Abstract
Greater amounts of physical activity (PA) and omega-3 fatty acids have both been independently associated with better cognitive performance. Because of the overlapping biological effects of omega-3 fatty acids and PA, fatty acid intake may modify the effects of PA on neurocognitive function. The present study tested this hypothesis by examining whether the ratio of serum omega-6 to omega-3 fatty acid levels would moderate the association between PA and executive and memory functions in 344 participants (Mean age = 44.42 years, SD = 6.72). The Paffenbarger Physical Activity Questionnaire (PPAQ), serum fatty acid levels, and performance on a standard neuropsychological battery were acquired on all subjects. A principal component analysis reduced the number of cognitive outcomes to three factors: n-back working memory, Trail Making test, and Logical Memory. We found a significant interaction between PA and the ratio of omega-6 to omega-3 fatty acid serum levels on Trail Making performance and n-back performance, such that higher amounts of omega-3 levels offset the deleterious effects of lower amounts of PA. These effects remained significant in a subsample (n=299) controlling for overall dietary fat consumption. There were no significant additive or multiplicative benefits of higher amounts of both omega-3 and PA on cognitive performance. Our results demonstrate that a diet high in omega-3 fatty acids might mitigate the effect of lower levels of PA on cognitive performance. This study illuminates the importance of understanding dietary and PA factors in tandem when exploring their effects on neurocognitive health.
Keywords: omega-3, physical activity, working memory, task-switching, logical memory, DHA
1. Introduction
Several modifiable behaviors influence cognitive performance throughout the lifespan. For example, physical activity (PA) is a modifiable behavior that influences brain and cognitive health. In children and adolescents, greater engagement in PA is associated with elevated cognitive performance and higher academic achievement scores [1, 2]. During mid-life, PA is associated with improved cognitive performance on tasks of memory, processing speed, and executive function [3, 4]. Furthermore, prospective and retrospective epidemiological studies suggest that PA during mid-life is predictive of cognitive outcomes in old age, and that increasing PA earlier in life may prevent or delay future cognitive impairment [5, 6]. Yet, beginning a physically active lifestyle in late adulthood is not futile; even modest amounts of PA in late life is sufficient for improving cognitive performance [7]. Improvements in cognitive function translate to PA-induced changes in brain morphology and function. For example, randomized controlled trials of PA suggest that it increases hippocampal [8] and prefrontal cortex [9] volume, as well as functional connectivity of hippocampal and prefrontal regions [10], and increases task-evoked brain activity [11, 12]. Cross-sectional studies of PA and physical fitness find similar patterns, suggesting a consistent effect on cognitive health in multiple populations and with multiple experimental designs.
In addition to the favorable effects of PA on cognitive function, other modifiable lifestyle factors may also contribute to cognitive function throughout the lifespan. For example, greater intake of long-chain, omega-3 polyunsaturated fatty acids (PUFA) was associated with better working memory, processing speed and cognitive flexibility in a sample of middle-aged adults [13]. In particular, higher exposure to docosahexaenoic acid (DHA), an omega-3 PUFA that is highly concentrated in the brain, has been associated with better performance on measures of executive function [13, 14]. In line with this evidence, neuroimaging studies have reported that greater levels of omega-3 PUFAs are related to fewer white matter hyperintensities and greater corticolimbic gray matter volume [15, 16]. Nonetheless, the effects of the omega-3 PUFA DHA on neurocognitive function appear to be less conclusive than the effects of PA.
In fact, several studies report little benefit of omega-3 intake on cognitive function [17–19], and initial randomized trials directly testing the effects of raised omega-3 intake have yielded only limited evidence of improved cognitive performance [20–28]., Some studies [22, 24, 29, 30] suggest that associations between omega-3 and cognitive performance may be domain specific with some cognitive functions (i.e., executive functions) being more sensitive to omega-3 than others. However, other studies have been more equivocal with respect to the cognitive domains affected in younger and mid-life adults. For example, a six-month DHA supplementation in healthy adults aged 18–45 years found improvements in response time for working memory tasks in men, but episodic memory tasks in women [23]. Furthermore, a 12-week DHA supplementation with healthy adults aged 18–35 years reported no significant improvements on any of the 15 neuropsychological tests administered [31]. It is possible that some of this heterogeneity may be explained by interactions between PUFAs and other lifestyle variables, such as PA.
On the molecular level, omega-3 and PA share some similar effects. For example, in rodents DHA supplementation rescues the effect of a DHA deficient diet on D2 receptors in the striatum [32] and loss of dopaminergic cells in the substantia nigra in models of Parkinson's Disease [33]. Likewise, rodent models demonstrate that PA affects dopaminergic function in reward pathways [34–36] and rescues dopamine depletion in hemi-parkinsonian models [34, 37]. Human studies of Parkinson's disease also show increased dopamine production and release [38] and cognitive and motor improvements with increased PA [35]. In addition to dopamine, both DHA supplementation and PA influence the expression of brain-derived neurotrophic factor (BDNF), which promotes synaptic plasticity, cell proliferation and cell survival in humans [8] and rodents [39]. Furthermore, both DHA [40–43] and PA [18, 44] have been associated with reduced β-amyloid (Aβ) plaque deposits, a putative cause of cognitive impairment and Alzheimer's Disease (AD). Finally, both PA and DHA may regulate the expression of inflammatory cytokines [45–47]; higher levels of which have been closely linked to a reduction in gray matter volume [48, 49] and impaired executive function [50] and memory [51] in humans.
Relative to the role of PUFA's in the inflammatory response, there is debate over the most appropriate method of quantifying arachidonic acid (AA; pro-inflammatory) and DHA (anti-inflammatory) levels in humans [52]. Since these n-6 and n-3 PUFAs are precursors of relatively pro- and anti-inflammatory eicosanoids, respectively [53], the n-6:n-3 ratio has been recommended as an index of DHA effectiveness. High ratios, reflecting a high proportion of AA to DHA, are associated with diminished physical health outcomes and increased incidence of inflammatory diseases [54]. Conversely, a low ratio has been associated with better cardiovascular and cognitive health [14, 19].
Because of the shared neurobiological and physiological effects of PA and DHA intake, several human and animal studies have speculated about the additive or multiplicative benefits that might arise from combining omega-3 supplementation with PA [55]. For example, PA may provide an avenue by which the effects of DHA on cellular integrity and cognitive function are enhanced [39, 55]. In rodents, the combination of PA and DHA supplementation (1.25% increase of DHA in standard rat chow) have additive effects on synaptic plasticity and membrane structure biomarkers in the dentate gyrus of the hippocampus, such that mice receiving both DHA supplementation and PA have greater levels of synaptic proteins than their counterparts not receiving PA [56]. However, these effects were not mirrored behaviorally. Instead, physical inactivity without DHA supplementation resulted in impaired learning compared to mice with DHA supplementation, PA, or both [56]. Studies in humans have not yet examined whether DHA levels moderate the effect of PA on cognitive performance in a similar way to that demonstrated in rodents.
The present study examined whether DHA omega-3 fatty acid levels moderate the effect of PA on executive function and working memory in humans. We expected effects to be specific to executive function and working memory domains because these areas have been shown to be sensitive to both PA [57] and omega-3 supplementation [13, 58] in prior studies. We reasoned that DHA omega-3 levels might moderate effects of PA in several ways. First, greater amounts of DHA might potentiate the effects of higher levels of PA on cognitive function. Such a finding would suggest that combining a diet high in omega-3 with a physically active lifestyle might prove more beneficial to cognitive function than either treatment by itself. However, an alternative outcome is that a deficiency in both PA and omega-3 PUFAs would result in reduced cognitive function. Such a finding might suggest that greater amounts of either omega-3 or PA could be sufficient for elevating cognitive function and that greater amounts of omega-3 could mitigate the deleterious effects of low amounts of PA.
2. Methods
2.1 Participants
Participants were middle-aged adults (30–54 years of age) recruited through the University of Pittsburgh Adult Health and Behavior (AHAB) project [58]. A total of 1379 participants were recruited via mass mail solicitation from communities of southwest Pennsylvania. Exclusion criteria for the AHAB project included a reported history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer treatment in the preceding year, major neurological disorders, schizophrenia or other psychotic illness, and current pregnancy or perimenopausal menstrual irregularities. Participants were required to speak English as their primary language for at least the past 5 years. From the AHAB registry cohort of 1379 individuals, 1295 were included in the AHAB study, where subsets of participants could elect to participate in one or more additional and smaller sub-studies that included, among other measures, blood samples for fatty acid analysis and dietary interviews if they met the following additional criteria: a) resting BP was < 180/110 mm Hg; b) body mass index (weight/height2) was < 40 kg/m2; c) mean daily alcohol consumption was ≤ 21 drinks per week (ethanol < 273 g/ wk); d) not currently taking any antihypertensive, diabetic, lipid-lowering, antiarrhythmic, glucocorticoid, weight-loss, or psychotropic medications. A total of 383 participants met these additional criteria and agreed to participate. From this sample we excluded 25 because of various technical difficulties in fatty acid assays, 4 from incomplete cognitive data, 5 because of an IQ<80, and 5 because they reported taking omega-3 supplements at the time of blood draw. Our final sample size for this study after these exclusions was 344. The study protocol was approved by the Institutional Review Board of the University of Pittsburgh (IRB numbers 0805006, 000535), and informed consent was obtained from all participants in accordance with the University IRB guidelines.
2.2 Measures
Participants completed multiple questionnaires assessing psychosocial, lifestyle and demographic information in addition to completing a physical exam.
2.2.1 Plasma Fatty Acid Serum Acquisition
A morning blood sample was obtained after an overnight fast. Following centrifugation, serum samples were stored at 28°C until analysis. Serum phospholipid fatty acid composition was determined by capillary GC as described elsewhere [59]. DHA and AA levels were expressed as percentages of the total fatty acid pool (weight or mol %). Intra- and interassay coefficients of variation were 2.0–9.2% and 1.9–9.6%, respectively, for all major serum fatty acids and polyunsaturated fatty acids [58]. Our primary analyses were conducted using the AA:DHA ratio since it has been the recommended approach in other studies [23, 60, 61], however for exploration in additional analyses we examined the predictive value of DHA and AA individually.
2.2.2 Cognitive Measures
A comprehensive battery of cognitive tests was administered to the participants in AHAB including the Wechsler Memory Scale, 3rd edition (WMS-III). From this cognitive battery we selected Trails A and B, spatial and letter n-back tasks, and logical memory from the WMS as tasks of interest (see below) because of a priori hypotheses that these cognitive tasks, and the domains that they measure, would be sensitive to both PA and DHA [62], and therefore be likely to show a DHA x PA interaction.
N-back task
This task was comprised of two parts, the letter n-back and the spatial n-back. In the letter n-back, participants viewed a series of letters presented sequentially for 500 ms each with an intertrial interval of 2000 ms. In the 1-back condition participants were asked to press a button if the letter currently displayed on the screen matched the letter previously displayed. If the letter did not match the previously presented letter, they were instructed to press a different button. In the 3-back condition participants were asked to determine if the letter currently displayed on the screen matched the letter that was displayed 3 letters prior. There were 56 trials presented (50% match, 50% non-match) for both 1-back and 3-back tasks. The outcome variable of interest was the number of correct responses for each task.
The spatial n-back task was similar to the letter n-back task except that spatial locations, rather than letters, were to be remembered. The participants viewed dots, presented sequentially, on the computer screen for 500 ms each with an intertrial interval of 2000 ms. Participants were instructed to respond when the dot appeared in the same location previously displayed (1-back) or in the same location as two trials before (2-back). Again, there were 56 trials per condition (50% match, 50% non-match). The number of correct responses was recorded and used as the outcome of interest.
The Trail Making test
This test measures processing speed (Trails A) and executive function, or task-switching (Trails B). In Trails A, participants were instructed to connect numbers 1–26 in numerical order as quickly as possible without lifting their pencil from the page. In Trails B, participants were instructed to alternate between connecting numbers and letters. Specifically, they were instructed to connect 1 to A, then A to 2, then 2 to B etc., without removing their pencil from the page. The time for completion was recorded and used as the primary outcome variable for each task. An additional difference measure of switching cost was calculated by subtracting Trails A time from Trails B time.
Logical Memory
This test is part of the WMS-III, designed to assess episodic memory. Participants were read a one-paragraph story and immediately after administration were asked to verbally recall any information from the story. In delayed recall, participants were asked to verbally recall information from the story 25–35 minutes after administration. The number of correctly recalled items was recorded. Participants were also given a recognition test (yes-no questions) after completion of the recall components. This widely used measure from the WMS battery is often used in diagnosis of memory problems and cognitive impairment [63, 64]. Further, both DHA and PA have been associated with performance on this task in prior studies [58].
2.2.3 Physical Activity Assessment
PA levels were assessed using the Paffenbarger Physical Activity Questionnaire (PPAQ). This widely used instrument was designed to assess daily and weekly activity from self-reported levels of activities of daily living (e.g., stairs climbed) and leisure activities requiring physical exertion (e.g., sports). This instrument has high reliability [65] and convergent validity with several objective measures of PA and fitness, including maximal oxygen uptake [66], dual-energy X-ray absorptiometry [67], and body mass index [68]. The Paffenbarger questionnaire is predictive of health conditions that are related to PA, including myocardial infarction [69], total cholesterol and fasting blood glucose [68], bone density [67], and inflammatory biomarkers [70]. Weekly kilocalories were calculated from participants' responses using criteria set by Paffenbarger [71].
2.2.4 Diet Recall
Two, unannounced 24-h diet recall interviews were conducted with each participant by telephone. The interviews used the Nutrition Data System for Research, a Windows-based dietary analysis program designed for the collection and analyses of 24-h dietary recalls (Nutrition Coordination Center at the University of Minnesota, http://www.ncc.umn.edu/)[72]. Consumption of fiber, folate, sodium, and saturated fat were expressed per 2000 kcal and used as indicators of diet quality. Dietary information such as calories consumed per day, calories from fat, and calories from saturated fats were calculated. This information was collected on a subset of the participants (n=299) and was used in secondary regression analyses with calories from saturated fat and total fat as covariates in the model (see Statistical Analyses section below).
2.3 Statistical Analyses
All variables were examined for normality, of which only kilocalories from the PPAQ was significantly skewed. This measure was then normalized by a log transformation prior to analysis. In an effort to identify significant covariates, bivariate Pearson correlations were conducted between demographic variables and the independent and dependent variables of interest. While each cognitive task was selected based on its ability to measure a domain of cognition known to be sensitive to PA and DHA, each task yielded multiple measures. As a result, there were a total of eight cognitive variables of interest. To reduce the number of dependent variables and account for multiple comparisons, we first subjected the eight cognitive measures to an exploratory factor analysis with varimax rotation. This resulted in three factors with eigenvalues >1 and corroborated by Scree test. The first factor (N-back) included the number of correct responses in the Spatial and Letter N-Back tasks and accounted for 30.19% of total variance. The second factor (Trails) included Trails B time and Trails B-A cost and accounted for 24.10% of total variance. The third factor (Logical Memory) included Logical Memory Recall and Recognition accuracy and accounted for 21.13% of total variance (Table 1). The resulting three factors were then used as dependent variables, each accounting for one domain of cognition of interest.
Table 1.
Factor Analysis
| N-Back Factor | Trail Making Factor | Logical Memory Factor | |
|---|---|---|---|
| Memory Recall | 0.934 | ||
| Memory Recognition | 0.952 | ||
|
| |||
| Trails B Time | 0.929 | ||
| Trails B – A Time | 0.946 | ||
|
| |||
| Letter 1-Back | 0.804 | ||
| Letter 3-Back | 0.675 | ||
| Spatial 1-Back | 0.830 | ||
| Spatial 2-Back | 0.708 | ||
Factor Analysis conducted using all eight cognitive outcome variables. Analyses were performed with varimax rotation where eigenvalues >1 and a threshold of 0.4 was used to determine the three factors: n-back (30.42% of total variance), Trail Making (24.23% of total variance), Logical Memory (23.75% of total variance).
Multiple regression was employed to test associations between PA and omega-3 levels on each of the three cognitive factors with age, sex, race, and years of education entered as covariates (see Results). A PA x omega-3 interaction term was modeled to examine whether AA:DHA ratios moderate the effects of PA on cognition. Main effects of both PA and AA:DHA were entered after the covariates, followed by the interaction term in the third model. A significant interaction (p<.05) between PA and AA:DHA was explored graphically and secondary analyses were conducted on the individual cognitive tests composing a factor that reached significance.
In a secondary analysis using a subsample (n=299 – see Table 2 for demographic information) with self-reported diet recall data, we examined whether the DHA x PA interactions remained significant after controlling for general dietary factors including total daily calories from fat and calories from saturated fat. These diet measures were used as covariates to control for the possibility that an overall poorer diet could be influencing or exacerbating results.
Table 2.
Demographics
| n-3 Sample (N=344) | Diet Sample (N=299) | Full AHAB Sample (N=1295) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 44.37 | 6.72 | 44.53 | 6.69 | 44.63 | 6.74 |
| Sex (% male) | 46.8% | 48.8% | 47.3% | |||
| Race (% Caucasian) | 88.4% | 89.0% | 83.5% | |||
| Years of Schooling | 15.97 | 2.65 | 16.01 | 2.71 | 15.71 | 2.84 |
| Smoking (% Smokers) | 12.2% | 18.7% | 16.4% | |||
| Ann. Household Income (mean range / % in range) | $35,000 – $49,999 | 20.3% | $35,000 – $49,999 | 18.6% | $35,000 – $49,999 | 15.3% |
| Drinks / Week | 2.92 | 4.23 | 2.93 | 4.30 | 3.80 | 7.50 |
| IQ | 115.78 | 10.35 | 115.80 | 10.29 | 116.09 | 12.79 |
| Kilocalories | 2447.91 | 1727.26 | 2408.07 | 1694.53 | 2416.47 | 1839.74 |
| AA | 8.76 | 1.94 | 8.50 | 1.82 | ||
| DHA | 1.74 | 0.86 | 1.62 | 0.74 | ||
| EPA | 0.50 | 0.36 | .050 | 0.38 | ||
| AA:DHA | 5.99 | 2.42 | 6.13 | 2.42 | ||
| Calories from Fat | 33.86 | 7.93 | ||||
| Calories from Saturated | 11.60 | 3.36 | ||||
| Fat | ||||||
|
| ||||||
| N-Back Factor | ||||||
|
| ||||||
| Letter 1-Back | 51.89 | 4.21 | 51.95 | 4.06 | 51.15 | 5.04 |
| Letter 3-Back | 38.68 | 6.53 | 38.51 | 6.60 | 37.93 | 6.96 |
| Spatial 1-Back | 50.09 | 5.08 | 50.09 | 4.95 | 49.26 | 6.10 |
| Spatial 2-Back | 41.50 | 6.62 | 41.43 | 6.63 | 40.70 | 7.47 |
|
| ||||||
| Trail Making Factor | ||||||
|
| ||||||
| Trail B Time | 52.72 | 18.29 | 53.45 | 18.85 | 55.67 | 22.14 |
| Trail B – A Time | 28.01 | 16.43 | 28.42 | 17.09 | 30.65 | 19.56 |
|
| ||||||
| Logical Memory Factor | ||||||
|
| ||||||
| Memory Recall | 29.22 | 8.15 | 29.09 | 8.36 | 28.37 | 8.05 |
| Memory Recognition | 26.97 | 2.44 | 26.92 | 2.48 | 26.67 | 2.52 |
Population Demographics: demographic information as well as descriptive statistics of cognitive performance for the full sample of participants
3. Results
3.1 Demographic analyses
We found no significant association between AA:DHA and sex or PA. More years of education was associated with lower AA:DHA ratios (r = −0.166, p = 0.002) and greater amounts of PA (r = 0.136, p = 0.012). PA was also related to race such that whites displayed higher amounts of PA than non-whites (t (342) = 3.482, p = 0.001). Age was not correlated with either AA:DHA or PA (both p>.05). No other associations reached significance (p > 0.05) (see Table 3).
Table 3.
Bivariate Associations Among Predictor Variables
| Kilocalories | AA:DHA ratio | |
|---|---|---|
| Age (r) | −0.040 | −0.038 |
| Sex (t) | 0.354 | 2.153* |
| Race (t) | 3.482 ** | −0.912 |
| Years of Schooling (r) | 0.136 * | −0.166 * |
Relationships between demographic information and independent variables: Values reported for age and years of schooling are results of Pearson's correlations; values reported for sex and race are results of independent samples t-tests.
p < 0.05
p < 0.001
3.2 Main effects of PA and AA:DHA on cognitive performance
Consistent with anticipated results, there was a significant association between PA and the N-Back factor (β = 0.178, t = 2.543, p = 0.01) such that greater amounts of PA were associated with better performance after controlling for age, sex, race, and years of education. In secondary analyses of the tasks composing the N-back factor, we found a significant main effect of PA on accuracy in the Letter 3-Back (β = 0.149, p = .007), Spatial 1-Back (β = 0.163, p = 0.003), and Spatial 2-back (β = 0.116, p = 0.032) tasks such that greater amounts of PA were associated with better performance. There were no significant associations between PA and the Trails factor (β = −0.093, t = −1.687, p = 0.092) or Logical Memory factor (β = 0.055, t = 1.103, p = 0.305).
Inconsistent with our predictions, there were no significant associations between serum AA:DHA ratio levels and any of the three factors examined: N-Back (β = 0.001, t = 0.018, p = 0.986), Trails (β = 0.076, t = 1.385, p = 0.167), or Logical Memory (β = −0.028, t= -0.531, p = 0.596) after controlling for variance associated with age, sex, race, and years of education. Similarly, no associations were found between cognitive performance and each PUFA individually. That is, there were no significant main effects of AA on any factor (all p>.30), nor was there a significant main effect of DHA for the N-back factor (β = 0.015, p = 0.776), Trail Making factor (β = −0.093, p = 0.089), or the Logical Memory factor (β = 0.021, p = 0.697).
3.3 AA:DHA moderates effects of PA on cognitive performance
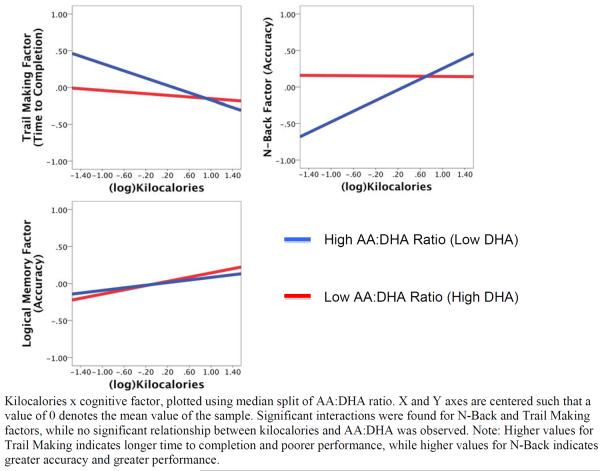
In support of our hypothesis, there was a significant interaction between serum AA:DHA ratio and PA on the N-back factor (β = 1.222, t = 2.145, p = 0.033) and the Trails factor (β= −1.478, t = −2.577, p = 0.010). In contrast, there was no significant interaction between PA and AA:DHA for the Logical Memory factor (β = 0.200, t = 0.354, p = 0.724). Decomposing these significant interactions revealed that individuals with higher AA:DHA ratios and lower amounts of PA performed more poorly than their peers with lower AA:DHA ratios or higher amounts of PA (see Figure 1).
Figure 1.
AA:DHA Ratio x Kilocalories Interaction with Cognitive Function
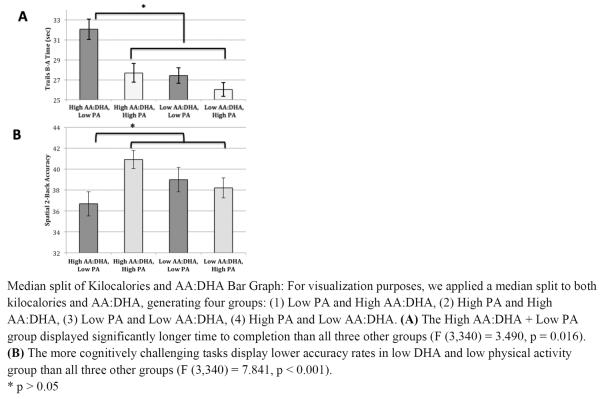
In secondary analyses, we found that the more challenging working memory conditions, the Letter 3-Back (β = 1.562, t = 2.720, p = 0.007) and Spatial 2-Back tasks (β = 1.503, t = 2.685, p = 0.008), were driving the N-back factor results such that lower AA:DHA ratios offset the association between lower PA and poorer performance. However, the combination of higher AA:DHA ratios (higher proportion of AA to DHA) with lower PA was associated with markedly decreased performance. In contrast, the interaction was not significant for the easier working memory conditions, the Letter 1-back (β = 1.011, t = 1.762, p = 0.079) and Spatial 1-back (β = 0.760, t = 1.330, p = 0.184) (see Figure 2B).
Figure 2.
Median split of Kilocalories and AA:DHA Bar Graph
Similarly, when examining the Trail Making Factor in secondary analyses, we found that both Trails B (β = −1.615, t = −2.889, p = 0.004) and Trails B-A (β = −1.596, t = −2.808, p = 0.005), displayed the same moderating relationship as the N-back (Figure 2A) such that lower AA:DHA ratios offset the association between lower PA and poorer performance.
In analyses of individual fatty acids, we found that AA did not moderate the effect of PA on the N-Back factor (β = −0.205, t = −0.353, p = 0.725), Trail Making factor (β = −0.579, t = − 0.963, p = 0.336), or Logical Memory factor (β = −0.027, t = −0.047, p = 0.963). In contrast, DHA significantly interacted with PA on the N-Back factor (β = −1.390, t = −2.467, p = 0.014). Decomposing this effect revealed significant interactions between DHA and PA for the Letter 3-Back (β = −1.169, t = −2.044, p = 0.042), Spatial 1-Back (β = −1.317, t = −2.336, p = 0.020), and Spatial 2-Back (β = −1.417, t = −2.554, p = 0.011) tasks. Similar to the factor scores, decomposing these interactions revealed that higher DHA levels offset the association between lower PA and poorer performance. There were no significant interactions between DHA and PA with the Trail Making factor (β = 0.795, t = 1.391, p = 0.165) or the Logical Memory Factor (β = 0.100, t = 0.178, p = 0.859) – see Table 4.
Table 4.
Omega-3 x Physical Activity Regression Results
| AA (N=344) | DHA (N=344) | |||||
|---|---|---|---|---|---|---|
| β | t | P | β | t | P | |
| N-Back Factor | −0.205 | −0.353 | 0.725 | −1.390 * | −2.467 | 0.014 |
| Letter 1-Back | 0.270 | 0.462 | 0.644 | −0.811 | −1.424 | 0.156 |
| Letter 3-Back | 0.241 | 0.409 | 0.683 | −1.169* | −2.044 | 0.042 |
| Spatial 1-Back | −0.706 | −1.218 | 0.224 | −1.317* | −2.336 | 0.020 |
| Spatial 2-Back | −0.015 | −0.026 | 0.979 | −1.417* | −2.554 | 0.011 |
|
| ||||||
| Trail Making Factor | −0.579 | −0.963 | 0.336 | 0.795 | 1.391 | 0.165 |
| Trails B Time | −0.517 | −0.899 | 0.369 | 0.986 | 1.769 | 0.078 |
| Trails B – A Time | −0.434 | −0.742 | 0.458 | 0.975 | 1.720 | 0.086 |
|
| ||||||
| Logical Memory Factor | −0.027 | −0.047 | 0.963 | 0.100 | 0.178 | 0.859 |
| Memory Recall | 0.064 | 0.114 | 0.909 | −0.269 | −0.491 | 0.624 |
| Memory Recognition | −0.289 | −0.503 | 0.615 | 0.821 | 1.464 | 0.144 |
Omega-3 x Physical Activity Regression Results: Moderation analyses included age, sex, education, and race as covariates. Analyses were conducted for AA and DHA separately, rather than AA:DHA.
p > 0.05
After adding calories from fat and calories from saturated fat into the regression model as covariates for a subset of the sample (n= 299), the results were unchanged (see Table 5 for regression results). Here too, the secondary analyses revealed that lower AA:DHA ratios offset the association between less PA and worse performance on the Trails and N-back tasks.
Table 5.
AA:DHA x Physical Activity Regression Results
| Full Sample (N=344) | Diet Sub-Sample (N=299) | |||||
|---|---|---|---|---|---|---|
| β | t | P | β | t | P | |
| N-Back Factor | 1.222 * | 2.145 | 0.033 | 0.140 * | 2.440 | 0.015 |
| Letter 1-Back | 1.011 | 1.762 | 0.079 | 0.130* | 2.248 | 0.025 |
| Letter 3-Back | 1.562** | 2.720 | 0.007 | 0.153** | 2.642 | 0.009 |
| Spatial 1-Back | 0.760 | 1.330 | 0.184 | 0.085 | 1.466 | 0.144 |
| Spatial 2-Back | 1.503** | 2.685 | 0.008 | 0.138* | 2.426 | 0.016 |
|
| ||||||
| Trail Making Factor | −1.478 ** | −2.577 | 0.010 | −0.141 * | −2.419 | 0.016 |
| Trails B Time | −1.615** | −2.889 | 0.004 | −0.159** | −2.808 | 0.005 |
| Trails B – A Time | −1.596** | −2.808 | 0.005 | −0.147* | −2.591 | 0.011 |
|
| ||||||
| Logical Memory Factor | 0.200 | 0.354 | 0.724 | -0.052 | −0.917 | 0.360 |
| Memory Recall | 0.516 | 0.935 | 0.350 | 0.058 | 1.024 | 0.307 |
| Memory Recognition | −0.810 | −1.432 | 0.153 | −0.075 | −1.310 | 0.191 |
Omega-3 x Physical Activity Regression Results: Moderation analyses included age, sex, education, and race as covariates. Total calories from saturated fat from diet and total calories from fat from diet were included in the moderation analyses as covariates in the sub-sample with available dietary data.
p > 0.05
p > 0.01
4. Discussion
We predicted that omega-3 PUFAs, as quantified by serum AA:DHA ratio levels, would moderate an association between PA and cognitive performance. The first hypothesis, based on animal research [56], was that a lower ratio of AA:DHA would magnify the positive association between higher amounts of PA and cognitive performance. We failed to find support for this hypothesis. That is, there was no evidence for an additive or multiplicative benefit of greater amounts of PA in combination with lower AA:DHA ratios on cognitive performance. Our second hypothesis, based on behavioral evidence from rodent studies [56], was that a high AA:DHA ratio would exacerbate the detrimental effects of physical inactivity on cognitive performance. Our results were consistent with this hypothesis, such that physical inactivity in combination with a high omega-6:omega-3 ratio was associated with impaired cognitive performance relative to individuals engaging in higher amounts of PA or with a low AA:DHA ratio. In summary, our results suggest that the undesirable effects of a physically inactive lifestyle on cognitive performance might be partially mitigated by greater intake of omega-3 fatty acids.
As previously discussed, PA increases cell proliferation and synaptogenesis in the hippocampus [73], increases expression of BDNF [74, 75], alters dopaminergic circuitry in the cortex [37, 76–78] and influences the expression and re-uptake of serotonin [36, 79, 80]. However, PA may also influence inflammatory pathways or influence brain function by improving vascular pathways. Importantly, DHA has also been found to influence BDNF, dopamine, serotonin, vascular reactivity, and anti-inflammatory pathways [17, 81, 82]. While it is not clear which molecular system(s) is contributing to the moderating effect of omega-3 on the association between PA and cognitive function, the parallels between the molecular pathways are hard to ignore.
DHA is critical for maintaining cell membrane structure, fluidity and ion permeability [39, 83] and is only synthesized in small amounts in humans, requiring it to be consumed to maintain recommended levels. Several studies have suggested that DHA might play an important role in cognitive function [58, 81, 83–85] because it is a primary fatty acid in the structure of the neuron membrane [84], influences inflammation, and is thought to be involved in cardiovascular and metabolic processes, all of which could influence cognitive performance [86]. Cross-sectional studies have reported results consistent with this reasoning, yet several interventions of omega-3 supplementation have failed to find strong evidence in favor of omega-3 intake as a treatment or prevention for cognitive decline (e.g., Alzheimer's disease)[87, 88]. In response to the null findings reported from randomized trials, there has been speculation that omega-3 interventions beginning earlier in life, administered for longer durations, at higher doses, or more careful screening of habitual dietary patterns, might provide a more revealing pattern. Our results complement this line of reasoning and go further to suggest that omega-3 levels should be assessed carefully alongside other modifiable factors (e.g., PA) when examining cognitive performance. Indeed, interactions between omega-3 and PA could explain some of the heterogeneous results linking DHA or DHA supplementation to cognitive performance.
Importantly, our results remained significant even when controlling for potentially confounding factors including age, sex, race, and years of education. Further, when controlling for fat consumption in a subset of the sample, the interaction between PA and AA:DHA remained significant. In addition, AA did not by itself moderate the association between PA and any of the cognitive factors or their components, while DHA by itself was a significant moderator of PA, although only for the N-Back factor. These results suggest that DHA was the primary component in the AA:DHA interaction with PA on the n-back task. It is intriguing to speculate why DHA by itself did not significantly interact with PA for the Trails factor while the AA:DHA did. In line with other studies, this effect suggests that the competition between AA and DHA, captured by the ratio, but not by the individual PUFAs, is critical when assessing its relation to cognitive performance. Moreover, this result suggests that it is primarily the AA:DHA ratio that is interacting with PA to influence executive function as measured by the Trails test.
There were several limitations to this study. First, given the cross-sectional design, we cannot draw causal conclusions about PA and omega-3 with respect to cognitive function. Hence, these analyses cannot ascertain whether poorer cognitive function leads to lower intake of DHA or less PA or whether lower DHA and PA lead to poorer cognitive function. To make causal conclusions, an intervention would need to be conducted in which supplementation of omega-3 and initiation of PA were randomized and systematically administered. Second, the Paffenbarger PA questionnaire is self-report, prone to subject bias [89] and may not reflect PA accurately. Yet, despite this limitation we were able to detect significant main effects of PA on n-back performance. Nonetheless, future studies could utilize actigraphy and other objective measures of PA and fitness to obtain more accurate assessments. Third, PUFA serum levels only reflect measures at the time of blood sampling. It is possible that the amount of PUFAs in brain tissue is not reflected by serum levels and instead may accumulate over time.
In summary, the present study revealed that AA:DHA levels moderate the association between PA and working memory and task-switching, such that high levels of DHA relative to AA mitigated the effects of lower levels of PA on performance. We failed to find additive effects of high levels of PA and high levels of DHA relative to AA on cognitive function. This study provides compelling initial evidence that dietary factors influence the association between PA and cognitive performance. Additionally, the results from this study have the potential for significant public health implications on recommended dietary and PA regimens.
Highlights.
Physical activity predicted cognition in healthy middle-aged adults
Serum omega-3 levels predicted cognition in healthy middle-aged adults
Working memory and task-switching are sensitive to physical activity levels
Working memory and task-switching are sensitive to serum DHA levels
Effects of physical activity on cognition are moderated by DHA:AA ratio
Acknowledgements
The authors would like to acknowledge Angus McDonald for his contribution to the development of the N-Back task. Additional thanks extend to the AHAB group for data collection and organization. This project was supported by US NIH grants PO1 40962 and T32 HL007560. KIE was supported by NIH grant R01 DK09172.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hillman CH, et al. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159(3):1044–54. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castelli DM, et al. Physical fitness and academic achievement in third- and fifth-grade students. J Sport Exerc Psychol. 2007;29(2):239–52. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- 3.Singh-Manoux A, et al. Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. Am J Public Health. 2005;95(12):2252–8. doi: 10.2105/AJPH.2004.055574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etnier JL, et al. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52(1):119–30. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Rovio S, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4(11):705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 6.Middleton LE, et al. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc. 2010;58(7):1322–6. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 8.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colcombe SJ, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 10.Voss MW, et al. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48(5):1394–406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colcombe SJ, et al. Neurocognitive aging and cardiovascular fitness: recent findings and future directions. J Mol Neurosci. 2004;24(1):9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- 12.Prakash RS, et al. Physical activity associated with increased resting-state functional connectivity in multiple sclerosis. J Int Neuropsychol Soc. 2011;17(6):986–97. doi: 10.1017/S1355617711001093. [DOI] [PubMed] [Google Scholar]
- 13.Kalmijn S, et al. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62(2):275–80. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- 14.Dullemeijer C, et al. n 3 fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr. 2007;86(5):1479–85. doi: 10.1093/ajcn/86.5.1479. [DOI] [PubMed] [Google Scholar]
- 15.Tan ZS, et al. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78(9):658–64. doi: 10.1212/WNL.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conklin SM, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421(3):209–12. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 17.Pistell PJ, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219(1–2):25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oksman M, et al. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis. 2006;23(3):563–72. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 19.de Lorgeril M, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343(8911):1454–9. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 20.Rogers PJ, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421–31. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 21.Freund-Levi Y, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63(10):1402–8. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 22.Antypa N, et al. Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J Psychopharmacol. 2009;23(7):831–40. doi: 10.1177/0269881108092120. [DOI] [PubMed] [Google Scholar]
- 23.Stonehouse W, et al. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr. 2013;97(5):1134–43. doi: 10.3945/ajcn.112.053371. [DOI] [PubMed] [Google Scholar]
- 24.Fontani G, et al. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur J Clin Invest. 2005;35(11):691–9. doi: 10.1111/j.1365-2362.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 25.Chiu CC, et al. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry, 2008. 32(6):1538–44. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 26.van de Rest O, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430–8. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 27.Dangour AD, et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr. 2010;91(6):1725–32. doi: 10.3945/ajcn.2009.29121. [DOI] [PubMed] [Google Scholar]
- 28.Giltay EJ, et al. No effects of n-3 fatty acid supplementation on serum total testosterone levels in older men: the Alpha Omega Trial. Int J Androl. 2012;35(5):680–7. doi: 10.1111/j.1365-2605.2012.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourre JM. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging. 2004;8(3):163–74. [PubMed] [Google Scholar]
- 30.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9(7):568–78. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson PA, et al. No effect of 12 weeks' supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–35 years. Br J Nutr. 2012;107(8):1232–43. doi: 10.1017/S000711451100403X. [DOI] [PubMed] [Google Scholar]
- 32.Davis PF, et al. Dopamine receptor alterations in female rats with diet-induced decreased brain docosahexaenoic acid (DHA): interactions with reproductive status. Nutr Neurosci. 2010;13(4):161–9. doi: 10.1179/147683010X12611460764282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bousquet M, et al. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson's disease. FASEB J. 2008;22(4):1213–25. doi: 10.1096/fj.07-9677com. [DOI] [PubMed] [Google Scholar]
- 34.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson's disease with severe neurodegeneration. BMC Neurosci. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil Neural Repair. 2009;23(6):600–8. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- 36.Speelman AD, et al. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7(9):528–34. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- 37.Petzinger GM, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27(20):5291–300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouchi Y, et al. Changes in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in Parkinson's disease. Brain. 2001;124(Pt 4):784–92. doi: 10.1093/brain/124.4.784. [DOI] [PubMed] [Google Scholar]
- 39.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155(3):751–9. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuede CM, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2009;35(3):426–32. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30(1):121–9. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichol KE, et al. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadoglou NP, et al. Effects of exercise training on the severity and composition of atherosclerotic plaque in apoE-deficient mice. J Vasc Res. 2011;48(4):347–56. doi: 10.1159/000321174. [DOI] [PubMed] [Google Scholar]
- 44.Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol. 2009;5(3):140–52. doi: 10.1038/ncpneuro1044. [DOI] [PubMed] [Google Scholar]
- 45.Kiecolt-Glaser JK, et al. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26(6):988–95. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rangel-Huerta OD, et al. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107(Suppl 2):S159–70. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 47.Rana JS, et al. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. Eur Heart J. 2011;32(3):336–44. doi: 10.1093/eurheartj/ehp010. [DOI] [PubMed] [Google Scholar]
- 48.Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. 2010;9(9):703–18. doi: 10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- 49.Marsland AL, et al. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64(6):484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wersching H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74(13):1022–9. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 51.Bettcher BM, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26(1):103–8. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klingler M, Koletzko B. Novel methodologies for assessing omega-3 fatty acid status - a systematic review. Br J Nutr. 2012;107(Suppl 2):S53–63. doi: 10.1017/S0007114512001468. [DOI] [PubMed] [Google Scholar]
- 53.Wallis JG, Watts JL, Browse J. Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem Sci. 2002;27(9):467. doi: 10.1016/s0968-0004(02)02168-0. [DOI] [PubMed] [Google Scholar]
- 54.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20(1):5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- 55.Gómez-Pinilla F, Feng C. Molecular Mechanisms for the Ability of Exercise Supporting Cognitive Abilities and Counteracting Neurological Disorders. 2012:25–43. [Google Scholar]
- 56.Chytrova G, Ying Z, Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. 2010;1341:32–40. doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith PJ, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–52. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muldoon MF, et al. Serum phospholipid docosahexaenonic acid is associated with cognitive functioning during middle adulthood. J Nutr. 2010;140(4):848–53. doi: 10.3945/jn.109.119578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42(1):7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]
- 60.Feart C, et al. Adherence to a Mediterranean diet and plasma fatty acids: data from the Bordeaux sample of the Three-City study. Br J Nutr. 2011;106(1):149–58. doi: 10.1017/S0007114510005805. [DOI] [PubMed] [Google Scholar]
- 61.Holub BJ, et al. Correlation of omega-3 levels in serum phospholipid from 2053 human blood samples with key fatty acid ratios. Nutr J. 2009;8:58. doi: 10.1186/1475-2891-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez-Pinilla F. Collaborative effects of diet and exercise on cognitive enhancement. Nutr Health. 2011;20(3–4):165–9. doi: 10.1177/026010601102000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larrabee GJ, et al. Construct validity of various memory testing procedures. J Clin Exp Neuropsychol. 1985;7(3):239–50. doi: 10.1080/01688638508401257. [DOI] [PubMed] [Google Scholar]
- 64.Troster AI, et al. The diagnostic utility of savings scores: differentiating Alzheimer's and Huntington's diseases with the logical memory and visual reproduction tests. J Clin Exp Neuropsychol. 1993;15(5):773–88. doi: 10.1080/01688639308402595. [DOI] [PubMed] [Google Scholar]
- 65.Ainsworth BE, et al. Accuracy of the College Alumnus Physical Activity Questionnaire. J Clin Epidemiol. 1993;46(12):1403–11. doi: 10.1016/0895-4356(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 66.Nowak K, et al. Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies. Eur J Cardiothorac Surg. 2010;37(4):859–63. doi: 10.1016/j.ejcts.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 67.Shedd KM, et al. Quantifying leisure physical activity and its relation to bone density and strength. Med Sci Sports Exerc. 2007;39(12):2189–98. doi: 10.1249/mss.0b013e318155a7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choo J, et al. Longitudinal relationship between physical activity and cardiometabolic factors in overweight and obese adults. Eur J Appl Physiol. 2010;108(2):329–36. doi: 10.1007/s00421-009-1203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chomistek AK, et al. Vigorous physical activity, mediating biomarkers, and risk of myocardial infarction. Med Sci Sports Exerc. 2011;43(10):1884–90. doi: 10.1249/MSS.0b013e31821b4d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McFarlin BK, et al. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci. 2006;61(4):388–93. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- 71.Paffenbarger RS, Jr., Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 72.Feskanich D, et al. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. doi: 10.1016/0169-2607(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 73.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 74.Vivar C, Potter MC, van Praag H. All About Running: Synaptic Plasticity, Growth Factors and Adult Hippocampal Neurogenesis. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stranahan AM, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19(10):951–61. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacRae PG, et al. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology (Berl) 1987;92(2):236–40. doi: 10.1007/BF00177922. [DOI] [PubMed] [Google Scholar]
- 77.Knab AM, et al. Altered dopaminergic profiles: implications for the regulation of voluntary physical activity. Behav Brain Res. 2009;204(1):147–52. doi: 10.1016/j.bbr.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fisher BE, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77(3):378–90. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 79.Wipfli B, et al. An examination of serotonin and psychological variables in the relationship between exercise and mental health. Scand J Med Sci Sports. 2011;21(3):474–81. doi: 10.1111/j.1600-0838.2009.01049.x. [DOI] [PubMed] [Google Scholar]
- 80.Dey S, Singh RH, Dey PK. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav. 1992;52(6):1095–9. doi: 10.1016/0031-9384(92)90465-e. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka K, et al. Effects of Docosahexaenoic Acid on Neurotransmission. Biomol Ther (Seoul) 2012;20(2):152–157. doi: 10.4062/biomolther.2012.20.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cardoso HD, et al. Omega-3 deficiency and neurodegeneration in the substantia nigra: Involvement of increased nitric oxide production and reduced BDNF expression. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbagen.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 83.Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007;12(3):207–27. [PubMed] [Google Scholar]
- 84.Wu A, Ying Z, Gomez-Pinilla F. The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma. 2011;28(10):2113–22. doi: 10.1089/neu.2011.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matchynski JJ, et al. Combinatorial treatment of tart cherry extract and essential fatty acids reduces cognitive impairments and inflammation in the mu-p75 saporin-induced mouse model of Alzheimer's disease. J Med Food. 2013;16(4):288–95. doi: 10.1089/jmf.2012.0131. [DOI] [PubMed] [Google Scholar]
- 86.Gomez-Pinilla F, Tyagi E. Diet and cognition: interplay between cell metabolism and neuronal plasticity. Curr Opin Clin Nutr Metab Care. 2013;16(6):726–33. doi: 10.1097/MCO.0b013e328365aae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazereeuw G, et al. Effects of omega-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging. 2012;33(7):1482, e17–29. doi: 10.1016/j.neurobiolaging.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012;6:CD005379. doi: 10.1002/14651858.CD005379.pub3. [DOI] [PubMed] [Google Scholar]
- 89.Simpson K. Validity and reliability of the Paffenbarger Physical Activity Questionnaire among Healthy Adults. University of Connecticut. 2011 doi: 10.1123/jpah.2013-0013. [DOI] [PubMed] [Google Scholar]