Abstract
Gastrointestinal manifestations of diabetes are common and a source of significant discomfort and disability. Diabetes affects almost every part of gastrointestinal tract from the esophagus to the rectum and causes a variety of symptoms including heartburn, nausea, vomiting, abdominal pain, diarrhea and constipation. Understanding the underlying mechanisms of diabetic gastroenteropathy is important to guide development of therapies for this common problem. Over recent years, the data regarding the pathophysiology of diabetic gastroenteropathy is expanding. In addition to autonomic neuropathy causing gastrointestinal disturbances the role of enteric nervous system is becoming more evident. In this review, we summarize the reported alterations in enteric nervous system including enteric neurons, interstitial cells of Cajal and neurotransmission in diabetic animal models and patients. We also review the possible underlying mechanisms of these alterations, with focus on oxidative stress, growth factors and diabetes induced changes in gastrointestinal smooth muscle. Finally, we will discuss recent advances and potential areas for future research related to diabetes and the ENS such as gut microbiota, micro-RNAs and changes in the microvasculature and endothelial dysfunction.
Keywords: Diabetes, Enteric nervous system, Enteric neurons, Smooth muscle, Interstitial cells of Cajal, Oxidative stress
Introduction
The enteric nervous system (ENS) is an independent network of neurons and glial cells that is responsible for controlling the gastrointestinal (GI) tract’s functions including motility, secretion and participation in immunoregulation1–3. ENS is structured as two major plexi, myenteric and submucosal, formed by small ganglia and neurons connected through bundles of nerves that run in the course of the entire GI tract. This system is connected to the central nervous system via sensory neurons that send afferent fibers conveying visceral sensations such as pain, stretch, fullness and nausea as well as efferent sympathetic and parasympathetic pathways that modulate motility, secretion and circulation4. In addition to the enteric neurons, interstitial cells of Cajal (ICC) are non-neuronal, non-glial cells that are present throughout the GI tract within multiple layers of the esophageal, gastric and intestinal wall including the myenteric plexus5. These cells function as the pacemaker, generating electrical activity resulting in the slow wave peristaltic movement of the intestine6, 7. Furthermore, they are involved in neurotransmission between enteric motor neurons, efferents from central nervous system and smooth muscle cells in the wall of GI tract8, 9.
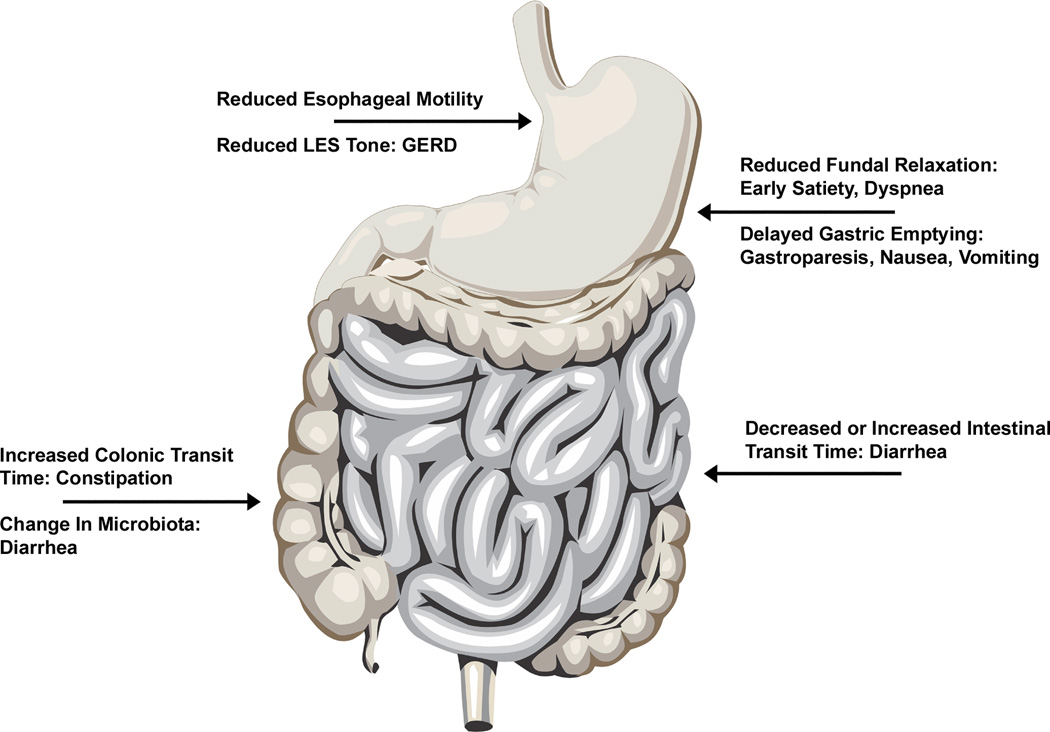
Diabetes mellitus (DM) is commonly associated with gastrointestinal symptoms such as nausea vomiting, diarrhea, abdominal pain and constipation10, 11. Diabetes affects almost all parts of the GI tract but the exact prevalence of diabetic gastroenteropathy is unclear12, 13. In the esophagus, DM is associated with decreased baseline tone of lower esophageal sphincter (LES), possibly leading to increased gastroesophageal reflux disorder (GERD)14. It has been reported that esophageal motility is delayed in patients with DM with impaired peristaltic movements in the esophagus15. The prevalence of silent esophageal dysmotility due to DM is reported to be higher than what is reported by the patients based on their symptoms16, 17. In the stomach, a wide variety of disturbances in the gastric motility as a result of DM has been reported. DM has been linked both to rapid gastric emptying, especially in the early stages of the disease18, and more commonly with delayed gastric emptying and gastroparesis17, 19. Impaired relaxation of the gastric fundus has been reported to be accountable for early satiety and dyspeptic symptoms20, 21. Electrophysiological studies have shown dysrhythmias of slow wave contraction, prolonged pyloric contractions, and impaired coordination between antrum and duodenum22–24. The effect of diabetes on small intestine and colonic functions is not as well studied. Increased prevalence of both constipation and diarrhea is reported in patients with DM. While initial data suggested that the intestinal transit time is slowed in animal models of DM leading to bacterial overgrowth and subsequently diarrhea25, further studies showed the presence of accelerated intestinal transit time in some models26. This accelerated intestinal transit was attributed to autonomic neuropathy and DM induced denervation of sympathetic nerve terminals. Colonic transit time is often increased and constipation is a common complaint in patients with DM27. The major changes in motility in various parts of the GI tract are shown in Figure 1.
Figure 1. Effects of diabetes on motility in various parts of gastrointestinal tract.
Diabetes affects almost all parts of GI tract. It can reduced the motility of esophagus and reduce the basal tone of LES. Gastroparesis and impaired gastric fundal relaxation are well known effects of diabetes on stomach. Both diarrhea and constipation are reported as GI manifestations of diabetes. GERD: gastroesophageal reflux disorder. LES: lower esophageal sphincter.
Our understanding of the underlying mechanisms of DM induced changes in GI tract has changed over the recent years. It was a widely accepted view that autonomic neuropathy is the underlying mechanism of gastrointestinal manifestations of DM; however, emerging evidence has suggested that other pathophysiologic factors also play an important role. Disturbances in the enteric nervous system and ICC, independent of autonomic nervous system, and smooth muscle myopathy are among these emerging mechanisms. In this review, we highlight recent studies using animal models and human tissue to investigate the effect of diabetes on inhibitory and excitatory neurons, population of ICC, interactions between enteric neurons and ICC in neurotransmission, and smooth muscles. Additionally, mechanism of altered survival of enteric neurons and ICC in DM such as oxidative stress and growth factors are elaborated.
Animal Models of Diabetic Neuropathy
Over the past few decades there have been several animal models developed to study diabetic neuropathy28. The most commonly used models of diabetic neuropathy include genetically modified29, 30, Streptozotocin-induced31 and high-fat diet induced mouse models32. One of the advantages of the classic Streptozotocin-induced diabetes model is that the animal develops a neuropathy similar to human diabetic neuropathy with features such as reduced size of nerve fibers, axon and myelin sheath and decreased nerve conduction velocities31. However, it has been suggested that toxic effects of Streptozotocin rather than diabetes is responsible for some degree of neuropathy observed in this model33. For studying human sensory neuropathy, one of the better models is the Streptozotocin-induced diabetic sensory neuropathy ddY mouse model, in which the mice have a lower sensory nerve conduction velocity, higher nociceptive threshold, hypoalgesia and unmyelinated fiber atrophy34. Spontaneously diabetic WBN/Kob rat model is another animal model used to study diabetic neuropathy with the advantage that it resembles the human pathogenesis of diabetic neuropathy with structural de-and remyelination in the sciatic and tibial nerves at 12 months without confounding effects of streptozotocin. At 20 months of age these mice have axonal degeneration, dystrophy and reduced myelinated fibers35. Similarly, the Nonobese diabetic (NOD) mouse model29, 30 and the Leptin-deficient ob/ob mouse model36 are consistent with the human pathogenesis of human peripheral diabetic neuropathy. Finally the high-fat diet-fed mouse model does demonstrate evidence of motor and sensory nerve conduction deficits and can be used as a model of obesity-related neuropathy32. In summary, some of the models most applicable to human diabetic neuropathy include the Streptozotocin-induced diabetic mouse models as well as the genetically modified NOD and ob/ob mouse models. These models have frequently been used to study diabetes induced enteric neuropathy.
Diabetes and autonomic neuropathy
The gastrointestinal tract is heavily connected to autonomic nervous system. Almost all parts of GI tract receive efferent connections from sympathetic and parasympathetic fibers and send afferents to the parasympathetic system. In the light of this interconnection and well-known autonomic neuropathy caused by diabetes, autonomic neuropathy was considered the origin of GI manifestations of DM. In diabetic patients, vagal nerve fibers show evidence of segmental demyelination and axonal degeneration both within myenteric and submucosal plexi and outside of the GI tract37, 38. Structural changes in axons of vagal fibers are seen in spontaneous diabetic rats39. In both patients and animal models of diabetes, the number of cells in motor vagal ganglions and sensory sympathetic ganglions is reduced40–42. However, the clinical correlation between GI symptoms and other evidence of autonomic neuropathy such as increased variability of R-R interval on electrocardiogram is controversial15, 43. Additionally, some studies have reported that although the number of neurons in the sympathetic and parasympathetic ganglions is reduced and there are structural changes in the axons, the overall density and morphology of vagal efferent fibers is not changed in animal models of diabetes44. It has been shown that vagal afferent fibers are closely related to ICC and express nNOS. A decrease in nNOS expression in the afferent vagal nerve has been reported in rat model of DM45. These findings suggest that most of the changes in diabetes in the autonomic nervous system might be related to the afferent arm of the gut-autonomic nervous system connection.
Diabetes and enteric neuropathy
The effect of DM on the population of enteric neurons is mostly studied in the rodent model of streptozotocin (STZ)-induced type I DM. Several of these studies have shown a reduction in number of enteric neurons in most parts of the GI tract including stomach46, ileum47, 48, cecum49, and colon48, 50, 51. Degenerative structural changes such as axonal swelling have also been observed as early as 2 weeks after the onset of diabetes52. Similar reduction in the number of enteric neurons has been shown in spontaneously diabetic rats53, 54 and non-obese diabetic (NOD) mice55, 56. Interestingly, DM might preferentially affect inhibitory neurons more than excitatory neurons. The population of nitrergic neurons is affected early after the onset of DM in animal models and expression of nNOS is reduced in diabetic animals while the population of cholinergic enteric neurons remains unaffected until later in the course of DM57. In a study of colonic tissue obtained from human subjects with DM, a decrease in the number of nitrergic neurons as well as neurons containing neuropeptide Y, another inhibitory neurotransmitter, but not in the number of cholinergic neurons has been reported58. Another study examined the population of nitrergic neurons in the appendix of 6 patients with type 1 DM and reported a significant decrease in the number of nNOS containing neurons within the myenteric plexus as well as muscular layers59.
Mechanism of Diabetic enteric neuropathy
The mechanism of degeneration of enteric neurons in DM is not clear. Some studies using STZ model and cell cultures have suggested increase in apoptosis due to hyperglycemia similar to the effect of hyperglycemia on other neuronal cell types41, 60, 61. Enteric neurons are shown to be glucose responsive and it is possible that hyperglycemia will activate apoptotic pathways by causing hypercalcemia due to increased activation of ATP-sensitive K+-channels62, 63.
Another mechanism suggested to play a role in decreased population of enteric neurons is decreased neuronal growth factors due to DM. Enteric neurons originate from vagal and sacral neural crest cells and they express Ret tyrosine kinase receptor which is essential for their differentiation, migration and survival. Glial cell line-derived neurotrophic factor (GDNF) is a growth factor that activates this receptor and through PI3K and MAPK pathways64–66. Diabetes is known to reduce the transport and function of nerve growth factors and it is possible that decreased survival of enteric neurons is accentuated by lack of enough growth factors. Furthermore, Insulin like growth factor-1 (IGF-1) and neurotrophin-3 (NT-3) have been reported to successfully reverse degenerative changes of nerves in animal models of diabetic neuropathy67–69. We have reported that hyperglycemia causes reduced activation of PI3K in cultured embryonic enteric neurons and in STZ –induced rat model of DM, leading to increased apoptosis of enteric neurons. In this experiment, GDNF was protective against adverse effects of hyperglycemia on enteric neurons and its addition to the culture led to significant reduction in apoptosis60. The number of enteric glial cells is also reduced in animal model of diabetes. It has been reported that the number of S-100 expressing enteric glial cells in the stomach of STZ-induced rat model of diabetes is reduced in correlation with development of delayed gastric emptying. Diabetic patients have high circulating levels of free fatty acids70. In mice fed with high fat diet and subsequently developed DM, the number of enteric glial cells was reduced in small intestine prior to loss of enteric neurons71. We and others have also shown that a diet high in fat can lead to intestinal enteric neuronal loss72, 73. Palmitic acid seems to play an important role in mediating this damage. Another growth factor signaling pathway that has reported to be altered in animal model of diabetes is transforming growth factor (TGF) beta. One recent study investigated the role of bone morphogenetic proteins (BMPs), a molecule involved in TGF beta signaling pathway and gut morphogenesis, in loss of enteric neurons in animal model of diabetes. In an experimental rat model of diabetes, abnormal BMP2 signaling was found in the myenteric plexus which was restored with insulin therapy. This suggests that the BMP2/SMAD signaling pathway is important in diabetic enteric neuropathy74.
Oxidative stress which results from excess of reactive oxygen species (ROS) has also been suggested as a potential mechanism of enteric neuron degeneration as a result of DM. In animal models of DM, increased oxidative stress has been shown to be associated with gastroparesis and treatment with antioxidants can prevent the development of gastroparesis75. In the high fat diet fed mouse model excessive palmitoylcarnitine formation leading to energy depletion and oxidative stress are felt to be one of the mechanisms underlying neuronal loss72, 73. Quercitin is a powerful antioxidant that has been shown to prevent the reduction in enteric neuronal numbers in the cecum of diabetic rats76. Examination of enteric ganglia obtained from colon biopsy specimen of diabetic patients through laser capture microdissection showed decreased levels of antioxidant GSH and increased expression of superoxide dismutase (SOD) mRNA which indicate increased oxidative stress. This increase in oxidative stress seems to preferentially affect the inhibitory nitrergic neurons, evident as decreased contractility in response to nitric oxide inhibitor. Addition of antioxidant lipoic acid to the cultured enteric neurons exposed to hyperglycemia reverses the reduction in PI3K activation, providing further evidence for increased oxidative stress due to hyperglycemia58. Furthermore, in vitro evidence suggests that GSH from enteric glia might have a protective effect on enteric neuronal survival77. In mouse models of diabetes, increased expression of antioxidants such as HO-1 can protect mice from developing gastroparesis75. In STZ-rat model of DM, nitrergic enteric neurons that also express HO-2 are more resistant to the effects of DM and are less likely to undergo apoptosis78. However, trials of antioxidants in diabetic patients have not been very successful in preventing or improving gastroparesis79, suggesting the need for more studies to develop the optimal therapeutic approach.
There are other suggested but not well-studied mechanisms for enteric neuropathy in diabetes. We will mention two of these mechanisms briefly here: accumulation of N-acetylaspartic acid and S-nitrosylation reactions. Normal hydrolysis of N-acetylaspartic acid (NAA) is important to maintain enteric neurons. Aspartoacylase (ASPA) is the enzyme responsible for hydrolysis of NAA and it has been shown that in an obesity-induced mice model of diabetes, the activity of this enzyme reduced, leading to accumulation of NAA and potentially contributing to loss of enteric neurons3, 80. One of the signaling pathways of NO is through S-nitrosothiol (SNO). In the central nervous system, aberrant S-nitrosylation of some molecular targets is implicated in CNS pathology but the role of abnormal SNO signaling in enteric nervous system is not clear. Recently, it has been shown that S-nitrosylation reactions may be involved in toxin-induced neurogenic inflammation in the colon81. The role of these factors in diabetes induced GI dysmotility remains to be elucidated through further research.
DM and Interstitial Cells of Cajal
ICC are non-neuronal cells that are present in multiple layers of GI tract’s wall and are named based on their location. For example, a group of ICC located in the myenteric plexus is named ICC-MY and a group of them located in muscular layer is called ICC-IM82. Recent evidence has suggested that these cells are different in ultra-structural morphology and function in addition to location5, 83. Development of ICC, like enteric neurons, is dependent on a tyrosine Kinase receptor, Kit84. Primary evidence for role of ICCs in control of GI motility came from animal models in which Kit receptors were blocked. These animals developed severe GI motility dysfunction with absence of slow phase peristaltic movements85, 86. Since then, ICCs became an area of interest in studies investigating abnormalities in GI motility, including those caused by DM and several other functions have been attributed them such as mediating neurotransmission between autonomic nervous system and enteric neurons or between enteric neurons and smooth muscles9, 87, 88.
Studies in animal model of DM and gastroparesis have shown that populations of ICC-MY and ICC-IM in gastric antrum and ICC-IM in gastric fundus are reduced in STZ model of DM as well as NOD model89, 90. The reduced population of ICC-IM in fundus is proposed to be associated with impaired relaxation of gastric fundus in diabetic animal, while reduced population of ICC-MY is associated with impaired slow wave peristaltic movements and delayed gastric emptying91. A case report of a patient with insulin-dependent DM with gastroparesis, demonstrated decreased population of ICC-IM in human, similar to animal models92. Foster et al reported the results of gastric biopsy from 14 patients with gastroparesis including 9 patients with DM and showed reduction in the number of ICC93. In 25 patients with DM and gastric cancer who underwent gastrectomy, study of the gastric tissue showed reduced number of ICC in the inner circular muscle layer but not the myenteric plexus94. Diminished population of ICCs has been reported in other parts of GI tract in animal model of diabetes including colon and small intestine. A case-series of 7 patients with DM showed reduced number of ICC in colonic tissue95. Yamamoto et al, using a db/db model of type 2 DM, showed reduced number of ICC in small intestine and colon in addition to stomach96.
In addition to being an independent pulse maker for slow wave movements of GI tract, ICC appear to be an important part of neurotransmission between motor neurons, efferent input from autonomic nervous system and muscle fibers. It has been proposed that both excitatory and inhibitory neurotransmission between enteric neurons and smooth muscles are dependent on presence of ICC-IM. Morphologically, there is close contact with synaptic specification between ICC and enteric neurons9, 88, 97. It has been shown that acetylcholine released from enteric motor neurons attaches to the Ach receptors of ICC-IM which in turn depolarize the smooth muscles through gap junctions8. The modulatory role of ICC-IM has also been shown in excitatory neurotransmission through substance P and neurokinin98. As for inhibitory neurotransmission, ICC-IM in distal esophagus and antrum of mice are surrounded by NO-containing nerves and mice model lacking ICC-IM doesn’t show any evidence of nitrergic transmission87. Therefore, it is possible that reduced population of ICC-IM impairs neurotransmission, adding to the disturbed motility caused by impaired pacemaker activity from ICC-MY. However, there are remaining questions about the degree that neurotransmission happening through ICC is important in controlling GI motility. One important reservation is that the number of ICC is considerably lower that smooth muscle cells, suggesting that neurotransmission cannot rely solely on ICC5.
Mechanism of DM induced changes in ICC
Similar to the enteric neurons, the mechanism by which DM affects ICCs remains to be fully elucidated. One interesting finding reported in the literature is that hyperglycemia alone is not enough to affect ICC and in fact lack of Insulin and Insulin-like growth factor-1 (IGF-1) plays the major role in reduced survival of ICC99. However, ICC lack receptors for insulin of IGF-1 and instead have receptors for stem cell factor (SCF). On the other hand, smooth muscle cells and enteric neurons have receptors for insulin and IGF-1. It has been suggested that ICC require SCF secreted by smooth muscle cells and enteric neurons in response to Insulin. Lack of insulin, reduced population of enteric neuron as the result of DM and myopathic changes in smooth muscle cells can all lead to decreased amount of CSF and consequently diminished survival of ICC100. While this finding might provide an explanation for long-term effects of diabetes on GI motility through its effect on population of ICC, it does not explain the reported effect of acute changes in serum glucose level on gastric motility. Hyperglycemia and hypoglycemia have both shown to change the rate of gastric emptying acutely101. This might be due to dysrhythmia in the pacemaker activity of ICCs or change in the contractibility of smooth muscle fibers.
DM and the balance of excitatory and inhibitory neurotransmitters
The main excitatory neurotransmitter of enteric nervous system is acetylcholine followed by neurokinin and substance P that are released by enteric motor neurons and have receptors on ICC-IM, mediating the contraction of smooth muscles102. Inhibitory neurotransmission in enteric nervous system happens through non-adrenergic non-cholinergic (NANC) pathway. Nitric oxide (NO) is the main inhibitory neurotransmitter, with neuronal nitric oxide (nNOS) being the rate controlling enzyme in its production within ENS103, 104. Studies of animal models with DM have demonstrated that the sources of nitrergic nerve terminals are both from intrinsic motor neurons and vagal and parasympathetic afferents. The distribution of nitrergic neurons is not equal throughout the GI tract, with them being more prevalent in the stomach and proximal parts of the intestine105, 106.
In animal models of DM, there is a gender specific reduction in the expression of nNOS and the production of NO within ENS107, 108. One study in STZ-induced diabetes in rats showed that there is reduction of NO in nerve terminals within the muscular layer of GI wall without decrease of nNOS positive neurons in parasympathetic ganglions. The authors suggested that there might be a diabetes-induced impairment of transport of nNOS from cell bodies to the nerve terminals56. The attenuation in expression of nNOS mRNA and production of NO in diabetic rats is associated with impaired relaxation of gastric muscles and subsequently decreased gastric capacity and probably delayed gastric emptying. In STZ-rat model of diabetes, depletion of neurotransmitter containing vesicles occurs before destruction of the enteric nerve terminals in the gastric tissue and application of electrical stimulation early in the course of the disease can partially reverse this process109. Animal models have also shown that reduction of nNOS expression in the stomach is a gradual process that happens earlier than loss of acetylcholine positive nerve endings and is reversible with early insulin treatment. A two-phase model of NO depletion has suggested in which during the first phase, expression of nNOS is reduced in a reversible fashion that if not treated, will eventually lead to irreversible loss of nitrergic neurons110, 111. Furthermore, DM can disturb the balanced expression of other neuropeptides such as vasoactive intestinal polypeptide (VIP), calcitonin gene-related peptide (CGRP) and substance P. In the ileum of diabetic rat, electrical stimulation failed to increase the release of VIP and CGRP, whereas endogenous release of acetylcholine, serotonin, and substance P was increased similar to normal animal112. The imbalance between expression of enteric neuropeptides is reported to be reversible with insulin treatment113.
Few studies in human patients with DM have confirmed the findings from animal models. Studying the gastric tissue obtained from patients with gastric cancer and DM revealed that the expression of nNOS is markedly decreased in the antrum, especially in the areas that have reduced density of ICC94. In another study, we examined the colonic tissue obtained during colonoscopy from patients with and without diabetes and demonstrated that the population of nNOS containing enteric neurons is reduced. In addition to NO, other inhibitory neurotransmitters such as neuropeptide Y (NPY) and vasoactive intestinal peptide (VIP) are also reported to be reduced in DM58.
Mechanism of DM induced changes in neurotransmitters
The mechanism of reduced expression of nNOS is not fully understood. Earlier data has shown that the loss of nitrergic nerves is independent of sympathetic and parasympathetic input. Only afferent vagal nerves appear to contain NO that located in close proximity with ICC-IM. It has been suggested that these afferent vagal nerve endings could be mechanoreceptors and their survival is mutually dependent on ICC-IM. Loss of ICC as a result of diabetes can cause the loss of afferent vagal nitrergic nerve endings and vice versa45. Another possibility is the loss of inhibitory enteric neurons that express high levels of nNOS. Evidence has suggested that inhibitory enteric neurons are more susceptible to the effects of diabetes and their population is reduced earlier in the course of the disease58. Others have proposed that non-enzymatic glycosylation of proteins that leads to production of advanced glycation end-products (AGEs) might play a role in reduced expression of nNOS, since inhibiting these products can prevent the depletion of NO from nerve terminals or even restore the depleted amount61, 114.
DM and GI smooth muscle
Contractility of smooth muscle cells is altered in animal models of DM. Myocytes isolated from GI tracts of STZ-induced rat show increased expression Na, K-ATPase, leading to increased intracellular calcium level115. In the same animal model, intracellular calcium binding proteins such as calmodulin and protein kinase C in intestinal smooth muscle cells are reduced. Similar changes are found in spontaneous diabetic rats, suggesting that diabetes alters smooth muscle contractility through changing the intracellular signaling pathways of intestinal myocytes116. Another study, using both STZ-induced DM and spontaneous DM models, suggested that impairment in the function of GTP-binding proteins might be important in altered contractility of gastric smooth muscles, providing further evidence for the importance of DM induced changes in the intracellular signaling pathways117. Myopathy and atrophy of gastric smooth muscle has also been reported100, 118
Another mechanism that is proposed to be involved in pathogenesis of DM-induced dysmotility is the change in phosphorylation of myosin light chain (MLC). Phosphorylation of MLC is an important regulatory mechanism in contraction of intestinal smooth muscles. Two important enzymes are involved in the process of phosphorylation (myosin light chain kinase (MLCK)) and dephosphorylation (myosin light chain phosphatase (MLCP)) of MLC. Targeted deletion of MLCK leads to significant disturbance in GI motility119. In a study of STZ-DM rats, the expression of MLCK was significantly reduced in the smooth muscles of pylorus and ileum. This effect was reversible with insulin treatment120.
Other Factors and New Directions
Autoimmunity
Lymphocytic infiltrates have been reported in MP of some animal models of DM52, but no clear correlation between presence of these infiltrates and DM-induced GI disturbance has been proved yet. In patients with DM, results have been contradictory. While inflammatory infiltrates have been reported in esophagus38 and autonomic ganglia121 of patients with diabetes, gastric specimens from diabetic patients with gastroparesis have not revealed any inflammation118. Efforts have been made to find functional antibodies that can act against enteric neurons or other parts of ENS in patients with diabetes and have been largely unsuccessful. There is a report of antibody against L-type calcium channels can disturb migrating myoelectric complex in mice122, but further studies are required to verify the role of autoimmunity in pathogenesis of enteric neuropathy in diabetic patients.
Endothelial Dysfunction
Enteric neurons within MP receive blood supply from adjacent capillaries. A recent study has shown that endothelial cells in the capillaries close the enteric neurons are damaged in diabetic animals, leading to change in the permeability of these capillaries as well as altered expression of adhesion molecules. The diabetes-induced change in the capillaries of wall of the gut is not uniform within various segment of GI tract and has a distribution that resembles the distribution of changes in enteric neurons and nitrergic transmission123. This evidence might suggest a role for endothelial dysfunction in pathogenesis of changes in ENS as a result of diabetes.
Microbiota
The effect of gut’s microbiota on GI motility is receiving more recognition due to recent research. It has been shown that elimination of microbiota causes dilatation of cecum as a result of abnormal motility that returns to normal when colonization of bacteria is allowed124. This change in GI motility has been attributed to change in neurotransmitters. It is well known that the composition of microbiota changes significantly in patients with DM and efforts have been made to explain the role of this change in pathogenesis of DM125. Some of the disturbances in GI motility such as diarrhea has been attributed to change in microbiota25, but the exact role of altered microbiota in diabetes induced GI dysmotility is not studies. It is possible that a mutual relation between GI motility and change in gut’s microbiata exists126. Decreased motility of GI tract can lead to change in the microbial flora of the gut while this change can alter neurotransmission within the gastric wall or even change the gastric motility through afferent signals to central nervous system127–129.
Micro-RNAs
Micro-RNAs are small non-coding RNAs that post-transcriptionally regulate protein synthesis130. The role of miRNAs in regulating GI motility and survival of enteric neurons is becoming more evident from the recent evidence. For example, we have recently shown that loss of enteric neurons caused by high fat diet is mediated through Mir-35772. Role of miRNAs in organ damage caused by diabetes as well as pathogenesis of diabetes is being actively explored, but there is no data regarding role of miRNAs in diabetic induced changes of ENS. miRNAs are also involved in proliferation and development of gastrointestinal smooth muscles131, 132. Future research is necessary to identify miRNAs that are involved in toxic effects of hyperglycemia or lack of insulin on enteric neurons and smooth muscles since these can be potential therapeutic targets.
Autophagy
Autophagy is referred to the process of collecting damaged proteins and organelles in to autophagosomes. The primary purpose of this process is to protect cells from damage caused by malfunctioning proteins and organelles, but it also can lead to cell death in certain circumstances133. Impairment of autophagy along with increased oxidative stress is suggested to be involved in pathogenesis of DM as well as end-organ damage caused by DM134. An association between impaired autophagy and increased apoptosis in dorsal root ganglia has been reported in STZ-rat model of diabetes135. The role of impaired autophagy in the pathogenesis of diabetic gastroenteropathy has not been directly studied. However, we have reported that impaired autophagy might be important in damage to the nitrergic neurons caused by high-fat diet72. It is possible that with increased oxidative stress and damage to the proteins and mitochondria, an impaired autophagy will exacerbate the problem and will lead to accelerated death of enteric neurons. Future research is required to test this hypothesis.
Challenges and Future perspectives
One of the key challenges in treatment of diabetic induced enteric neuropathy is identifying new modifiable risk factors. There are risk factors that have been known for a while and strategies have been developed to modify them. These types of risk factors might include but are not limited to hypertriglyceridemia, obesity, dyslipidemia, and consumption of high-fat diet136. However, addressing these factors alone has not been sufficient to prevent enteric neuropathy caused by diabetes, suggesting that other factors are playing parts that are largely neglected in treatment protocols. For example, a recent study showed a beneficial effect of using human TNF-α receptor antibody in preventing diabetic neuropathy using a rodent model137. This study suggests that inflammation can play a key role in the pathogenesis of diabetic neuropathy. Oxidative stress is another important factor that is increased in diabetes and can lead to DNA damage, ER stress, mitochondrial complex dysfunction and neuronal apoptosis. The cells involved can include neurons, glia, vascular endothelial cells and can lead to the activation of macrophages leading to neuronal dysfunction and neuropathy138. Given the chronic progressive nature of the diabetic neuropathy, new treatment strategies may need to target multiple pathways contributing to the disease, including a combination of antioxidants, nerve growth factors and improvement of hyperglycemia and hyperlipidemia. Recently targeting ER stress was demonstrated to be beneficial in the treatment of diabetic neuropathy139. Novel research strategies need to focus on identifying potential inflammatory mediators of neuropathy as well as studying the role of non-coding RNAs (miRNAs and lncRNAs) in the pathogenesis of diabetic enteric neuropathy and their therapeutic potential72. Finally given the recent findings of the role of gut microbiota in contributing to the development of the metabolic syndrome, more research will need to be done in looking for potential changes in gut microbiota leading to progression of diabetic enteric neuropathy126.
Conclusion
Recent developments in our understanding of how diabetes affects the enteric nervous system has caused a paradigm shift in how we think about GI manifestations of DM. Autonomic neuropathy is no longer considered the sole cause of GI dysmotility in DM and importance of enteric neurons, ICC, smooth muscle and enteric microbiota is becoming more evident. However, translating these findings into clinical treatment has been challenging. For example, while oxidative stress has been shown to play an important role in DM-induced apoptosis of enteric neurons and ICC140, use of antioxidants with oral supplementation has not proved efficacious79. This calls for more research to find more creative approaches in delivering antioxidants to the enteric nervous system. Some steps have already been taken by using gene therapy in animal models to increase expression of antioxidants such as HO-175.
Another interesting recent development is a new line of research investigating the bidirectional interaction of microbiata and enteric motility126. Diabetes is known to change enteric microbiata and researchers were interested in this change from the perspective of DM pathophysiology. However, it has shown that some species of gut microbiota can be used to decrease oxidative stress. Additionally, metabolites produced by some of the species can stimulate or inhibit enteric nervous system and modulate GI motility126. Methods of altering microbi0ta in a favorable way to prevent and treat GI complication of DM remains to be fully developed with further research.
Another important question is how well the findings in animal models translate into human patients? So far, only few studies have used human tissues from diabetic patients to test the hypothesis driven from animal models58, 92–95. Although the findings of these studies have been mostly confirmatory of animal findings that diabetes reduces the number of enteric neurons and ICCs, there is no longitudinal data available to elucidate the chain of events that lead to GI complications of DM. Furthermore, there is no data to help predict which patient will develop GI manifestations and what the correlation between glucose control and GI dysmotility is.
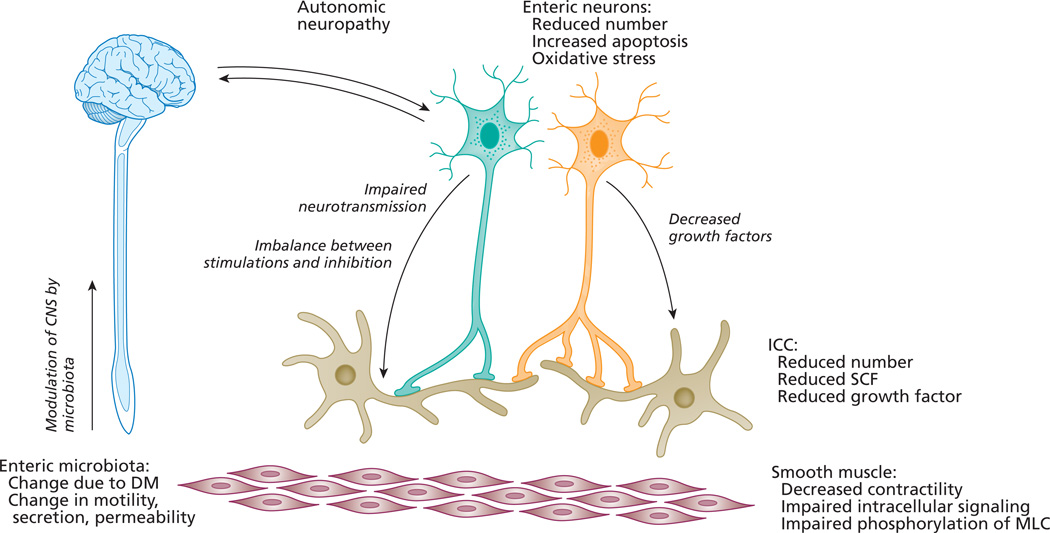
In summary, DM significantly alters the microenvironment of enteric neurons and ICC, leading to decreased survival of the cells. Change of the microenvironment is multifactorial and includes increased oxidative stress, alteration of enteric microbiata, reduction of growth factors, change in intracellular signaling pathways and post-transcription regulatory factors such as miRNAs, and endothelia dysfunction (Figure 2). The interaction between these factors is complex, but each of them offers the potential for new therapeutic approaches.
Figure 2. Potential mechanisms involving the ENS in impaired gastrointestinal motility in DM.
Mechanism of diabetes induced gastrointestinal enteric neuropathy is complex and multifactorial. Autonomic neuropathy can affect both afferent and efferent connections between enteric nervous system and central nervous system. Reduced number of enteric neurons is caused by increased oxidative stress and increased apoptosis. The number of interstitial cells of Cajal in is also reduced, possibly due to lack of growth factors. There myopathic changes in smooth muscle and decreased contractility due to impaired signaling pathways. Diabetes induced changes in gut microbiota can modulate the CNS-gut interaction. ICC: interstitial cells of Cajal, SCF: stem cell factor, MLC: myosine light chain, DM: diabetes mellitus, CNS: central nervous system.
References
- 1.Costa M, Brookes SJ. The enteric nervous system. Am J Gastroenterol. 1994;89:S129–S137. [PubMed] [Google Scholar]
- 2.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 3.Surendran S. Upregulation of N-acetylaspartic acid alters inflammation, transcription and contractile associated protein levels in the stomach and smooth muscle contractility. Mol Biol Rep. 2009;36:201–206. doi: 10.1007/s11033-007-9167-2. [DOI] [PubMed] [Google Scholar]
- 4.Lundgren O, Svanvik J, Jivegard L. Enteric nervous system. I. Physiology and pathophysiology of the intestinal tract. Dig Dis Sci. 1989;34:264–283. doi: 10.1007/BF01536062. [DOI] [PubMed] [Google Scholar]
- 5.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 7.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 8.Ward SM, Beckett EA, Wang X, et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147–159. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 11.Maleki D, Locke GR, 3rd, Camilleri M, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808–2816. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- 12.Ordog T, Hayashi Y, Gibbons SJ. Cellular pathogenesis of diabetic gastroenteropathy. Minerva Gastroenterol Dietol. 2009;55:315–343. [PMC free article] [PubMed] [Google Scholar]
- 13.Sellin JH, Chang EB. Therapy Insight: gastrointestinal complications of diabetes--pathophysiology and management. Nat Clin Pract Gastroenterol Hepatol. 2008;5:162–171. doi: 10.1038/ncpgasthep1054. [DOI] [PubMed] [Google Scholar]
- 14.Nishida T, Tsuji S, Tsujii M, et al. Gastroesophageal reflux disease related to diabetes: Analysis of 241 cases with type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:258–265. doi: 10.1111/j.1440-1746.2003.03288.x. [DOI] [PubMed] [Google Scholar]
- 15.Kinekawa F, Kubo F, Matsuda K, et al. Relationship between esophageal dysfunction and neuropathy in diabetic patients. Am J Gastroenterol. 2001;96:2026–2032. doi: 10.1111/j.1572-0241.2001.03862.x. [DOI] [PubMed] [Google Scholar]
- 16.Talley NJ. Diabetic gastropathy and prokinetics. Am J Gastroenterol. 2003;98:264–271. doi: 10.1111/j.1572-0241.2003.07268.x. [DOI] [PubMed] [Google Scholar]
- 17.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–829. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 18.Choi KM, Zhu J, Stoltz GJ, et al. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1039–G1045. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 19.Intagliata N, Koch KL. Gastroparesis in type 2 diabetes mellitus: prevalence, etiology, diagnosis, and treatment. Curr Gastroenterol Rep. 2007;9:270–279. doi: 10.1007/s11894-007-0030-3. [DOI] [PubMed] [Google Scholar]
- 20.Samsom M, Roelofs JM, Akkermans LM, et al. Proximal gastric motor activity in response to a liquid meal in type I diabetes mellitus with autonomic neuropathy. Dig Dis Sci. 1998;43:491–496. doi: 10.1023/a:1018894520557. [DOI] [PubMed] [Google Scholar]
- 21.Undeland KA, Hausken T, Aanderud S, et al. Lower postprandial gastric volume response in diabetic patients with vagal neuropathy. Neurogastroenterol Motil. 1997;9:19–24. doi: 10.1046/j.1365-2982.1997.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen JD, Lin Z, Pan J, et al. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538–1545. doi: 10.1007/BF02087897. [DOI] [PubMed] [Google Scholar]
- 23.Hasler WL, Soudah HC, Dulai G, et al. Mediation of hyperglycemia-evoked gastric slow-wave dysrhythmias by endogenous prostaglandins. Gastroenterology. 1995;108:727–736. doi: 10.1016/0016-5085(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 24.Jebbink RJ, Samsom M, Bruijs PP, et al. Hyperglycemia induces abnormalities of gastric myoelectrical activity in patients with type I diabetes mellitus. Gastroenterology. 1994;107:1390–1397. doi: 10.1016/0016-5085(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 25.Sellin JH, Hart R. Glucose malabsorption associated with rapid intestinal transit. Am J Gastroenterol. 1992;87:584–589. [PubMed] [Google Scholar]
- 26.Rosa-e-Silva L, Troncon LE, Oliveira RB, et al. Rapid distal small bowel transit associated with sympathetic denervation in type I diabetes mellitus. Gut. 1996;39:748–756. doi: 10.1136/gut.39.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imaeda K, Takano H, Koshita M, et al. Electrical properties of colonic smooth muscle in spontaneously non-insulin-dependent diabetic rats. J Smooth Muscle Res. 1998;34:1–11. doi: 10.1540/jsmr.34.1. [DOI] [PubMed] [Google Scholar]
- 28.Islam MS. Animal models of diabetic neuropathy: progress since 1960s. J Diabetes Res. 2013;2013:149452. doi: 10.1155/2013/149452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt RE, Dorsey DA, Beaudet LN, et al. Non-obese diabetic mice rapidly develop dramatic sympathetic neuritic dystrophy: a new experimental model of diabetic autonomic neuropathy. Am J Pathol. 2003;163:2077–2091. doi: 10.1016/S0002-9440(10)63565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homs J, Ariza L, Pages G, et al. Comparative study of peripheral neuropathy and nerve regeneration in NOD and ICR diabetic mice. J Peripher Nerv Syst. 2011;16:213–227. doi: 10.1111/j.1529-8027.2011.00345.x. [DOI] [PubMed] [Google Scholar]
- 31.Jakobsen J, Lundbaek K. Neuropathy in experimental diabetes: an animal model. Br Med J. 1976;2:278–279. doi: 10.1136/bmj.2.6030.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obrosova IG, Ilnytska O, Lyzogubov VV, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of"healthy" diet and aldose reductase inhibition. Diabetes. 2007;56:2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 33.Davidson E, Coppey L, Lu B, et al. The roles of streptozotocin neurotoxicity and neutral endopeptidase in murine experimental diabetic neuropathy. Exp Diabetes Res. 2009;2009:431980. doi: 10.1155/2009/431980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami T, Iwanaga T, Ogawa Y, et al. Development of sensory neuropathy in streptozotocin-induced diabetic mice. Brain Behav. 2013;3:35–41. doi: 10.1002/brb3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yagihashi S, Wada R, Kamijo M, et al. Peripheral neuropathy in the WBN/Kob rat with chronic pancreatitis and spontaneous diabetes. Lab Invest. 1993;68:296–307. [PubMed] [Google Scholar]
- 36.Drel VR, Mashtalir N, Ilnytska O, et al. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- 37.Guy RJ, Dawson JL, Garrett JR, et al. Diabetic gastroparesis from autonomic neuropathy: surgical considerations and changes in vagus nerve morphology. J Neurol Neurosurg Psychiatry. 1984;47:686–691. doi: 10.1136/jnnp.47.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith B. Neuropathology of the oesophagus in diabetes mellitus. J Neurol Neurosurg Psychiatry. 1974;37:1151–1154. doi: 10.1136/jnnp.37.10.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yagihashi S, Sima AA. Diabetic autonomic neuropathy in BB rat. Ultrastructural and morphometric changes in parasympathetic nerves. Diabetes. 1986;35:733–743. doi: 10.2337/diab.35.7.733. [DOI] [PubMed] [Google Scholar]
- 40.Carroll SL, Byer SJ, Dorsey DA, et al. Ganglion-specific patterns of diabetes-modulated gene expression are established in prevertebral and paravertebral sympathetic ganglia prior to the development of neuroaxonal dystrophy. J Neuropathol Exp Neurol. 2004;63:1144–1154. doi: 10.1093/jnen/63.11.1144. [DOI] [PubMed] [Google Scholar]
- 41.Guo C, Quobatari A, Shangguan Y, et al. Diabetic autonomic neuropathy: evidence for apoptosis in situ in the rat. Neurogastroenterol Motil. 2004;16:335–345. doi: 10.1111/j.1365-2982.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 42.Tay SS, Wong WC. Short- and long-term effects of streptozotocin-induced diabetes on the dorsal motor nucleus of the vagus nerve in the rat. Acta Anat (Basel) 1994;150:274–281. doi: 10.1159/000147630. [DOI] [PubMed] [Google Scholar]
- 43.Clouse RE, Lustman PJ. Gastrointestinal symptoms in diabetic patients: lack of association with neuropathy. Am J Gastroenterol. 1989;84:868–872. [PubMed] [Google Scholar]
- 44.Yoshida MM, Schuffler MD, Sumi SM. There are no morphologic abnormalities of the gastric wall or abdominal vagus in patients with diabetic gastroparesis. Gastroenterology. 1988;94:907–914. doi: 10.1016/0016-5085(88)90546-x. [DOI] [PubMed] [Google Scholar]
- 45.Regalia J, Cai F, Helke C. Streptozotocin-induced diabetes and the neurochemistry of vagal afferent neurons. Brain Res. 2002;938:7–14. doi: 10.1016/s0006-8993(02)02456-3. [DOI] [PubMed] [Google Scholar]
- 46.Fregonesi CE, Miranda-Neto MH, Molinari SL, et al. Quantitative study of the myenteric plexus of the stomach of rats with streptozotocin-induced diabetes. Arq Neuropsiquiatr. 2001;59:50–53. doi: 10.1590/s0004-282x2001000100011. [DOI] [PubMed] [Google Scholar]
- 47.Alves AM, Alves EP, Fregonesi CE, et al. Morphoquantitative aspects of NADH-diaphorase myenteric neurons in the ileum of diabetic rats treated with acetyl-L-carnitine. Anat Histol Embryol. 2006;35:13–18. doi: 10.1111/j.1439-0264.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 48.Domenech A, Pasquinelli G, De Giorgio R, et al. Morphofunctional changes underlying intestinal dysmotility in diabetic RIP-I/hIFNbeta transgenic mice. Int J Exp Pathol. 2011;92:400–412. doi: 10.1111/j.1365-2613.2011.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanoni JN, de Miranda Neto MH, Bazotte RB, et al. Morphological and quantitative analysis of the neurons of the myenteric plexus of the cecum of streptozotocin-induced diabetic rats. Arq Neuropsiquiatr. 1997;55:696–702. doi: 10.1590/s0004-282x1997000500004. [DOI] [PubMed] [Google Scholar]
- 50.Du F, Wang L, Qian W, et al. Loss of enteric neurons accompanied by decreased expression of GDNF and PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil. 2009;21:1229-e1114. doi: 10.1111/j.1365-2982.2009.01379.x. [DOI] [PubMed] [Google Scholar]
- 51.Furlan MM, Molinari SL, Miranda Neto MH. Morphoquantitative effects of acute diabetes on the myenteric neurons of the proximal colon of adult rats. Arq Neuropsiquiatr. 2002;60:576–581. doi: 10.1590/s0004-282x2002000400012. [DOI] [PubMed] [Google Scholar]
- 52.Diani AR, Gerritsen GC, Stromsta S, et al. A study of the morphological changes in the small intestine of the spontaneously diabetic Chinese hamster. Diabetologia. 1976;12:101–109. doi: 10.1007/BF00428973. [DOI] [PubMed] [Google Scholar]
- 53.Pereira RV, Tronchini EA, Tashima CM, et al. L-glutamine supplementation prevents myenteric neuron loss and has gliatrophic effects in the ileum of diabetic rats. Dig Dis Sci. 2011;56:3507–3516. doi: 10.1007/s10620-011-1806-8. [DOI] [PubMed] [Google Scholar]
- 54.Yoneda S, Kadowaki M, Kuramoto H, et al. Enhanced colonic peristalsis by impairment of nitrergic enteric neurons in spontaneously diabetic rats. Auton Neurosci. 2001;92:65–71. doi: 10.1016/S1566-0702(01)00317-4. [DOI] [PubMed] [Google Scholar]
- 55.Surendran S, Kondapaka SB. Altered expression of neuronal nitric oxide synthase in the duodenum longitudinal muscle-myenteric plexus of obesity induced diabetes mouse: implications on enteric neurodegeneration. Biochem Biophys Res Commun. 2005;338:919–922. doi: 10.1016/j.bbrc.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 56.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:803. doi: 10.1172/jci8273c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demedts I, Masaoka T, Kindt S, et al. Gastrointestinal motility changes and myenteric plexus alterations in spontaneously diabetic biobreeding rats. J Neurogastroenterol Motil. 2013;19:161–170. doi: 10.5056/jnm.2013.19.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chandrasekharan B, Anitha M, Blatt R, et al. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–138. e126. doi: 10.1111/j.1365-2982.2010.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller SM, Narasimhan RA, Schmalz PF, et al. Distribution of interstitial cells of Cajal and nitrergic neurons in normal and diabetic human appendix. Neurogastroenterol Motil. 2008;20:349–357. doi: 10.1111/j.1365-2982.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 60.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cellek S, Qu W, Schmidt AM, et al. Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: a new insight into selective nitrergic neuropathy in diabetes. Diabetologia. 2004;47:331–339. doi: 10.1007/s00125-003-1298-y. [DOI] [PubMed] [Google Scholar]
- 62.Liu M, Seino S, Kirchgessner AL. Identification and characterization of glucoresponsive neurons in the enteric nervous system. J Neurosci. 1999;19:10305–10317. doi: 10.1523/JNEUROSCI.19-23-10305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moriyama R, Tsukamura H, Kinoshita M, et al. In vitro increase in intracellular calcium concentrations induced by low or high extracellular glucose levels in ependymocytes and serotonergic neurons of the rat lower brainstem. Endocrinology. 2004;145:2507–2515. doi: 10.1210/en.2003-1191. [DOI] [PubMed] [Google Scholar]
- 64.Heuckeroth RO, Lampe PA, Johnson EM, et al. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasan S, Anitha M, Mwangi S, et al. Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNF-stimulated survival. Mol Cell Neurosci. 2005;29:107–119. doi: 10.1016/j.mcn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 67.Delaney CL, Russell JW, Cheng HL, et al. Insulin-like growth factor-I and over-expression of Bcl-xL prevent glucose-mediated apoptosis in Schwann cells. J Neuropathol Exp Neurol. 2001;60:147–160. doi: 10.1093/jnen/60.2.147. [DOI] [PubMed] [Google Scholar]
- 68.Leinninger GM, Backus C, Uhler MD, et al. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004;18:1544–1546. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- 69.Middlemas A, Delcroix JD, Sayers NM, et al. Enhanced activation of axonally transported stress-activated protein kinases in peripheral nerve in diabetic neuropathy is prevented by neurotrophin-3. Brain. 2003;126:1671–1682. doi: 10.1093/brain/awg150. [DOI] [PubMed] [Google Scholar]
- 70.Qi R, Yang W, Chen J. Role of enteric glial cells in gastric motility in diabetic rats at different stages. J Huazhong Univ Sci Technolog Med Sci. 2013;33:496–500. doi: 10.1007/s11596-013-1148-1. [DOI] [PubMed] [Google Scholar]
- 71.Stenkamp-Strahm C, Patterson S, Boren J, et al. High-fat diet and age-dependent effects on enteric glial cell populations of mouse small intestine. Auton Neurosci. 2013;177:199–210. doi: 10.1016/j.autneu.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nezami BG, Mwangi SM, Lee JE, et al. MicroRNA 375 Mediates Palmitate-Induced Enteric Neuronal Damage and High-Fat Diet-Induced Delayed Intestinal Transit in Mice. Gastroenterology. doi: 10.1053/j.gastro.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voss U, Sand E, Olde B, et al. Enteric neuropathy can be induced by high fat diet in vivo and palmitic Acid exposure in vitro. PLoS One. 2013;8:e81413. doi: 10.1371/journal.pone.0081413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Honore SM, Zelarayan LC, Genta SB, et al. Neuronal loss and abnormal BMP/Smad signaling in the myenteric plexus of diabetic rats. Auton Neurosci. 2011;164:51–61. doi: 10.1016/j.autneu.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. 2064, e2051–e2052. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferreira PE, Lopes CR, Alves AM, et al. Diabetic neuropathy: an evaluation of the use of quercetin in the cecum of rats. World J Gastroenterol. 2013;19:6416–6426. doi: 10.3748/wjg.v19.i38.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdo H, Derkinderen P, Gomes P, et al. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J. 2010;24:1082–1094. doi: 10.1096/fj.09-139519. [DOI] [PubMed] [Google Scholar]
- 78.Shotton HR, Lincoln J. Diabetes only affects nitric oxide synthase-containing myenteric neurons that do not contain heme oxygenase 2. Brain Res. 2006;1068:248–256. doi: 10.1016/j.brainres.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 79.Golbidi S, Ebadi SA, Laher I. Antioxidants in the treatment of diabetes. Curr Diabetes Rev. 2011;7:106–125. doi: 10.2174/157339911794940729. [DOI] [PubMed] [Google Scholar]
- 80.Surendran S, Matalon R, Tyring SK. Upregulation of aspartoacylase activity in the duodenum of obesity induced diabetes mouse: implications on diabetic neuropathy. Biochem Biophys Res Commun. 2006;345:973–975. doi: 10.1016/j.bbrc.2006.04.179. [DOI] [PubMed] [Google Scholar]
- 81.Savidge TC. S-nitrosothiol signals in the enteric nervous system: lessons learnt from big brother. Front Neurosci. 2011;5:31. doi: 10.3389/fnins.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–658. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanani M, Farrugia G, Komuro T. Intercellular coupling of interstitial cells of cajal in the digestive tract. Int Rev Cytol. 2005;242:249–282. doi: 10.1016/S0074-7696(04)42006-3. [DOI] [PubMed] [Google Scholar]
- 84.Maeda H, Yamagata A, Nishikawa S, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 85.Huizinga JD, Thuneberg L, Kluppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 86.Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480(Pt 1):91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ward SM, Morris G, Reese L, et al. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 88.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–682. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Long QL, Fang DC, Shi HT, et al. Gastro-electric dysrhythm and lack of gastric interstitial cells of cajal. World J Gastroenterol. 2004;10:1227–1230. doi: 10.3748/wjg.v10.i8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 91.Wang XY, Huizinga JD, Diamond J, et al. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095-e1092. doi: 10.1111/j.1365-2982.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 92.He CL, Szurszewski JH, Farrugia G, et al. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–434. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 93.Forster J, Damjanov I, Lin Z, et al. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–108. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–1087. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 95.Nakahara M, Isozaki K, Hirota S, et al. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666–670. doi: 10.1046/j.1440-1746.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto T, Watabe K, Nakahara M, et al. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660–667. doi: 10.1111/j.1440-1746.2008.05326.x. [DOI] [PubMed] [Google Scholar]
- 97.Beckett EA, Takeda Y, Yanase H, et al. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193–206. doi: 10.1002/cne.20746. [DOI] [PubMed] [Google Scholar]
- 98.Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol. 2004;556:521–530. doi: 10.1113/jphysiol.2003.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horvath VJ, Vittal H, Ordog T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528–1533. doi: 10.2337/diabetes.54.5.1528. [DOI] [PubMed] [Google Scholar]
- 100.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 101.Kong MF, Horowitz M. Gastric emptying in diabetes mellitus: relationship to blood-glucose control. Clin Geriatr Med. 1999;15:321–338. [PubMed] [Google Scholar]
- 102.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 103.Brehmer A, Schrodl F, Neuhuber W. Morphology of VIP/nNOS-immunoreactive myenteric neurons in the human gut. Histochem Cell Biol. 2006;125:557–565. doi: 10.1007/s00418-005-0107-8. [DOI] [PubMed] [Google Scholar]
- 104.De Giorgio R, Parodi JE, Brecha NC, et al. Nitric oxide producing neurons in the monkey and human digestive system. J Comp Neurol. 1994;342:619–627. doi: 10.1002/cne.903420409. [DOI] [PubMed] [Google Scholar]
- 105.Jarvinen MK, Wollmann WJ, Powrozek TA, et al. Nitric oxide synthase-containing neurons in the myenteric plexus of the rat gastrointestinal tract: distribution and regional density. Anat Embryol (Berl) 1999;199:99–112. doi: 10.1007/s004290050213. [DOI] [PubMed] [Google Scholar]
- 106.Roman V, Bagyanszki M, Krecsmarik M, et al. Spatial pattern analysis of nitrergic neurons in the developing myenteric plexus of the human fetal intestine. Cytometry A. 2004;57:108–112. doi: 10.1002/cyto.a.10112. [DOI] [PubMed] [Google Scholar]
- 107.Gangula PR, Maner WL, Micci MA, et al. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725–G733. doi: 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takahashi T, Nakamura K, Itoh H, et al. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–1544. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 109.Li C, Liu S, Guan Y, et al. Long pulse gastric electrical stimulation induces regeneration of myenteric plexus synaptic vesicles in diabetic rats. Neurogastroenterol Motil. 2010;22:453–461. e108. doi: 10.1111/j.1365-2982.2009.01420.x. [DOI] [PubMed] [Google Scholar]
- 110.Cellek S. Point of NO return for nitrergic nerves in diabetes: a new insight into diabetic complications. Curr Pharm Des. 2004;10:3683–3695. doi: 10.2174/1381612043382792. [DOI] [PubMed] [Google Scholar]
- 111.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–2362. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 112.Belai A, Lincoln J, Burnstock G. Lack of release of vasoactive intestinal polypeptide and calcitonin gene-related peptide during electrical stimulation of enteric nerves in streptozotocin-diabetic rats. Gastroenterology. 1987;93:1034–1040. doi: 10.1016/0016-5085(87)90567-1. [DOI] [PubMed] [Google Scholar]
- 113.Belai A, Calcutt NA, Carrington AL, et al. Enteric neuropeptides in streptozotocin-diabetic rats; effects of insulin and aldose reductase inhibition. J Auton Nerv Syst. 1996;58:163–169. doi: 10.1016/0165-1838(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 114.Jeyabal PV, Kumar R, Gangula PR, et al. Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol Motil. 2008;20:253–261. doi: 10.1111/j.1365-2982.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 115.Touw K, Chakraborty S, Zhang W, et al. Altered calcium signaling in colonic smooth muscle of type 1 diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G66–G76. doi: 10.1152/ajpgi.00183.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takahashi T, Kojima Y, Tsunoda Y, et al. Impaired intracellular signal transduction in gastric smooth muscle of diabetic BB/W rats. Am J Physiol. 1996;270:G411–G417. doi: 10.1152/ajpgi.1996.270.3.G411. [DOI] [PubMed] [Google Scholar]
- 117.Lin S, Kajimura M, Takeuchi K, et al. Alterations of GTP-binding proteins (Gsalpha and Gq/11alpha) in gastric smooth muscle cells from streptozotocin-induced and WBN/Kob diabetic rats. Dig Dis Sci. 2000;45:1517–1524. doi: 10.1023/a:1005596407496. [DOI] [PubMed] [Google Scholar]
- 118.Moscoso GJ, Driver M, Guy RJ. A form of necrobiosis and atrophy of smooth muscle in diabetic gastric autonomic neuropathy. Pathol Res Pract. 1986;181:188–194. doi: 10.1016/S0344-0338(86)80009-7. [DOI] [PubMed] [Google Scholar]
- 119.He WQ, Peng YJ, Zhang WC, et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008;135:610–620. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu W, Feng P. Myosin light chain kinase is involved in the mechanism of gastrointestinal dysfunction in diabetic rats. Dig Dis Sci. 2012;57:1197–1202. doi: 10.1007/s10620-012-2041-7. [DOI] [PubMed] [Google Scholar]
- 121.Duchen LW, Anjorin A, Watkins PJ, et al. Pathology of autonomic neuropathy in diabetes mellitus. Ann Intern Med. 1980;92:301–303. doi: 10.7326/0003-4819-92-2-301. [DOI] [PubMed] [Google Scholar]
- 122.Jackson MW, Gordon TP, Waterman SA. Disruption of intestinal motility by a calcium channel-stimulating autoantibody in type 1 diabetes. Gastroenterology. 2004;126:819–828. doi: 10.1053/j.gastro.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 123.Bodi N, Talapka P, Poles MZ, et al. Gut region-specific diabetic damage to the capillary endothelium adjacent to the myenteric plexus. Microcirculation. 2012;19:316–326. doi: 10.1111/j.1549-8719.2012.00164.x. [DOI] [PubMed] [Google Scholar]
- 124.Yoshiya K, Lapchak PH, Thai TH, et al. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1020–G1030. doi: 10.1152/ajpgi.00239.2011. [DOI] [PubMed] [Google Scholar]
- 125.Bagyanszki M, Bodi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes. 2012;3:80–93. doi: 10.4239/wjd.v3.i5.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barbara G, Stanghellini V, Brandi G, et al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 128.Dass NB, John AK, Bassil AK, et al. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19:66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 129.Malbert CH. The ileocolonic sphincter. Neurogastroenterol Motil. 2005;17(Suppl 1):41–49. doi: 10.1111/j.1365-2982.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 130.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 131.Park C, Yan W, Ward SM, et al. MicroRNAs dynamically remodel gastrointestinal smooth muscle cells. PLoS One. 2011;6:e18628. doi: 10.1371/journal.pone.0018628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanders KM, Koh SD, Ro S, et al. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–645. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gonzalez CD, Lee M-S, Marchetti P, et al. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy. 2011;7:2–11. doi: 10.4161/auto.7.1.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Towns R, Kabeya Y, Yoshimori T, et al. Sera from patients with type 2 diabetes and neuropathy induce autophagy and colocalization with mitochondria in SY5Y cells. Autophagy. 2005;1:163–170. doi: 10.4161/auto.1.3.2068. [DOI] [PubMed] [Google Scholar]
- 136.Callaghan BC, Cheng HT, Stables CL, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi X, Chen Y, Nadeem L, et al. Beneficial effect of TNF-alpha inhibition on diabetic peripheral neuropathy. J Neuroinflammation. 2013;10:69. doi: 10.1186/1742-2094-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 139.O'Brien PD, Hinder LM, Sakowski SA, et al. ER stress in diabetic peripheral neuropathy: A new therapeutic target. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5807. [DOI] [PubMed] [Google Scholar]
- 140.Kashyap P, Farrugia G. Oxidative stress: key player in gastrointestinal complications of diabetes. Neurogastroenterol Motil. 2011;23:111–114. doi: 10.1111/j.1365-2982.2010.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]