155/2012/271606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weischenfeldt J, Porse B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH protocols. 2008;2008 doi: 10.1101/pdb.prot5080. pdb prot5080. [DOI] [PubMed] [Google Scholar]
- 60.Hitosugi T, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang R, et al. WAVE1 regulates Bcl-2 localization and phosphorylation in leukemia cells. Leukemia. 2010;24:177–186. doi: 10.1038/leu.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang D, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu M, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 64.Tang D, et al. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13:701–711. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.